Abstract
Median arcuate ligament syndrome is a clinical condition in which the median arcuate ligament causes compression and narrowing of the celiac artery. It has been reported that collateral pathways, which is developed by the decrease of blood flow from the celiac artery, facilitates the formation of aneurysms. Aneurysms around the pancreas in particular require aggressive therapeutic intervention, because a rupture can be fatal. We herein report two cases of pancreaticoduodenal aneurysms associated with median arcuate ligament syndrome treated by coil embolization and median arcuate ligament incision. Case 1 required a hybrid procedure in which median arcuate ligament incision and coil embolization were performed simultaneously. In Case 2, the median arcuate ligament incision was performed about 3 months after emergency endovascular hemostasis for hemorrhagic duodenal ulcer. In both cases, there were no major postoperative complications and no recurrence of aneurysm. Median arcuate ligament incision may be effective to prevent organ ischemia and aneurysm recurrence after coil embolization of intra-abdominal aneurysms associated with median arcuate ligament syndrome.
Keywords: Median arcuate ligament syndrome, Pancreaticoduodenal aneurysm, Coil embolization, Median arcuate ligament incision
Introduction
Median arcuate ligament syndrome (MALS) is a clinical condition in which the median arcuate ligament (MAL) causes compression and stenosis of the celiac artery [1], [2], [3], [4], [5]. It is presumed that decreased blood flow through the celiac artery in MALS lead to development of collateral pathways in the celiac artery-superior mesenteric artery arcade and formation of aneurysms. Pancreaticoduodenal aneurysm is particularly important because even as small as 2 mm aneurysm can rupture and be fatal [6], [7], [8]. Endovascular embolization has been recommended as the first choice for treatment of the aneurysms. While, there is currently no clear evidence whether invasive surgery for MAL incision should be performed for MALS with pancreaticoduodenal aneurysm [9]. In the current report, we present 2 cases of pancreaticoduodenal aneurysms with MALS treated by coil embolization and MAL incision.
Case presentation
Case 1
An abnormal mass in the pancreatic head was pointed out by health check in a 49-year-old woman without a significant previous medical history of any particular diseases. Contrast-enhanced computed tomography (CT) scan showed severe stenosis at the celiac artery, and 2 pancreaticoduodenal artery (PDA) aneurysms of 10 mm and 32 mm in diameter (Fig. 1). Based on the above, pancreaticoduodenal aneurysms with MALS were diagnosed. To prevent rupture of these aneurysms, endovascular embolization was planned. It was assumed that liver ischemia might occur after endovascular embolization of these aneurysms because hepatic arterial blood flow from the superior mesenteric artery (SMA) decreased after embolization. Wherein, catheter angiography was scheduled to evaluate blood flow and consider adequate treatment based on the results. Catheter angiography was performed using a 5.2-French balloon catheter (TERUMO CLINICAL SUPPLY CO., Ltd., Gifu, Japan) and a 4.2-French pigtail catheter (Goodman Co., Ltd., Aichi, Japan). Superior mesenteric arteriography and aortography are shown in Figure 2A and B. Aortography, performed with balloon occlusion of PDA from the caudal side of aneurysm, showed weak delineation of the celiac artery and its branches. As a result, it was indicated that hepatic arterial blood flow mostly depended on the SMA through the PDA. If aneurysm embolization in the PDA was performed, liver ischemia might have occurred. Therefore, hybrid surgery in which coil embolization of the aneurysm was planned after MAL incision to increase hepatic arterial blood flow from the celiac artery. In the laparotomy, the celiac artery was exposed up to the origin, and the MAL was released (Fig. 2C). The hepatic artery blood flow was evaluated by Doppler ultrasonography at the umbilical portion. Although the peak flow of the hepatic artery was 52.2 ± 3.0 cm/s without gastroduodenal artery (GDA) cramping, it decreased into 23.4 ± 3.2 cm/s with GDA cramping. After the MAL incision, it recovered up to 46.8 ± 7.7 cm/s with GDA cramping. Subsequently, coil embolization was started in the supine position, using the right femoral artery approach. The pancreaticoduodenal artery aneurysm was confirmed by superior mesenteric arteriography (Fig. 2D) using a 4-French catheter (Medikit Co., Ltd., Tokyo, Japan). A LANTERN Microcatheter (Penumbra, Alameda, CA) was then inserted into the vicinity of the aneurysm, and the 2 aneurysms were embolized using a total of 15 microcoils, one Penumbra occlusion device (POD) System (Penumbra) 6 mm × 50 cm and eight POD PACKING COIL (Penumbra) (5 × 60 cm, 3 × 45 cm) and 6 Ruby coil COMPLEX STANDARD (Penumbra) (3 × 28 mm × 60 cm, 3 × 24 mm × 60 cm). Superior mesenteric arteriography and celiac arteriography after coil embolization are shown in Figure 2E and F. After embolization of the aneurysm, the hepatic arterial blood flow was 54.5 ± 0.7 cm/s, which did not decrease comparing with that before embolization. The operation time was about 6 hours, and the amount of blood loss was approximately 5 mL. At 5 postoperative days (POD), CT revealed the dilation of the celiac artery and no findings of liver ischemia. The patient was uneventfully discharged at 6 POD. Recurrence of aneurysm have not been observed 20 months after surgery.
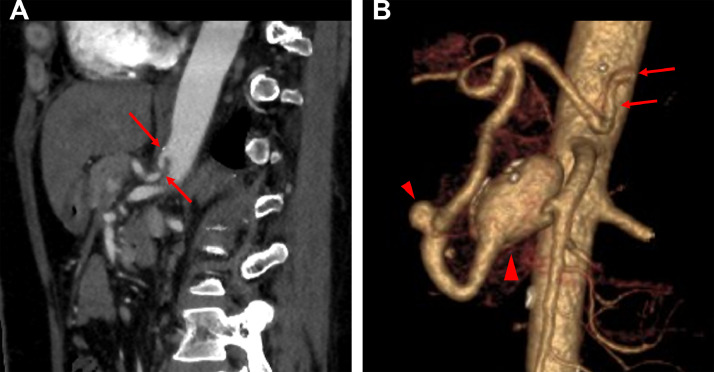
Fig. 1.
CT images of Case 1. (A, B) Sagittal contrast enhanced CT and volume rendering (VR) images show severe stenosis at the celiac artery (arrows). VR image also shows two aneurysms (arrowheads) in the PDA (10 mm, 32 mm).
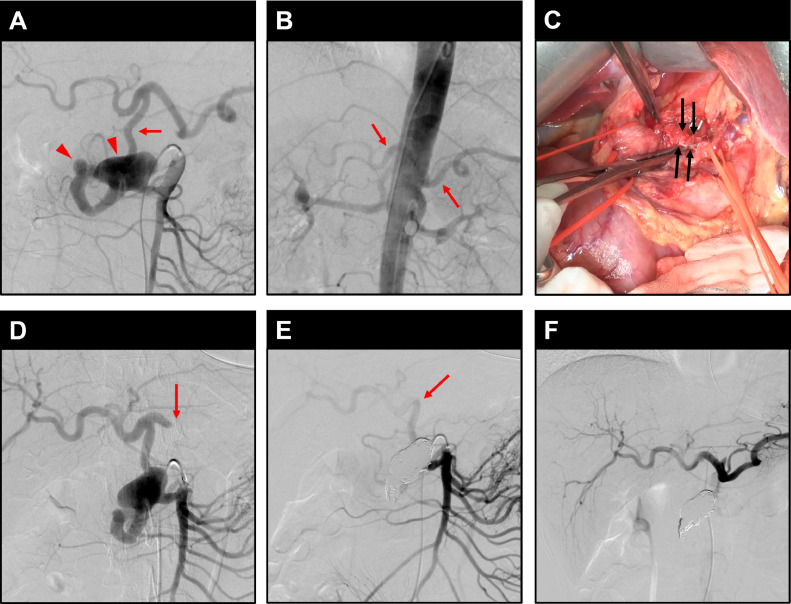
Fig. 2.
Angiography images of Case 1. (A) and (B) are images for evaluation of blood flow before hybrid surgery. (C) is a photograph of the post-MAL incision. (D)–(F) are images for endovascular embolization. (A) Angiography of the SMA shows two pancreaticoduodenal aneurysms (arrowheads) and a dilated PDA (arrow), through which blood flow to the liver and spleen was supplied. (B) Aortography with balloon blockage of the PDA shows a small branch of the celiac artery (arrows). (C) The MAL, which is a hard fibrous tissue continuous with the crura of the diaphragm, was identified and incised (arrows). (D) After MAL incision, superior mesenteric angiography shows that the distal splenic artery was no longer depicted (arrow) and the CHA was slightly thicker. (E) Superior mesenteric arteriography after embolization of aneurysm shows no visualized aneurysm. The CHA and part of the PDA are slightly delineated through other peripancreatic arcades (arrow). (F) After MAL incision and aneurysm embolization, the hepatic and splenic arteries can be clearly seen on celiac arteriography.
Case 2
The patient is a 58-year-old male with a history of hypertension and mild thrombocytopenia. Repeated hematemesis had occurred 10 days prior to transport to our hospital, and 2 days later, a bleeding duodenal ulcer was diagnosed by upper endoscopy, and emergency laparotomy was performed. Afterwards, the patient returned to the hospital because of melena. At that time, rebleeding from the duodenal ulcer was observed, and the patient was transferred to our hospital. Contrast-enhanced CT showed a stenosis at the celiac artery and a pancreaticoduodenal aneurysm near the duodenal ulcer, suggesting a pancreaticoduodenal aneurysm complicated by MALS and a hemorrhagic ulcer formed by its rupture or draining (Fig. 3). Emergency endovascular embolization was performed to treat the aneurysm. Embolization was performed in the supine position using the right femoral artery approach. Selection of the celiac artery was difficult due to stenosis, but we managed to select it with a 4-French catheter (Medikit Co). A Carnelian Si Microcatheter (Tokai Medical Products, Inc., Aichi, Japan) was inserted close to the pancreaticoduodenal aneurysm to confirm the blood vessels traveling around the aneurysm (Fig. 4A). The afferent and efferent arteries were included and embolized with a total of 10 microcoils; seven Target XL 360 Soft Coils (Stryker Japan K.K., Tokyo, Japan) (1 × 8 mm × 30 cm, 2 × 7 mm × 20 cm, 1 × 6 mm × 20 cm, 2 × 5 mm × 15 cm, 1 × 4 mm × 12 cm), and three Target XXL 360 coils (Stryker Japan K.K.) (1 × 14 mm × 50 cm, 1 × 12 mm × 45 cm, 1 × 10 mm × 40 cm; Fig. 4B). The procedure time was about 2 hours and 30 minutes, and blood loss was minimal. The proper hepatic artery was depicted by superior mesenteric angiography after embolization (Fig. 4C), therefore, we presumed that hepatic arterial blood flow was maintained to some extent and did not perform an emergency MAL incision. The patient underwent a MAL incision about 3 months after the embolization. In the laparotomy, the celiac artery was exposed to the aorta to identify the MAL, which was then released. Blood flow in the common hepatic artery (CHA) and splenic artery was evaluated by Doppler ultrasonography before and after the incision, and both showed improvement in blood flow after the incision. The operation time was about 4 hours, and the blood loss was 150 mL. Postoperatively, thrombocytopenia occurred but improved with a total of 20 units of platelet transfusion. No other major complications occurred, and the patient was discharged on the 9 POD. Recurrence of aneurysm have not been observed 13 months after surgery.
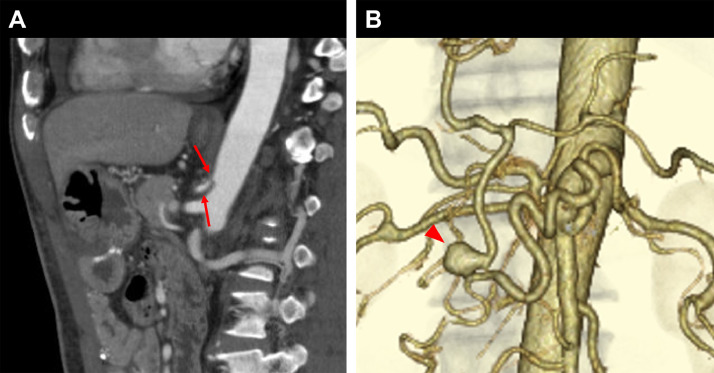
Fig. 3.
CT images of Case 2. (A) Sagittal contrast enhanced CT image shows stenosis at the celiac artery (arrows). (B) VR image shows an aneurysm (arrowhead) in the PDA (10 mm).
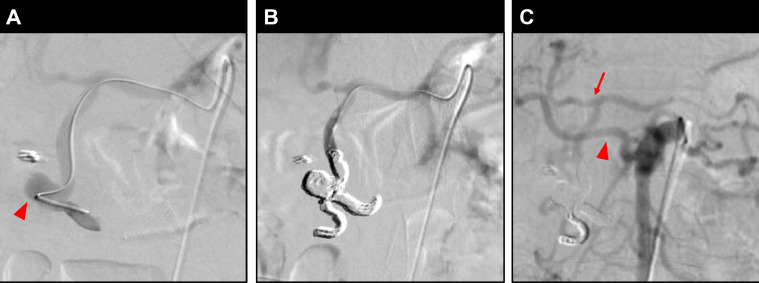
Fig. 4.
Angiography images of Case 2. (A) Angiography of a microcatheter inserted through the celiac artery and advanced to the distal part of the aneurysm revealed a 10-mm unruptured aneurysm (arrowhead). (B) Celiac arteriography after aneurysm embolization shows no visualized aneurysm. (C) Superior mesenteric arteriography after aneurysm embolization also shows no visualized aneurysm. The CHA and part of the PDA (arrow) are depicted through other peripancreatic arcades. In addition, the right hepatic artery (arrowhead) is bifurcated from the SMA.
Discussion
Harjola reported the first case of MALS in 1963, and Dunbar further described it as a clinical syndrome in 1965 [1], [2], [3], [4]. MAL is a fibrous tissue that connects the two legs of the diaphragm and is generally located at the level of the 12th thoracic vertebra. Typically, the celiac artery branches off the abdominal aorta between the 11th thoracic vertebra and the 1st lumbar vertebra, but it is known that the branching position varies greatly depending on the person [5,10]. Therefore, the celiac artery and MAL sometimes overlap. MALS is a disease in which the celiac artery is compressed by MAL, resulting in stenosis and various symptoms, and is broadly defined to include people without subjective symptoms [6]. These symptoms are thought to be caused by stimulation of the celiac plexus or organ ischemia due to celiac artery stenosis [1,[9], [10], [11]]. The frequency of symptomatic patients is about 0.4% of the total population; 1.76%-8% if asymptomatic patients are included. It has been reported that 87% of patients with MALS are asymptomatic, and MALS is more common in females than in males, with a male to female ratio of 1:6 [1,6,9,10,12]. Regarding the relationship between MALS and pancreaticoduodenal aneurysms, Sutton and Lawton first suggested in 1973 that occlusion or stenosis of the celiac artery is the basic cause of true pancreaticoduodenal aneurysms [7,13]. In 2006, Murata et al reported that stenosis of the celiac artery increased blood flow in the pancreaticoduodenal arcade and developed collateral pathways, and that the stress caused by the subsequent chronic increase in blood flow makes the arterial wall vulnerable, resulting in the formation of an aneurysm [14]. Pancreaticoduodenal aneurysms (excluding pseudoaneurysms due to inflammation, etc.) account for about 2% of all visceral aneurysms, but it has been reported that 50%-74% of these aneurysms coexist with stenosis or occlusion of the celiac artery, such as MALS [10]. There is also a close relationship between the development of the collateral tract of the celiac artery-superior mesenteric artery arcade and aneurysm formation due to MALS. Heo et al reported that among patients with MALS, 46%-80% had significant collateral tract development and 24% had aneurysm formation. In this report, collateral tracts were also found in the CHA and left gastric artery, and the peripancreatic vascular network including the PDA was the most frequent at 82.4% [6]. Pancreaticoduodenal aneurysms are associated with a high risk of rupture regardless of size, and therapeutic intervention should be considered promptly after diagnosis. In recent years, endovascular aneurysm embolization has become the first choice of treatment because it is minimally invasive compared to surgery and allows simultaneous diagnosis and treatment [6], [7], [8]. In the presence of celiac artery stenosis due to MALS, revascularization procedures such as MAL incision, bypass, or stenting should be considered. In Case 1, it was desirable not only to embolize the aneurysm but also to reduce the risk of recurrence as much as possible, given the patient's young age. However, angiography before embolization showed that hepatic artery blood flow was expected to be supplied from the SMA through the peripancreatic vascular network, and the risk of organ ischemia due to aneurysm embolization was high. Therefore, we performed a hybrid treatment of simultaneous coil embolization and MAL incision, and as a result, the operation was completed without organ ischemia. In Case 2, endovascular embolization was performed urgently because of a bleeding ulcer caused by compression of the aneurysm. Then no immediate additional surgical treatment was performed because blood flow from the celiac artery was maintained to some extent before and after the embolization. However, during the follow-up, a MAL incision was performed to prevent recurrence of the aneurysm. In both cases, the postoperative course was uneventful, and no recurrence of aneurysm was observed in Case 1 or Case 2 after follow-up periods of 20 months and 13 months, respectively. As for the effect of MAL incision on preventing recurrence, we expect that the risk of recurrence was reduced due to the reduction of hemodynamic stress, but some studies have reported that the risk of aneurysm recurrence remains the same even without hemodynamic modification. [9] Therefore, further study into this effect is required. It is necessary to continue to follow up on the patients in the current report in case of aneurysm recurrence.
Conclusion
We presented 2 cases of pancreaticoduodenal aneurysms with MALS treated with coil embolization and MAL incision. Our results suggest that MAL incision was effective in preventing organ ischemia after coil embolization. The MAL incision may also be effective in reducing the risk of aneurysm recurrence due to the reduction of hemodynamic stress, but there is still insufficient evidence for this, and further investigation is needed.
Patient consent statement
Informed consent has been obtained from the patients for the publication of this case report.
Footnotes
Competing Interests: The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Jimenez JC, Harlander-Locke M, Dutson EP. Open and laparoscopic treatment of median arcuate ligament syndrome. J Vasc Surg. 2012;56:869–873. doi: 10.1016/J.JVS.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 2.Kim EN, Lamb K, Relles D, Moudgill N, DiMuzio PJ, Eisenberg JA. Median arcuate ligament syndrome - review of this rare disease. JAMA Surg. 2016;151:471–477. doi: 10.1001/jamasurg.2016.0002. [DOI] [PubMed] [Google Scholar]
- 3.Harjola PT. A rare obstruction of the coeliac artery. Report of a case. Ann Chir Gynaecol Fenn. 1963;52:547–550. [PubMed] [Google Scholar]
- 4.Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol. 1965;95:731–744. doi: 10.2214/AJR.95.3.731. [DOI] [PubMed] [Google Scholar]
- 5.Loukas M, Pinyard J, Vaid S, Kinsella C, Tariq A, Tubbs RS. Clinical anatomy of celiac artery compression syndrome: a review. Clin Anat. 2007;20:612–617. doi: 10.1002/CA.20473. [DOI] [PubMed] [Google Scholar]
- 6.Heo S, Kim HJ, Kim B, Lee JH, Kim J, Kim JK. Clinical impact of collateral circulation in patients with median arcuate ligament syndrome. Diagn Interv Radiol. 2018;24:181–186. doi: 10.5152/dir.2018.17514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chivot C, Rebibo L, Robert B, Regimbeau JM, Yzet T. Ruptured pancreaticoduodenal artery aneurysms associated with celiac stenosis caused by the median arcuate ligament: a poorly known etiology of acute abdominal pain. Eur J Vasc Endovasc Surg. 2016;51:295–301. doi: 10.1016/j.ejvs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 8.Bonardelli S, Spampinato B, Ravanelli M, Cuomo R, Zanotti C, Paro B, et al. The role of emergency presentation and revascularization in aneurysms of the peripancreatic arteries secondary to celiac trunk or superior mesenteric artery occlusion. J Vasc Surg. 2020;72:46S–55S. doi: 10.1016/j.jvs.2019.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Dyches RP, Eaton KJ, Smith HF. The roles of celiac trunk angle and vertebral origin in median arcuate ligament syndrome. Diagnostics. 2020;10 doi: 10.3390/diagnostics10020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaszczewski P, Leszczyński J, Elwertowski M, Maciąg R, Chudziński W, Gałązka Z. Combined treatment of multiple splanchnic artery aneurysms secondary to median arcuate ligament syndrome: a case study and review of the literature. Am J Case Rep. 2020;21:1–6. doi: 10.12659/AJCR.926074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rongies-Kosmol M, Jakimowicz T. Celiac artery compression syndrome. Mini-review. Acta Angiol. 2015;21:21–24. doi: 10.5603/AA.2015.0005. [DOI] [Google Scholar]
- 12.Baskan O, Kaya E, Gungoren FZ, Erol C. Compression of the celiac artery by the median arcuate ligament: multidetector computed tomography findings and characteristics. Can Assoc Radiol J. 2015;66:272–276. doi: 10.1016/j.carj.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Sutton D, Lawton G. Coeliac stenosis or occlusion with aneurysm of the collateral supply. Clin Radiol. 1973;24:49–53. doi: 10.1016/S0009-9260(73)80114-X. [DOI] [PubMed] [Google Scholar]
- 14.Murata S, Tajima H, Fukunaga T, Abe Y, Niggemann P, Onozawa S, et al. Management of Pancreaticoduodenal Artery Aneurysms: Results of Superselective Transcatheter Embolization. Am J Roentgenol. 2006;187:290–298. doi: 10.2214/AJR.04.1726. [DOI] [PubMed] [Google Scholar]