Abstract
Epilepsy is a common and severe brain disease affecting >65 million people worldwide. Recent studies have shown that kinesin superfamily motor protein 17 (KIF17) is expressed in neurons and is involved in regulating the dendrite-targeted transport of N-methyl-D-aspartate receptor subtype 2B (NR2B). However, the effect of KIF17 on epileptic seizures remains to be explored. We found that KIF17 was mainly expressed in neurons and that its expression was increased in epileptic brain tissue. In the kainic acid (KA)-induced epilepsy mouse model, KIF17 overexpression increased the severity of epileptic activity, whereas KIF17 knockdown had the opposite effect. In electrophysiological tests, KIF17 regulated excitatory synaptic transmission, potentially due to KIF17-mediated NR2B membrane expression. In addition, this report provides the first demonstration that KIF17 is modified by SUMOylation (SUMO, small ubiquitin-like modifier), which plays a vital role in the stabilization and maintenance of KIF17 in epilepsy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-022-00888-9.
Keywords: Epilepsy, KIF17, NR2B, SUMOylation
Introduction
Epilepsy is a common and severe brain disorder characterized by recurrent seizures that affects >65 million people worldwide [1–3]. Structural, genetic, infectious, metabolic, immune, and unknown etiologies may contribute to epileptic seizures [2], and dozens of antiepileptic drugs have been used in the clinic. Nevertheless, approximately one-third of patients with epilepsy develop intractable epilepsy, a new modality that needs to be explored, but its pathogenesis remains unclear [3]. However, the synchronous abnormal discharge of brain neurons caused by an imbalance between excitatory and inhibitory conductance is certainly a common mechanism in various types of epileptic seizures [4]. Therefore, an understanding of the mechanism regulating this balance is needed to explore new antiepileptic targets.
The N-methyl-D-aspartate receptor (NMDAR), an ionotropic glutamate receptor, plays a vital role in excitatory neurotransmission in the central nervous system [5]. Specifically, NMDARs, which are highly permeable to Ca2+ and Ca2+ influx, are critical for synaptogenesis, synaptic plasticity, learning, and memory [6]. The abnormal expression, transport, and synaptic input of NMDAR subunits affect normal physiological functions and constitute important pathophysiological mechanisms in Alzheimer's disease, epilepsy, and other neurological diseases [7, 8]. The NMDAR is a heterotetramer consisting of two types of subunits, either GluN1 and GluN2 or GluN2 and GluN3 [5]. Among these subunits, NR2B (one of the forms of GluN2) is expressed at high levels in the cortex and hippocampus and is one of the most important subunits, and newly-synthesized NR2B must be transported to dendrites and localized to synapses before playing a physiological role [9]. In addition, multiple mutations in the GRIN2B (glutamate ionotropic receptor NMDA type subunit 2B) gene have been linked to epilepsy [10, 11]. The kinesin superfamily of proteins (KIFs) are expressed at high levels in mammalian neurons and transport organelles, protein complexes, and mRNA to specific locations along microtubules by hydrolyzing adenine triphosphate [12]. In the nervous system, KIF17, a member of the kinesin superfamily, interacts with and transports dendritic proteins, including the K+ channel Kv4.2, the kainate receptor, and NR2B, along microtubules to their dendritic destinations [13–15]. In particular, NR2B is the main neuronal cargo of KIF17, and deletion of the Kif17 gene in mice inhibits NR2B transport and reduces the availability of synaptic NR2B [16]. In contrast, transgenic mice overexpressing Kif17 exhibit up-regulated hippocampal NR2B protein levels and improved spatial learning and memory [17]. Given this circumstantial evidence, we aimed to investigate the role of KIF17 in seizures, which has not been studied to date.
SUMOylation, a post-translational modification, regulates the location, stability, and activity of target proteins by covalently attaching the small ubiquitin-like modifier (SUMO) protein SUMO1, SUMO2, or SUMO3 to a lysine residue of target proteins [18, 19]. In addition, this process is mediated by an enzymatic cascade consisting of the activating enzyme E1, the conjugating enzyme E2, and the ligating enzyme E3 [19]. The importance of SUMOylation for neuronal development, the neuronal stress response, synaptic transmission, and plasticity has been widely described [20, 21], and SUMOylation of the neuronal K+ channel Kv7, synapsin Ia, and other proteins has revealed a relationship between SUMOylation and epilepsy [22, 23].
In this study, we determined the distribution of KIF17 in epileptic brain tissue and its subcellular localization. The expression of KIF17 in the brain tissue of patients with epilepsy and animal models was assessed. Interfering with the endogenous expression of KIF17 using a lentivirus clarified the effect of KIF17 on epileptic behavior and electrophysiology. We also applied dendritic spine detection and immunoprecipitation to explore the potential mechanisms through which KIF17 modulates epileptic seizures. And we further investigated the role of SUMOylation of KIF17 in epilepsy. In conclusion, our experiments revealed the distribution and subcellular localization of KIF17 and explored its potential regulatory mechanism in epileptic seizures.
Materials and Methods
Epilepsy Model, Drug Administration, and Local Field Potential (LFP) Recordings
Eight-week-old male C57BL/6 mice (20 g –30 g) from the Laboratory Animal Center of Chongqing Medical University were reared in a standard environment with a 12/12-h light/dark cycle, a temperature of 22°C, and free access to water and food.
The chronic spontaneous epilepsy model was induced through an intrahippocampal injection of kainic acid (KA). In the viral intervention experiments, epileptic models were established 1 month after viral injection. Briefly, mice were anesthetized with 1% pentobarbital and fixed on a stereotaxic apparatus (RWD Life Science Co., Ltd., Shenzhen, China). Then, 1.0 nmol of KA (Sigma–Aldrich, St. Louis, USA) dissolved in 50 nL of saline was injected into the unilateral hippocampus (anteroposterior, −1.6 mm; mediolateral, −1.5 mm; dorsoventral, −1.5 mm) at 100 nL/min. Two hours after the injection, non-convulsive status epilepticus (SE) was terminated with diazepam, and the mice were then subjected to video surveillance for behavioral tests for one month. In addition, as previously described [24], LFPs were recorded one month after SE.
An acute seizure model was induced by intraperitoneal administration of KA at 25 mg/kg, and the severity of the seizures was assessed using the modified Racine scale within 1 h after KA injection [25].
For SUMOylation inhibitor administration, the mice received bilateral intrahippocampal injections of 2-D08 (Sigma–Aldrich; SML1052) or vehicle (0.5% dimethyl sulfoxide) at 1 days and 14 days after SE. The doses were based on a previous study [26].
Lentiviral Vector Construction and Intrahippocampal Virus Injections
A short hairpin RNA (shRNA) with the targeting sequence 5′-CGCAGACAACAATTACGAT-3′ directed against Kif17 (GeneChem, Shanghai, China) was carried by a lentiviral vector (GV248: hU6-MCS-Ubiquitin-EGFP-IRES-puromycin), designated LV-sh-KIF17, and used to reduce the endogenous hippocampal KIF17 level. The lentiviral vector (GV358: Ubi-MCS-3FLAG-SV40-EGFP-IRES-puromycin) containing the whole Kif17 complementary DNA (cDNA) sequence is designated LV-KIF17 hereafter. The control vectors Con-LV-KIF17 and Con-shRNA were constructed with the same promoters used for LV-KIF17 and LV-sh-KIF17, respectively.
Mice were fixed on a stereotaxic apparatus after anesthesia. Using a 5-μL microsyringe (Hamilton, Reno, USA), 2 μL of virus particles was injected into the bilateral hippocampus (anteroposterior, −1.6 mm; mediolateral, ±1.5 mm; dorsoventral, −1.5 mm) at 200 nL/min, and the needle was left in place for another 15 min.
Whole-Cell Patch-Clamp Recordings
Whole-cell patch-clamp recordings were made as previously described [27]. After anesthetization with 1% pentobarbital sodium, male C57BL/6 mice were sacrificed for the preparation of brain slices. Transverse hippocampal slices (400 μm) were cut in a cold sterile slice solution [in mmol/L: 2 MgCl2, 2 CaCl2, 2.5 KCl, 1.25 KH2PO4, 26 NaHCO3, 220 sucrose, and 10 glucose (pH 7.4) bubbled with 95% O2/5% CO2] (Leica VT1200S; Nussloch, Germany). The slices were allowed to recover in a storage chamber containing Mg2+-free artificial cerebrospinal fluid [ACSF (in mmol/L); 2.5 KCl, 125 NaCl, 2 CaCl2, 1.25 KH2PO4, 26 NaHCO3, and 25 glucose (pH 7.4) bubbled with 95% O2/5% CO2] at 34°C for 1 h. The slices were fully submerged in flowing Mg2+-free ACSF (4 mL/min) at room temperature (RT) for the recordings.
To record spontaneous action potentials (sAPs), glass pipette electrodes were filled with an internal solution of the following composition (in mmol/L): 60 K2SO4, 40 HEPES, 4 MgCl2·6H2O, 60 N-methyl-D-glucamine, 0.5 BAPTA, 12 phosphocreatine, 2 Na2ATP, and 0.2 Na3GTP. Neuronal sAPs were recorded at the resting membrane potential with a whole-cell patch-clamp in current-clamp mode.
Excitatory postsynaptic currents (EPSCs) were recorded as the membrane potential was held at −70 mV in voltage-clamp mode using glass pipette electrodes filled with an internal solution of the following composition (in mmol/L): 10 CsCl2, 130 CsMeSO4, 4 NaCl, 1 MgCl2, 10 HEPES, 1 EGTA, 5 MgATP, 0.5 Na3GTP, 12 phosphocreatine, and 5 NMG (pH 7.4) at 280–290 mOsm. Miniature EPSCs (mEPSCs) were recorded with 1 μmol/L tetrodotoxin (TTX), and 100 μmol/L picrotoxin (PTX) was added to the internal solution to block the γ-aminobutyric acid type A receptor. In addition, spontaneous EPSCs (sEPSCs) were measured in the presence of 100 μmol/L PTX.
For micro-inhibitory postsynaptic current (mIPSC) recordings, glass pipette electrodes were filled with the following internal solution (in mmol/L): 1 MgCl2, 1 EGTA, 100 CsCl, 10 HEPES, 5 MgATP, 0.5 Na3GTP, 12 phosphocreatine, and 30 N-methyl-D-glucamine (NMG) (pH 7.4) at 280 mOsm–290 mOsm. The mIPSCs were recorded in the presence of 20 μmol/L 6,7-dinitroquinoxaline-2,3(1H,4H)-dione, 50 μmol/L D-2-amino-5-phosphonovaleric acid, and 1 μmol/L TTX in voltage-clamp mode at a holding potential of −70 mV.
For recordings of the paired-pulse ratio (PPR), the membrane potential was held at −70 mV in the presence of 100 μmol/L PTX. The interval between paired stimuli was 50 ms. The PPRs were measured as the ratio of the second peak amplitude to the first peak amplitude.
Immunofluorescence Staining
Frozen sections were prepared using previously reported methods [28]. The sections were equilibrated to RT, and antigens were retrieved with sodium citrate restoration solution. The samples were then treated with phosphate-buffered saline (PBS) containing 0.4% Triton X-100 for 10 min and blocked with 10% goat serum in PBS for 30 min. For cellular immunofluorescence staining, primary neurons on day 18 in vitro (DIV 18) were fixed in PBS containing 4% paraformaldehyde and 4% sucrose for 30 min at 37°C. After permeabilization with 0.3% Triton X-100 in PBS for 30 min at 37°C, the coverslips were treated with 10% goat serum for 1 h. The samples were incubated overnight at 4°C with the following primary antibodies: anti-KIF17 (Proteintech, Wuhan, China; Cat No. 14615-1-AP), anti-GFAP (Abcam, Cambridge, UK; ab4648), anti-NeuN (Millipore, Billerica, USA; NBP192693PE), anti-VGLUT1 (Santa Cruz, Texas, USA; sc-377425), anti-GAD67 (Abcam; ab26116), anti-PSD95 (Santa Cruz; sc-32290), anti-NR2B (Proteintech; Cat No. 21920-1-AP), and anti-GFP (Proteintech; Cat No. 50430-2-AP). The next day, the samples were washed three times with PBS and incubated with fluorophore-labeled secondary antibodies [DyLight 488, goat anti-rabbit IgG (Abbkine, Wuhan, China; A23220), DyLight 594, goat anti-mouse IgG (Abbkine; A23410), or DyLight 405, goat anti-rabbit IgG (Abbkine; A23120)] at RT for 1 h. The images were captured with a laser-scanning confocal microscope (Zeiss, Oberkochen, Germany) and analyzed using ZEN (Oberkochen, Germany) or ImageJ software (Rawak Software, Inc., Germany) for the assessment of co-localization. The spines were analyzed using ImageJ software.
Human Brain Tissue Collection
Briefly, seven cortical specimens from patients with temporal lobe epilepsy (TLE) and seven cortical specimens from patients with head trauma were collected from the First Affiliated Hospital of Chongqing Medical University and reviewed by two neuropathologists. Specimen collection was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University, and informed consent forms were signed by the patients or their immediate family members. All patients with TLE met the International League Against Epilepsy 2001 seizure classification and diagnostic criteria [29]. Table S1 summarizes the clinical characteristics of the patients and controls.
Western Blotting
Mouse hippocampal and cortical tissue, human brain specimens, or primary neurons were lysed in radioimmunoprecipitation assay lysis buffer containing a protease inhibitor cocktail (MCE; New Jersey, USA; Cat. No.: HY-K0010) and centrifuged at 16,000×g and 4°C for 15 min to obtain total protein. Following the manufacturer’s instructions (SM-005; Invent Biotechnologies, Minnesota, USA), mouse hippocampi were used for membrane protein extraction. Proteins were separated on SDS–PAGE gels (6% or 8%) and transferred to 0.45-μm polyvinylidene difluoride membranes (Millipore). The membranes were subsequently blocked with 5% nonfat dry milk at RT for 1 h and then incubated with primary antibodies overnight at 4°C. After three 5-min washes with Tris-buffered saline containing 0.15% Tween-20 (TBST), the membrane was incubated with a peroxidase-conjugated secondary antibody (Proteintech, Cat No. SA00001-2) at RT for 1 h and then subjected to three 5-min washes with TBST. The Fusion Imaging System was used for imaging. The following primary antibodies were used: rabbit anti-KIF17 (1:1000; Proteintech; Cat No. 14615-1-AP), anti-NR2B (Proteintech; Cat No. 21920-1-AP), anti-SUMO1 (1:1000; Proteintech; Cat No. 10329-1-AP), anti-ATP1A1 (Proteintech; Cat No. 14418-1-AP), and anti-GAPDH (1:5000; Proteintech; Cat No. 10494-1-AP).
Immunoprecipitation
Bilateral mouse hippocampal tissue were used for immunoprecipitation. The tissue lysates were first incubated with 30 µL of Protein A/G magnetic beads (MCE; Cat. No.: HY-K0202) at 4°C for 2 h to reduce nonspecific binding. Subsequently, 0.5 µg of rabbit IgG, 2 µg of the KIF17 antibody (Proteintech; Cat No. 14615-1-AP), 2 µg of the NMDAR2B antibody (Proteintech; Cat No. 21920-1-AP), or 2 µg of the SUMO1 antibody (Proteintech; Cat No. 10329-1-AP) were incubated with 40 μL of Protein A/G magnetic beads at 4°C for 2 h and washed four times with PBS containing 0.05% Triton X-100 (0.05% PBST). The pretreated protein sample was then mixed with the antibody-protein A/G magnetic bead complex, incubated at 4°C for 2 h, and washed four times with 0.05% PBST. The sample protein was mixed with 1× loading buffer and heated at 95°C for 10 min for denaturation and elution; after magnetic separation, the protein samples were collected for Western blot analysis.
Primary Neuron Culture
The day before the experiment, the coverslips were coated with a poly-L-lysine solution, washed with sterilized deionized water, and dried before use. The brains of C57BL/6 mice (P0–P1) were dissected, and the tissue was mechanically minced and treated with 0.25% trypsin (37°C, 5% CO2) for 10 min. After centrifugation at 1000 r/min for 5 min, the cells were re-suspended in DMEM (Dulbecco’s Modified Eagle Medium)/F12 containing 10% fetal bovine serum and 1% penicillin/streptomycin and counted with a blood cytometer. The cells were plated in 6-well plates at 50 × 104 cells/well for dendritic spine counting or 100 × 104 cells/well for Western blotting and qRT-PCR (quantitative real-time PCR). After incubation under 5% CO2 at 37°C for 4 h, the plating medium was replaced by Neurobasal maintenance medium supplemented with 2% B27, 1% penicillin/streptomycin, and 2 mmol/L glutamine. Half of the medium was replaced with fresh medium every 2 days after plating.
On day 3, the culture medium was replaced with the maintenance medium containing LV-sh-KIF17 or the corresponding vectors. Forty-eight hours after infection, the medium was replaced with fresh medium. The cells were prepared for immunofluorescence staining at DIV 18.
On day 6, the neurons were treated with 0.1% dimethyl sulfoxide or the SUMOylation inhibitor 2-D08 for 24 h based on previous research [30], and the cells were collected for Western blotting or qRT–PCR on DIV 7.
Reverse Transcription–quantitative RT–PCR
Total RNA was extracted from 2-D08-treated primary neurons using an RNAsimple Total RNA Kit (Tiangen; #DP419; Beijing, China), and the RNA was reverse-transcribed into cDNA according to the manufacturer’s instructions (Vazyme; R223-01; Nanjing, China). qRT-PCR was applied with a ChamQ SYBR qPCR Master Mix (Vazyme; Q341-02) kit for 40 cycles (95°C for 5 s and 55°C for 30 s). The following primers were used:
Gapdh-forward: GGTTGTCTCCTGCGACTTCA
Reverse: TGGTCCAGGGTTTCTTACTCC
Kif17-forward: ATCAAGAACAAGCCACGCATTA
Reverse: TCAGCCACAGCCACATCAG
Statistical Analyses
Student’s t-test was used for comparisons between two groups, and one-way analysis of variance (ANOVA) was used for comparisons among four groups. Statistical analyses were performed using GraphPad Prism software (version 8.0; GraphPad Software Inc., San Diego, USA). Differences (mean ± SEM) were considered statistically significant if P < 0.05.
Ethical Approval and Consent to Participate
The study protocol involving human subjects was approved by the Medical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. All animal procedures were approved by the Medical Ethics Committee of Chongqing Medical University and were conducted under the guidelines of Animal Research: Reporting In Vivo Experiments.
Results
KIF17 Distribution in the Epileptic Brain
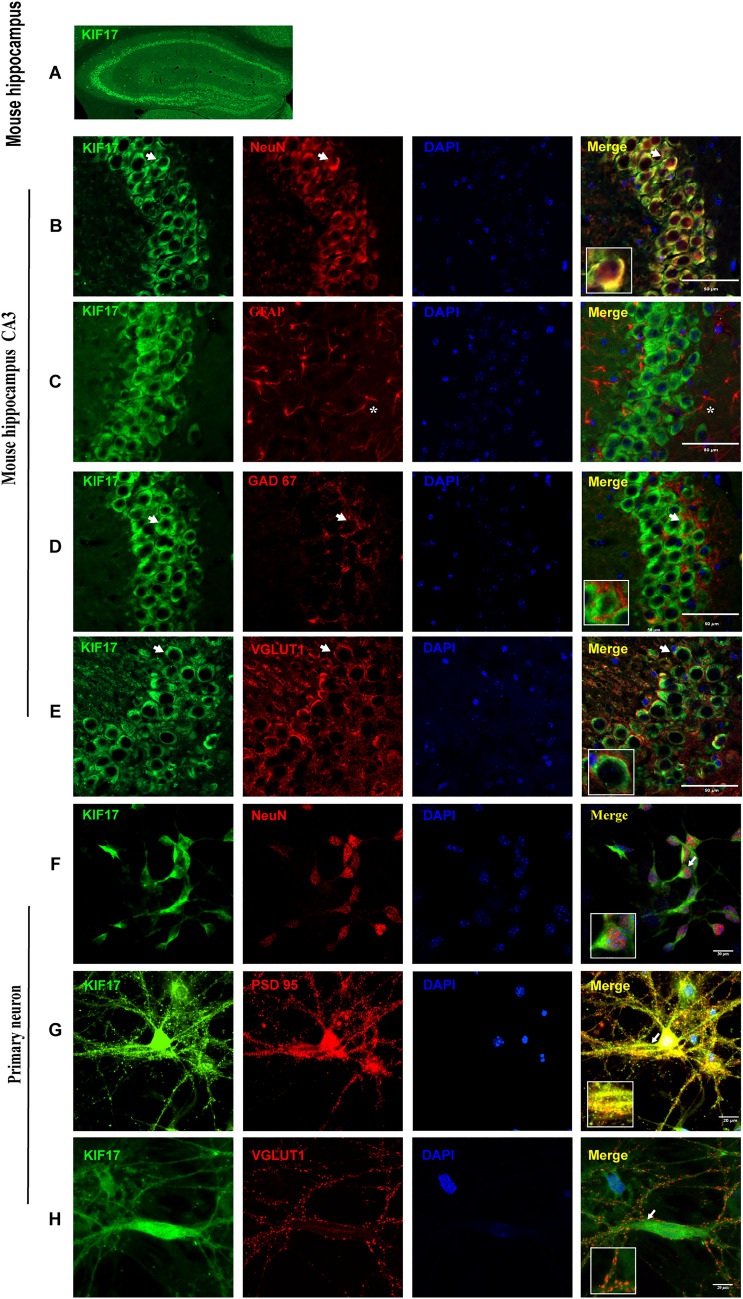
Previous studies have suggested that KIF17 is expressed in neurons [13–15], but its distribution in the hippocampus, which is closely associated with epilepsy, has not been reported. We defined the distribution of KIF17 in a KA-induced epilepsy model through immunofluorescence staining. We found that KIF17 was expressed at high levels in the pyramidal cell layer and the dentate gyrus (Fig. 1A). Furthermore, KA-treated and control mice were subjected to immunofluorescence analysis to characterize the cellular localization of KIF17. Specifically, in both epileptic and control hippocampi, KIF17 mainly co-localized with neuron-specific nucleoprotein (NeuN; a neuronal marker) and rarely with glial fibrillary acidic protein (GFAP; an astrocyte marker) (Figs 1B, C, and S1A, B). KIF17 co-localized with glutamate decarboxylase 67 (GAD67; a GABAergic neuronal marker) and vesicular glutamate transporter 1 (VGLUT1; a glutamate neuronal marker) (Figs 1D, E and S1C, D). The results from the immunofluorescence analysis of the cortex of the control and epileptic mice were consistent with those of the hippocampus (Figs. S2 and S3). We assessed the expression of KIF17 in cultured primary neurons to explore its subcellular localization. As shown in Fig. 1F, KIF17 co-localized with the neuronal marker NeuN. In addition, the staining results showed that KIF17 mostly co-localized with postsynaptic density protein 95 (PSD95; a marker of postsynaptic specialization) and exhibited limited co-localization with VGLUT1 (Fig. 1G, H). The immunofluorescence results revealed that KIF17 is mainly expressed in neurons, including excitatory glutamatergic neurons and GABAergic neurons, particularly in their postsynaptic area.
Fig. 1.
Localization of KIF17 in epileptic mouse brain and subcellular localization. A Distribution of KIF17 in the mouse hippocampus. B, C KIF17 co-localizes with NeuN but rarely with GFAP (*astrocytes). D, E KIF17 co-localizes with GAD67 and VGLUT1. Scale bars, 50 μm. F–H In neurons at DIV 18, KIF17 co-localizes with NeuN (F) and PSD95 (G) but not with VGLUT1 (H). Scale bars, 20 μm (arrows indicate positive cells).
Increased Expression of KIF17 in the Epileptic Brain
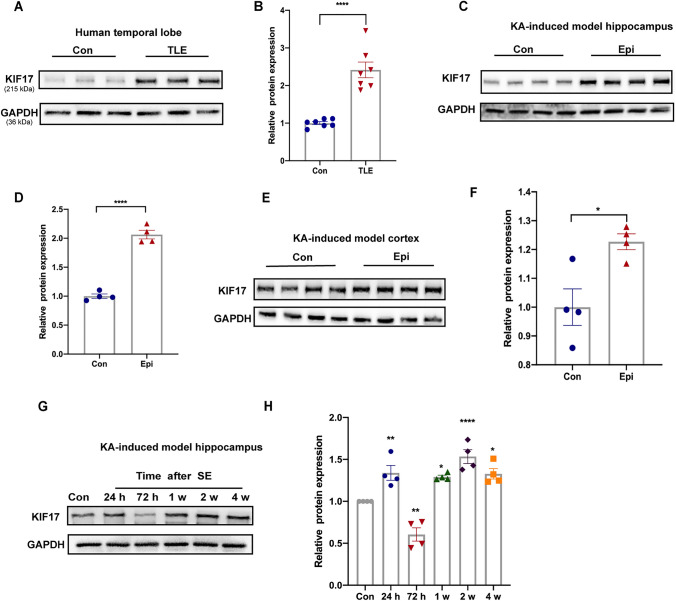
We investigated the level of the KIF17 protein in individuals with epilepsy to further confirm the changes in KIF17 level in epilepsy. We first compared the expression of KIF17 in temporal lobe tissue from patients with TLE and controls. Western blot analysis showed increased expression of KIF17 in patients with TLE compared with controls (Fig. 2A, B). To further confirm the trend of KIF17 in epilepsy, we assessed the KIF17 protein level in a mouse model of KA-induced chronic spontaneous epilepsy. KIF17 expression was increased in the cortex and particularly the hippocampus of mice with KA-induced epilepsy (Fig. 2C–F). Considering the significant change in KIF17 levels in the epileptic hippocampus, we subsequently examined the dynamic change in KIF17 levels in the hippocampus during epilepsy. Compared with the control group, the level of the KIF17 protein began to increase on the first day after SE and reached a peak on day 14 (Fig. 2G, H). However, KIF17 expression showed a decreasing trend on day 3 after SE (Fig. 2G, H). Taken together, the results indicated that KIF17 expression is significantly increased in subjects with chronic spontaneous epilepsy and show the importance of KIF17 in the development of epilepsy.
Fig. 2.
KIF17 expression in brain tissue from patients and mice with epilepsy. A, B KIF17 protein is increased in the brain tissue from patients with TLE (n = 7). C–F Representative Western blot images and statistics of KIF17 expression in the hippocampus and cortex of epileptic (Epi) and control (Con) mice. G, H Changes in KIF17 expression over time during epilepsy (n = 4; w, week). Data are presented as the mean ± SEM, *P < 0.05, **P < 0.01, ****P < 0.0001, independent Student’s t-test.
KIF17 Modulates Epileptic Seizure Activity
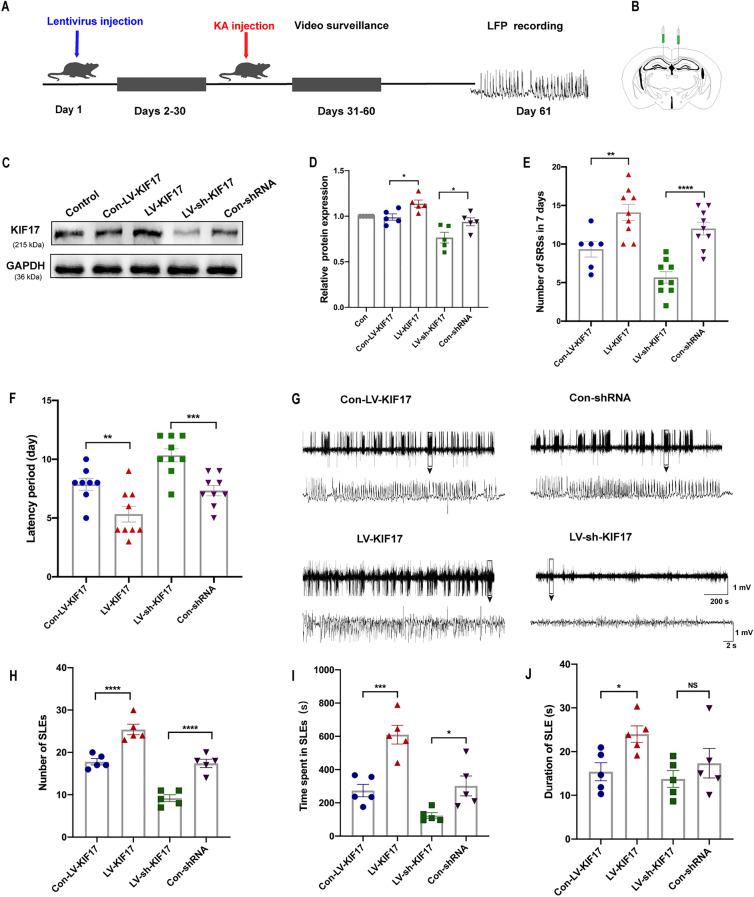
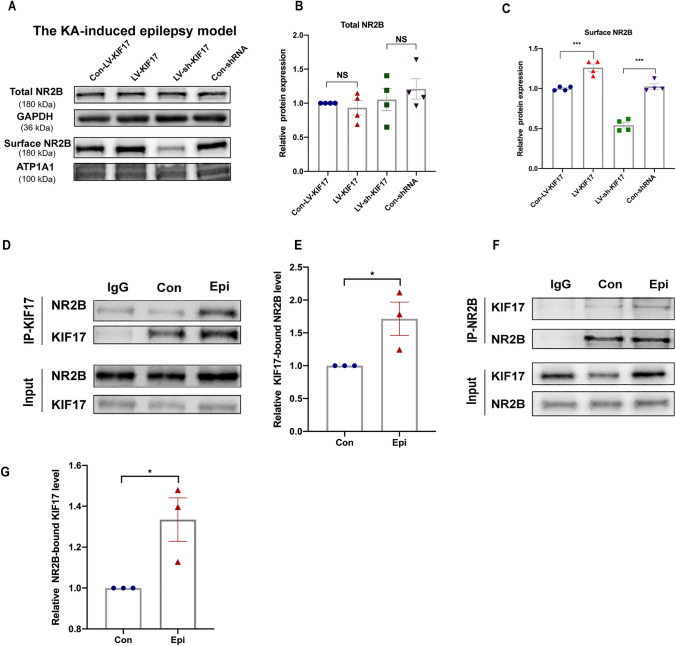
Given the changes in KIF17 expression in epilepsy, we subsequently determined whether a change in KIF17 expression affects spontaneous epilepsy. Behavioral detection and LFP recordings were made to investigate whether the up-regulation and down-regulation of KIF17 exerted specific effects on epileptic activity and seizure-like discharges in the KA-induced model. We first interfered with endogenous KIF17 expression by stereotactically injecting a lentivirus into the hippocampus. Four weeks after the virus injection, Western blot analysis revealed that the KIF17 protein levels in the LV-KIF17 group were higher than those in the Con-LV-KIF17 group. The protein level in the LV-sh-KIF17 group was significantly lower than that in the Con-shRNA group (Fig. 3C, D). These results demonstrated that lentivirus injection effectively altered endogenous KIF17 expression.
Fig. 3.
KIF17 modulates chronic epileptic seizure activity. A The timeline of behavioral experiments. KA is injected into the hippocampus one month after lentivirus injection, and local field potentials are recorded 30 days after video surveillance. B Image of a viral injection site. C, D The lentivirus successfully reduces hippocampal KIF17 protein expression (n = 5). E, F In the mouse model, the numbers of SRSs (n = 6 in the Con-LV-KIF17 group, n = 9 in the other three groups) and the latencies (n = 8 in the Con-LV-KIF17 group, n = 9 in the other three groups) of the four groups. G Representative LFPs in the four groups. H–J Numbers of SLEs, time spent experiencing SLEs, and duration of SLEs over a 30-min period (n = 5). Data are presented as the mean ± SEM, NS means P > 0.05,*P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.0001, one-way ANOVA followed by LSD-t test.
We then injected KA into the hippocampus to investigate the changes in chronic epileptic seizure behavior. After SE induction, videos of each group were recorded for one month. We analyzed the number of spontaneous seizures (SRSs) seven days after SE (days 24–30), as shown in Fig. 3E and F. The mice in the LV-KIF17 group had a shorter SRS latency and an increased occurrence of SRSs than the control group. The LV-sh-KIF17 group exhibited a longer incubation period and a significantly reduced frequency of SRSs compared with the corresponding control group. One month after SE, the LFPs of all the groups were recorded. We analyzed seizure-like events (SLEs) for 30 min (Fig. 3G), and the LV-KIF17 group spent a longer total time experiencing SLEs and exhibited more seizure-like discharges than the control group (Fig. 3H, I). Moreover, the LV-sh-KIF17 group spent a clearly shorter total time experiencing SLEs and exhibited a markedly lower frequency of SLEs than the control group (Fig. 3H, I). In addition, the duration of SLEs in the LV-KIF17 group was longer than that in the control group but did not differ from that in the LV-sh-KIF17 group (Fig. 3J).
In experimental animal models, the irreversible brain damage and neurological deficits caused by SE are the pathophysiological basis of chronic spontaneous seizures [31, 32]. This means that the frequency of spontaneous seizures is directly correlated with the severity of SE [33–35]. Given that intervention with KIF17 affects spontaneous seizures, we further investigated the changes in acute seizure behavior. As shown in Fig. S4, we found that the overexpression of KIF17 shortened the time to reach SE compared with that of the control group (Fig. S4A); in addition, the LV-KIF17 group showed significantly faster seizure progression than the control group (Fig. S4B, C). Inhibition of KIF17 expression yielded the opposite result (Fig. S4A–C). However, the difference did not reach statistical significance. This means that KIF17 shortened the latency and increased the susceptibility to acute epileptic seizures. Overall, the results indicated that KIF17 overexpression aggravates epileptic seizure activity, whereas the down-regulation of KIF17 has the opposite effect.
KIF17 Regulates Neuronal Excitability in the Hippocampal CA1 Region and the Growth of Dendritic Spines
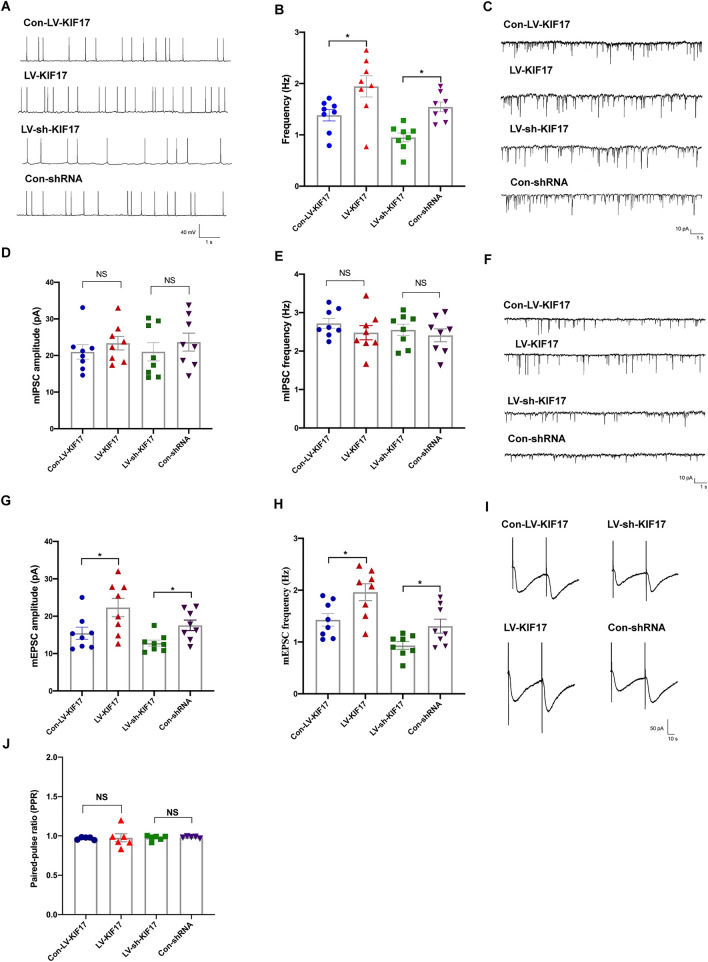
Whole-cell patch-clamp recordings from CA1 pyramidal neurons in mouse hippocampal sections were made four weeks after lentivirus infection to evaluate the effect of KIF17 on neuronal electrical activity. First, we recorded sAPs to measure neuronal excitability. The sAP frequency in the LV-KIF17-treated group was significantly higher than that in the Con-LV- KIF17-treated group; moreover, a markedly lower frequency of sAPs was found in the LV-sh-KIF17-treated group than in the Con-shRNA group (Fig. 4A, B). We then recorded mEPSCs and mIPSCs to explore whether KIF17 is involved in the regulation of excitatory and inhibitory transmission in neurons. As shown in Fig. 4C–E, neither the up-regulation nor the down-regulation of KIF17 expression affected the frequency or amplitude of mIPSCs. However, the hippocampal knockdown of KIF17 decreased the frequency and amplitude of mEPSCs compared with the control group; moreover, compared with the control group, KIF17 overexpression increased the frequency and amplitude of mEPSCs (Fig. 4F–H). Because intervention with KIF17 affected both the frequency and amplitude of mEPSCs, we further examined the PPR, a widely-used measure of presynaptic release probability [36]. As shown in Fig. 4I and J, no significant differences in the PPR were detected between the groups. In addition, we also recorded the sEPSCs in each group to further confirm that different KIF17 levels affect excitatory transmission. The results showed that the overexpression of KIF17 increased the amplitude of sEPSCs compared with the control group and that the inhibition of KIF17 produced the opposite result (Fig. S5A, B), but the mean differences were not statistically significant. Moreover, the sEPSC frequency did not differ between the experimental groups (Fig. S5C). The results indicated that KIF17 regulates the excitability of neurons mainly by modulating excitatory synaptic transmission rather than inhibiting inhibitory synaptic transmission. These findings also indicate the electrophysiological mechanism of the involvement of KIF17 in epilepsy.
Fig. 4.
KIF17 regulates excitatory synaptic transmission. A–H Representative traces of sAPs (A), mIPSCs (C), and mEPSCs (F), and summary of the frequency of sAPs (B) and the frequency and amplitude of mIPSCs (D–E) and mEPSCs (G–H) (n = 8). I, J Representative traces and summary of the PPR (n = 5). Data are presented as the mean ± SEM, NS means P > 0.05,*P < 0.05, one-way ANOVA followed by LSD-t test.
The studies indicated that the change in the frequency of mEPSCs may be caused by an increase in the presynaptic release probability [37]; however, an increase in excitatory synapses could also increase the frequency of mEPSCs [38]. Due to the difference in the mEPSC frequency and the lack of changes in the PPR among the groups, we examined the effect of interfering with KIF17 expression in cultured primary neurons on the maturation of dendritic spines that form excitatory synapses with presynaptic axons and receive input from presynaptic axons [39, 40]. Compared with the control group, the number of dendritic spines in the LV-KIF17 group was increased (Fig. S6A, B), and in the LV-sh-KIF17 group was decreased (Fig. S6A, B). This finding demonstrates that the corresponding change in mEPSC frequency is due to the variation in the number of synapses caused by interference with endogenous KIF17 expression.
KIF17 Affects the Membrane Expression of the NMDA Receptor Subunit NR2B in the Hippocampus
Previous studies have shown that the transport of NR2B is regulated by a microtubule-associated trafficking complex consisting of the KIF17, APBA1 (amyloid-beta A4 precursor protein-binding family A member 1), CASK (calcium/calmodulin-dependent serine protein kinase), and mLin7 proteins [41] and that the lack of KIF17 leads to decreases in synaptic NR2B levels and the amplitude of NMDAR-mediated EPSCs [16]. Based on these findings, we evaluated the expression of NR2B in the epileptic hippocampus after lentivirus intervention to explore the possible mechanism through which KIF17 affects EPSCs. First, we assessed the NR2B levels in the postsynaptic density region of primary cultured neurons after interfering with endogenous KIF17 expression. As shown in Fig. S7A and B, compared with the control group, the co-localization of NR2B and PSD95 was increased after KIF17 overexpression, whereas inhibition of KIF17 produced the opposite result. This finding suggested that KIF17 affects the distribution of NR2B in the excitatory synapses. We then assessed the total and membrane NR2B levels in epileptic hippocampal tissue after lentivirus intervention. The Western blot results showed that neither the up-regulation nor the down-regulation of KIF17 affected the total protein level of the NR2B subunit (Fig. 5A, B). However, the amount of NR2B on the membrane in the LV-KIF17 group was significantly higher than that in the Con-LV-KIF17 group (Fig. 5A, C); in addition, the amount of NR2B in the LV-sh-KIF17 group was lower than that in the Con-shRNA group (Fig. 5A, C). These findings suggested that changes in KIF17 expression do not affect the total amount of NR2B in the epileptic environment but do affect its membrane transport. Furthermore, we evaluated the number of KIF17/NR2B complexes in the epileptic environment by co-immunoprecipitation. The interaction between KIF17 and NR2B was significantly higher in the epileptic hippocampus (Fig. 5D–G), consistent with the expression model of NR2B in the epileptic hippocampus after intervention in endogenous KIF17 expression. This finding suggests that increased KIF17 increases the membrane transport of NR2B in the epileptic environment. Therefore, we hypothesized that KIF17 mediates the changes in excitability in epilepsy by regulating NR2B transport to alter its content on the membrane of neurons.
Fig. 5.
Total and surface NR2B expression in hippocampal tissue from KIF17-treated epileptic mice; quantification of the KIF17/NR2B complex in epileptic mice. A–C Representative immunoblotting images of the surface and total NR2B expression in the KA model and statistics of total and surface NR2B expression (B and C) (n = 4). D–G Quantitative co-immunoprecipitation for detecting the KIF17/NR2B complex in controls and mice with epilepsy (n = 3). Data are presented as the mean ± SEM, NS means P > 0.05,*P < 0.05, ***P < 0.005, one-way ANOVA followed by LSD-t test (B and C) and independent Student’s t-test (E and G).
KIF17 is SUMOylated by SUMO1, and this SUMOylation is Increased in Response to Epilepsy
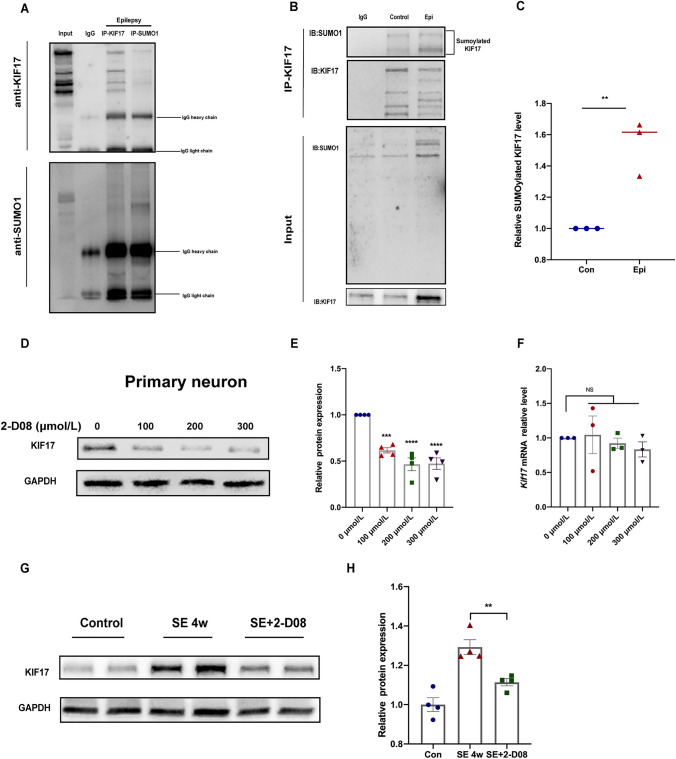
KIF17 is rapidly degraded by the ubiquitin–proteasome system in response to NMDA-mediated activity, and SUMOylation reportedly plays a role in regulating the stability of substrate proteins [42, 43]. We sought to explore whether SUMOylation plays a role in regulating the stability of KIF17 in epilepsy. First, as shown in Fig. 6A, an interaction between KIF17 and SUMO1 was detected in the hippocampus of epileptic mice, and the SUMOylation level of KIF17, which is mediated by SUMO1, was higher in the hippocampus of subjects with chronic epilepsy (Fig. 6B, C), which suggested that the SUMOylation of KIF17 is increased in epilepsy. In cultured hippocampal primary neurons, the SUMOylation inhibitor 2-D08 decreased the protein but not the mRNA level of KIF17 in a dose-dependent manner (Fig. 6D–F). Moreover, a hippocampal stereotactic injection of 2-D08 significantly reduced KIF17 expression in mice with KA-induced chronic epilepsy (Fig. 6G and H). In other words, both in vivo and in vitro experiments showed that the KIF17 protein level is positively correlated with its SUMOylation level, which suggested that SUMOylation of the KIF17 protein increases its stability during epilepsy to some extent.
Fig. 6.
SUMOylation of KIF17 stabilizes the protein in epilepsy. A SUMO1 interacts with KIF17. B, C The level of SUMOylated KIF17 is increased in epilepsy (n = 3). D–F Cultured primary neurons were treated with 2-D08, and immunoblotting (n = 4) or qRT-PCR (n = 3) was then performed. G, H Hippocampal KIF17 expression after treatment with 2-D08 (n = 4). Data are presented as the mean ± SEM, NS means P > 0.05,**P < 0.01, ***P < 0.005, independent Student’s t-test (C and H), and one-way ANOVA followed by LSD-t test (E and F).
Discussion
In this study, we first found that KIF17 was expressed in neurons and increased in epileptic brain tissue. We then discovered that KIF17 regulated seizure activity and excitatory synaptic transmission in an epilepsy model, possibly due to the regulation of dendritic spine maturation and NR2B membrane trafficking mediated by KIF17. We also provided the first demonstration that KIF17 is modified by SUMO1-mediated SUMOylation and that this process plays a role in maintaining the stability of the KIF17 protein in epilepsy.
TLE is the most common type of medically refractory epilepsy [44], and hippocampal sclerosis is an important biomarker of TLE [45, 46]. However, for practical and ethical reasons, KIF17 expression in the hippocampus cannot be compared between patients with TLE and controls. As one of the animal models of epilepsy, the KA-induced model largely reproduces events corresponding to those in humans with TLE, including the behavioral and molecular events of epilepsy [47], and these findings provide direct evidence for the study of KIF17 in epilepsy. In the hippocampus of epileptic mice, KIF17 is expressed at high levels in the pyramidal cell layer and dentate gyrus region, which is the most important region involved in TLE, as manifested in histopathology by neuronal loss and reactive gliosis [45]. Further immunofluorescence staining showed that KIF17 was expressed in glutamatergic and GABAergic neurons but rarely in astrocytes. Moreover, subcellular localization analysis showed that KIF17 mainly co-localized with the excitatory postsynaptic marker PSD95 rather than the presynaptic marker VGLUT1. The distribution and subcellular localization revealed a correlation between KIF17 and the development of epilepsy. Furthermore, the KIF17 protein levels were increased in brain tissue of patients with TLE and the hippocampus and cortex of KA-treated mice. In addition, immunoblotting results suggested dynamic changes in KIF17 levels in the hippocampus during epilepsy. Specifically, the KIF17 protein level began to increase one day after SE and reached a peak 14 days later. Interestingly, KIF17 expression decreased on the third day after SE, possibly in response to rapid degradation by the ubiquitin–proteasome system mediated by NMDA activity [42] or some feedback mechanism to some extent. In addition, spontaneous seizures were aggravated by overexpression and reduced by inhibition of KIF17. This may have some connection to the effect of KIF17 intervention on the induction and development of acute epileptic seizures. On the one hand, this study provides direct evidence for the involvement of KIF17 in epilepsy, and on the other hand, hippocampal KIF17 overexpression increases the severity of seizures rather than causing seizures directly, which suggests that the increased expression of KIF17 in the brains of patients and mice with epilepsy might be secondary to epilepsy, and increases in KIF17 expression further aggravate epileptic seizures. In conclusion, our findings indicate that KIF17 is involved in the development of epilepsy, but a causal relationship between KIF17 and the occurrence of epilepsy remains difficult to distinguish based on the behavioral and expression data obtained in our study.
Various transcriptomic and genome-specific functional studies, as well as multiple in vivo and in vitro experiments, have shown the importance of the genes involved in synaptic transmission and its regulation in the pathogenesis of epilepsy [48]. Since KIF17 is involved in the regulation of synaptic function [49, 50], we focused on the effects of KIF17 on neuronal excitability and synaptic transmission. The mEPSCs and mIPSCs in isolated hippocampal slices were recorded in an Mg2+-free model of epilepsy. The overexpression and inhibition of KIF17 increased and decreased the frequency and amplitude of mEPSCs, respectively, but did not affect mIPSCs, which was consistent with the effect of KIF17 intervention on the amplitude of sEPSCs. This finding suggests that activated KIF17 increases neuronal excitability by increasing excitatory synaptic transmission. Although the modulation of KIF17 resulted in changes in the frequency and amplitude of mEPSCs, the PPR was not affected. In general, the amplitude of mEPSCs reflects a postsynaptic receptor effect, whereas the variation in frequency is related to a presynaptic effect [37]. The excitatory synapses of hippocampal pyramidal neurons, most of which are located on dendritic spines, are critical for normal synaptic transmission and are closely associated with the pathophysiology of epilepsy [51, 52]. Changes in their number may also lead to changes in the frequency of mEPSCs. KIF17 altered the number of dendritic spines, which may partially explain the trend of changes in mEPSCs.
Although the molecular mechanism by which KIF17 regulates excitatory synaptic transmission in the epileptic environment remains unclear, numerous studies have investigated the physiological effects of KIF17 on NMDA-dependent excitatory currents and the transport capacity of the NR2B subunit in neuronal dendrites [16, 53, 54]. In addition, NMDARs change dynamically in response to neuronal activity, and the regulated insertion and removal of NMDARs are critical for the maintenance of synaptic transmission in the mammalian central nervous system [55, 56]. One of the main subunits of NMDAR, the NR2B subunit has been shown to be associated with epilepsy [57]. Here, we focused on the distribution of NR2B in neurons. We found that in the epileptic hippocampus, KIF17 overexpression increased the surface NR2B expression but did not change its total expression. This finding suggested an underlying correlation between KIF17-mediated NR2B membrane expression and the regulation of excitatory synaptic transmission in the epileptic environment. In neurons, secondary to synaptic stimulation, the NR2B/KIF17 complex is up-regulated on demand through cAMP response element-binding protein activity, and this up-regulation leads to increases in NR2B transport and synaptic accumulation [53]. In our study, co-immunoprecipitation showed that the NR2B/KIF17 complex was up-regulated in the epileptic environment. This finding implies that KIF17 leads to increases in NR2B transport and membrane surface accumulation secondary to epileptic activity, and this change may be the underlying mechanism through which KIF17 regulates the epileptic activity and neural excitability.
The above data suggest that KIF17 expression is up-regulated in response to epileptic stimulation and may aggravate epileptic seizures through different mechanisms. In addition, it has been reported that a few post-translational modifications by Ca2+/calmodulin-dependent kinase II (CaMKII), protein kinase A, and ubiquitin affect the functions of KIF17 [42, 50]. For instance, the ability of KIF17 to bind/release NR2B-containing vesicles is affected by the phosphorylation of KIF17 by CaMKII, a process triggered by NMDA-mediated activity [58]. This suggests that the regulation of the function of KIF17 is of great importance for epileptic seizures. We focused on SUMOylation, a highly transient post-translational modification that affects substrate functions including stability, cellular localization, and interactions with other proteins, and plays a vital role in neurological disorders such as epilepsy and Alzheimer's disease [59]. The SUMOylation of synapses and synaptic proteins, including glutamate kainate receptor subunit 2, metabolic glutamate receptor type 7, and syntaxin1A, is reportedly involved in neuronal differentiation and synapse formation [20, 60, 61]. Local ubiquitin–proteasome degradation of KIF17 occurs in dendrites following NMDAR activation [42], but researchers have not clearly determined whether SUMOylation is involved in this process. The present study provides the first demonstration that SUMO1 mediates the SUMOylation of KIF17, which may be a substrate for SUMOylation. Co-immunoprecipitation revealed increased SUMOylation of KIF17 in the epileptic environment. Treatment with the SUMOylation inhibitor 2-D08 decreased the expression of KIF17 both in vivo and in vitro, which suggested that SUMOylation plays an important role in the maintenance of KIF17 stability. In conclusion, the SUMOylation of KIF17 is important for maintaining the stability of KIF17 in the epileptic state, which may exacerbate seizures.
In summary, through pathological, behavioral, and electrophysiological explorations, we revealed the role and mechanism of KIF17 in regulating NR2B function and neuronal excitability and provide a potential target for epilepsy therapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81873788, 81922023, 82001378, and 82171440), the Chongqing Natural Science Foundation Project (cstc2019jcyj-msxmX0184), and the Fifth Senior Medical Talents Program of Chongqing for Young and Middle-aged.
Conflict of interest
The authors claim that there are no conflicts of interest.
Footnotes
Yan Liu and Xin Tian contributed equally to this work.
Contributor Information
Xuefeng Wang, Email: xfyp@163.com.
Fei Xiao, Email: feixiao81@126.com.
References
- 1.Devinsky O, Vezzani A, O'Brien T, Jetté N, Scheffer I, Curtis M, et al. Epilepsy. Nat Rev Dis Primers. 2018;4:18024. doi: 10.1038/nrdp.2018.24. [DOI] [PubMed] [Google Scholar]
- 2.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon CS, Dykeman J, et al. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology. 2017;88:296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staley K. Molecular mechanisms of epilepsy. Nat Neurosci. 2015;18:367–372. doi: 10.1038/nn.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: Many regulators, many consequences. Neuroscientist. 2013;19:62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collingridge GL, Isaac JTR, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- 7.Mellone M, Gardoni F. Modulation of NMDA receptor at the synapse: Promising therapeutic interventions in disorders of the nervous system. Eur J Pharmacol. 2013;719:75–83. doi: 10.1016/j.ejphar.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 8.Chen K, Yang LN, Lai C, Liu D, Zhu LQ. Role of Grina/Nmdara1 in the central nervous system diseases. Curr Neuropharmacol. 2020;18:861–867. doi: 10.2174/1570159X18666200303104235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–426. doi: 10.1038/nrn2153. [DOI] [PubMed] [Google Scholar]
- 10.Smigiel R, Kostrzewa G, Kosinska J, Pollak A, Stawinski P, Szmida E, et al. Further evidence for GRIN2B mutation as the cause of severe epileptic encephalopathy. Am J Med Genet A. 2016;170:3265–3270. doi: 10.1002/ajmg.a.37887. [DOI] [PubMed] [Google Scholar]
- 11.Hu C, Chen WJ, Myers SJ, Yuan HJ, Traynelis SF. Human GRIN2B variants in neurodevelopmental disorders. J Pharmacol Sci. 2016;132:115–121. doi: 10.1016/j.jphs.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miki H, Okada Y, Hirokawa N. Analysis of the kinesin superfamily: Insights into structure and function. Trends Cell Biol. 2005;15:467–476. doi: 10.1016/j.tcb.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Chu PJ, Rivera JF, Arnold DB. A role for Kif17 in transport of Kv4.2. J Biol Chem. 2006;281:365–373. doi: 10.1074/jbc.M508897200. [DOI] [PubMed] [Google Scholar]
- 14.Kayadjanian N, Lee HS, Piña-Crespo J, Heinemann SF. Localization of glutamate receptors to distal dendrites depends on subunit composition and the kinesin motor protein KIF17. Mol Cell Neurosci. 2007;34:219–230. doi: 10.1016/j.mcn.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- 16.Yin XL, Takei Y, Kido MA, Hirokawa N. Molecular motor KIF17 is fundamental for memory and learning via differential support of synaptic NR2A/2B levels. Neuron. 2011;70:310–325. doi: 10.1016/j.neuron.2011.02.049. [DOI] [PubMed] [Google Scholar]
- 17.Wong RWC, Setou M, Teng JL, Takei Y, Hirokawa N. Overexpression of motor protein KIF17 enhances spatial and working memory in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:14500–14505. doi: 10.1073/pnas.222371099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunz K, Piller T, Müller S. SUMO-specific proteases and isopeptidases of the SENP family at a glance. J Cell Sci. 2018;131:jcs211904. doi: 10.1242/jcs.211904. [DOI] [PubMed] [Google Scholar]
- 19.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 20.Henley JM, Seager R, Nakamura Y, Talandyte K, Nair J, Wilkinson KA. SUMOylation of synaptic and synapse-associated proteins: An update. J Neurochem. 2021;156:145–161. doi: 10.1111/jnc.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schorova L, Martin S. Sumoylation in synaptic function and dysfunction. Front Synaptic Neurosci. 2016;8:9. doi: 10.3389/fnsyn.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qi YT, Wang JX, Bomben VC, Li DP, Chen SR, Sun H, et al. Hyper-SUMOylation of the Kv7 potassium channel diminishes the M-current leading to seizures and sudden death. Neuron. 2014;83:1159–1171. doi: 10.1016/j.neuron.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang LTH, Craig TJ, Henley JM. SUMOylation of synapsin Ia maintains synaptic vesicle availability and is reduced in an autism mutation. Nat Commun. 2015;6:7728. doi: 10.1038/ncomms8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sada N, Lee SN, Katsu T, Otsuki T, Inoue T. Epilepsy treatment. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–1367. doi: 10.1126/science.aaa1299. [DOI] [PubMed] [Google Scholar]
- 25.Lévesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013;37:2887–2899. doi: 10.1016/j.neubiorev.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh H, Auguadri L, Battaglia S, Simone Thirouin Z, Zemoura K, Messner S, et al. Several posttranslational modifications act in concert to regulate gephyrin scaffolding and GABAergic transmission. Nat Commun. 2016;7:13365. doi: 10.1038/ncomms13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Tian X, Xu DM, Zheng FS, Lu X, Zhang YK, et al. GPR40 modulates epileptic seizure and NMDA receptor function. Sci Adv. 2018;4:eaau357. doi: 10.1126/sciadv.aau2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y, Zhang YK, Zheng FS, Yang Y, Xu X, Wang W, et al. Expression of Glypican-4 in the brains of epileptic patients and epileptic animals and its effects on epileptic seizures. Biochem Biophys Res Commun. 2016;478:241–246. doi: 10.1016/j.bbrc.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 29.Engel J., Jr A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: Report of the ILAE task force on classification and terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou LW, Zheng LS, Hu KS, Wang X, Zhang RH, Zou YZ, et al. SUMOylation stabilizes hSSB1 and enhances the recruitment of NBS1 to DNA damage sites. Signal Transduct Target Ther. 2020;5:80. doi: 10.1038/s41392-020-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang YL, Wei PH, Yan F, Luo YM, Zhao GG. Animal models of epilepsy: A phenotype-oriented review. Aging Dis. 2022;13:215–231. doi: 10.14336/AD.2021.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lothman EW, Bertram EH., 3rd Epileptogenic effects of status epilepticus. Epilepsia. 1993;34:S59–S70. doi: 10.1111/j.1528-1157.1993.tb05907.x. [DOI] [PubMed] [Google Scholar]
- 33.Puttachary S, Sharma S, Tse K, Beamer E, Sexton A, Crutison J, et al. Immediate epileptogenesis after kainate-induced status epilepticus in C57BL/6J mice: Evidence from long term continuous video-EEG telemetry. PLoS One. 2015;10:e0131705. doi: 10.1371/journal.pone.0131705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maroso M, Balosso S, Ravizza T, Iori V, Wright CI, French J, et al. Interleukin-1β biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iori V, Iyer AM, Ravizza T, Beltrame L, Paracchini L, Marchini S, et al. Blockade of the IL-1R1/TLR4 pathway mediates disease-modification therapeutic effects in a model of acquired epilepsy. Neurobiol Dis. 2017;99:12–23. doi: 10.1016/j.nbd.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 37.Sala C, Piëch V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/S0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 38.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290:1364–1368. doi: 10.1126/science.290.5495.1364. [DOI] [PubMed] [Google Scholar]
- 39.Gray EG. Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature. 1959;183:1592–1593. doi: 10.1038/1831592a0. [DOI] [PubMed] [Google Scholar]
- 40.Rochefort NL, Konnerth A. Dendritic spines: From structure to in vivo function. EMBO Rep. 2012;13:699–708. doi: 10.1038/embor.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr Res. 2010;119:198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwata S, Morikawa M, Takei Y, Hirokawa N. An activity-dependent local transport regulation via degradation and synthesis of KIF17 underlying cognitive flexibility. Sci Adv. 2020;6:eabc355. doi: 10.1126/sciadv.abc8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hegde AN. Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. Prog Neurobiol. 2004;73:311–357. doi: 10.1016/j.pneurobio.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 44.Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:630853. doi: 10.1155/2012/630853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ren E, Curia G. Synaptic reshaping and neuronal outcomes in the temporal lobe epilepsy. Int J Mol Sci. 2021;22:3860. doi: 10.3390/ijms22083860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thom M. Review: hippocampal sclerosis in epilepsy: A neuropathology review. Neuropathol Appl Neurobiol. 2014;40:520–543. doi: 10.1111/nan.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Ari Y. Cell death and synaptic reorganizations produced by seizures. Epilepsia. 2001;42:5–7. doi: 10.1046/j.1528-1157.2001.042suppl.3005.x. [DOI] [PubMed] [Google Scholar]
- 48.di Paolo A, Garat J, Eastman G, Farias J, Dajas-Bailador F, Smircich P, et al. Functional genomics of axons and synapses to understand neurodegenerative diseases. Front Cell Neurosci. 2021;15:686722. doi: 10.3389/fncel.2021.686722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuen EY, Jiang Q, Feng J, Yan Z. Microtubule regulation of N-methyl-D-aspartate receptor channels in neurons. J Biol Chem. 2005;280:29420–29427. doi: 10.1074/jbc.M504499200. [DOI] [PubMed] [Google Scholar]
- 50.Hanus C, Kochen L, Tom Dieck S, Racine V, Sibarita JB, Schuman EM, et al. Synaptic control of secretory trafficking in dendrites. Cell Rep. 2014;7:1771–1778. doi: 10.1016/j.celrep.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helm MS, Dankovich TM, Mandad S, Rammner B, Jähne S, Salimi V, et al. A large-scale nanoscopy and biochemistry analysis of postsynaptic dendritic spines. Nat Neurosci. 2021;24:1151–1162. doi: 10.1038/s41593-021-00874-w. [DOI] [PubMed] [Google Scholar]
- 52.Rossini L, de Santis D, Mauceri RR, Tesoriero C, Bentivoglio M, Maderna E, et al. Dendritic pathology, spine loss and synaptic reorganization in human cortex from epilepsy patients. Brain. 2021;144:251–265. doi: 10.1093/brain/awaa387. [DOI] [PubMed] [Google Scholar]
- 53.Yin XL, Feng X, Takei Y, Hirokawa N. Regulation of NMDA receptor transport: A KIF17-cargo binding/releasing underlies synaptic plasticity and memory in vivo. J Neurosci. 2012;32:5486–5499. doi: 10.1523/JNEUROSCI.0718-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guillaud L, Setou M, Hirokawa N. KIF17 dynamics and regulation of NR2B trafficking in hippocampal neurons. J Neurosci. 2003;23:131–140. doi: 10.1523/JNEUROSCI.23-01-00131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 56.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: Mechanisms and functional implications. Curr Opin Neurobiol. 2012;22:496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Möddel G, Jacobson B, Ying Z, Janigro D, Bingaman W, González-Martínez J, et al. The NMDA receptor NR2B subunit contributes to epileptogenesis in human cortical dysplasia. Brain Res. 2005;1046:10–23. doi: 10.1016/j.brainres.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 58.Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: A molecular model of kinesin-cargo release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- 59.Chen X, Zhang YH, Wang QQ, Qin YY, Yang XY, Xing ZC, et al. The function of SUMOylation and its crucial roles in the development of neurological diseases. FASEB J. 2021;35:e21510. doi: 10.1096/fj.202002702R. [DOI] [PubMed] [Google Scholar]
- 60.Chamberlain SEL, González-González IM, Wilkinson KA, Konopacki FA, Kantamneni S, Henley JM, et al. SUMOylation and phosphorylation of GluK2 regulate kainate receptor trafficking and synaptic plasticity. Nat Neurosci. 2012;15:845–852. doi: 10.1038/nn.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Craig TJ, Anderson D, Evans AJ, Girach F, Henley JM. SUMOylation of Syntaxin1A regulates presynaptic endocytosis. Sci Rep. 2016;5:17669. doi: 10.1038/srep17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.