Abstract
Microglia are involved in the inflammatory response and retinal ganglion cell damage in glaucoma. Here, we investigated how microglia proliferate and migrate in a mouse model of chronic ocular hypertension (COH). In COH retinas, the microglial proliferation that occurred was inhibited by the P2X7 receptor (P2X7R) blocker BBG or P2X7R knockout, but not by the P2X4R blocker 5-BDBD. Treatment of primary cultured microglia with BzATP, a P2X7R agonist, mimicked the effects of cell proliferation and migration in COH retinas through the intracellular MEK/ERK signaling pathway. Transwell migration assays showed that the P2X4R agonist CTP induced microglial migration, which was completely blocked by 5-BDBD. In vivo and in vitro experiments demonstrated that ATP, released from activated Müller cells through connexin43 hemichannels, acted on P2X7R to induce microglial proliferation, and acted on P2X4R/P2X7R (mainly P2X4R) to induce microglial migration. Our results suggest that inhibiting the interaction of Müller cells and microglia may attenuate microglial proliferation and migration in glaucoma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12264-022-00833-w.
Keywords: Glaucoma, Chronic ocular hypertension, Retinal microglia, Proliferation, Migration, P2X7R/P2X4R, Müller cells
Introduction
Glaucoma is a disease resulting in irreversible blindness; it is characterized by apoptotic death of retinal ganglion cells (RGCs). Although the pathogenesis of glaucoma is not completely understood, it is commonly believed that retinal glial cells are activated, and the activated glial cells participate in RGC damage by releasing a variety of harmful factors [1–5].
In the vertebrate retina, there are two types of glial cells: microglia and macroglia (Müller cells and astrocytes). Under normal conditions, retinal microglia are mostly distributed in the inner plexiform layer (IPL) and the outer plexiform layer (OPL). As antigen-presenting cells, microglia monitor the retinal microenvironment, migrate extensively, clear cellular debris, and participate in the immune-mediated network, thus maintaining retinal homeostasis [5, 6]. Müller cells are the principal macroglial cells in the retina. Their nuclei are located in the inner nuclear layer and their processes span the entire thickness of the retina, and support the functioning and metabolism of retinal neurons [2, 7]. Astrocytes are highly aggregated in the optic nerve head, playing intrinsic protective and supportive roles for RGCs [8].
In the glaucomatous retina, microglia are activated [9–13], as evidenced by up-regulated expression of translocator protein, a biomarker of microglial activation [14]. Activated microglia undergo a morphological change from a ramified to an amoeboid shape; at the same time, the cells show enhanced proliferation and migration to the ganglion cell layer (GCL). These microglia gathering in the GCL induce RGC damage by releasing pro-inflammatory factors, such as tumor necrosis factor-α (TNF-α), interleukins (ILs), and nitric oxide (NO) [12]. However, the mechanisms underlying microglial proliferation and migration to the GCL are largely unknown. There is evidence demonstrating that the interaction of Müller cells and microglia augments the retinal inflammatory response in a positive-feedback manner in retinal injury, in which ATP may be an important mediator [15–17]. Müller cells are potential sources of extracellular ATP [18]. In a chronic ocular hypertension (COH) experimental glaucoma model, we have shown that Müller cells are activated, which is characterized by up-regulated expression of glial fibrillary acidic protein [19, 20]. Activated Müller cells may promote ATP release [21]. Therefore, in this study, we investigated whether and how ATP regulates the proliferation and migration of retinal microglia in glaucoma.
Materials and Methods
Animals
All animal experiments were carried out according to the National Institutes of Health (NIH) guidelines for the Care and Use of Laboratory Animals and the guidelines of Fudan University on the Ethical Use of Animals and were approved by the Institutes of Brain Science of Fudan University. P2X7R−/− mice were a generous gift from Dr. Yuqiu Zhang at Fudan University. Male C57BL/6 mice (weighing 18–20 g) were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and MacGreen mice from The Jackson Laboratory (Hancock County, USA). All mice were housed on a 12-h light/dark schedule, with standard food and water provided ad libitum.
Mouse COH Model
The mouse COH model was produced following the procedure previously described [22]. Briefly, under an OPMI VISU 140 microscope (Carl Zeiss, Jena, Germany), micro-magnetic beads (2 μL, diameter ~10 μm, BioMag®Superparamagnetic Iron Oxide, Bangs Laboratories, Inc., IN, USA) were microinjected into the anterior chamber of anesthetized mice. Sham-operated mice were injected with 2 μL of 0.9% NaCl.
Under general anesthesia (2% pentobarbital sodium, 40 mg/kg, i.p.), intraocular pressure (IOP) was measured using a handheld digital tonometer (TonoLab, Icare, Finland). The average value of five consecutive measurements with a deviation of < 5% was accepted. All measurements were performed in the morning (09:00–10:00) to avoid possible circadian differences. The IOPs of both eyes were measured before the injection (d0), and then on the next day of the operation (G1d), days 2, 3, 4, and 7 (G2d, G3d, G4d, and G1w), and weekly thereafter (G2w–G6w).
Immunofluorescence and Quantification of Microglia in the Retina
Immunofluorescence was assessed as previously described [23–25]. In brief, the eyes of anesthetized mice were removed quickly after perfusion fixation, further fixed with 4% paraformaldehyde in 0.1 mol/L phosphate buffer (pH 7.4) for 4 h at 4°C, and then dehydrated with graded sucrose solutions at 4°C. The eyecups were embedded in OCT compound (Tissue Tek, Torrance, USA), and vertically sectioned at 14 μm on a freezing microtome (Leica, Nussloch, Germany). After blocking in 10% normal donkey serum (v/v) (Sigma-Aldrich, St. Louis, USA) in phosphate-buffered saline (PBS) plus 0.1% Triton X-100 for 2 h at room temperature, the retinal sections were incubated with the primary antibodies polyclonal goat-anti-Iba1 (ab5076, 1:1000 dilution, Abcam, Cambridge, USA), monoclonal rabbit-anti-GS (MAB302, 1:1000, Millipore, Billerica, USA), or monoclonal mouse-anti-Cx43 (SAB4200819, 1:1000, Sigma-Aldrich) overnight at 4°C. The secondary antibodies were Alexa Fluor 488 AffiniPure donkey anti-goat IgG, Alexa Fluor 488 AffiniPure donkey anti-rabbit IgG, or Alexa Fluor Cy3 AffiniPure donkey anti-mouse IgG (1:500, Jackson ImmunoResearch Laboratories, Inc., West Grove, USA). Signals were visualized with a confocal laser scanning microscope (Fluroview 1000, Olympus, Monolith, Tokyo, Japan) through a 40× objective.
Microglia were counted on vertical retinal sections following the procedure previously reported [23], with some modifications. In a single preparation, the whole retina was cut vertically into 128 sections 14 μm thick. The first 8 sections were sequentially collected on 8 different glass slides, and the next 8 sections were collected in the same way, and so on. Therefore, on each of the 8 slides, there was a total of 16 sections covering different zones of the whole retina. We randomly chose one section to count all Iba1-positive microglia in the 16 sections.
Primary Retinal Microglia and Müller Cell Culture
Primary retinal microglia and Müller cells were cultured following procedures previously described [25, 26]. Briefly, retinas of newborn C57BL/6 mice (5 days old) were digested with 0.25% trypsin (Thermo Fisher Scientific, Rockford, USA). Dissociated retinal cells were then cultured in 75 cm2 flasks containing Dulbecco’s modified Eagle’s medium nutrient mixture F12 (DMEM/F12, Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific), 100 U/mL penicillin, and 100 μg/mL streptomycin in a humidified 5% CO2 incubator at 37°C. Microglial purification was conducted when mixed retinal cells were cultured for 11–13 days. Microglia, mainly growing on top of the cell layer, were detached by forcibly flapping the flasks. The detached cells, which were 95% microglia, were seeded into 24-well plates or the transwell system. The cells remaining in the flasks were purified Müller cells, and the third generation of these cells was used for experiments. The purity of microglia and Müller cells were determined by positive staining of ionized Ca2+-binding adaptor molecule 1 (Iba1) and glutamine synthase (GS), respectively.
5-ethynyl-2′-deoxyuridine (EdU) Labeling, Immunofluorescence, and Quantification
The proliferation of primary cultured microglia was assayed by the EdU labeling method as described previously [27, 28], with some modifications. In short, EdU was added to the culture medium at a concentration of 10 μmol/L. After labeling, microglia (1 × 103) were fixed and permeabilized followed by washing two to three times with PBS. The EdU-labeled microglia were assessed by immunocytochemistry with a Click-iT™ EdU Alexa Fluor™ 594 imaging kit (Thermo Fisher Scientific). Signals were visualized with a confocal laser scanning microscope through a 20× objective. Experiments were performed in triplicate. In each sample, we randomly chose 8 fields for counting the number of EdU and DAPI double-labeled cells.
Transwell Migration/co-culture Assay
Cell migration was analyzed using Costar Transwell plates (5 μm pore size, 3421, Corning, Bedford, USA), as described previously [29, 30], with some modifications. In brief, primary microglia at a density of 1 × 103 in 200 µL of serum-free medium were placed in the upper chamber, and complete medium (500 μL) with 10% fetal bovine serum was added to the lower chamber. After incubation for 6 or 24 h in the presence or absence of the migration-inducing factors (100 μmol/L BzATP, 1 mmol/L CTP or Müller cells), microglia attached to the other side of the insert were fixed in 90% ethanol and stained with 0.1% crystal violet. Migrated cells were observed and counted under a Nikon Eclipse Ti inverted microscope (Nikon, Tokyo, Japan) through a 20× objective. In each well, at least 5 different fields were chosen at random, and the number of migrated cells was averaged from these fields. Experiments were performed in triplicate.
Interaction between Müller cells and microglia was analyzed using Costar Transwell plates (0.4 μm pore size; 3470, Corning), as described previously [31]. Müller cells were seeded onto the upper insert of a 24-well Transwell at a density of 5 × 105/mL, and microglia were placed on the lower chamber. After co-culture, changes of microglia were measured by Western blot or EdU labeling. Experiments were performed in triplicate.
Western Blot Analysis
Western blotting was performed according to previous studies [20, 24]. Total proteins of retinas or cultured cells were extracted using the lysis buffer, and protein concentrations were determined using a standard bicinchoninic acid assay kit (Pierce Biotechnology, Rockford, USA). After separation by SDS-PAGE gel, transfer to a PVDF membrane, and blocking in non-fat powdered milk, the samples were incubated with the primary antibodies polyclonal goat-anti-Iba1 (ab5076; 1:1000 dilution; Abcam), monoclonal rabbit-anti-GS (MAB302; 1:1000; Millipore), monoclonal mice-anti-Cx43 (SAB4200819; 1:1000; Sigma-Aldrich), monoclonal mice-anti-p-ERK1/2 (M9692; 1:1000; Sigma-Aldrich), or polyclonal rabbit-anti-ERK1/2 (M5670; 1:1000; Sigma-Aldrich). The secondary antibodies used in this study were horseradish peroxidase-conjugated anti-goat, anti-rabbit, anti-mouse IgG (705-165-003, 711-165-152, and 715-165-151; 1:800; Jackson ImmunoResearch Laboratories). The blots were visualized with the ECL enhanced chemifluorescent reagent (Thermo Scientific) and an Odyssey near-infrared imaging scanner (FluorChem E System, Protein Simple, San Francisco, USA). Experiments were performed in triplicate.
Flow Cytometry Assay
The retinas from anesthetized MacGreen mice were digested in an enzyme buffer containing 1 mg/mL collagenase D (#11088858001, Roche, Switzerland) and 30 U/mL DNase I (#18047019, Thermo Fisher Scientific, USA) in Hank’s balanced salt solution for 60 min at 37°C, during which gentle shaking was applied every 15 min. Retinal cells were carefully dissociated using a fire-polished pipette and filtered through a 70-μm cell strainer (#352350, Corning). Retinal microglia carrying the GFP signal were detected by fluorescence-activated cell sorting (BD FACSCalibur, BD, USA) and analyzed by FlowJo V10.0 software (TreeStar, Ashland, USA).
Statistical Analysis
Data are expressed as the mean ± SEM and analyzed using SPSS 19.0 (SPSS Inc.©, Armonk, New York, USA). One-way ANOVA with Bonferroni’s post hoc test (multiple comparisons) was used. P < 0.05 was considered statistically significant.
Results
P2X7R Mediates Microglial Proliferation in COH Retina
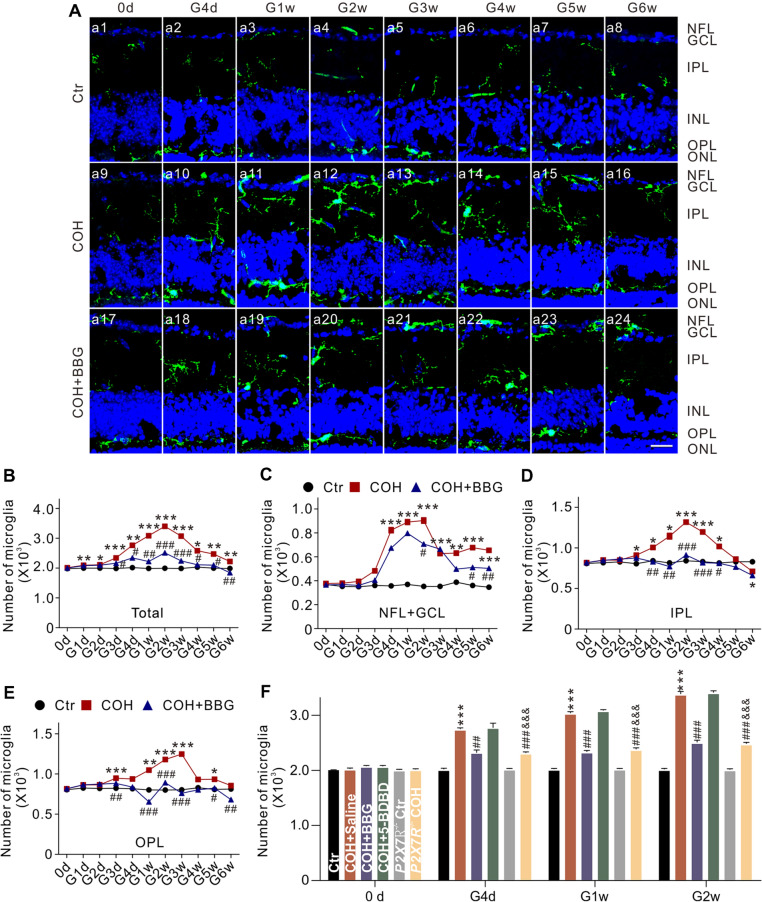
The mouse COH model was successfully produced, and the changes of IOP were similar to our previous report [22] (Fig. S1). In this model, we quantified the dynamic changes in the number of microglia in a set of vertical retinal sections. In control mice, microglia were mainly distributed in the IPL and OPL (Fig. 1A, a1). IOP elevation induced an increase in the number of microglia, and their migration to the GCL and the nerve fiber layer (NFL) (Fig. 1A, a9–a16). As shown in Fig. 1B, the number of microglia started to increase at G1d (n = 6, P < 0.01), peaked at G2w (n = 6, P < 0.001), and then declined but remaining higher than the controls through G6w (n = 6, P < 0.05). We also counted the number of microglia in each of the NFL + GCL, IPL, and OPL. The patterns of increase in the numbers of microglia in these areas were similar to that of the total number in COH retinas (Fig. 1C–E). In addition, we explored changes in the number of microglia in COH retinas from MacGreen mice, in which the green fluorescent protein (GFP) was highly expressed in retinal microglia, using flow cytometry. The results showed that IOP elevation indeed increased the percentage of GFP-positive microglia in the total number of retinal cells (Fig. S2). Mouse retinal microglia are known to express two purinergic receptor subtypes, the P2X7 receptor (P2X7R) and P2X4R, but only P2X7R has been reported to be involved in RGC injury [32, 33]. Overexpression of P2X7R in rat primary hippocampal cultures drives microglial activation and proliferation [34]. We examined whether P2X7R is involved in the IOP-induced change of microglia. Intraperitoneal injection of brilliant blue G (BBG), an antagonist of P2X7R, significantly, but not completely, inhibited the IOP elevation-induced increase in the total number of microglia (Fig. 1A, a17–a24, and Fig. 1B) (n = 6, P < 0.05). It is interesting that the increase in the number of microglia in the IPL and OPL was completely blocked (n = 6, P < 0.05) (Fig. 1D, E), but only partially reduced in the NFL + GCL (n = 6, P < 0.05 (Fig. 1C). Since microglia of the mouse retina also express P2X4R, we tested whether this receptor is also be involved in microglial proliferation. However, intravitreal injection of 5-BDBD, a P2X4R inhibitor, did not affect the increase in the number of microglia in COH retinas (Fig. 1F) (n = 6, P >0.05). Furthermore, in COH retinas from P2X7R-knockout (P2X7R−/−) mice, the increase in the number of microglia was significantly suppressed (Fig. 1F) (n = 6, P < 0.001). These results suggest that P2X7R indeed mediates the proliferation of microglia in the COH retina, but is unlikely to be involved in their migration.
Fig. 1.
P2X7R mediates the increased number of microglia in COH retinas. A Confocal laser microphotographs of vertical retinal sections stained with the antibody against Iba1 (green), showing the changes in the number of microglia in control (Ctr) (a1–a8), and in COH retinas at different post-operational times (G0d–G6w) without (COH group) (a9–a16) or with BBG injection (COH + BBG group) (a17–a24) (scale bar, 10 μm). B Average numbers of microglia under different conditions as shown in A. C–E Average numbers of microglia in the NFL + GCL (C), IPL (D), and OPL (E) groups under different conditions as shown in A. n = 6 –10. *P < 0.05, **P < 0.01, and ***P < 0.001 vs Ctr; #P < 0.05, ##P < 0.01 and ###P < 0.001 vs COH group. F Average numbers of microglia in COH retinas at different post-operational times (G0d–G2w) with intravitreal injections of saline (COH + Saline), BBG (COH + BBG), 5-BDBD (COH + 5-BDBD), or P2X7R knockout (P2X7R−/− COH). n = 6. ***P < 0.001 vs Ctr, ##P < 0.01 and ###P < 0.001 vs COH + Saline, &&&P < 0.001 vs P2X7R−/− Ctr. COH, chronic ocular hypertension; NFL, nerve fiber layer; GCL, ganglion cell layer; IPL, inner plexiform layer; OPL, outer plexiform layer; ONL, outer nuclear layer.
We then measured changes in the protein levels of Iba1 and P2X7R in COH retinas at G2w, and found that the levels of Iba1 were significantly increased in COH retinas, and this was inhibited by BBG (10 μmol/L) [n = 6, P < 0.001 vs COH; P < 0.05 vs control (Ctr)], but not by 5-BDBD (10 μmol/L) (n = 6, P >0.05 vs COH; P < 0.001 vs Ctr) (Fig. 2A, B). P2X7R knockout did not affect the levels of Iba1. In COH retinas from P2X7R−/− mice, the levels of Iba1 were slightly increased, but were significantly lower than those from COH retinas (n = 6, P < 0.001 vs COH; P < 0.05 vs P2X7R−/− Ctr) (Fig. 2A, B). The changes of P2X7R protein levels were similar to those of Iba1 except in P2X7R−/− mice (Fig. 2A, C). We have shown that P2X4R expression in COH retinas does not significantly change, while in P2X7R-knockout mice P2X4R expression increases in a compensatory manner in COH retinas [35]. These findings further demonstrate that microglial proliferation in the retinas of COH mice is mediated by P2X7R, but not P2X4R.
Fig. 2.
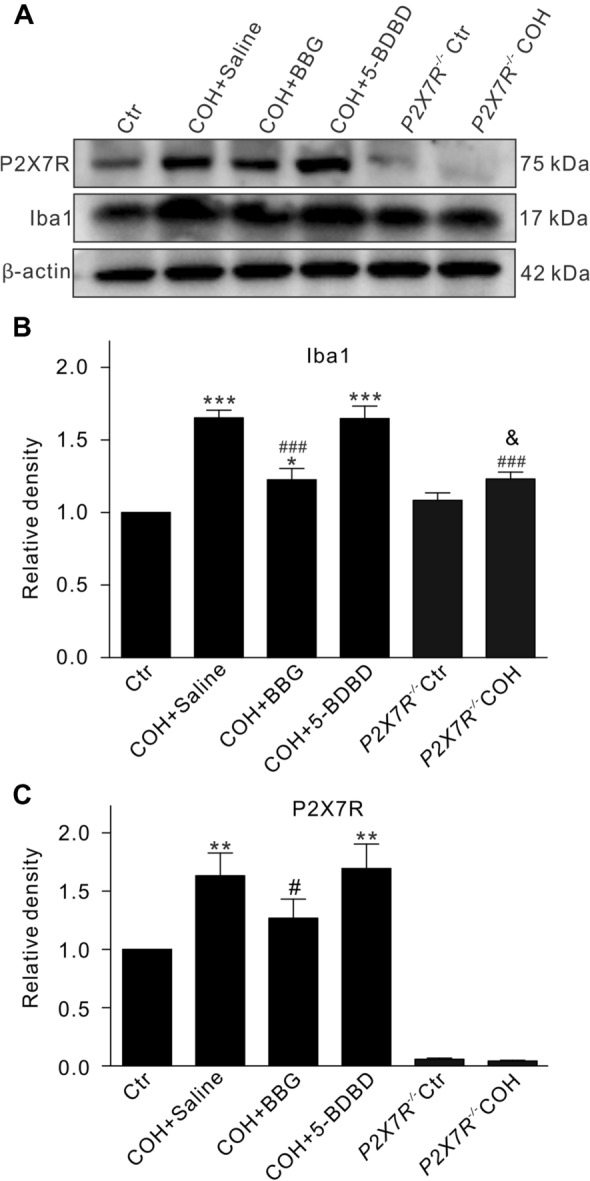
Changes in protein levels of Iba1 and P2X7R in retinas of COH mice. A Representative immunoblots showing the changes in Iba1 and P2X7R levels in retinas from control (Ctr) mice or COH mice with intravitreal injection of saline (COH + Saline), BBG (COH + BBG), 5-BDBD (COH + 5-BDBD), or P2X7R knockout (P2X7R−/− COH). B, C Average densitometric quantification of immunoreactive bands of Iba1 (B) and P2X7R (C) under different conditions as shown in A. All data are normalized to their corresponding β-actin and then to Ctr. n = 6 per group. *P < 0.05, **P < 0.01, and ***P < 0.001 vs Ctr; #P < 0.05 and ###P < 0.001 vs COH + Saline; &P < 0.05 vs P2X7R−/− Ctr.
We then explored how ATP/P2X7R mediates the microglial proliferation in the COH retina. Previous studies have shown that MEK/extracellular signal-regulated protein kinase (ERK) signaling is involved in microglial proliferation [36, 37]. We tested the possible involvement of this signaling in retinal microglia proliferation in primary purified cultured cells by EdU labeling, which showed that microglia treated with the P2X7R agonist BzATP (100 μmol/L) showed increased proliferation (n = 3, P < 0.001 vs Ctr) (Fig. 3Aa6, B), which was reduced by co-application of BBG (10 μmol/L) (n = 3, P < 0.01 vs Ctr and P < 0.001 vs BzATP alone) (Fig. 3Aa7, B). BzATP treatment also induced morphological changes of microglia (Fig. 3Aa10, B), indicative of microglial activation. Co-application of the MEK/ERK antagonist PD98059 (10 μmol/L) significantly inhibited the BzATP-induced microglial proliferation (n = 3, P < 0.001 vs Ctr and P < 0.001 vs BzATP alone) (Fig. 3Aa8, B). It is noted that stimulation of P2X7R also induced microglial activation, which resulted in morphological changes of microglia (Fig. 3Aa10), similar to our previous study [35]. Inhibition of P2X7R by BBG reversed these changes (Fig. 3Aa11). Although inhibition of ERK1/2 activation blocked microglial proliferation, their morphological changes were not completely blocked by the ERK1/2 inhibitor PD98059 (Fig. 3Aa12). We then examined the changes in protein levels of p-ERK1/2 and ERK1/2 in microglia by Western blotting. Figure 3C and D show that BzATP treatment increased the p-ERK/ERK ratio (n = 3, P < 0.001 vs Ctr), which was reversed by co-application of BBG (n = 3, P < 0.001 vs BzATP alone). The MEK/ERK antagonist PD98059 also reduced the BzATP-induced increase of the p-ERK/ERK ratio (n = 3, P < 0.001 vs Ctr; P < 0.001 vs BzATP alone). In contrast, CTP (1 mmol/L), a P2X4R agonist, treatment of primary cultured microglia had no effect on their proliferation (Fig. S3). Moreover, intravitreal injection of BzATP (100 μmol/L, 2 μL) induced an increase in the number of Iba1-positive microglia (n = 6–9, P < 0.05 vs Ctr), while injection of CTP (1 mmol/L, 2 μL) failed to change the number of microglia (n = 6, P >0.05 vs Ctr) (Fig. S4). These results indicate that the ATP/P2X7R/MEK/ERK signaling pathway mediates microglial proliferation in the COH retina.
Fig. 3.
Activation of P2X7R induces retinal microglia proliferation. A Representative images showing double immunofluorescent staining of Iba1 (green) and EdU (red) in primary cultured microglia in controls (Ctr) (a5), and after treatment with BzATP (a6), BzATP + BBG (a7), and BzATP + PD98059 (a8). Nuclei are stained with DAPI. a9–a12 are enlarged images of the white squares in a5–8. Scale bars, 50 μm. B Bar charts summarizing the average relative number of Iba1 and EdU double-labeled microglia under different conditions. All data are normalized to Ctr. n = 3 biological replicates × 3 technical replicates. C Representative immunoblots showing the changes in protein levels of p-ERK1/2 and ERK1/2 in primary cultured microglia in controls (Ctr), and after treatment with BzATP, BzATP + BBG, or BzATP + PD98059. D Ratios of p-ERK/ERK under different conditions as shown in C. All data are normalized to Ctr. n = 3 biological replicates × 3 technical replicates. ***P < 0.001 vs Ctr; ###P < 0.001 vs BzATP alone.
Activated Müller Cells Mediate Microglial Proliferation by Releasing ATP in COH Retina
Our previous studies have shown that Müller cell activation is mediated by group I metabotropic glutamate receptor I (mGluR I) in the COH retina [20, 24]. Activated Müller cells release ATP through connxin43 (Cx43) hemichannels [18, 21, 38, 39]. Indeed, treatment of primary cultured Müller cells with DHPG (100 μmol/L), an mGluR I agonist, intravitreal injection of DHPG (100 μmol/L, 2 μL), or IOP elevation in COH retina increased the expression of Cx43. which was mainly localized at the endfeet of Müller cells, suggesting that there may be a higher ATP concentration in the NFL + GCL (Fig. S5). Although mGluR I is also expressed in microglia [40, 41], their treatment with DHPG (100 μmol/L) for different times did not affect the number of EdU-labeled microglia (n = 3, P >0.05 vs Ctr), suggesting that activation of mGluR I has no direct effect on microglial proliferation (Fig. S6). However, intravitreal injection of DHPG (100 μmol/L, 2 μL) to activate Müller cells [19, 20] significantly increased the total number of microglia at 4 days to 2 weeks after the injection (n = 6, P all < 0.001 vs saline-injected group), indicating that activated Müller cells induce microglial proliferation (Fig. 4). Co-injection of the Cx43 blocker Gap26 (200 μmol/L, 2 μL) or BBG (10 μmol/L, 2 μL) reversed the DHPG-induced changes in the total number of microglia (n = 6, P < 0.01 or 0.001 vs DHPG alone), while co-injection of 5-BDBD (10 μmol/L, 2 μL) failed to do so (Fig. 4). These results indicate that activated Müller cells may induce microglial proliferation by releasing ATP, which then acts on P2X7Rs expressed on microglia.
Fig. 4.
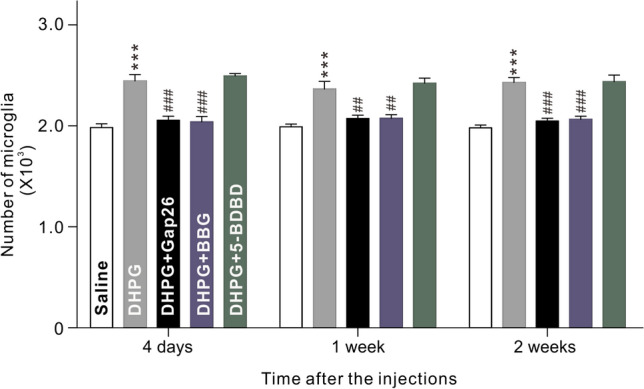
Activation of Müller cells induces proliferation of microglia through ATP/P2X7R. Total numbers of microglia in vertical retinal sections taken from mice injected intravitreally with saline (control), DHPG, DHPG + Gap26, DHPG + BBG, or DHPG + 5-BDBD at 4 days, 1 week, and 2 weeks after injection. n = 6 per group. ***P < 0.001 vs Saline, ##P < 0.01 and ###P < 0.001 vs DHPG alone.
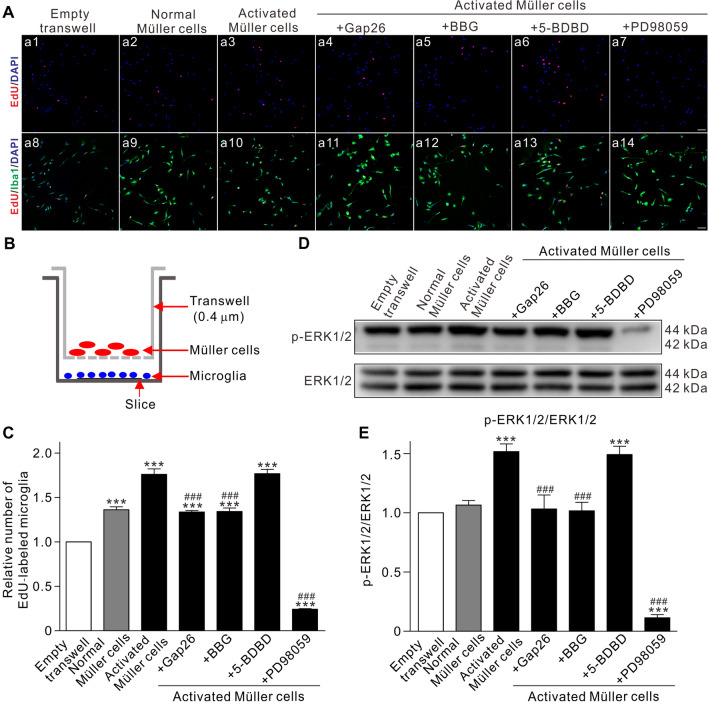
A transwell co-culture system was used to further confirm the Müller cell-mediated proliferation of microglia. As shown in Fig. 5A and C, co-culture of purified primary retinal microglia with normal Müller cells resulted in an increase in microglial proliferation (n = 3, P < 0.001 vs Empty transwell), which was further boosted when co-cultured with Müller cells activated by 100 μmol/L DHPG (n = 3, P < 0.001 vs Empty transwell, and P < 0.001 vs normal Müller cells). When activated Müller cells were pre-treated with Gap26 (200 μmol/L) and then co-cultured with microglia, activated Müller cell-induced microglial proliferation was blocked (n = 3, P < 0.001 vs activated Müller cells alone). Similarly, when microglia were pre-treated with BBG (10 μmol/L) or PD98059 (10 μmol/L) and then co-cultured with activated Müller cells, the microglial proliferation was reversed (n = 3, P all < 0.001 vs activated Müller cells alone), while pre-treatment with 5-BDBD (10 μmol/L) did not affect microglial proliferation (n = 3, P >0.05 vs activated Müller cells alone) (Fig. 5A, C). Changes in the ratio of p-ERK1/2 to ERK1/2, tested by Western blotting, were basically similar to those of microglial proliferation (Fig. 5D, E). These results provide further evidence showing that activated Müller cells induce microglial proliferation through the Cx43/ATP/P2X7R/MEK/ERK pathway in the COH retina. It should be noted that in this study, the in vitro experiments were done in the presence of serum. The higher level of basal p-ERK1/2 may be a result of stimulation from the serum.
Fig. 5.
Activated Müller cells induce proliferation in primary cultured microglia via the ATP/P2X7R/MEK/ERK pathway. A Representative images showing double immunofluorescent staining of Iba1 (green) and EdU (red) in primary cultured microglia in the transwell co-culture system. Microglia are co-cultured with Empty Transwell (control) (a8), normal Müller cells (a9), activated Müller cells without (a10) or with Gap26 pre-treatment (a11), BBG (a12), 5-BDBD (a13), and PD98059 (a14). Nuclei are stained with DAPI. Scale bars, 50 μm. B Diagrammatic sketch showing the transwell co-culture system. C Relative average numbers of Iba1 and Edu double-labeled microglia under different conditions as shown in A. D Representative immunoblots showing the changes in protein levels of p-ERK1/2 and ERK1/2 in primary cultured microglia under different treatments similar to A. E Average ratios of p-ERK/ERK. All data are normalized to the Empty Transwell group. n = 3 biological replicates × 3 technical replicates. ***P < 0.001 vs Empty Transwell, ###P < 0.001 vs Activated Müller cells group.
P2X4R/P2X7R Mediates Microglial Migration in Retina of COH Mice
The results in Fig. 1 showed that BBG completely blocked the increase in the number of microglia in the IPL and OPL, but partially blocked the increase in the NFL + GCL, suggesting that microglia migrate to this area, so we explored the mechanisms of microglial migration by transwell assay. As shown in Fig. 6A and 6B, BzATP (100 μmol/L) treatment of primary cultured microglia for 6 h induced a significant increase in cell migration (n = 3, P < 0.001 vs Ctr), which was partially inhibited by pre-application of BBG (10 μmol/L) (n = 3, P < 0.05 vs BzATP alone; P < 0.001 vs Ctr). CTP (1 mmol/L) treatment of primary cultured microglia for 6 h also induced a significant increase of cell migration (n = 3, P < 0.001 vs Ctr), which was completely blocked by 5-BDBD (10 μmol/L) (n = 3, P < 0.001 vs CTP alone; P >0.05 vs Ctr) (Fig. 6C, D). Furthermore, in COH retinas, the number of microglia in the NFL + GCL were counted after different treatments (Fig. 6E). The changes in microglial number were similar to that shown in Fig. 1 when BBG (10 μmol/L, 2 μL) was injected. Similarly, in COH retinas from P2X7R−/− mice, the number of microglia was also significantly reduced (n = 6, P < 0.001 vs COH saline). Interestingly, intravitreal injection of 5-BDBD (10 μmol/L, 2 μL) significantly reduced the number of microglia in the NFL + GCL (n = 6, P < 0.01 vs COH saline) (Fig. 6F). These results indicate that the increase of microglial in the NFL + GCL may be due to both cell proliferation and migration in COH mice, and this is mediated by both P2X7R and P2X4R, the latter being predominant.
Fig. 6.
P2X4R/P2X7R mediates microglial migration in the COH retina. A Representative images of transwell migration assays showing changes in microglial migration in control (Ctr), BzATP, and BzATP+BBG treatment groups (scale bar, 20 μm). B Average relative migration of microglia under different conditions as shown in A. C Representative images showing changes in microglial migration in control (Ctr), CTP, and CTP + 5-BDBD treatment groups, respectively (scale bar, 20 μm). D Average relative migration of microglia under different conditions as shown in C. All data are normalized to the Ctr group. n = 3 biological replicates × 3 technical replicates. ***P < 0.001 vs Ctr; #P < 0.05 vs CTP. E Confocal laser microphotographs of vertical retinal sections stained with the antibody against Iba1 (green), showing changes in the numbers of microglia in control (Ctr) (e1), and COH retinas after intravitreal injection of saline (COH saline) (e2) or BBG (COH + BBG) (e3), or 5-BDBD (COH + 5-BDBD) (e4), and in P2X7R-knockout mice without COH (P2X7R−/− Ctr) (e5) or with COH (P2X7R−/− COH) (e6) (scale bar, 10 μm). F Average numbers of microglia in the NFL + GCL under different conditions as shown in E. n = 6 per group. ***P < 0.001 vs Ctr; ##P < 0.01 and ###P < 0.001 vs COH + Saline; &&&P < 0.001 vs P2X7R−/− Ctr.
Activated Müller Cells Mediate Microglial Migration in COH Retina
We then tested whether activated Müller cells mediate microglial migration by counting the number of microglia in the NFL + GCL. Intravitreal injection of DHPG (100 μmol/L, 2 μL) in normal mice significantly increased the number of microglia in the NFL + GCL [n = 6, P < 0.001 vs Ctr (saline)], and this was completely blocked by co-application of Gap26 (200 μmol/L, 2 μL) (n = 6, P < 0.01 vs DHPG alone) or BBG (10 μmol/L, 2 μL) (n = 6, P < 0.001 vs DHPG group alone), and partially blocked by co-application of 5-BDBD (10 μmol/L, 2 μL) (n = 6, P < 0.001 vs DHPG alone) (Fig. 7A, B). These results indicate that ATP, released from activated Müller cells through the Cx43 hemichannel, induces the accumulation of microglia (including proliferation and migration) in the NFL + GCL through activating P2X7R/P2X4R.
Fig. 7.
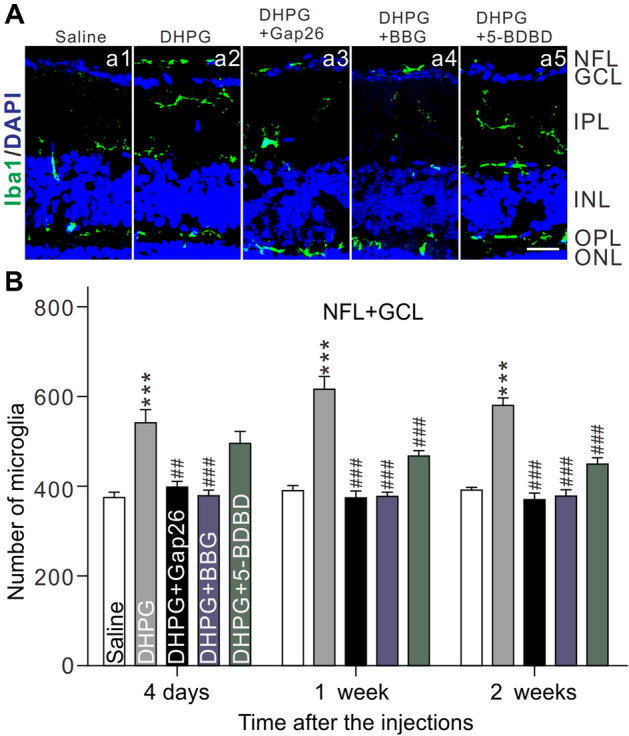
Activation of Müller cells induces microglia migration in retinas through P2X4R/P2X7R. A Representative microphotographs of vertical retinal sections stained with the antibody against Iba1 (green), showing changes in the numbers of microglia in retinas after intravitreal injection of saline (control) (a2), DHPG (a2), DHPG + Gap26 (a3), DHPG + BBG (a4), or DHPG + 5-BDBD (a5) (scale bar, 10 μm). B Average numbers of microglia in the NFL + GCL under different conditions as shown in A. n = 6. ***P < 0.001 vs Saline group, ###P < 0.001 vs DHPG alone.
The transwell chemotaxis assay was applied to further confirm the effect of activated Müller cells on microglial migration. As shown in Fig. 8A–C, no change of microglial migration was found when co-cultured with normal Müller cells for 24 h (n = 3, P >0.05 vs Empty group), while a significant increase in microglial migration occurred when co-cultured with Müller cells activated by 100 μmol/L DHPG (n = 3, P < 0.001 vs Empty group). Activated Müller cells were pre-treated with Gap26 (200 μmol/L) for 2 h (Activated Müller cells + Gap26 group) or microglia were pre-treated with 5-BDBD (10 μmol/L) for 2 h (Activated Müller cells + 5-BDBD group), and then these cells were co-cultured. The increased microglia migration was no longer found (n = 3, P all < 0.001 vs Activated Müller cell group) (Fig. 8A, C). However, BBG (10 μmol/L) pre-treatment of microglia did not block the effect of activated Müller cells (n = 3, P >0.05 vs Activated Müller cells group; P < 0.001 vs Empty group) (Fig. 8A, C).
Fig. 8.
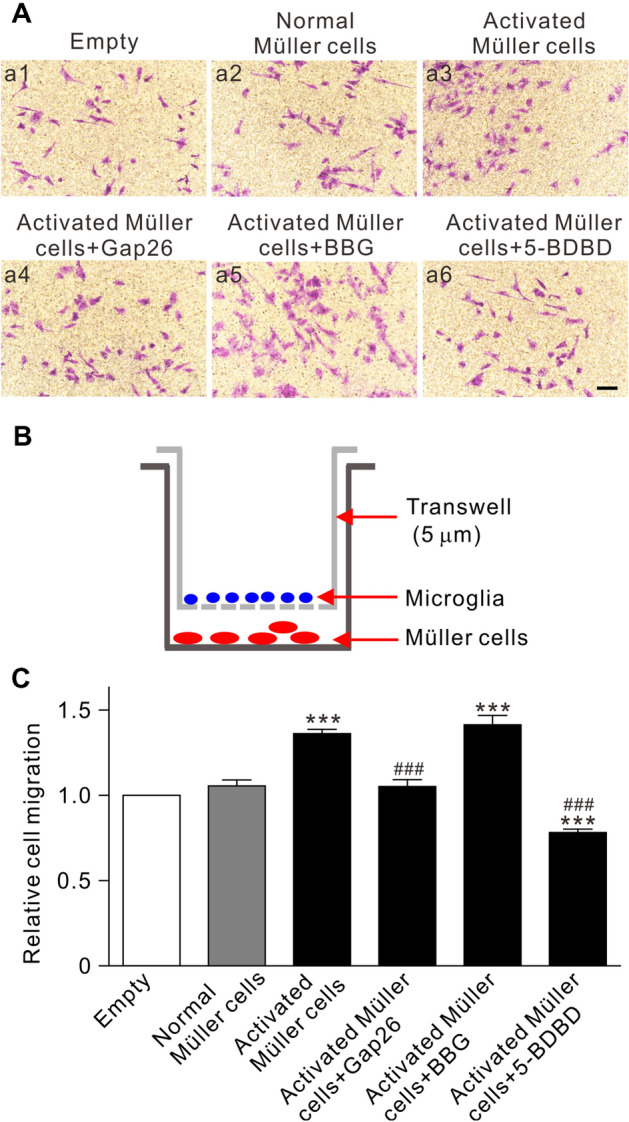
Activated Müller cells mediate the migration of primary cultured microglia. A Representative images showing changes in the numbers of primary cultured microglia migrating in the transwell co-culture system. Microglia are co-cultured with Empty (control) (a1), normal Müller cells (a2), activated Müller cells without (a3) or with pre-treatment of Gap26 (a4), BBG (a5), and 5-BDBD (a6) (scale bar, 20 μm). B Diagrammatic sketch showing the transwell migration assay system. C Average relative migration of microglia under different conditions as shown in A. All data are normalized to the Empty group. n = 3 biological replicates × 3 technical replicates. ***P < 0.001 vs Empty group, ###P < 0.001 vs Activated Müller cell group.
Discussion
ATP/P2X7R/MEK/ERK Pathway Mediates Microglial Proliferation in COH Retina
Neuroinflammation is one of the important factors in the pathogenesis of glaucoma [42], and is mainly mediated by activated retinal glial cells. Activated retinal glial cells promote widespread inflammatory responses by releasing excessive pro-inflammatory factors, such as TNF-α, ILs, and NO, thus aggravating a cytotoxic reaction and RGC injury [1–5, 43]. As the resident immune cells in the retina, microglia are activated in the glaucomatous retina, and these activated glial cells are the main source of inflammatory factors [9–11, 13, 44]. Microglial proliferation has also been reported in retinas with experimental glaucoma [28, 45]. Similarly, in this study, we showed that IOP elevation in COH mice induced an increase in the number of EdU-labeled microglia, suggestive of cell proliferation. This may be one of the important factors for the retinal inflammatory response in glaucoma. We provide evidence in vivo and in vitro demonstrating that it was ATP, released by the activated Müller cells, that induced microglial proliferation through activating P2X7Rs. First, intraperitoneal or intravitreal injection of the P2X7R blocker BBG significantly inhibited the proliferation of microglia induced by IOP elevation. Knockout of P2X7R had a similar effect. Although P2X4Rs were also expressed in microglia, this receptor was unlikely to be involved in microglial proliferation because the P2X4R blocker 5-BDBD had almost no effect on the increased number of microglia in COH retinas (Fig. 1). It should be noted that either BBG or knockout of P2X7R did not completely inhibit the proliferation of microglia, suggesting that other factors may be involved in the proliferation in addition to the ATP/P2X7R signaling pathway. On the other hand, previous studies have shown that under some pathological conditions or eliminating retinal resident microglia, peripheral monocytes/macrophages or residual microglia in the optic nerve can infiltrate the retina [46, 47]. Therefore, the increased number of Iba1+ cells in the NFL/GCL in the COH retina may include infiltrated peripheral monocytes/macrophages. These issues will be explored in the future. Second, in the normal retina, intravitreal injection of the P2X7R agonist BzATP, but not the P2X4R agonist CTP, mimicked the effect of COH on microglial proliferation (Fig. S3). Indeed, it has been reported that overexpression of P2X7R in rat primary hippocampal cultures is enough to induce cell proliferation [34]. Elevated expression of P2X7R was found in our COH retinas. Furthermore, intravitreal injection of DHPG to activate Müller cells increased the proliferation of microglia, which was inhibited by Gap26 and BBG, but not by 5-BDBD (Fig. 4). This was further confirmed in the transwell co-culture system in vitro (Fig. 5). Moreover, in line with previous reports [36, 37], our results showed that the MEK/ERK signaling pathway is involved in the proliferation of microglia in COH retinas. It is interesting that the MEK/ERK signaling blocker PD98059 not only completely inhibited the P2X7R-mediated microglial proliferation, but also reduced the number of microglia to levels lower than that of controls. We speculate that basal levels of MEK/ERK in microglia play an important role in maintaining normal cell proliferation. MEK/ERK activation may be mediated by a Ca2+-dependent pathway. In a recent study, we have shown that activation of P2X7R increases intracellular Ca2+ levels [35]. Evidence also shows that stimulation of P2X7R elevates the levels of intracellular Ca2+, thus activating the MEK/ERK signaling pathway [48, 49].
P2X4R/P2X7R Mediates Microglial Migration in COH Retina
Cell migration is a complex process that plays an important role in a variety of physiological and pathological processes [50]. In this study, we showed that microglia migrated to the NFL + GCL in the COH retina, in which glaucoma-induced RGCs occurred, similar to other reports [12]. The migrated and proliferated microglia in the NFL + GCL, together with microglial activation, may enhance phagocytosis, and they may secrete cytokines, chemokines, and neurotoxins, thus contributing to RGC damage. Our present results showed that both P2X4R and P2X7R participated in the microglial migration to the NFL + GCL in experimental glaucoma, P2X4R dominating, as evidenced by in vivo and in vitro experiments. First, in the COH retinas, BBG or P2X7R knockout completely blocked the increased number of microglia in the IPL and OPL, but partially in the NFL+GCL (Figs 1 and 6), suggesting that the increased number of microglia in the NFL + GCL may be due to both the migration and proliferation of cells. Intravitreal injection of 5-BDBD did not inhibit the total number of microglia (Fig. 1), but partially reduced the number in the NFL + GCL (Fig. 6). Considering the fact that P2X4R does not participate in the proliferation of microglia, it is likely that P2X4R mediated the migration of microglia in the COH retina. Second, the transwell chemotaxis assay showed that CTP treatment significantly increased the migration of primary cultured microglia, and the effects were fully reversed by 5-BDBD. Interestingly, the P2X7R agonist BzATP also induced microglial migration, but was only slightly suppressed by BBG (Fig. 6). This result suggests that P2X7R is also involved in microglia migration to a certain extent. Another possibility is that BzATP may have a weak effect on P2X4Rs [51, 52]. Nevertheless, the P2X4R is the dominant factor that mediates the migration of microglia. Similarly, P2X4R-mediated chemotaxis has been reported in primary cultured microglia from the rat cerebral cortex [53]. Finally, activated Müller cells mediated microglial migration through releasing ATP. We showed that the expression of Cx43 was increased at the endfeet of activated Müller cells, and this may lead to an ATP concentration gradient to induce microglial migration to the NFL + GCL (Fig. S4). We did find that intravitreal injection of DHPG induced an increase in the number of microglia in the NFL + GCL that was completely inhibited by Gap26 and partially inhibited by 5-BDBD (Fig. 7). In the transwell chemotaxis assay, activated Müller cells significantly increased microglial migration, which was completely inhibited by Gap26 and 5-BDBD. Indeed, in the retina Cx43 is mainly expressed in glial cells; it consists of both connexon and hemichannel. Under pathological conditions, including glaucoma and diabetic retinopathy, up-regulated Cx43 expression and hemichannel opening have been implicated in retinal neuronal death through activating glial cells, dysfunction of vascular integrity, and retinal edema [54–57]. A question is how Müller cells sense mechanical information in the COH retina. In the retina, Müller cells express mechanosensitive ion channels, such as transient vanilloid isoform 4 and pannexin-1.We speculate that IOP elevation can activate these channels, thus mediating Ca2+ influx and inducing ATP release [58–61]. In addition, we have previously demonstrated that in the COH retina, Müller cell gliosis is mediated by activation of mGluR I through the intracellular Ca2+-dependent PLC/IP3-ryanodine/PKC signaling pathway [20]. Treatment of purified cultured Müller cells with the mGluR I agonist DHPG induced an increase in ATP release, which is mediated by the mGluR5/Gq/PI-PLC/PKC signaling pathway [21]. Therefore, it is possible that increased PKC activity when mGluR I is activated may regulate Cx43-mediated ATP release. The detailed mechanisms will be explored in future studies. It should be noted that BBG completely blocked the DHPG-induced increase in the number of microglia in the NFL + GCL and 5-BDBD did not significantly reduce the number at 4 days after the DHPG injection (Fig. 7). In addition, in the transwell chemotaxis assay, BBG did not affect the microglial migration induced by pre-activated Müller cells (Fig. 8). We speculate that activated Müller cells induced by DHPG injection mainly induce microglial proliferation at day 4, while at 1 and 2 weeks, both the proliferation and migration of microglia occur and proliferation may be the primary event. It should be noted that in the central nervous system, P2Y12Rs may play important roles in microglial activation and translocation, in addition to P2X7R [53, 62, 63]. Although P2X12R is also expressed in mouse retinal microglia [64], it seems unlikely that this receptor is involved in microglial proliferation and migration because the P2X7R antagonist BBG and the P2X4R antagonist 5-BDBD completely blocked the activated Müller cell-induced microglial proliferation and migration, respectively. In addition, the migration of microglia needs complex interactions between cells and intercellular molecules, such as chemokines [12], which may be elucidated in future studies.
Apoptotic death of RGCs is a fundamental characteristic of glaucoma. We have previously shown RGC apoptotic death in mouse COH experimental glaucoma [22]. It should be noted that ATP released from Müller cells can directly activate P2X7Rs expressed on RGCs and trigger RGC death [21, 65, 66], in addition to the induction of microglial proliferation. Increasing evidence has demonstrated that glial responses (such as activation, proliferation, migration, and interaction among glial cells) in glaucomatous retinas are involved in RGC damage [1–4, 12, 16, 17, 67]. Many studies, including our work [35], have shown that P2X7R plays an important role in microglial activation [68, 69] and the interaction of Müller cells and microglia, which could aggravate the retinal inflammatory response and RGC injury in the COH retina [33, 35]. Therefore, in our opinion, P2X7Rs expressed in both RGCs and microglia play an important role in glaucoma pathology. Inhibition of Müller cell activation by the mGluR5 antagonist MPEP or inhibition of microglial activation/proliferation by the P2X7R antagonist BBG indeed reduces RGC apoptosis in the COH retina [35]. In a rat COH model, there is also evidence showing that the P2X7R antagonist BBG reduces RGC apoptosis by inhibiting microglial activation [69]. In purified cultured Müller cells, the mGluR I agonist DHPG treatment induces an increase in ATP release, which up-regulates P2X7R expression and induces RGC death [21, 67]. All these studies, together with our present work, suggest that activation of Müller cells in the COH retina induces microglial proliferation and migration through ATP-P2X7/P2X4R signaling pathways, thus contributing to RGC damage.
In summary, our results demonstrate that in the retinas of COH experimental glaucoma mice, activated Müller cells release ATP through Cx43 hemichannel; in turn, ATP acts on P2X7Rs to induce microglial proliferation through the intracellular MEK/ERK signaling pathway. At the same time, ATP acts on P2X4Rs and P2X7Rs (mainly P2X4Rs) to induce microglial migration. Inhibiting the interaction of Müller cells and microglia may be an effective strategy to attenuate microglial proliferation and migration, thus reducing the RGC damage in glaucoma.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
We thanks Dr. Xiong-Li Yang for helpful discussion and critical comments on the manuscript. This work was supported by grants from the National Natural Science Foundation of China (81790642 and 31872765), the Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), ZJ Lab, and the Shanghai Center for Brain Science and Brain-Inspired Technology.
Conflict of interest
The authors declare no potential conflict of interest.
References
- 1.Bringmann A, Reichenbach A. Role of Muller cells in retinal degenerations. Front Biosci. 2001;6:E72–E92. doi: 10.2741/Bringman. [DOI] [PubMed] [Google Scholar]
- 2.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, et al. Cellular signaling and factors involved in Müller cell gliosis: Neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–451. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Cuenca N, Fernández-Sánchez L, Campello L, Maneu V, de la Villa P, Lax P, et al. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog Retin Eye Res. 2014;43:17–75. doi: 10.1016/j.preteyeres.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Silverman SM, Wong WT. Microglia in the retina: Roles in development, maturity, and disease. Annu Rev Vis Sci. 2018;4:45–77. doi: 10.1146/annurev-vision-091517-034425. [DOI] [PubMed] [Google Scholar]
- 6.Rathnasamy G, Foulds WS, Ling EA, Kaur C. Retinal microglia - A key player in healthy and diseased retina. Prog Neurobiol. 2019;173:18–40. doi: 10.1016/j.pneurobio.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Vecino E, Rodriguez FD, Ruzafa N, Pereiro X, Sharma SC. Glia-neuron interactions in the mammalian retina. Prog Retin Eye Res. 2016;51:1–40. doi: 10.1016/j.preteyeres.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Reichenbach A, Bringmann A. Glia of the human retina. Glia. 2020;68:768–796. doi: 10.1002/glia.23727. [DOI] [PubMed] [Google Scholar]
- 9.Bordone MP, González Fleitas MF, Pasquini LA, Bosco A, Sande PH, Rosenstein RE, et al. Involvement of microglia in early axoglial alterations of the optic nerve induced by experimental glaucoma. J Neurochem. 2017;142:323–337. doi: 10.1111/jnc.14070. [DOI] [PubMed] [Google Scholar]
- 10.Bosco A, Steele MR, Vetter ML. Early microglia activation in a mouse model of chronic glaucoma. J Comp Neurol. 2011;519:599–620. doi: 10.1002/cne.22516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebneter A, Casson RJ, Wood JP, Chidlow G. Microglial activation in the visual pathway in experimental glaucoma: Spatiotemporal characterization and correlation with axonal injury. Invest Ophthalmol Vis Sci. 2010;51:6448–6460. doi: 10.1167/iovs.10-5284. [DOI] [PubMed] [Google Scholar]
- 12.Langmann T. Microglia activation in retinal degeneration. J Leukoc Biol. 2007;81:1345–1351. doi: 10.1189/jlb.0207114. [DOI] [PubMed] [Google Scholar]
- 13.Yuan L, Neufeld AH. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64:523–532. doi: 10.1002/jnr.1104. [DOI] [PubMed] [Google Scholar]
- 14.Wang MH, Wang X, Zhao L, Ma WX, Rodriguez IR, Fariss RN, et al. Macroglia-microglia interactions via TSPO signaling regulates microglial activation in the mouse retina. J Neurosci. 2014;34:3793–3806. doi: 10.1523/JNEUROSCI.3153-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chong RS, Martin KR. Glial cell interactions and glaucoma. Curr Opin Ophthalmol. 2015;26:73–77. doi: 10.1097/ICU.0000000000000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang MH, Wong WT. Microglia-Müller cell interactions in the retina. Adv Exp Med Biol. 2014;801:333–338. doi: 10.1007/978-1-4614-3209-8_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang MH, Ma WX, Zhao L, Fariss RN, Wong WT. Adaptive Müller cell responses to microglial activation mediate neuroprotection and coordinate inflammation in the retina. J Neuroinflammation. 2011;8:173. doi: 10.1186/1742-2094-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Müller cells. J Neurosci. 2001;21:2215–2223. doi: 10.1523/JNEUROSCI.21-07-02215.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao F, Li F, Miao YY, Xu LJ, Zhao Y, Li Q, et al. Involvement of the MEK-ERK/p38-CREB/c-fos signaling pathway in Kir channel inhibition-induced rat retinal Müller cell gliosis. Sci Rep. 2017;7:1480. doi: 10.1038/s41598-017-01557-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji M, Miao YY, Dong LD, Chen J, Mo XF, Jiang SX, et al. Group I mGluR-mediated inhibition of Kir channels contributes to retinal Müller cell gliosis in a rat chronic ocular hypertension model. J Neurosci. 2012;32:12744–12755. doi: 10.1523/JNEUROSCI.1291-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue B, Xie YT, Xue Y, Hu N, Zhang GW, Guan HJ, et al. Involvement of P2X 7 receptors in retinal ganglion cell apoptosis induced by activated Müller cells. Exp Eye Res. 2016;153:42–50. doi: 10.1016/j.exer.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Zhang ML, Zhao GL, Hou Y, Zhong SM, Xu LJ, Li F, et al. Rac1 conditional deletion attenuates retinal ganglion cell apoptosis by accelerating autophagic flux in a mouse model of chronic ocular hypertension. Cell Death Dis. 2020;11:734. doi: 10.1038/s41419-020-02951-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Miao YY, Wang XH, Wang ZF. Elevation of p-NR2A(S1232) by Cdk5/p35 contributes to retinal ganglion cell apoptosis in a rat experimental glaucoma model. Neurobiol Dis. 2011;43:455–464. doi: 10.1016/j.nbd.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Dong LD, Gao F, Wang XH, Miao YY, Wang SY, Wu Y, et al. GluA2 trafficking is involved in apoptosis of retinal ganglion cells induced by activation of EphB/EphrinB reverse signaling in a rat chronic ocular hypertension model. J Neurosci. 2015;35:5409–5421. doi: 10.1523/JNEUROSCI.4376-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu ST, Zhong SM, Li XY, Gao F, Li F, Zhang ML, et al. EphrinB/EphB forward signaling in Müller cells causes apoptosis of retinal ganglion cells by increasing tumor necrosis factor alpha production in rat experimental glaucomatous model. Acta Neuropathol Commun. 2018;6:111. doi: 10.1186/s40478-018-0618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao F, Li F, Miao YY, Dong LD, Zhang SH, Wu JH, et al. Group I metabotropic glutamate receptor agonist DHPG modulates Kir4.1 protein and mRNA in cultured rat retinal Müller cells. Neurosci Lett. 2015;588:12–17. doi: 10.1016/j.neulet.2014.12.048. [DOI] [PubMed] [Google Scholar]
- 27.Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Gonçalves N, et al. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. J Neuroinflammation. 2013;10:16. doi: 10.1186/1742-2094-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inman DM, Horner PJ. Reactive nonproliferative gliosis predominates in a chronic mouse model of glaucoma. Glia. 2007;55:942–953. doi: 10.1002/glia.20516. [DOI] [PubMed] [Google Scholar]
- 29.Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp 2014, 88: 51046. [DOI] [PMC free article] [PubMed]
- 30.Zhu K, Hu X, Chen H, Li F, Yin N, Liu AL, et al. Downregulation of circRNA DMNT3B contributes to diabetic retinal vascular dysfunction through targeting miR-20b-5p and BAMBI. EBioMedicine. 2019;49:341–353. doi: 10.1016/j.ebiom.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiong GY, Yang LH, Chen Y, Fan ZN. Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution. Am J Transl Res. 2015;7:2262–2269. [PMC free article] [PubMed] [Google Scholar]
- 32.Ho T, Vessey KA, Fletcher EL. Immunolocalization of the P2X4 receptor on neurons and glia in the mammalian retina. Neuroscience. 2014;277:55–71. doi: 10.1016/j.neuroscience.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 33.Zhang YJ, Xu Y, Sun Q, Xue SD, Guan HJ, Ji M. Activation of P2X7R- NLRP3 pathway in Retinal microglia contribute to Retinal Ganglion Cells death in chronic ocular hypertension (COH) Exp Eye Res. 2019;188:107771. doi: 10.1016/j.exer.2019.107771. [DOI] [PubMed] [Google Scholar]
- 34.Monif M, Reid CA, Powell KL, Smart ML, Williams DA. The P2X7 receptor drives microglial activation and proliferation: A trophic role for P2X7R pore. J Neurosci. 2009;29:3781–3791. doi: 10.1523/JNEUROSCI.5512-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X, Zhao GL, Xu MX, Zhou H, Li F, Miao YY, et al. Interplay between Müller cells and microglia aggravates retinal inflammatory response in experimental glaucoma. J Neuroinflammation. 2021;18:303. doi: 10.1186/s12974-021-02366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvo M, Zhu N, Grist J, Ma ZZ, Loeb JA, Bennett DL. Following nerve injury neuregulin-1 drives microglial proliferation and neuropathic pain via the MEK/ERK pathway. Glia. 2011;59:554–568. doi: 10.1002/glia.21124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang W, Ji P, Dow KE. Corticotropin-releasing hormone induces proliferation and TNF-alpha release in cultured rat microglia via MAP kinase signalling pathways. J Neurochem. 2003;84:189–195. doi: 10.1046/j.1471-4159.2003.01544.x. [DOI] [PubMed] [Google Scholar]
- 38.Uckermann O, Wolf A, Kutzera F, Kalisch F, Beck-Sickinger AG, Wiedemann P, et al. Glutamate release by neurons evokes a purinergic inhibitory mechanism of osmotic glial cell swelling in the rat retina: Activation by neuropeptide Y. J Neurosci Res. 2006;83:538–550. doi: 10.1002/jnr.20760. [DOI] [PubMed] [Google Scholar]
- 39.Voigt J, Grosche A, Vogler S, Pannicke T, Hollborn M, Kohen L, et al. Nonvesicular release of ATP from rat retinal glial (Müller) cells is differentially mediated in response to osmotic stress and glutamate. Neurochem Res. 2015;40:651–660. doi: 10.1007/s11064-014-1511-z. [DOI] [PubMed] [Google Scholar]
- 40.Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI, Faden AI. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia. 2009;57:550–560. doi: 10.1002/glia.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loane DJ, Stoica BA, Pajoohesh-Ganji A, Byrnes KR, Faden AI. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J Biol Chem. 2009;284:15629–15639. doi: 10.1074/jbc.M806139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM. Inflammation in Glaucoma: From the back to the front of the eye, and beyond. Prog Retin Eye Res. 2021;83:100916. doi: 10.1016/j.preteyeres.2020.100916. [DOI] [PubMed] [Google Scholar]
- 43.Yuan L, Neufeld AH. Tumor necrosis factor-alpha: A potentially neurodestructive cytokine produced by glia in the human glaucomatous optic nerve head. Glia. 2000;32:42–50. doi: 10.1002/1098-1136(200010)32:1<42::AID-GLIA40>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Madeira MH, Boia R, Santos PF, Ambrósio AF, Santiago AR. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediators Inflamm. 2015;2015:673090. doi: 10.1155/2015/673090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lozano DC, Choe TE, Cepurna WO, Morrison JC, Johnson EC. Early optic nerve head glial proliferation and jak-stat pathway activation in chronic experimental glaucoma. Invest Ophthalmol Vis Sci. 2019;60:921–932. doi: 10.1167/iovs.18-25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang YB, Xu Z, Xiong SS, Qin GR, Sun FF, Yang J, et al. Dual extra-retinal origins of microglia in the model of retinal microglia repopulation. Cell Discov. 2018;4:9. doi: 10.1038/s41421-018-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paschalis EI, Lei FY, Zhou CX, Kapoulea V, Thanos A, Dana R, et al. The role of microglia and peripheral monocytes in retinal damage after corneal chemical injury. Am J Pathol. 2018;188:1580–1596. doi: 10.1016/j.ajpath.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsao HK, Chiu PH, Sun SH. PKC-dependent ERK phosphorylation is essential for P2X7 receptor-mediated neuronal differentiation of neural progenitor cells. Cell Death Dis. 2013;4:e751. doi: 10.1038/cddis.2013.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stefano L, Rössler OG, Griesemer D, Hoth M, Thiel G. P2X7 receptor stimulation upregulates Egr-1 biosynthesis involving a cytosolic Ca2+ rise, transactivation of the EGF receptor and phosphorylation of ERK and Elk-1. J Cell Physiol. 2007;213:36–44. doi: 10.1002/jcp.21085. [DOI] [PubMed] [Google Scholar]
- 50.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/S0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 51.Asatryan L, Ostrovskaya O, Lieu D, Davies DL. Ethanol differentially modulates P2X4 and P2X7 receptor activity and function in BV2 microglial cells. Neuropharmacology. 2018;128:11–21. doi: 10.1016/j.neuropharm.2017.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burnstock G. Introduction to the special issue on purinergic receptors. Adv Exp Med Biol. 2017;1051:1–6. doi: 10.1007/5584_2017_12. [DOI] [PubMed] [Google Scholar]
- 53.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 54.Danesh-Meyer HV, Zhang J, Acosta ML, Rupenthal ID, Green CR. Connexin43 in retinal injury and disease. Prog Retin Eye Res. 2016;51:41–68. doi: 10.1016/j.preteyeres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Kerr NM, Johnson CS, Zhang J, Eady EK, Green CR, Danesh-Meyer HV. High pressure-induced retinal ischaemia reperfusion causes upregulation of gap junction protein connexin43 prior to retinal ganglion cell loss. Exp Neurol. 2012;234:144–152. doi: 10.1016/j.expneurol.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 56.Danesh-Meyer HV, Kerr NM, Zhang J, Eady EK, O’Carroll SJ, Nicholson LFB, et al. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain. 2012;135:506–520. doi: 10.1093/brain/awr338. [DOI] [PubMed] [Google Scholar]
- 57.Huang X, Wang N, Xiao X, Li S, Zhang Q. A novel truncation mutation in GJA1 associated with open angle glaucoma and microcornea in a large Chinese family. Eye (Lond) 2015;29:972–977. doi: 10.1038/eye.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, et al. From mechanosensitivity to inflammatory responses: New players in the pathology of glaucoma. Curr Eye Res. 2014;39:105–119. doi: 10.3109/02713683.2013.836541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryskamp DA, Jo AO, Frye AM, Vazquez-Chona F, MacAulay N, Thoreson WB, et al. Swelling and eicosanoid metabolites differentially gate TRPV4 channels in retinal neurons and glia. J Neurosci. 2014;34:15689–15700. doi: 10.1523/JNEUROSCI.2540-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jo AO, Ryskamp DA, Phuong TTT, Verkman AS, Yarishkin O, MacAulay N, et al. TRPV4 and AQP4 channels synergistically regulate cell volume and calcium homeostasis in retinal Müller glia. J Neurosci. 2015;35:13525–13537. doi: 10.1523/JNEUROSCI.1987-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lakk M, Yarishkin O, Baumann JM, Iuso A, Križaj D. Cholesterol regulates polymodal sensory transduction in Müller glia. Glia. 2017;65:2038–2050. doi: 10.1002/glia.23213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jing F, Zhang YX, Long T, He W, Qin GC, Zhang DK, et al. P2Y12 receptor mediates microglial activation via RhoA/ROCK pathway in the trigeminal nucleus caudalis in a mouse model of chronic migraine. J Neuroinflammation. 2019;16:217. doi: 10.1186/s12974-019-1603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eyo UB, Mo MS, Yi MH, Murugan M, Liu JT, Yarlagadda R, et al. P2Y12R-dependent translocation mechanisms gate the changing microglial landscape. Cell Rep. 2018;23:959–966. doi: 10.1016/j.celrep.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, Liu JJ, Xu AM, Heiduschka P, Eter N, Chen CZ. Expression of purinergic receptors on microglia in the animal model of choroidal neovascularisation. Sci Rep. 2021;11:12389. doi: 10.1038/s41598-021-91989-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell CH, Lu WN, Hu HL, Zhang XL, Reigada D, Zhang M. The P2X(7) receptor in retinal ganglion cells: A neuronal model of pressure-induced damage and protection by a shifting purinergic balance. Purinergic Signal. 2009;5:241–249. doi: 10.1007/s11302-009-9142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xia JS, Lim JC, Lu WN, Beckel JM, Macarak EJ, Laties AM, et al. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7receptors. J Physiol. 2012;590:2285–2304. doi: 10.1113/jphysiol.2012.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xue Y, Xie YT, Xue B, Hu N, Zhang GW, Guan HJ, et al. Activated Müller cells involved in ATP-induced upregulation of P2X7 receptor expression and retinal ganglion cell death. Biomed Res Int. 2016;2016:9020715. doi: 10.1155/2016/9020715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campagno KE, Lu WN, Jassim AH, Albalawi F, Cenaj A, Tso HY, et al. Rapid morphologic changes to microglial cells and upregulation of mixed microglial activation state markers induced by P2X7 receptor stimulation and increased intraocular pressure. J Neuroinflammation. 2021;18:217. doi: 10.1186/s12974-021-02251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong LD, Hu YH, Zhou L, Cheng XL. P2X7 receptor antagonist protects retinal ganglion cells by inhibiting microglial activation in a rat chronic ocular hypertension model. Mol Med Rep. 2018;17:2289–2296. doi: 10.3892/mmr.2017.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.