Abstract
Background
Metabolic syndrome and related metabolic disturbances represent a state of low-grade inflammation, which accelerates insulin resistance, type 2 diabetes (T2D) and cardiovascular disease (CVD) progression. Among antidiabetic medications, sodium glucose co-transporter (SGLT) 2 inhibitors are the only agents which showed remarkable reductions in heart failure (HF) hospitalizations and major cardiovascular endpoints (MACE) as well as renal endpoints regardless of diabetes status in large randomized clinical outcome trials (RCTs). Although the exact mechanisms underlying these benefits are yet to be established, growing evidence suggests that modulating inflammation by SGLT2 inhibitors may play a key role.
Scope of review
In this manuscript, we summarize the current knowledge on anti-inflammatory effects of SGLT2 inhibitors as one of the mechanisms potentially mediating their cardiovascular (CV) benefits. We introduce the different metabolic and systemic actions mediated by these agents which could mitigate inflammation, and further present the signalling pathways potentially responsible for their proposed direct anti-inflammatory effects. We also discuss controversies surrounding some of these mechanisms.
Major conclusions
SGLT2 inhibitors are promising anti-inflammatory agents by acting either indirectly via improving metabolism and reducing stress conditions or via direct modulation of inflammatory signalling pathways. These effects were achieved, to a great extent, in a glucose-independent manner which established their clinical use in HF patients with and without diabetes.
Keywords: SGLT2 inhibitors, Inflammation, Metabolism, Heart failure, Cardiovascular disease
List of abbreviations
- ACEI
angiotensin-converting enzyme inhibitors
- ADP
adenosine diphosphate
- AGE
advanced glycation end-products
- AngII
angiotensin II
- AMP
adenosine monophosphate
- AMPK
5′ adenosine monophosphate-activated protein kinase
- AP
activator protein
- ARB
angiotensin II receptor blockers
- ASC
apoptosis-associated speck-like protein
- ATM
adipose tissue macrophages
- ATP
adenosine triphosphate
- CAD
coronary artery disease
- Cana
canagliflozin
- cGMP
cyclic guanosine monophosphate
- COX
cyclooxygenase
- CV
cardiovascular
- CVD
cardiovascular disease
- DAMP
damage associated molecular patterns
- Dapa
dapagliflozin
- EAT
epicardial adipose tissue
- EMA
European Medicines Agency
- Empa
empagliflozin
- Ertu
ertugliflozin
- eNOS
endothelial nitric oxide synthase
- FDA
Food and Drug Administration
- FFA
Free fatty acids
- FOXO3a
forkhead box O3a
- FOXP3
forkhead box P3
- GLP-1 RA
glucagon-like peptide 1 receptor agonists
- GSK3β
glycogen synthase kinase 3β
- HbA1c
haemoglobin A1c
- HDAC
histone deacetylase
- HF
heart failure
- HFpEF
HF with preserved ejection fraction
- HFrEF
HF with reduced ejection fraction
- Hk
human kidney
- Hs-CRP
high sensitivity C- reactive protein
- HUVEC
human umbilical vein endothelial cells
- ICAM
intracellular adhesion molecule
- IFN
Interferon
- IGF
insulin-like growth factor
- IKK
IκB kinase
- IL
interleukin
- iNOS
inducible NOS
- Ipra
ipragliflozin
- IRF
interferon regulatory factor
- IRS
insulin receptor substrate
- JAK
Janus kinase
- JNK
c-Jun N-terminal kinases
- LPS
lipopolysaccharide
- Luseo
luseogliflozin
- LV
left ventricular
- MACE
major cardiovascular endpoints
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemoattractant protein
- MMP
matrix metalloproteinases
- MRI
magnetic resonance imaging
- MT
melatonin membrane receptor
- mTOR
mammalian target of rapamycin
- NAD+
nicotinamide adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- NLRP3
nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3
- NO
nitric oxide
- PAI
plasminogen activator inhibitor
- PAMP
pathogen associated molecular patterns
- PGC
peroxisome proliferator-activated receptor gamma coactivator
- PI3K
phosphatidylinositide 3-kinase
- PKG
protein kinase G
- PKR
protein kinase R
- PTEN
phosphorylated phosphatase and tension homologue
- PVAT
perivascular adipose tissue
- RAS
renin-angiotensin system
- RCTs
randomized clinical outcome trials
- ROS
reactive oxygen species
- SGLT
sodium glucose co-transporter
- SIRT
sirtuin
- SOD
superoxide dismutase
- Sota
sotagliflozin
- STAT
signal transducer and activator of transcription
- STZ
streptozotocin
- T2D
type 2 diabetes
- TCA
tricarboxylic acid
- TGF
tumour growth factor
- TLR
toll-like receptors
- TNF
tumour necrosis factor
- Tofo
tofogliflozin
- UCP
uncoupling protein
- VCAM
vascular cell adhesion molecule
- WAT
white adipose tissue
- XO
xanthine oxidase
- ZDF
Zucker diabetic fatty
- βOH
β-hydroxybutyrate
1. Introduction
Obesity and related metabolic disorders are leading global burdens which are rapidly increasing owing to the rise in sedentary lifestyle and high caloric diet [1]. Since obesity is a strong risk factor for T2D, a parallel increase in the numbers of diabetic patients have been observed which is expected to reach 366 million in 2030 [2]. Both, T2D and obesity play a major role in the development of CVD [3], which is considered the major cause of death worldwide [4].
HF is one of the most common and serious CV complications in diabetes, which is associated with a poor prognosis [5,6]. Development of HF in diabetic patients is in fact twice as frequent as in non-diabetics [7]. Even though the incidence of HF correlates to the level of haemoglobin A1c (HbA1c) [8], lowering blood glucose by conventional anti-hyperglycaemic agents failed to show convincing evidence in reducing HF risk. In fact, all glucagon-like peptide-1 receptor agonists (GLP-1 RA) have shown at least non-inferiority and some showed significant reductions in 3-point MACE (composite of CV death, nonfatal myocardial infarction, or nonfatal stroke) in patients with T2D and high CV risk in several large RCTs [9]. Several mechanisms have been postulated to explain these CV benefits including possible direct and indirect anti-inflammatory actions [10]. However, despite these potential mechanisms, GLP-1 RA only caused modest and inconsistent reductions in HF hospitalization rate [11]. To date, SGLT2 inhibitors are the only glucose lowering agents that reduce HF risk based on the outcomes of large RCTs, despite the fact that SGLT2 expression in the heart is quite unlikely [12].
SGLT2 inhibitors are a novel class of antidiabetic drugs which cause insulin-independent glucose lowering by reducing renal glucose reabsorption and thus enhancing glycosuria [13]. The EMPA-REG outcome trial was the first RCT to show HF and other CV outcome benefits among SGLT2 inhibitors [14]. In this trial, patients with T2D and established CVD showed reduced risk of 3-point MACE by 14% driven by a significant reduction of death from CV causes, CV death by 38%, all-cause mortality by 32%, and HF hospitalization by 35% within the first few weeks of treatment. Similar reductions in HF risk were observed with subsequent trials i.e. CANVAS for canagliflozin, DECLARE-TIMI 58 for dapagliflozin and VERTIS CV for ertugliflozin, showing ca. 30% reduction of HF hospitalization compared to placebo which confirm the role of SGLT2 inhibitors in preventing or delaying HF onset [[15], [16], [17]]. SOLOIST-WHF was the first trial to suggest that the potential of SGLT2 inhibitors lies beyond HF prevention [18]. Sotagliflozin reduced CV death, HF hospitalization and urgent HF visits (HR, 0.67; 95% CI, 0.52 to 0.85) in diabetic patients with recent worsening HF. Recently, this therapeutic role of SGLT2 inhibitors was further supported in DAPA-HF and EMPEROR- Reduced, where dapagliflozin and empagliflozin reduced the composite of worsening HF (i.e. hospitalisation or urgent visit for HF) and CV mortality regardless of diabetes status of HF with reduced ejection fraction (HFrEF) patients [19,20]. These benefits were driven by 30% reductions in the first or recurrent HF hospitalizations [21]. Accordingly, SGLT2 inhibitors are now established as first line agents for HFrEF management in recent guidelines [22]. In 2021, EMPEROR-Preserved has established, for the first time, the CV benefits of SGLT2 inhibitors in HF with preserved ejection fraction (HFpEF) patients with or without diabetes with similar reductions in the primary endpoint as shown in EMPEROR-Reduced [23]. The ongoing large trial; DELIVER, is expected to provide further therapeutic implications in this patient population [24]. In a press release, however, it was announced that top-line results from the study showed reductions in the primary end point [25].
Apart from their diuretic and natriuretic effects [26], several novel hypotheses have been proposed to explain the early remarkable CV benefits of SGLT2 inhibitors. These include increased erythropoiesis [27], improved cardiac remodelling [28], improved ion balance and mitochondrial energetics [12] and inhibition of sympathetic stimulation [29]. While the exact mechanisms are still under debate, the anti-inflammatory effects of SGLT2 inhibitors have come more into focus based on recent findings obtained mostly from animal and cell culture studies, as discussed in the next sections.
2. Inflammation links obesity, diabetes and heart failure
Obesity and related metabolic disturbances are currently considered as conditions of chronic low-grade inflammation, which contributes to the incidence and progression of insulin resistance [30]. The expanded visceral white adipose tissue (WAT) in obesity represents not only a massive lipid storage depot, but also a major source of proinflammatory cytokines (adipokines) [[31], [32], [33], [34]]. Supportive evidence of the role of inflammation in obesity-associated metabolic disorders arises from the promising results of salicylates, anakinra and infliximab in ameliorating hyperglycaemia [[35], [36], [37], [38], [39]]. Free fatty acids (FFA) released from adipose tissues into the systemic circulation bind to toll-like receptors (TLR) – 2 and 4 in metabolic cells such as adipose tissue, liver and muscles, activating downstream kinases; c-Jun N-terminal kinases (JNK), IκB kinase (IKK) and protein kinase R (PKR) [40]. These kinases mediate serine phosphorylation of insulin receptor substrate (IRS)-1 resulting in insulin resistance. Ultimately, these kinases activate inflammatory transcription factors; nuclear factor-κB (NF-κB), activator protein (AP)-1 and interferon regulatory factor (IRF) contributing to inflammation. The activation of nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing (NLRP) 3 inflammasome can further contribute to the inflammatory status [41], which may be explained by the reduced 5′ adenosine monophosphate-activated protein kinase (AMPK) signalling by elevated FFA levels [42]. Moreover, hypoxia resulting from the rapid expanding adipose tissue is associated with increased macrophage infiltration with a phenotypic conversion from the M2 type expressing anti-inflammatory cytokines to the proinflammatory M1 [43,44].
The increased proinflammatory cytokines from these pathways together with FFA further promote insulin resistance by establishing a positive feedback loop of inflammation [30]. Normally, pancreatic β-cells can adapt initially to the reduced insulin response by increasing their proliferation and insulin secretion, a mechanism which is compromised in later stages owing to increased β cell apoptosis leading to overt T2D [45]. The resulting hyperglycaemia plays a major role in inflammation and diabetic vascular complications which were shown to be also mediated by oxidative stress [46,47]. This state of chronic low-grade inflammation contributes to the progression of CVD by inducing endothelial dysfunction and atherosclerosis or by causing direct myocardial damage in the absence of CVD (or diabetic cardiomyopathy) [48,49].
In this regard, SGLT2 inhibitors were able to ameliorate markers of inflammation in clinical and in vivo studies, which may possibly contribute to their CV benefits. Studies demonstrated reductions of a large set of pro-inflammatory cytokines with empagliflozin including interleukin (IL)-6, tumour necrosis factor (TNF), monocyte chemoattractant protein (MCP)-1, Interferon (IFN)- γ, P-selectin and intercellular adhesion molecule (ICAM)-1 in the hearts of Zucker diabetic fatty (ZDF) rats [50,51]. In T2D patients, treatment with canagliflozin was associated with lower serum levels of leptin and IL-6 with higher adiponectin levels compared to glimepiride [52], while reductions in high sensitivity C- reactive protein (hs-CRP) and myeloperoxidase with concomitant increase in anti-inflammatory IL-10 were observed with empagliflozin [53].
These anti-inflammatory properties of SGLT2 inhibitors could be a result of general effects on metabolism, oxidative stress and renin-angiotensin system (RAS) signalling or from its direct action on inflammatory signalling pathways.
3. General effects of SGLT2 inhibitors
3.1. Reduction of oxidative stress
Reactive oxygen species (ROS) are formed from redox reactions or electron excitation [54]. In physiological levels, they play an important role in the regulation of cell signalling, autophagy, immunity and cellular differentiation [55]. However, when the production of ROS exceeds the detoxification ability of the antioxidant defence mechanism, detrimental effects on lipids, proteins, lipoproteins and DNA can occur contributing to the loss of important cellular functions, cell damage and apoptosis, in a phenomenon defined as oxidative stress [56]. In the heart, ROS overproduction occurs primarily due to altered mitochondrial functions, enhanced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and xanthine oxidase (XO) activities or due to uncoupling of endothelial nitric oxide synthase (eNOS) [57]. On the other hand, depletion of antioxidants such as superoxide dismutase (SOD), catalase, glutathione peroxidase, nicotinamide adenine dinucleotide (NAD+) and glutathione have also contributed to CVD and HF.
There is a direct link between ROS accumulation and stimulation of inflammatory pathways. ROS can mediate endothelial dysfunction and atherosclerotic plaque formation by activating NF-κB mediated expression of several cytokines including endothelin-1 [58], vascular cell adhesion molecule (VCAM)-1 and ICAM-1 which promote macrophage infiltration and vascular inflammation [59]. Owing to eNOS uncoupling, oxidative stress enhances inducible NOS (iNOS) production to compensate for the decreased nitric oxide (NO) production [60], a potent vasodilator which is necessary to maintain endothelial functions, prevent leucocyte adhesion and atherosclerosis [61], while maintaining normal cardiac contractions [62]. However, excess NO binds to superoxide (O2 •−) to form peroxynitrite (ONOO•−), which could be further involved in lipid peroxidation [63], foam cell formation and atherosclerosis [64]. In the myocardium of HFrEF, ROS stimulate mitogen-activated protein kinase (MAPK) mediated NF-κB and AP-1 transcription factors which promotes release of inflammatory cytokines, cellular apoptosis and activation of matrix metalloproteinases (MMP), which contribute to collagen degradation and left ventricular (LV) dilatation [65]. In HFpEF, on the other hand, ROS mediated eNOS inactivation reduces nitric oxide-cyclic guanosine monophosphate-protein kinase G (NO-cGMP-PKG) signalling and thus leads to titin hypophosphorylation, predisposing to myocardial stiffness and diastolic dysfunction [66].
Several studies have reported the ability of SGLT2 inhibitors in mitigating oxidative stress either by inducing metabolic changes (as will be mentioned in the next sections) or by acting as an antioxidant per se.
Dapagliflozin reduced the levels of the antioxidant DJ-1 and Nrf2 that was elevated as a compensatory mechanism to neutralize the excessive lipid peroxides produced in a Parkinson's disease rat model [67], while it reduced ROS and its associated apoptosis and NF-κB activation. In postinfarcted rats, dapagliflozin reduced myocardial superoxide and nitrotyrosine levels which resulted in activation of the signal transducer and activator of transcription (STAT) 3 signalling pathway [68]. STAT3 is a key regulator of macrophage polarization, which upon activation by dapagliflozin enhanced anti-inflammatory M2 macrophage expression and IL-10 release, whereas cardiac fibrosis was attenuated.
Furthermore, SGLT2 inhibitors act on the main sources of ROS in the heart. Empagliflozin improved mitochondrial function in rats with LV dysfunction by enhancing the expression of peroxisome proliferator-activated receptor gamma coactivator (PGC) 1-α, an important mediator of mitochondrial bioenergetics [69]. On the other hand, SGLT2 inhibitors downregulated NADPH oxidase expression in-vitro and in-vivo [69,70] with subsequent reduction of H2O2 and superoxide levels [70], while it restored oxidant-antioxidant balance by attenuating overexpressed SOD. In LV tissue samples of HFpEF patients, empagliflozin increased NO-cGMP-PKG signalling leading to enhanced titin phosphorylation with subsequent reduction in cardiac stiffness and LV dysfunction [[71], [72], [73]]. At the same time, empagliflozin enhanced endothelial relaxation and function by downregulating ICAM-1, VCAM-1, TNF, and IL-6 levels [71,72]. These effects have been attributed to the inhibition of eNOS uncoupling and its associated oxidative stress, which could be related to the direct activation of SOD [73].
3.2. Reduction of glucotoxicity
Large prospective cohort studies have repeatedly shown the strong association between the indices of glycemia and risk of CVD and mortality in diabetic patients [[74], [75], [76]], which was supported by evidence indicating the role of hyperglycaemia in promoting endothelial dysfunction [[77], [78], [79]] and atherosclerosis in-vivo [80]. Oxidative stress has been proposed as the link between hyperglycaemia and its vascular damage [81,82].
Glucose is normally metabolized by tricarboxylic acid (TCA) cycle to produce adenosine triphosphate (ATP) molecules required for various cellular functions [82]. The electrons generated from the mitochondrial electron transport chain are then transferred to oxygen to be reduced to water. However, in hyperglycaemia, more glucose enters the TCA cycle causing the excess electrons to be captured by coenzyme Q and finally generating superoxide. Increasing superoxide levels by hyperglycaemia stimulates mechanisms of glucotoxicity [83], all of which activate NADPH oxidase and further enhance ROS production. Thus, contributing to induced inflammation and potential risk of CVD.
Whether the anti-inflammatory effects of SGLT2 inhibitors could be partly explained by reduction of hyperglycaemia has been the subject of a considerable debate.
In ZDF rats, empagliflozin improved endothelial function by ameliorating the activation of advanced glycation end-products (AGE) to their receptors and the associated oxidative stress and inflammation in whole blood and aortic tissues [50]. This has been attributed to the glucose lowering effects and improved glucose utilization by empagliflozin. Similar results were reported in the aortic tissues and blood of streptozotocin (STZ) treated rats [84] as well as aortic rings and perivascular adipose tissue (PVAT) of STZ treated ApoE−/− mice [85]. Glucose normalization by empagliflozin in STZ-treated mice mediated atherosclerosis regression in aortic roots [86], while it significantly reduced cardiac and coronary arterial fibrosis and improved aortic endothelial function in db/db mice [87], which have been explained by the alleviation of hyperglycaemia and associated oxidative stress. Similarly, tofogliflozin attenuated tubulointerstitial inflammation in diabetic mice via normalization of blood glucose levels [88].
On the other hand, several studies have excluded glycaemic control as the sole mechanism behind SGLT2 inhibitors benefits. First, some of these studies have utilized non-diabetic models of CVD which were generated using lipopolysaccharide (LPS) [[89], [90], [91]] or angiotensin II (AngII) [92,93] as inflammatory stimulants or arterial ligation to induce ischemia [68,69,94] without altering blood glucose levels. In this view, recent RCTs such as DAPA-HF, EMPEROR-Reduced and EMPEROR-Preserved showed CV benefits in HF patients without diabetes which support the glucose-independent mechanisms of SGLT2 inhibitors [19,20,23]. Second, in-vitro experiments which were carried out on myocardial cells [70] - currently known as non-expressors of SGLT2-ensure that glucose transport into the cells is not affected by SGLT2 inhibition. Third, superior anti-inflammatory effects have been reported with SGLT2 inhibitors compared with other anti-diabetic agents with similar glucose lowering effects [95,96].
In fact, lowering glucose levels has substantially reduced the risk of microvascular complications in diabetic patients [97,98], while the benefits regarding macrovascular complications are still uncertain. In a meta-analysis, intensive glycaemic control reduced risk of non-fatal myocardial infarction and coronary heart disease by about 15% without affecting rate of stroke, HF and all-cause mortality [99]. In agreement with that, another meta-analysis comprised of four large RCTs; VADT, UKPDS, ACCORD and ADVANCE reported glucose-lowering associated reduction in MACE without affecting all-cause mortality [100]. In fact, the ACCORD trial showed an increased mortality rate with intensive therapy in diabetic patients [101], while UKPDS was the only trial among the four to show a 13% reduction in death from any cause which was manifested over 10 years of follow-up [102]. This could be related to the healthier patient population, less intensive glycaemic control and longer follow-up period utilized in this trial as compared to others [103]. On the other hand, the benefits observed by SGLT2 inhibitors in the recent RCTs diverge in the first few weeks of treatment which contradicts the long-term period required for glycaemic control to manifest improved CV outcomes as observed with other antidiabetic agents in the UKPDS trial.
3.3. Reduction of hyperuricemia
Uric acid is the end product of endogenous and dietary purine metabolism which is disposed mainly through the kidneys [104]. Elevation of serum uric acid could be attributed to the imbalance between uric acid production and excretion, which are regulated by several enzymes such as the xanthine oxidase (XO). In the context of HF and CVD, the incidence of hyperuricemia is common owing to the reduction of uric acid excretion and increasing its production in HF [105,106], the increased use of diuretics and low dose aspirin which stimulate uric acid tubular reabsorption [107], and the association of hyperuricemia to common CV risk factors e.g. diabetes, hypertension and insulin resistance [[108], [109], [110]].
Elevated serum uric acid was shown to be correlated to inflammatory markers in several clinical studies [[111], [112], [113]]. Activated inflammatory pathways could be a result of the underlying elevated oxidative stress caused by uric acid stimulation of NADPH oxidase [[114], [115], [116]], which is believed to be mediated by uric acid activation of RAS in some in-vitro studies [117,118]. In mice with unilateral ureteral obstruction, high uric acid levels enhanced fibrosis through activation of ROS/NLRP3/IL-1B signalling which was reversed by allopurinol (XO inhibitor) administration [119]. Similarly, allopurinol reversed ROS mediated uric acid activation of NLRP3 inflammasome that was responsible for endothelial injury in a chronic kidney disease rat model [120]. Moreover, elevated uric acid levels directly stimulated NF-κB mediated pro-inflammatory cytokine release in mouse kidneys and in-vitro, which was blocked by inhibiting tubular uric acid transporters [121]. The same effect of uric acid on NF-κB was associated with increased incidence of dyslipidaemia and hyperglycaemia in rats [122] and was responsible for reduced NO levels and endothelial dysfunction in human umbilical vein endothelial cells (HUVEC) [123]. Exposure to uric acid increased CRP-mediated human vascular smooth muscle cell migration and reduction of NO from HUVEC, which could be partially explained by MAPK activation [124]. Incubation of the cells with probenecid, an inhibitor of uric acid cellular entry, reversed these effects.
On this background, elevated uric acid levels have been associated with increased CV risk, mortality and incidence of HF in several large cohort studies [[125], [126], [127]]. Thus, it is not surprising to find that several studies have attributed the improved CV and HF outcomes to lowering uric acid levels after administration of XO inhibitors in patients with high CV risk [128,129].
SGLT2 inhibitors have been shown to reduce the levels of circulating uric acid regardless of diabetic status [130,131] and in patients with CV risk [27,132]. Since these agents reduce glucose transport through SGLT2, the resulting high glucose concentration in proximal tubules facilitates glucose exchange with intracellular uric acid through GLUT9, thus increasing uric acid elimination [133]. In this view, attenuation of hyperuricemia by SGLT2 inhibitors has been proposed as a potential mechanism of their CV benefits via reducing oxidative stress, inflammation, endothelial dysfunction and fibrosis [134].
Conversely, other studies have failed to show any improvement in CV or HF outcomes upon treatment with XO inhibitors [[135], [136], [137]]. In fact, patients treated with these agents might even show a trend of increasing CV mortality and hospitalization [135,138]. This evidence supports the fact that elevated uric acid is just a biomarker of oxidative stress and does not stimulate ROS or inflammatory cytokine release per se which precipitate myocardial injury [139]. In pro-oxidant conditions like in CVD, eNOS is uncoupled and becomes unable to produce NO, which beside its vasculoprotective effects plays an important role in ROS quenching [140]. Instead, XO level is upregulated to act as an alternative source of NO production together with a parallel increase in uric acid levels [141]. Thus, inhibition of XO deprives the body from an important antioxidant and NO source which could contribute to increased CV risk.
SGLT2 inhibitors, on the other hand, elicit a fasting-like state probably due to glycosuria, which in turn activates sirtuin (SIRT)1, an enzyme which plays an important role in the protection against oxidative stress and inflammation [142]. SIRT1 restores eNOS ability to produce NO [143] and consequently lowers XO and its associated uric acid levels [144]. Therefore, it can be hypothesized that the CV benefits of SGLT2 inhibitors are mainly mediated by the indirect activation of SIRT1 antioxidant and anti-inflammatory pathways, rather than by lowering serum uric acid.
3.4. Enhancing ketonemia
In diabetic failing hearts, there is an increased reliance on FFA oxidation for energy production owing to reduced glucose utilization on the basis of insulin resistance [145]. Although FFA produce more ATP than glucose, this comes with the price of increased oxygen consumption and ROS generation which can further exacerbate HF [146]. Against this background, enhancing ketogenesis by SGLT2 inhibitors may in part explain their cardioprotective effect, since ketone bodies are more energy efficient than FFA; yielding more ATP molecules per molecule of oxygen utilized [147]. Ferannini et al. have proposed that empagliflozin-associated glycosuria is responsible for the reduction of the insulin/glucagon ratio, which enhances hepatic FFA oxidation and subsequent elevation of circulating β-hydroxybutyrate (βOHB) [148] to be eventually utilized by the failing heart as a metabolic stress defence [149]. This hypothesis was experimentally proved in non-diabetic animal models of HFrEF, where enhanced myocardial utilization of ketone bodies by empagliflozin was associated with attenuation of adverse cardiac remodelling [69,150] and diastolic dysfunction [73]. In that light, βOHB also improved cardiac output, ejection fraction and reduced systemic vascular resistance in HFrEF patients [151]. Interestingly, Lopaschuk and Verma have proposed a counterargument, stating that increased βOHB by empagliflozin is probably due to reduced myocardial ketone body utilization, a process which could be maladaptive on long term [152].
Regardless of the mechanism of increase, it was suggested that elevated cardiac βOHB increased histone acetylation and expression of oxidative stress resistant factors; melatonin membrane receptor (MT)2 and forkhead box O3a (FOXO3a) by inhibiting histone deacetylase (HDAC) [153]. Beside its antioxidant effect, HDAC inhibition by βOHB can also mitigate cardiac and extra cardiac inflammation. Rats treated with valproic acid, an HDAC inhibitor, showed reduced ventricular levels of NF-κB, TNF, IL-1β and ROS which attenuated cardiac hypertrophy and fibrosis [154]. Another HDAC inhibitor, suberoylanilide hydroxamic acid, reduced a diverse range of inflammatory cytokines, which diminished cardiovascular fibrosis and stiffness in hypertensive rats [155]. Acetylation of the MAPK phosphatase-1 by HDAC inhibitors attenuated MAPK signalling in-vitro, which was associated with reduced inflammation and mortality in LPS-treated mice [156]. Furthermore, HDAC inhibitors enhanced production of anti-inflammatory regulatory T cells through the acetylation of forkhead box P3 (FOXP3), a key transcription factor for regulatory T cells development [157]. These inhibitors also enhanced macrophage polarization to the anti-inflammatory M2 phenotype [158] through modulation of the glycogen synthase kinase 3β/phosphorylated phosphatase and tension homologue/phosphatidylinositide 3-kinase or GSK3β/PTEN/PI3K signalling pathway [159].
Enhanced adiponectin expression by βOHB was associated with reduced pro-inflammatory cytokines in 3T3-L1 adipocytes, mediated by the direct epigenetic modification of βOHB on histone H3K9 of the adiponectin gene [160].
Furthermore, modulation of the NLRP3 inflammasome by βOHB has been reported in several studies [[161], [162], [163]]. Youm et al. was the first to show the impact of βOHB on the NLRP3- inflammasome and its mediated secretion of IL-1β in human macrophages and mice [164]. Later, Byrne et al. showed the important role of high βOHB in inhibition of NLRP3 inflammasome activation with subsequent reduction in pro-inflammatory cytokine levels and macrophage infiltration into cardiac tissues of HF mice [165]. In this study, elevated βOHB was associated with reduced cardiac remodelling and improved diastolic filling parameters.
Thus, inhibiting HDAC and NLRP3 inflammasome or enhancing adiponectin expression by elevated βOHB could contribute to the anti-inflammatory effect of SGLT2 inhibitors. However, only limited studies have shown the impact of SGLT2 inhibitor-induced ketonemia on inflammation and associated CV risk.
Recently, Kim et al. showed that empagliflozin attenuated NLRP3 inflammasome activation and the secretion of IL-1β which could be attributed to the elevated levels of βOHB in macrophages of diabetic patients with CVD [166]. In a HFpEF mouse model, elevated βOHB by empagliflozin inhibited mitochondrial protein hyperacetylation resulting in reduced NLRP3 inflammasome assembly and subsequent cytokine release [167]. These effects were associated with reduced BNP levels, cardiac fibrosis and stiffness, while they improved exercise tolerance.
In diabetic patients with history of CVD, elevated βOHB resulted in improved left ventricular diastolic functions which was reflected by reduced E/e’ in echocardiography after treatment with tofogliflozin [168]. The anti-inflammatory and antioxidant effects of increased βOHB might explain, at least partially, the improvement in cardiac function in these patients and potential reduction of their HF risk.
3.5. Reduction of adipose tissue mass and associated pro-inflammatory cytokine release
Obese patients with metabolic syndrome are characterized by WAT hypertrophy in the face of increased demand of energy storage together with impaired angiogenesis, hypoxia and adipocyte apoptosis leading to macrophage infiltration and inflammation [169]. Subsequently, adipose tissue differentiation is impaired which predisposes to ectopic fat accumulation in tissues such as the heart and blood vessels. Therefore, obesity-induced inflammation can promote CVD progression through the secretion of pro-inflammatory cytokine into the circulation from distant adipose tissue depots in an endocrine manner or from the adjacent PVAT and epicardial adipose tissue (EAT) through paracrine release [170].
PVAT surrounds large arteries and veins as well as small resistant vessels, while it is completely absent from the cerebral circulation [171]. These adipose tissues regulate vascular tone in healthy conditions by releasing several relaxing factors such as adiponectin, NO and hydrogen sulphide. In obesity, however, PVAT loses its anticontractile functions which can predispose to endothelial dysfunction and atherogenesis. In PVAT dysfunction, NO production is reduced owing to enhanced eNOS uncoupling which subsequently contributes to ultimate generation of superoxide [172,173] promoting vasoconstriction and vascular remodelling [174]. Proinflammatory adipokines such as leptin and resistin can further exacerbate oxidative stress in the vascular endothelium by activating NADPH oxidase [175]. Furthermore, PVAT release of visfatin and resistin was associated with enhanced ICAM-1 and VCAM-1 expression which can mediate leucocyte infiltration of vascular endothelial cells and atherosclerosis [176,177]. In clinical studies, PVAT inflammation predisposed to coronary artery vasospasm and increased atherosclerotic burden in patients with vasospastic angina and coronary artery disease (CAD), respectively [178,179].
EAT lies in close proximity to the heart i.e. between the myocardium and the pericardial visceral layer [180] acting as an energy source to the underlying myocytes by providing FFA, while it exerts antioxidant effect by the released adiponectin [181]. Like PVAT, the accumulated EAT in obesity releases various proinflammatory mediators such as leptin, resistin and other adipokines with reduced adiponectin production [182]. These changes were associated with increased myocardial inflammation promoting cardiac fibrosis and remodelling, which can contribute to impaired myocardial contractility [183]. In a similar manner, EAT can influence the underlying coronary vasculature promoting atherogenesis and microvascular rarefaction [184,185]. In this regard, EAT contributed to increased left ventricular mass and worse diastolic functions in HF patients [[186], [187], [188]].
SGLT2 inhibitors have shown consistent reductions in body weight of 1–3 kg in the first weeks of treatment [189] even in obese patients without T2D [190], which could be maintained up to 4 years [191]. Although the impact of weight loss on HF outcomes has been so far seen sceptically [192], the reduction of visceral fat content associated with dapagliflozin and ipragliflozin reduced LV mass compared to placebo and metformin, respectively, which highlights the impact of VAT inflammation on CV remodelling [28,193].
On the same note, T2D patients treated with SGLT2 inhibitors exhibited reduced EAT mass [[194], [195], [196]], which was assumed to be independent of glycaemic control in some studies [197,198]. This was confirmed by the positive findings observed even in non-obese, non-diabetic HFrEF patients using cardiac magnetic resonance imaging (MRI) [199]. The observed EAT reductions were much greater than the changes (if any) in body weight, VAT and subcutaneous fat. This was attributed to the high turn-over rate of FFA in EAT which could explain their higher sensitivity to SGLT2 inhibitors. Loss of EAT by SGLT2 inhibitors showed parallel reductions in pro-inflammatory cytokines [195,196], which might explain the observed reductions of interstitial myocardial fibrosis and aortic stiffness by empagliflozin in nondiabetic patients with HFrEF [200]. These findings contributed to improved diastolic functions, pulsatile load and ventricular pressure. Conversely, Gaborit et al. showed no change in EAT volume after treatment with empagliflozin in T2D patients [201], which could be attributed to the different cardiac imaging modalities used as compared to the previously mentioned studies [202]. This emphasizes the importance of utilizing more sensitive multi-slice volumetric approaches for EAT measurement using cardiac MRI instead of the single-slice methods such as computed tomography [203]. However, Gaborit et al. did not exclude the possibility of improved EAT phenotype by empagliflozin that could positively influence cardiac functions [201]. In this regard, dapagliflozin improved EAT stromal cell differentiation, reduced pro-inflammatory cytokines and enhanced EAT secretome contributing to the healing of endothelial cells in patients undergoing cardiac surgery without affecting EAT mass [204].
On the other hand, few studies showed paradoxical effects of SGLT2 inhibitors on PVAT in vivo. Luseogliflozin attenuated neointimal hyperplasia after wire injury in mice receiving high fat diet by reducing adipocyte size of PVAT which was associated with increased adiponectin levels and reduced macrophage infiltration [205]. Mori et al. also showed that ipragliflozin reduced inflammation, adipocyte apoptosis and macrophage accumulation in abdominal PVAT of obese diabetic mice resulting in suppression of adverse vascular remodelling [206]. Surprisingly, ipragliflozin in this study increased adipocyte size and lipid storage capacity owing to increased insulin sensitivity which was designated as “healthy adipose tissue expansion”. Another study, however, questioned this cardioprotective effect of SGLT2 inhibitors in a rat model of metabolic syndrome [207], since tofogliflozin failed to enhance PVAT-mediated vasorelaxation with no associated improvement in cardiac function and heart weight.
The mechanisms behind adipose tissue loss with SGLT2 inhibitors cannot be simply explained by caloric loss due to glycosuria [208]. Alternatively, the low insulin/glucagon ratio mediated by the reduced plasma glucose levels with SGLT2 inhibitors shifts energy metabolism towards fat utilization, fatty acid oxidation and ketogenesis in T2D patients [209], which could be AMPK dependent [210]. In obese mice, empagliflozin was associated with enhanced WAT browning by increasing the expression of uncoupling protein (UCP)-1 [208], a protein responsible for energy dissipation and thermogenesis in brown adipose tissue [211]. Together with increased adiponectin levels, enhanced M2-macrophage polarization and its associated sympathetic stimulation could be the mechanisms behind empagliflozin-mediated UCP-1 expression [208]. Sawada et al. showed that in high fat fed mice, tofogliflozin promoted glycogen depletion which subsequently mediated, at least partly, sympathetic stimulation via liver-brain-adipose-neural axis [212]. The activation of this neurological pathway was associated with enhanced lipolysis in WAT and eventually weight loss.
3.6. Attenuation of RAS signalling
RAS plays a key role in the development of hypertension and CVD which is mediated, to a great extent, by the pro-inflammatory effects of AngII signalling pathway [213]. Initially, AngII sets the stage for the inflammatory process by enhancing vascular permeability through increasing BP or by releasing prostaglandins and vascular endothelial growth factors [214]. Subsequently, the role of AngII in leucocyte recruitment to the vascular wall comes into play. AngII increases proinflammatory cytokine and chemokine release together with the expression of adhesion molecules such as E-selectin, ICAM-1 and VCAM-1 thus enhancing leucocyte endothelial interaction. Thus, AngII can contribute to endothelial dysfunction and progression of atherosclerosis [215,216]. Furthermore, AngII is involved in the production of growth and fibrotic mediators which can augment thrombus formation such as plasminogen activator inhibitor (PAI)-1 [217], or mediate vascular remodelling and hypertension by promoting tumour growth factor (TGF)-β/Smad signalling [218]. The underlying mechanism of the inflammatory effects of AngII is primarily attributed to enhanced ROS production through AngII mediated activation of NADPH oxidase [219] and XO [220] or its associated mitochondrial dysfunction [221]. Further, AngII enhances NF-κB activity by promoting its DNA binding or by inducing its nuclear translocation through the degradation of its inhibitor IκB [222]. Against this background, inhibition of AngII signalling by angiotensin-converting enzyme inhibitors (ACEI) or angiotensin II receptor blockers (ARB) blunted the expression of pro-inflammatory cytokines in patients with CAD [223], hypertension [224] and HF [225] which could explain the decreased mortality and CV benefits of these agents in high risk patients [226,227].
Similarly, the deleterious effects of RAS activation were attenuated by the administration of SGLT2 inhibitors in diabetic and non-diabetic models. Empagliflozin blocked in-vivo AngII mediated inflammation by inhibiting NF-κB and MAPK activation, which consequently attenuated macrophage infiltration, neovascularization and endothelial dysfunction in the suprarenal aorta [92]. In kidney tissue of diabetic mice, treatment with dapagliflozin was associated with reduced urinary AngII levels, corresponding decrease in ROS and enhanced antioxidant expression compared to voglibose, the comparator antidiabetic agent [96]. These effects contributed to reduced inflammatory cell infiltration, tubulointerstitial fibrosis and collagen accumulation. Since both antidiabetic agents had similar glucose lowering effects, the superior antioxidant and anti-inflammatory benefits of dapagliflozin were mainly related to a glucose-independent mechanism. Similar renal protective effects have been demonstrated by canagliflozin in AngII treated mice [228]. In this study, however, RAS activation was associated with enhanced SGLT2 expression in kidney cells in-vitro and in-vivo, which was oxidative stress mediated. Thus, the protective effects of canagliflozin in this study are assumed to be a consequence of direct inhibition of SGLT2 rather than an off-target effect.
Although the expression of SGLT2 in endothelial cells is controversial, Park et al. interestingly showed that AngII enhanced NADPH oxidase activity via its action on type 1 angiotensin receptor which ultimately increased the expression of SGLT1 and SGLT2 in rat endothelial cells [229]. This signalling pathway was associated with endothelial dysfunction resulting from reduction in eNOS levels and subsequent NO release together with enhanced expression of VCAM-1, MCP-1 and tissue factor. Sotagliflozin and empagliflozin reversed these effects, highlighting the possible contribution of SGLT2 in mediating RAS outcomes. Attenuation of inflammation by SGLT2 inhibitors might be responsible for the inhibition of AngII stimulated TGF-β/Smad signalling in rats, thus ameliorating cardiac fibrosis, remodelling and diastolic dysfunction [93].
Inhibition of RAS by SGLT2 inhibitor, however, has been challenged by some studies. In patients with T2D, SGLT2 inhibitors contributed to enhanced RAS activity after 30 days, possibly as a compensatory mechanism to their natriuretic and osmotic diuretic effects [230]. Similar results were reported in diabetic patients with canagliflozin and dapagliflozin [231,232], while renin and aldosterone levels were not changed by dapagliflozin in another study of T2D [233].
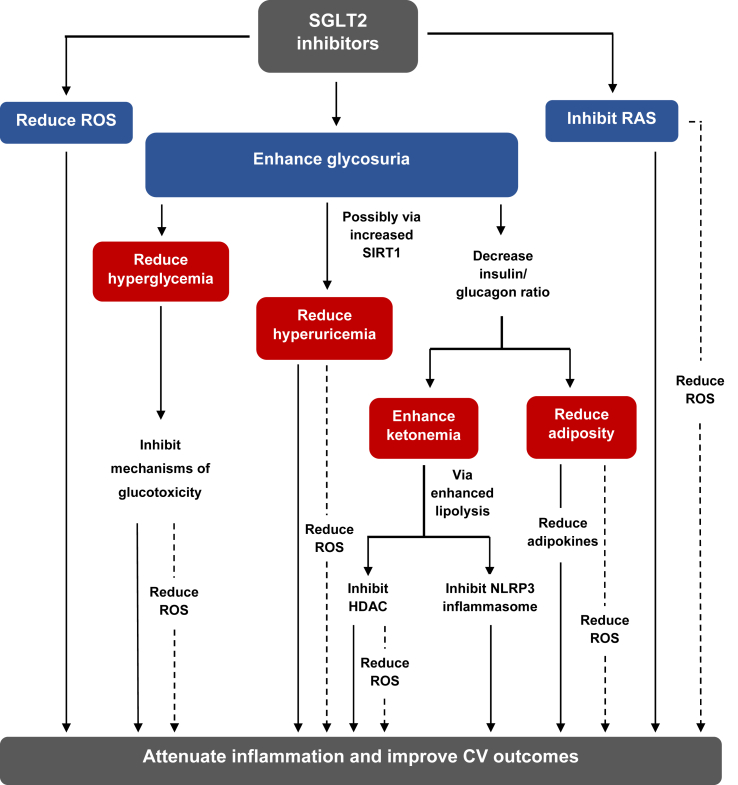
Figure 1 summarizes the possible metabolic and systemic alterations which could be related to the indirect anti-inflammatory effects of SGLT2 inhibitors.
Figure 1.
General effects of SGLT2 inhibitors indirectly contributing to reduced inflammation. Apart from being glucose lowering agents, SGLT2 inhibitors act by modulating other metabolic pathways through their glycosuric effects which mediate reduction of uric acid, increased ketone bodies and adipose tissue loss. Systemically, they interfere with ROS generation and RAS signalling by acting on their major sources. SGLT2 inhibitors can, therefore, indirectly attenuate inflammatory signalling via these mechanisms which might contribute to their established CV benefits. Abbreviations: HDAC, histone deacetylase; NLRP3, nucleotide-binding domain leucine-rich-containing family, pyrin domain-containing-3; RAS, renin-angiotensin system; ROS, reactive oxygen species; SGLT2, sodium glucose co-transporter 2; SIRT1, sirtuin1.
4. Effects of SGLT2 inhibitors on inflammatory signalling pathways
4.1. AMPK activation
AMPK is the fuel gauge of the cell, which under stress conditions, reduces energy consumption and enhances compensatory production of ATP resulting in increased ATP/adenosine diphosphate (ADP) ratio [234]. Therefore, AMPK can regulate major metabolic pathways of glucose, lipids and proteins and is considered as a key player in autophagy. The anti-inflammatory role of AMPK has been demonstrated in diabetic patients treated with metformin which showed AMPK mediated inhibition of NLRP3 inflammasome and IL-1β release [235]. In obese mice, AMPK deficiency enhanced macrophage polarization to the pro-inflammatory M1 type [236]. With respect to vascular cells, AMPK activation reduced TNF-stimulated NF-κB activity [237] and the subsequent expression of adhesion molecules [238,239] in cultured endothelial cells. Again with metformin, the activation of AMPK contributed to reduced NF-κB, iNOS and cyclooxygenase (COX)-2 expressions in vascular smooth muscle cells [240]. Furthermore, incubating fibroblasts and endothelial cells with IL-6 stimulated Janus kinase (JAK)–STAT pathway mediated inflammation, which was inhibited upon activation of AMPK by salicylate and metformin [241]. Thus, enhancing the AMPK pathway is associated with inhibition of vascular inflammation and endothelial dysfunction, which could contribute, at least partly, to less cardiac remodelling and myocardial dysfunction [[242], [243], [244], [245]].
SGLT2 inhibitors have been shown to act as AMPK activators through inhibition of complex I of the mitochondrial respiratory chain, which subsequently increases adenosine monophosphate (AMP) and ADP content [210]. Elevated AMP/ADP bind to the γ subunit of AMPK which then activates its phosphorylation at threonine 172.
In diabetic models, SGLT2 inhibitors showed anti-inflammatory effects through activation of AMPK signalling. Dapagliflozin reduced NF-κB nuclear translocation in renal tubular human kidney (HK)-2 cells [246], while it reduced NLRP3 inflammasome activation and progression of diabetic nephropathy in mice [247], which were AMPK mediated. Through the same pathway, empagliflozin reduced the expression of IL-6, TNF and MCP-1 in the hearts of diabetic rats [51], while in cultured human cells canagliflozin reduced IL-1β stimulated IL-6 and MCP-1 secretion probably through the inhibition of facilitative glucose uptake [248].
However, other in-vitro and in-vivo studies showed that reduced glucose levels by SGLT2 inhibitors cannot be the only mechanism explaining the AMPK-mediated anti-inflammatory effects. Inflammatory cytokine levels were attenuated [91,249] and the expression of M2 macrophages was enhanced [249] upon treatment with SGLT2 inhibitors in an AMPK- dependent manner in LPS- stimulated in-vitro and in-vivo models. Furthermore, the reduction of NLRP3 inflammasome by dapagliflozin through AMPK activation in the hearts of diabetic mice has been successfully replicated in cardiomyocytes in-vitro [90]. These AMPK- anti-inflammatory effects possibly contributed to inhibition of pro-fibrotic TGF-β/Smad signalling [250], reduced ventricular remodelling and improved cardiac function [90,250].
Autophagy is an important process for cellular homeostasis, by which misfolded proteins, damaged organelles and pathogens are captured by autophagosomes to be degraded by lysosomal proteases [251]. Accordingly, dysregulation of this process can predispose to inflammation [252,253] and can contribute to CVD [[254], [255], [256]]. Autophagy is regulated, among others, by AMPK/the mammalian target of rapamycin (mTOR) signalling [257]. Thus, modulation of this pathway restores autophagy and can contribute to the anti-inflammatory effects of AMPK activators [258,259]. Likewise, empagliflozin enhanced autophagy by stimulating AMPK and inhibiting mTOR in a mouse model of liver disease [260,261], accompanied by a concomitant reduction of IL-17/IL-23 release [261]. Moreover, the improvement of cardiac function by empagliflozin in-vivo was attributed to autophagosome accumulation and enhanced autophagic flux mediated by AMPK/mTOR pathway [262].
4.2. Inhibition of NLRP3 inflammasome activation
The NLRP3 inflammasome is a macromolecular protein complex which is comprised of 3 main components: NLRP3, apoptosis-associated speck-like protein (ASC) and pro-caspase-1 [263]. In response to danger signals, the inflammasome triggers pro-inflammatory cytokine secretion in two steps [264]. Priming is the first step of the inflammasome activation, where cellular debris and pathogens, also known as damage- or pathogen associated molecular patterns (DAMP or PAMP), bind to TLR and induce downstream NF-κB mediated expression of NLRP3 and pro-IL-1β. The second signal of DAMP or PAMP trigger several possible pathways such as enhanced K+ efflux and lysosomal degradation, which finally leads to inflammasome assembly and conversion of pro-caspase-1 into its active form. The cleavage of pro-IL-1β and pro-IL-18 by active caspase-1 to IL-1β and IL-18 prompts the start of the inflammatory cascade.
Accumulated lipids in blood vessels can lead to NLRP3 activation which predisposes to atherosclerosis by impairing endothelial dysfunction and promoting coagulation [265]. The cell debris released from ischemic cardiac injury afterwards act as DAMP which trigger NLRP3/IL-1β signalling and acute inflammation leading to leucocytes infiltration to the myocardium and triggering further myocardial damage [266]. Tissue healing is initiated when the inflammasome stimulates IL-1β release in myofibroblasts, promoting collagen accumulation, cardiac fibrosis and remodelling. Moreover, IL-1 β has been considered as cardio-depressant; impairing contractility and inducing LV systolic dysfunction which further worsens HF [267]. It is worth mentioning that the role of NLRP3 inflammasome activation in cardiotoxicity and non-ischemic injuries has also been discussed [268,269]. Thus, it is no surprise that mice deficient of inflammasome components were more resistant to developing atherosclerosis [270], while inflammasome inhibitors contributed to reducing infarct size and improving cardiac function in different in-vivo models [271]. Furthermore, deficiency of NLRP3 in mice resulted in lower LV dilation and fibrosis, while it showed better survival compared to wild type [272]. In HF patients, administration of anakinra (IL-1 β inhibitor) improved peak oxygen consumption and exercise capacity [267,273].
Direct effects of SGLT2 inhibitors on NLRP3 inflammasome activation have been established in non-diabetic mice via modulation of intracellular Ca2+ levels. Although not commonly mentioned, Ca2+ mobilization was considered an important activator of NLRP3 inflammasome starting from the assembly step until IL-1 β release [274]. Recently, it has been shown that extracellular Ca2+ can activate the inflammasome and IL-1β release through the induction of calcium sensing receptor signalling [275]. On this account, empagliflozin improved systolic and diastolic functions in HFrEF mice, while it reduced cardiac fibrosis, mass and remodelling as well as diastolic dysfunction in HFpEF rats [94]. This was attributed to the inhibitory effect of empagliflozin on the priming and activation of the inflammasome and the resulting expression of inflammatory cytokines in-vivo, ex-vivo and in-vitro, mediated by lowering intracellular Ca2+ levels. Since the intracellular levels of both Na+ and Ca2+ are interrelated [276], empagliflozin was able to reduce Ca2+levels in cardiomyocytes of HF mice by the attenuation of late Na + current and its intracellular accumulation [277]. This caused inhibition of NLRP3 inflammasome activation and improved functional recovery. Similar reduction in NLRP3 mediated inflammation was observed with empagliflozin in cardiomyocytes treated with doxorubicin (a dose-dependent cardiotoxic agent), which might be explained, although not explicitly stated, by lowering Ca2+ content [278].
4.3. Promoting M2 macrophage polarization
Following tissue injury, innate immune response is initiated where macrophages release inflammatory cytokines that stimulate differentiation and activation of fibroblasts and other cells necessary for tissue repair and wound healing [279]. Macrophages then take another form which supresses inflammation and ensures normal tissue function and structure. Thus, macrophages have two distinct phenotypes, M1 and M2 [280], where the abundant subtype can be decided by the nature of the surrounding microenvironment [281]. The M1 proinflammatory subtype releases TNF, IL-6 and IL-1β, while M2 releases anti-inflammatory cytokines and growth factors such as IL-10, TGF-β and insulin-like growth factor (IGF)-1 [282]. In the heart, aberrant inflammatory response can hinder post myocardial injury repair mechanism and can predispose to cardiac remodelling [283]. Therefore, targeting the direction of macrophage polarization towards the anti-inflammatory type or reducing the levels of pro-inflammatory M1 can be considered as therapeutic strategies to improve healing process and reduce fibrosis, remodelling and risk of HF [284,285].
SGLT2 inhibitors have been shown to enhance macrophage polarization from M1 to M2 in several studies. However, further research is needed to examine this mechanism in the context of CVD and myocardial injury.
LPS- stimulated human and murine macrophages showed increased numbers of M1 subtype and M1/M2 ratio, the effect which was completely reversed upon dapagliflozin treatment in a glucose-independent manner [89,286]. The same results were replicated by canagliflozin in LPS induced mice and macrophages [287]. The reduction of M1 phenotype was associated with attenuation of TNF, IL-6 and IL-1β release which alleviated lung injury. Against this background, the enhanced M1 to M2 polarization reported in T2D patients might be explained by a direct effect of empagliflozin rather than by its glucose lowering alone [288].
Adipose tissue macrophages (ATM) play a critical role in aggravating inflammation and insulin resistance [289], that could be extended to ectopic fat tissues around blood vessels and heart promoting CVD, as previously discussed. Strategies to promote the anti-inflammatory ATM phenotype have been utilized to improve insulin sensitivity and reduce inflammation in obesity [[290], [291], [292]]. In high fat-fed mice empagliflozin enhanced M2 ATM levels in WAT, while it reduced M1-mediated inflammatory cytokines release together with reduced phosphorylation of JNK and extracellular signal-regulated kinase (ERK)1/2 [208], p38 MAPK and NF-κB [293]. Additionally, empagliflozin inhibited infiltration of CD3+, CD4+ and CD8+ T cells in WAT, which is considered a critical step in M1 ATM recruitment. Interestingly, modulation of ATM polarization by ipragliflozin contributed to healthy adipose tissue expansion which was attributed to enhanced insulin signalling [294].
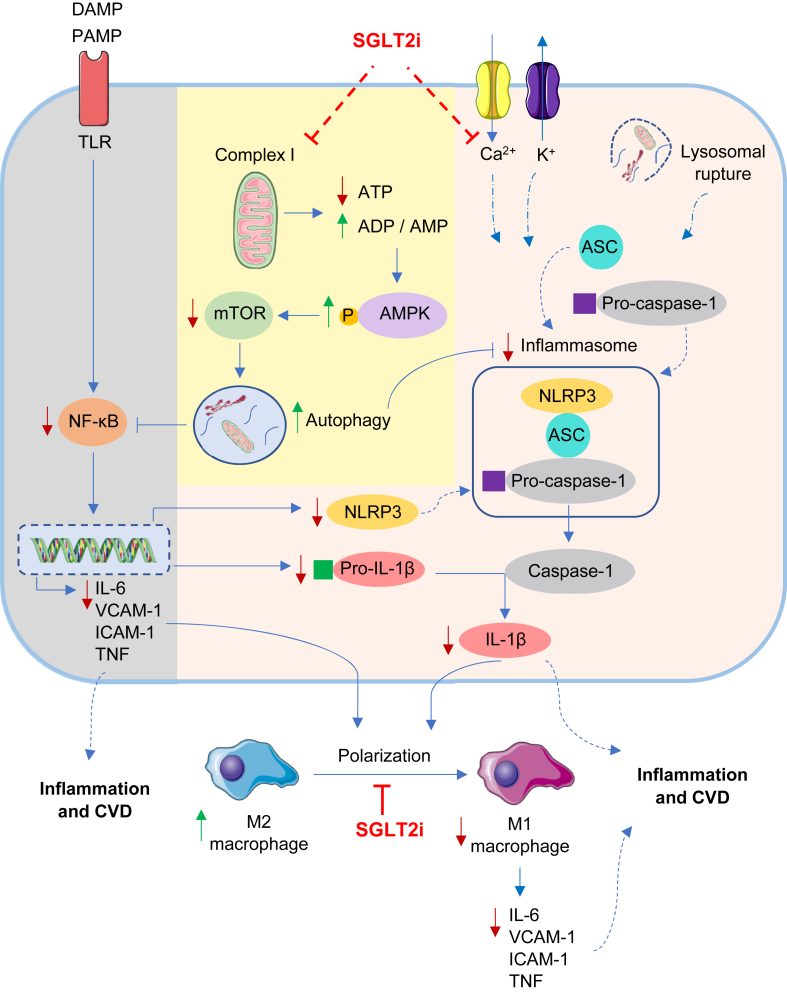
Potential inflammatory pathways that could be altered by SGLT2 inhibitors are presented in Figure 2.
Figure 2.
Inflammatory signalling pathways as potential targets of SGLT2 inhibitors. SGLT2 inhibitors supress the mitochondrial complex I eventually predisposing to the induction of AMPK/mTOR/autophagy signalling. Autophagy directly inhibits inflammasome activation, while it also attenuates NF-κB mediated release of several inflammatory cytokines together with NLRP3, an essential component of the inflammasome. Increased intracellular Ca2+, enhanced K+ efflux and lysosomal degradation can signal inflammasome assembly and activation. On that note, SGLT2 inhibitors reduce intracellular Ca2+ levels which can contribute to the inhibition of inflammasome activation and subsequent attenuation of IL-1β release. The overall reduction of inflammatory cytokines enhances M2 macrophage production and reduce their polarization to the pro-inflammatory M1 subtype, a process which was also directly modulated by SGLT2 inhibitors. These direct actions on inflammation can explain the CV benefits of SGLT2 inhibitors. Blue arrows, represent the normal signalling pathway; red arrows, represent decreased expression upon SGLT2 inhibitor treatment; green arrows, represent enhanced expression. ADP, adenosine diphosphate; AMP, adenosine monophosphate; AMPK, AMP-activated protein kinase; ASC, apoptosis-associated speck-like protein; ATP, adenosine triphosphate; CVD, cardiovascular disease; DAMP, damage-associated molecular patterns; ICAM-1, intercellular adhesion molecule-1; IL-1β, interleukin-1β; IL-6, interleukin-6; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-κB; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; PAMP, pathogen associated molecular patterns; SGLT2i, sodium glucose co-transporter 2 inhibitors; TNF, tumour necrosis factor; VCAM-1, vascular cell adhesion molecule-1.
Table 1. summarizes the different SGLT2 inhibitors available in the market and the respective evidence, if any, which supports the anti-inflammatory effects of each member.
Table 1.
A summary of the currently available SGLT2 inhibitors and the respective evidence, if any, of their anti-inflammatory mechanism.
| Member |
Empa [295] C23H27ClO7 |
Dapa [296] C21H25ClO6 |
Cana [297] C24H25FO5S |
Ertu [298] C22H25ClO7 |
Sota [299] C21H25ClO5S |
Ipra [300] C21H21FO5S |
Luseo [301] C23H30O6S |
Tofo [302] C22H26O6 |
| Approval | FDA [303] EMA [304] |
FDA [305] EMA [306] |
FDA [307] EMA [308] |
FDA [309] EMA [310] |
EMA for type 1 diabetes [311] | Only in Japan, South Korea and Russia [312] | Only in Japan [313] | Only in Japan [314] |
| Dosing regimen | 10/25 mg once daily | 5/10 mg once daily | 100/300 mg once daily | 5/15 mg once daily | 200 mg once or twice daily | 50/100 mg dose once daily | 2.5/5 mg once daily | 20 mg once daily |
| Anti-inflammatory mechanism | ||||||||
| Reduce ROS and hyperglycaemia mediated inflammation |  |
|||||||
| Reduce adipose tissue volume and associated adipokines |  |
|||||||
| Reduce AngII mediated inflammatory mediators/macrophage infiltration/fibrosis |  |
 |
||||||
| Enhance ketone mediated NLRP3 inflammasome inactivation | ||||||||
| Enhance M2 macrophage polarization |  |
 |
||||||
| Enhance AMPK mediated reduction of inflammatory mediators | ||||||||
| Inactivation of NLRP3 inflammasome | ||||||||
References of the anti-inflammatory mechanisms are colour coded in  for animal models,
for animal models,  for in-vitro and ex-vivo studies,
for in-vitro and ex-vivo studies,  for human data and
for human data and  for studies with combined models.
for studies with combined models.
AngII, angiotensin II; AMPK, 5′ adenosine monophosphate activated protein kinase; Cana, canagliflozin; Dapa, dapagliflozin; Ertu, ertugliflozin; EMA, European Medicines Agency; Empa, empagliflozin; FDA, Food and Drug Administration; Ipra, ipragliflozin; Luseo, luseogliflozin; NLRP3, nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3; ROS, reactive oxygen species; SGLT2, sodium glucose co-transporter 2; Sota, sotagliflozin; Tofo, tofogliflozin.
5. Summary
Growing evidence has shown the importance of SGLT2 inhibitors in attenuating inflammation in several in-vitro and in-vivo studies, which could possibly explain in part the reduced risk of plaque formation, endothelial dysfunction, cardiac fibrosis and ventricular remodelling. However, further clinical trials are needed to determine whether these anti-inflammatory effects are associated with the reduction of HF hospitalization and CV mortality observed with SGLT2 inhibitors in recent RCTs.
In fact, most of the evidence available relates these anti-inflammatory effects to the systemic and metabolic improvements of SGLT2 inhibitors, which were, to a great extent, dependent on their glucosuric effects. Although SGLT2 inhibitors are mainly investigated in the setting of T2D, it is becoming increasingly evident that the CV benefits observed are not exclusively related to the associated glycaemic control and reduced glucotoxicity. Furthermore, lowering of uric acid by SGLT2 inhibitors is probably not cardioprotective per se, but it is rather a reflection of oxidative stress mitigation via promoting the antioxidant SIRT1 expression or through direct inhibition of the main ROS generating mechanisms in the heart. Oxidative stress and inflammation could also be mediated by RAS activation, which was reversed upon SGLT2 inhibitor administration. Reduction of ectopic fat deposition by SGLT2 inhibitors is associated with reduced proinflammatory cytokine and adipokine release, which directly influence cardiac and vascular remodelling. Rather being a super fuel, a hypothesis which is still under debate, βOHB has been established as a specific HDAC and NLRP3 inflammasome inhibitor, which may explain the antioxidant and anti-inflammatory effects of ketonemia associated with this drug class.
Although based on limited number of studies to date, the reported CV benefits could partially be a consequence of direct influence on proinflammatory signalling pathways. SGLT2 inhibitors contributed to enhanced AMPK phosphorylation which was associated with downstream inhibition of inflammatory mediators. Activation of autophagy mediated by AMPK/mTOR signalling could explain these effects. Apart from AMPK activation, SGLT2 inhibitors contributed to inhibition of NLRP3 inflammasome priming and assembly, possibly via modulation of Ca2+ signalling. Enhancing macrophage polarization to the M2 subtype could attribute to SGLT2 inhibitor anti-inflammatory actions, although further studies are needed to establish these findings in cardiac tissues.
In summary, among other mechanisms cardioprotective effects and major improvement of HF outcomes observed with SGLT2 inhibitors are possibly related to both direct and indirect mitigation of inflammatory signalling pathways independent of glycaemic control.
Acknowledgement
The first author was supported by the German Academic Exchange Service (DAAD) – German Egyptian Research Long-Term Scholarship (GERLS) Programme.
Conflict of interest
None declared
References
- 1.Verma S., Hussain M.E. Obesity and diabetes: an update. Diabetes & Metabolic Syndrome. 2017;11(1):73–79. doi: 10.1016/j.dsx.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Ginsberg H.N., MacCallum P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. Journal of the Cardiometabolic Syndrome. 2009;4(2):113–119. doi: 10.1111/j.1559-4572.2008.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer P.E., Hill J.A. Obesity, diabetes, and cardiovascular diseases: a compendium. Circulation Research. 2016;118(11):1703–1705. doi: 10.1161/CIRCRESAHA.116.308999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.N.d Cardiovascular diseases (CVDs) https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 5.McMurray J.J.V., Gerstein H.C., Holman R.R., Pfeffer M.A. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. The Lancet. Diabetes & Endocrinology. 2014;2(10):843–851. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 6.Packer M. Heart failure: the most important, preventable, and treatable cardiovascular complication of type 2 diabetes. Diabetes Care. 2018;41(1):11–13. doi: 10.2337/dci17-0052. [DOI] [PubMed] [Google Scholar]
- 7.Nichols G.A., Gullion C.M., Koro C.E., Ephross S.A., Brown J.B. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27(8):1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 8.Erqou S., Lee C.-T.C., Suffoletto M., Echouffo-Tcheugui J.B., de Boer R.A., van Melle J.P., et al. Association between glycated haemoglobin and the risk of congestive heart failure in diabetes mellitus: systematic review and meta-analysis. European Journal of Heart Failure. 2013;15(2):185–193. doi: 10.1093/eurjhf/hfs156. [DOI] [PubMed] [Google Scholar]
- 9.Sheahan K.H., Wahlberg E.A., Gilbert M.P. An overview of GLP-1 agonists and recent cardiovascular outcomes trials. Postgraduate Medical Journal. 2020;96(1133):156–161. doi: 10.1136/postgradmedj-2019-137186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vergès B., Charbonnel B. After the LEADER trial and SUSTAIN-6, how do we explain the cardiovascular benefits of some GLP-1 receptor agonists? Diabetes & Metabolism. 2017;43(Suppl 1):2S3-12. doi: 10.1016/S1262-3636(17)30067-8. 12. [DOI] [PubMed] [Google Scholar]
- 11.Scheen A.J. GLP-1 receptor agonists and heart failure in diabetes. Diabetes & Metabolism. 2017;43(Suppl 1):2S13–12S19. doi: 10.1016/S1262-3636(17)30068-X. [DOI] [PubMed] [Google Scholar]
- 12.Bertero E., Prates Roma L., Ameri P., Maack C. Cardiac effects of SGLT2 inhibitors: the sodium hypothesis. Cardiovascular Research. 2018;114(1):12–18. doi: 10.1093/cvr/cvx149. [DOI] [PubMed] [Google Scholar]
- 13.Vallon V., Thomson S.C. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215–225. doi: 10.1007/s00125-016-4157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S., et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 15.Neal B., Perkovic V., Matthews D.R. Canagliflozin and cardiovascular and renal events in type 2 diabetes. New England Journal of Medicine. 2017;377(21):2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 16.Wiviott S.D., Raz I., Bonaca M.P., Mosenzon O., Kato E.T., Cahn A., et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 17.Cannon C.P., Pratley R., Dagogo-Jack S., Mancuso J., Huyck S., Masiukiewicz U., et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. New England Journal of Medicine. 2020;383(15):1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt D.L., Szarek M., Steg P.G., Cannon C.P., Leiter L.A., McGuire D.K., et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. New England Journal of Medicine. 2021;384(2):117–128. doi: 10.1056/NEJMoa2030183. [DOI] [PubMed] [Google Scholar]
- 19.McMurray J.J.V., Solomon S.D., Inzucchi S.E., Køber L., Kosiborod M.N., Martinez F.A., et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. New England Journal of Medicine. 2019;381(21):1995–2008. doi: 10.1056/NEJMoa1911303. [DOI] [PubMed] [Google Scholar]
- 20.Packer M., Anker S.D., Butler J., Filippatos G., Pocock S.J., Carson P., et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. New England Journal of Medicine. 2020;383(15):1413–1424. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- 21.Zannad F., Ferreira J.P., Pocock S.J., Anker S.D., Butler J., Filippatos G., et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396(10254):819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 22.McDonagh T.A., Metra M., Adamo M., Gardner R.S., Baumbach A., Böhm M., et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. European Heart Journal. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 23.Anker S.D., Butler J., Filippatos G., Ferreira J.P., Bocchi E., Böhm M., et al. Empagliflozin in heart failure with a preserved ejection fraction. New England Journal of Medicine. 2021;385(16):1451–1461. doi: 10.1056/NEJMoa2107038. [DOI] [PubMed] [Google Scholar]
- 24.Solomon S.D., de Boer R.A., DeMets D., Hernandez A.F., Inzucchi S.E., Kosiborod M.N., et al. Dapagliflozin in heart failure with preserved and mildly reduced ejection fraction: rationale and design of the DELIVER trial. European Journal of Heart Failure. 2021;23(7):1217–1225. doi: 10.1002/ejhf.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.N.d. FARXIGA met primary endpoint in DELIVER Phase III trial, reducing risk of cardiovascular death or worsening heart failure in patients with preserved ejection fraction. https://www.astrazeneca-us.com/media/press-releases/2022/farxiga-met-primary-endpoint-in-deliver-phase-III-trial.html
- 26.Verma S., McMurray J.J.V., Cherney D.Z.I. The metabolodiuretic promise of sodium-dependent glucose cotransporter 2 inhibition: the search for the sweet spot in heart failure. JAMA Cardiology. 2017;2(9):939–940. doi: 10.1001/jamacardio.2017.1891. [DOI] [PubMed] [Google Scholar]
- 27.Inzucchi S.E., Zinman B., Fitchett D., Wanner C., Ferrannini E., Schumacher M., et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care. 2018;41(2):356–363. doi: 10.2337/dc17-1096. [DOI] [PubMed] [Google Scholar]
- 28.Brown A.J.M., Gandy S., McCrimmon R., Houston J.G., Struthers A.D., Lang C.C. A randomized controlled trial of dapagliflozin on left ventricular hypertrophy in people with type two diabetes: the DAPA-LVH trial. European Heart Journal. 2020;41(36):3421–3432. doi: 10.1093/eurheartj/ehaa419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herat L.Y., Magno A.L., Rudnicka C., Hricova J., Carnagarin R., Ward N.C., et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC. Basic to Translational Science. 2020;5(2):169–179. doi: 10.1016/j.jacbts.2019.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H., Ballantyne C.M. Metabolic inflammation and insulin resistance in obesity. Circulation Research. 2020;126(11):1549–1564. doi: 10.1161/CIRCRESAHA.119.315896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park H.S., Park J.Y., Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Research and Clinical Practice. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Tsigos C., Kyrou I., Chala E., Tsapogas P., Stavridis J.C., Raptis S.A., et al. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism Clinical and Experimental. 1999;48(10):1332–1335. doi: 10.1016/s0026-0495(99)90277-9. [DOI] [PubMed] [Google Scholar]
- 33.Fontana L., Eagon J.C., Trujillo M.E., Scherer P.E., Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 34.Curat C.A., Wegner V., Sengenès C., Miranville A., Tonus C., Busse R., et al. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49(4):744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 35.Williamson R.T. On the treatment of glycosuria and diabetes mellitus with sodium salicylate. British Medical Journal. 1901;1(2100):760–762. doi: 10.1136/bmj.1.2100.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleischman A., Shoelson S.E., Bernier R., Goldfine A.B. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31(2):289–294. doi: 10.2337/dc07-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsen C.M., Faulenbach M., Vaag A., Vølund A., Ehses J.A., Seifert B., et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. New England Journal of Medicine. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 38.Larsen C.M., Faulenbach M., Vaag A., Ehses J.A., Donath M.Y., Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32(9):1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonzalez-Gay M.A., De Matias J.M., Gonzalez-Juanatey C., Garcia-Porrua C., Sanchez-Andrade A., Martin J., et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clinical & Experimental Rheumatology. 2006;24(1):83–86. [PubMed] [Google Scholar]
- 40.Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annual Review of Immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 41.Vandanmagsar B., Youm Y.-H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L., et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature Medicine. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wen H., Gris D., Lei Y., Jha S., Zhang L., Huang M.T.-H., et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nature Immunology. 2011;12(5):408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Rourke R.W., White A.E., Metcalf M.D., Olivas A.S., Mitra P., Larison W.G., et al. Hypoxia-induced inflammatory cytokine secretion in human adipose tissue stromovascular cells. Diabetologia. 2011;54(6):1480–1490. doi: 10.1007/s00125-011-2103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujisaka S., Usui I., Ikutani M., Aminuddin A., Takikawa A., Tsuneyama K., et al. Adipose tissue hypoxia induces inflammatory M1 polarity of macrophages in an HIF-1α-dependent and HIF-1α-independent manner in obese mice. Diabetologia. 2013;56(6):1403–1412. doi: 10.1007/s00125-013-2885-1. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes C.J. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307(5708):380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 46.Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Advances in Cardiology. 2008;45:1–16. doi: 10.1159/000115118. [DOI] [PubMed] [Google Scholar]
- 47.Esposito K., Nappo F., Marfella R., Giugliano G., Giugliano F., Ciotola M., et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106(16):2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 48.Frati G., Schirone L., Chimenti I., Yee D., Biondi-Zoccai G., Volpe M., et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovascular Research. 2017;113(4):378–388. doi: 10.1093/cvr/cvx011. [DOI] [PubMed] [Google Scholar]
- 49.Paneni F., Beckman J.A., Creager M.A., Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. European Heart Journal. 2013;34(31):2436–2443. doi: 10.1093/eurheartj/eht149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steven S., Oelze M., Hanf A., Kröller-Schön S., Kashani F., Roohani S., et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biology. 2017;13:370–385. doi: 10.1016/j.redox.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aragón-Herrera A., Feijóo-Bandín S., Otero Santiago M., Barral L., Campos-Toimil M., Gil-Longo J., et al. Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats. Biochemical Pharmacology. 2019;170 doi: 10.1016/j.bcp.2019.113677. [DOI] [PubMed] [Google Scholar]
- 52.Garvey W.T., Van Gaal L., Leiter L.A., Vijapurkar U., List J., Cuddihy R., et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism Clinical and Experimental. 2018;85:32–37. doi: 10.1016/j.metabol.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 53.Iannantuoni F., M. de Marañon A., Diaz-Morales N., Falcon R., Bañuls C., Abad-Jimenez Z., et al. The SGLT2 inhibitor empagliflozin ameliorates the inflammatory profile in type 2 diabetic patients and promotes an antioxidant response in leukocytes. Journal of Clinical Medicine. 2019;8(11):1814. doi: 10.3390/jcm8111814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nature Reviews Molecular Cell Biology. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 55.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Molecular Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., et al. Oxidative stress: harms and benefits for human health. Oxidative Medicine and Cellular Longevity. 2017;2017 doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. Treating oxidative stress in heart failure: past, present and future. European Journal of Heart Failure. 2019;21(4):425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Minchenko A.G., Stevens M.J., White L., Abatan O.I., Komjáti K., Pacher P., et al. Diabetes-induced overexpression of endothelin-1 and endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. Federation of American Societies for Experimental Biology Journal. 2003;17(11):1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 59.Kim S.-R., Bae Y.-H., Bae S.-K., Choi K.-S., Yoon K.-H., Koo T.H., et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochimica et Biophysica Acta. 2008;1783(5):886–895. doi: 10.1016/j.bbamcr.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 60.Gliozzi M., Scicchitano M., Bosco F., Musolino V., Carresi C., Scarano F., et al. Modulation of nitric oxide synthases by oxidized LDLs: role in vascular inflammation and atherosclerosis development. International Journal of Molecular Sciences. 2019;20(13):3294. doi: 10.3390/ijms20133294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Förstermann U., Sessa W.C. Nitric oxide synthases: regulation and function. European Heart Journal. 2012;33(7):829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Massion P.B., Feron O., Dessy C., Balligand J.-L. Nitric oxide and cardiac function: ten years after, and continuing. Circulation Research. 2003;93(5):388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- 63.Rubbo H., Radi R., Trujillo M., Telleri R., Kalyanaraman B., Barnes S., et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. Journal of Biological Chemistry. 1994;269(42):26066–26075. [PubMed] [Google Scholar]
- 64.Esterbauer H., Wäg G., Puhl H. Lipid peroxidation and its role in atherosclerosis. British Medical Bulletin. 1993;49(3):566–576. doi: 10.1093/oxfordjournals.bmb.a072631. [DOI] [PubMed] [Google Scholar]
- 65.Hori M., Nishida K. Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovascular Research. 2009;81(3):457–464. doi: 10.1093/cvr/cvn335. [DOI] [PubMed] [Google Scholar]
- 66.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. Journal of the American College of Cardiology. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 67.Arab H.H., Safar M.M., Shahin N.N. Targeting ROS-dependent AKT/GSK-3β/NF-κB and DJ-1/nrf2 pathways by dapagliflozin attenuates neuronal injury and motor dysfunction in rotenone-induced Parkinson's disease rat model. ACS Chemical Neuroscience. 2021;12(4):689–703. doi: 10.1021/acschemneuro.0c00722. [DOI] [PubMed] [Google Scholar]
- 68.Lee T.-M., Chang N.-C., Lin S.-Z. Dapagliflozin, a selective SGLT2 Inhibitor, attenuated cardiac fibrosis by regulating the macrophage polarization via STAT3 signaling in infarcted rat hearts. Free Radical Biology & Medicine. 2017;104:298–310. doi: 10.1016/j.freeradbiomed.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 69.Yurista S.R., Silljé H.H.W., Oberdorf-Maass S.U., Schouten E.-M., Pavez Giani M.G., Hillebrands J.-L., et al. Sodium-glucose co-transporter 2 inhibition with empagliflozin improves cardiac function in non-diabetic rats with left ventricular dysfunction after myocardial infarction. European Journal of Heart Failure. 2019;21(7):862–873. doi: 10.1002/ejhf.1473. [DOI] [PubMed] [Google Scholar]
- 70.Xing Y.-J., Liu B.-H., Wan S.-J., Cheng Y., Zhou S.-M., Sun Y., et al. A SGLT2 inhibitor dapagliflozin alleviates diabetic cardiomyopathy by suppressing high glucose-induced oxidative stress in vivo and in vitro. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.708177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolijn D., Pabel S., Tian Y., Lódi M., Herwig M., Carrizzo A., et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovascular Research. 2021;117(2):495–507. doi: 10.1093/cvr/cvaa123. [DOI] [PubMed] [Google Scholar]
- 72.Paneni F., Sciarretta S., Costantino S. Tackling myocardial oxidative stress with empagliflozin: are we big enough to fight heart failure with preserved ejection fraction? Cardiovascular Research. 2021;117(2):343–345. doi: 10.1093/cvr/cvaa196. [DOI] [PubMed] [Google Scholar]
- 73.Santos-Gallego C.G., Requena-Ibanez J.A., San Antonio R., Garcia-Ropero A., Ishikawa K., Watanabe S., et al. Empagliflozin ameliorates diastolic dysfunction and left ventricular fibrosis/stiffness in nondiabetic heart failure: a multimodality study. JACC. Cardiovascular Imaging. 2021;14(2):393–407. doi: 10.1016/j.jcmg.2020.07.042. [DOI] [PubMed] [Google Scholar]
- 74.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gerstein H.C., Pogue J., Mann J.F.E., Lonn E., Dagenais G.R., McQueen M., et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia. 2005;48(9):1749–1755. doi: 10.1007/s00125-005-1858-4. [DOI] [PubMed] [Google Scholar]
- 76.Eeg-Olofsson K., Cederholm J., Nilsson P.M., Zethelius B., Svensson A.-M., Gudbjörnsdóttir S., et al. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR) Journal of Internal Medicine. 2010;268(5):471–482. doi: 10.1111/j.1365-2796.2010.02265.x. [DOI] [PubMed] [Google Scholar]
- 77.Chen Y., Wang L., Pitzer A.L., Li X., Li P.-L., Zhang Y. Contribution of redox-dependent activation of endothelial Nlrp3 inflammasomes to hyperglycemia-induced endothelial dysfunction. Journal of Molecular Medicine (Berlin) 2016;94(12):1335–1347. doi: 10.1007/s00109-016-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nieuwdorp M., van Haeften T.W., Gouverneur M.C.L.G., Mooij H.L., van Lieshout M.H.P., Levi M., et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes. 2006;55(2):480–486. doi: 10.2337/diabetes.55.02.06.db05-1103. [DOI] [PubMed] [Google Scholar]
- 79.Williams S.B., Goldfine A.B., Timimi F.K., Ting H.H., Roddy M.A., Simonson D.C., et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97(17):1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 80.Kunjathoor V.V., Wilson D.L., LeBoeuf R.C. Increased atherosclerosis in streptozotocin-induced diabetic mice. Journal of Clinical Investigation. 1996;97(7):1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nishikawa T., Edelstein D., Du X.L., Yamagishi S., Matsumura T., Kaneda Y., et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404(6779):787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 82.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circulation Research. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 84.Oelze M., Kröller-Schön S., Welschof P., Jansen T., Hausding M., Mikhed Y., et al. The sodium-glucose Co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One. 2014;9(11) doi: 10.1371/journal.pone.0112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ganbaatar B., Fukuda D., Shinohara M., Yagi S., Kusunose K., Yamada H., et al. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. European Journal of Pharmacology. 2020;875 doi: 10.1016/j.ejphar.2020.173040. [DOI] [PubMed] [Google Scholar]
- 86.Pennig J., Scherrer P., Gissler M.C., Anto-Michel N., Hoppe N., Füner L., et al. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Scientific Reports. 2019;9(1) doi: 10.1038/s41598-019-54224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin B., Koibuchi N., Hasegawa Y., Sueta D., Toyama K., Uekawa K., et al. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovascular Diabetology. 2014;13:148. doi: 10.1186/s12933-014-0148-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z., Murakoshi M., Ichikawa S., Koshida T., Adachi E., Suzuki C., et al. The sodium–glucose cotransporter 2 inhibitor tofogliflozin prevents diabetic kidney disease progression in type 2 diabetic mice. FEBS Open Bio. 2020;10(12):2761–2770. doi: 10.1002/2211-5463.13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Abdollahi E., Keyhanfar F., Delbandi A.-A., Falak R., Hajimiresmaiel S.J., Shafiei M. Dapagliflozin exerts anti-inflammatory effects via inhibition of LPS-induced TLR-4 overexpression and NF-κB activation in human endothelial cells and differentiated macrophages. European Journal of Pharmacology. 2022;918 doi: 10.1016/j.ejphar.2021.174715. [DOI] [PubMed] [Google Scholar]
- 90.Ye Y., Bajaj M., Yang H.-C., Perez-Polo J.R., Birnbaum Y. SGLT-2 inhibition with dapagliflozin reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic cardiomyopathy in mice with type 2 diabetes. Further augmentation of the effects with saxagliptin, a DPP4 inhibitor. Cardiovascular Drugs and Therapy. 2017;31(2):119–132. doi: 10.1007/s10557-017-6725-2. [DOI] [PubMed] [Google Scholar]
- 91.Xu C., Wang W., Zhong J., Lei F., Xu N., Zhang Y., et al. Canagliflozin exerts anti-inflammatory effects by inhibiting intracellular glucose metabolism and promoting autophagy in immune cells. Biochemical Pharmacology. 2018;152:45–59. doi: 10.1016/j.bcp.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 92.Ortega R., Collado A., Selles F., Gonzalez-Navarro H., Sanz M.-J., Real J.T., et al. SGLT-2 (Sodium-Glucose cotransporter 2) inhibition reduces ang II (angiotensin II)-Induced dissecting abdominal aortic aneurysm in ApoE (apolipoprotein E) knockout mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2019;39(8):1614–1628. doi: 10.1161/ATVBAHA.119.312659. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y., Lin X., Chu Y., Chen X., Du H., Zhang H., et al. Dapagliflozin: a sodium-glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFβ1/Smad signaling. Cardiovascular Diabetology. 2021;20(1):121. doi: 10.1186/s12933-021-01312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Byrne N.J., Matsumura N., Maayah Z.H., Ferdaoussi M., Takahara S., Darwesh A.M., et al. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (Nucleotide-Binding domain-like receptor protein 3) inflammasome activation in heart failure. Circulation. Heart Failure. 2020;13(1) doi: 10.1161/CIRCHEARTFAILURE.119.006277. [DOI] [PubMed] [Google Scholar]
- 95.Han J.H., Oh T.J., Lee G., Maeng H.J., Lee D.H., Kim K.M., et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE -/- mice fed a western diet. Diabetologia. 2017;60(2):364–376. doi: 10.1007/s00125-016-4158-2. [DOI] [PubMed] [Google Scholar]
- 96.Shin S.J., Chung S., Kim S.J., Lee E.-M., Yoo Y.-H., Kim J.-W., et al. Effect of sodium-glucose Co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nathan D.M., Genuth S., Lachin J., Cleary P., Crofford O., Davis M., et al. Diabetes Control. Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 98.Shichiri M., Kishikawa H., Ohkubo Y., Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–B29. [PubMed] [Google Scholar]
- 99.Ray K.K., Seshasai S.R.K., Wijesuriya S., Sivakumaran R., Nethercott S., Preiss D., et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 100.Hayward R.A., Reaven P.D., Wiitala W.L., Bahn G.D., Reda D.J., Ge L., et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2015;372(23):2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 101.Gerstein H.C., Miller M.E., Byington R.P., Goff D.C., Bigger J.T., Buse J.B., et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. New England Journal of Medicine. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. New England Journal of Medicine. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 103.Low Wang C.C., Hess C.N., Hiatt W.R., Goldfine A.B. Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes – mechanisms, management, and clinical considerations. Circulation. 2016;133(24):2459–2502. doi: 10.1161/CIRCULATIONAHA.116.022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maiuolo J., Oppedisano F., Gratteri S., Muscoli C., Mollace V. Regulation of uric acid metabolism and excretion. International Journal of Cardiology. 2016;213:8–14. doi: 10.1016/j.ijcard.2015.08.109. [DOI] [PubMed] [Google Scholar]
- 105.Ochiai M.E., Barretto A.C.P., Oliveira M.T., Munhoz R.T., Morgado P.C., Ramires J.A.F. Uric acid renal excretion and renal insufficiency in decompensated severe heart failure. European Journal of Heart Failure. 2005;7(4):468–474. doi: 10.1016/j.ejheart.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 106.Gladden J.D., Zelickson B.R., Wei C.-C., Ulasova E., Zheng J., Ahmed M.I., et al. Insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free Radical Biology & Medicine. 2011. Novel;51(11):1975–1984. doi: 10.1016/j.freeradbiomed.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ben Salem C., Slim R., Fathallah N., Hmouda H. Drug-induced hyperuricaemia and gout. Rheumatology. 2017;56(5):679–688. doi: 10.1093/rheumatology/kew293. [DOI] [PubMed] [Google Scholar]
- 108.Ali N., Miah R., Hasan M., Barman Z., Mou A.D., Hafsa J.M., et al. Association between serum uric acid and metabolic syndrome: a cross-sectional study in Bangladeshi adults. Scientific Reports. 2020;10(1):7841. doi: 10.1038/s41598-020-64884-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cirillo P., Sato W., Reungjui S., Heinig M., Gersch M., Sautin Y., et al. Uric acid, the metabolic syndrome, and renal disease. Journal of the American Society of Nephrology. 2006;17(12 Suppl 3):S165–S168. doi: 10.1681/ASN.2006080909. [DOI] [PubMed] [Google Scholar]
- 110.Feig D.I., Kang D.-H., Johnson R.J. Uric acid and cardiovascular risk. New England Journal of Medicine. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tomiyama H., Shiina K., Vlachopoulos C., Iwasaki Y., Matsumoto C., Kimura K., et al. Involvement of arterial stiffness and inflammation in hyperuricemia-related development of hypertension. Hypertension. 2018;72(3):739–745. doi: 10.1161/HYPERTENSIONAHA.118.11390. [DOI] [PubMed] [Google Scholar]
- 112.Krishnan E. Interaction of inflammation, hyperuricemia, and the prevalence of hypertension among adults free of metabolic syndrome: NHANES 2009-2010. Journal of American Heart Association. 2014;3(2) doi: 10.1161/JAHA.113.000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhou Y., Zhao M., Pu Z., Xu G., Li X. Relationship between oxidative stress and inflammation in hyperuricemia. Medicine. 2018;97(49) doi: 10.1097/MD.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verzola D., Ratto E., Villaggio B., Parodi E.L., Pontremoli R., Garibotto G., et al. Uric acid promotes apoptosis in human proximal tubule cells by oxidative stress and the activation of NADPH oxidase NOX 4. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Z., Sheng Y., Liu C., Li K., Huang X., Huang J., et al. Nox4 has a crucial role in uric acid-induced oxidative stress and apoptosis in renal tubular cells. Molecular Medicine Reports. 2016;13(5):4343–4348. doi: 10.3892/mmr.2016.5083. [DOI] [PubMed] [Google Scholar]
- 116.Milanesi S., Verzola D., Cappadona F., Bonino B., Murugavel A., Pontremoli R., et al. Uric acid and angiotensin II additively promote inflammation and oxidative stress in human proximal tubule cells by activation of toll-like receptor 4. Journal of Cellular Physiology. 2019;234(7):10868–10876. doi: 10.1002/jcp.27929. [DOI] [PubMed] [Google Scholar]
- 117.Corry D.B., Eslami P., Yamamoto K., Nyby M.D., Makino H., Tuck M.L. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. Journal of Hypertension. 2008;26(2):269–275. doi: 10.1097/HJH.0b013e3282f240bf. [DOI] [PubMed] [Google Scholar]
- 118.Zhang J., Zhang Y., Wu Q., Chen B. Uric acid induces oxidative stress via an activation of the renin-angiotensin system in 3T3-L1 adipocytes. Endocrine. 2015;48(1):135–142. doi: 10.1007/s12020-014-0239-5. [DOI] [PubMed] [Google Scholar]
- 119.Braga T.T., Forni M.F., Correa-Costa M., Ramos R.N., Barbuto J.A., Branco P., et al. Soluble uric acid activates the NLRP3 inflammasome. Scientific Reports. 2017;7 doi: 10.1038/srep39884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yin W., Zhou Q.-L., OuYang S.-X., Chen Y., Gong Y.-T., Liang Y.-M. Uric acid regulates NLRP3/IL-1β signaling pathway and further induces vascular endothelial cells injury in early CKD through ROS activation and K+ efflux. BMC Nephrology. 2019;20(1):319. doi: 10.1186/s12882-019-1506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou Y., Fang L., Jiang L., Wen P., Cao H., He W., et al. Uric acid induces renal inflammation via activating tubular NF-κB signaling pathway. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu W., Xu Y., Shao X., Gao F., Li Y., Hu J., et al. Uric acid produces an inflammatory response through activation of NF-κB in the hypothalamus: implications for the pathogenesis of metabolic disorders. Scientific Reports. 2015;5 doi: 10.1038/srep12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhen H., Gui F. The role of hyperuricemia on vascular endothelium dysfunction. Biomedical Reports. 2017;7(4):325–330. doi: 10.3892/br.2017.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kang D.-H., Park S.-K., Lee I.-K., Johnson R.J. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. Journal of the American Society of Nephrology. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 125.Chang C.-C., Wu C.-H., Liu L.-K., Chou R.-H., Kuo C.-S., Huang P.-H., et al. Association between serum uric acid and cardiovascular risk in nonhypertensive and nondiabetic individuals: the Taiwan I-Lan Longitudinal Aging Study. Scientific Reports. 2018;8(1):5234. doi: 10.1038/s41598-018-22997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huang H., Huang B., Li Y., Huang Y., Li J., Yao H., et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. European Journal of Heart Failure. 2014;16(1):15–24. doi: 10.1093/eurjhf/hft132. [DOI] [PubMed] [Google Scholar]
- 127.Rahimi-Sakak F., Maroofi M., Rahmani J., Bellissimo N., Hekmatdoost A. Serum uric acid and risk of cardiovascular mortality: a systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovascular Disorders. 2019;19(1):218. doi: 10.1186/s12872-019-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saito H., Tanaka K., Iwasaki T., Oda A., Watanabe S., Kanno M., et al. Xanthine oxidase inhibitors are associated with reduced risk of cardiovascular disease. Scientific Reports. 2021;11(1):1380. doi: 10.1038/s41598-020-80835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bredemeier M., Lopes L.M., Eisenreich M.A., Hickmann S., Bongiorno G.K., d'Avila R., et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovascular Disorders. 2018;18(1):24. doi: 10.1186/s12872-018-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhao Y., Xu L., Tian D., Xia P., Zheng H., Wang L., et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes, Obesity and Metabolism. 2018;20(2):458–462. doi: 10.1111/dom.13101. [DOI] [PubMed] [Google Scholar]
- 131.Wilcox C.S., Shen W., Boulton D.W., Leslie B.R., Griffen S.C. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. Journal of American Heart Association. 2018;7(4) doi: 10.1161/JAHA.117.007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Griffin M., Rao V.S., Ivey-Miranda J., Fleming J., Mahoney D., Maulion C., et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation. 2020;142(11):1028–1039. doi: 10.1161/CIRCULATIONAHA.120.045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chino Y., Samukawa Y., Sakai S., Nakai Y., Yamaguchi J., Nakanishi T., et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharmaceutics & Drug Disposition. 2014;35(7):391–404. doi: 10.1002/bdd.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bailey C.J. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes. Obesity and Metabolism. 2019;21(6):1291–1298. doi: 10.1111/dom.13670. [DOI] [PubMed] [Google Scholar]
- 135.Hare J.M., Mangal B., Brown J., Fisher C., Freudenberger R., Colucci W.S., et al. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. Journal of the American College of Cardiology. 2008;51(24):2301–2309. doi: 10.1016/j.jacc.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 136.Givertz M.M., Anstrom K.J., Redfield M.M., Deswal A., Haddad H., Butler J., et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation. 2015;131(20):1763–1771. doi: 10.1161/CIRCULATIONAHA.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ju C., Lai R.W.C., Li K.H.C., Hung J.K.F., Lai J.C.L., Ho J., et al. Comparative cardiovascular risk in users versus non-users of xanthine oxidase inhibitors and febuxostat versus allopurinol users. Rheumatology. 2020;59(9):2340–2349. doi: 10.1093/rheumatology/kez576. [DOI] [PubMed] [Google Scholar]
- 138.White W.B., Chohan S., Dabholkar A., Hunt B., Jackson R. Cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular comorbidities. American Heart Journal. 2012;164(1):14–20. doi: 10.1016/j.ahj.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 139.Packer M. Uric acid is a biomarker of oxidative stress in the failing heart: lessons learned from trials with allopurinol and SGLT2 inhibitors. Journal of Cardiac Failure. 2020;26(11):977–984. doi: 10.1016/j.cardfail.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 140.Förstermann U., Xia N., Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circulation Research. 2017;120(4):713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 141.Peleli M., Zollbrecht C., Montenegro M.F., Hezel M., Zhong J., Persson E.G., et al. Enhanced XOR activity in eNOS-deficient mice: effects on the nitrate-nitrite-NO pathway and ROS homeostasis. Free Radical Biology & Medicine. 2016;99:472–484. doi: 10.1016/j.freeradbiomed.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Packer M. Cardioprotective effects of sirtuin-1 and its downstream effectors: potential role in mediating the heart failure benefits of SGLT2 (Sodium-Glucose cotransporter 2) inhibitors. Circulation. Heart Failure. 2020;13(9) doi: 10.1161/CIRCHEARTFAILURE.120.007197. [DOI] [PubMed] [Google Scholar]
- 143.Mattagajasingh I., Kim C.-S., Naqvi A., Yamamori T., Hoffman T.A., Jung S.-B., et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(37):14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Huang X.-F., Li H.-Q., Shi L., Xue J.-Y., Ruan B.-F., Zhu H.-L. Synthesis of resveratrol analogues, and evaluation of their cytotoxic and xanthine oxidase inhibitory activities. Chemistry and Biodiversity. 2008;5(4):636–642. doi: 10.1002/cbdv.200890059. [DOI] [PubMed] [Google Scholar]
- 145.Abel E.D., O'Shea K.M., Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(9):2068–2076. doi: 10.1161/ATVBAHA.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lopaschuk G.D., Ussher J.R., Folmes C.D.L., Jaswal J.S., Stanley W.C. Myocardial fatty acid metabolism in health and disease. Physiological Reviews. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 147.Mudaliar S., Alloju S., Henry R.R. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG outcome study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–1122. doi: 10.2337/dc16-0542. [DOI] [PubMed] [Google Scholar]
- 148.Ferrannini E., Mark M., Mayoux E. CV protection in the EMPA-REG outcome trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39(7):1108–1114. doi: 10.2337/dc16-0330. [DOI] [PubMed] [Google Scholar]
- 149.Horton J.L., Davidson M.T., Kurishima C., Vega R.B., Powers J.C., Matsuura T.R., et al. The failing heart utilizes 3-hydroxybutyrate as a metabolic stress defense. JCI Insight. 2019;4(4) doi: 10.1172/jci.insight.124079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Santos-Gallego C.G., Requena-Ibanez J.A., San Antonio R., Ishikawa K., Watanabe S., Picatoste B., et al. Empagliflozin ameliorates adverse left ventricular remodeling in nondiabetic heart failure by enhancing myocardial energetics. Journal of the American College of Cardiology. 2019;73(15):1931–1944. doi: 10.1016/j.jacc.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 151.Nielsen R., Møller N., Gormsen L.C., Tolbod L.P., Hansson N.H., Sorensen J., et al. Cardiovascular effects of treatment with the ketone body 3-hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139(18):2129–2141. doi: 10.1161/CIRCULATIONAHA.118.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lopaschuk G.D., Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metabolism. 2016;24(2):200–202. doi: 10.1016/j.cmet.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 153.Shimazu T., Hirschey M.D., Newman J., He W., Shirakawa K., Le Moan N., et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339(6116):211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cardinale J.P., Sriramula S., Pariaut R., Guggilam A., Mariappan N., Elks C.M., et al. HDAC inhibition attenuates inflammatory, hypertrophic, and hypertensive responses in spontaneously hypertensive rats. Hypertension. 2010;56(3):437–444. doi: 10.1161/HYPERTENSIONAHA.110.154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iyer A., Fenning A., Lim J., Le G.T., Reid R.C., Halili M.A., et al. Antifibrotic activity of an inhibitor of histone deacetylases in DOCA-salt hypertensive rats. British Journal of Pharmacology. 2010;159(7):1408–1417. doi: 10.1111/j.1476-5381.2010.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cao W., Bao C., Padalko E., Lowenstein C.J. Acetylation of mitogen-activated protein kinase phosphatase-1 inhibits Toll-like receptor signaling. Journal of Experimental Medicine. 2008;205(6):1491–1503. doi: 10.1084/jem.20071728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang L., de Zoeten E.F., Greene M.I., Hancock W.W. Immunomodulatory effects of deacetylase inhibitors: therapeutic targeting of FOXP3+ regulatory T cells. Nature Reviews. Drug Discovery. 2009;8(12):969–981. doi: 10.1038/nrd3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu C., Li A., Leng Y., Li Y., Kang J. Histone deacetylase inhibition by sodium valproate regulates polarization of macrophage subsets. DNA and Cell Biology. 2012;31(4):592–599. doi: 10.1089/dna.2011.1401. [DOI] [PubMed] [Google Scholar]
- 159.Wang G., Shi Y., Jiang X., Leak R.K., Hu X., Wu Y., et al. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3β/PTEN/Akt axis. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(9):2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nishitani S., Fukuhara A., Shin J., Okuno Y., Otsuki M., Shimomura I. Metabolomic and microarray analyses of adipose tissue of dapagliflozin-treated mice, and effects of 3-hydroxybutyrate on induction of adiponectin in adipocytes. Scientific Reports. 2018;8(1):8805. doi: 10.1038/s41598-018-27181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Goldberg E.L., Asher J.L., Molony R.D., Shaw A.C., Zeiss C.J., Wang C., et al. β-Hydroxybutyrate deactivates neutrophil NLRP3 inflammasome to relieve gout flares. Cell Reports. 2017;18(9):2077–2087. doi: 10.1016/j.celrep.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hutton H.L., Ooi J.D., Holdsworth S.R., Kitching A.R. The NLRP3 inflammasome in kidney disease and autoimmunity. Nephrology. 2016;21(9):736–744. doi: 10.1111/nep.12785. [DOI] [PubMed] [Google Scholar]
- 163.Yuan X., Wang L., Bhat O.M., Lohner H., Li P.-L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: antioxidant action of butyrate. Redox Biology. 2018;16:21–31. doi: 10.1016/j.redox.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Youm Y.-H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nature Medicine. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Byrne N.J., Soni S., Takahara S., Ferdaoussi M., Al Batran R., Darwesh A.M., et al. Chronically elevating circulating ketones can reduce cardiac inflammation and blunt the development of heart failure. Circulation. Heart Failure. 2020;13(6) doi: 10.1161/CIRCHEARTFAILURE.119.006573. [DOI] [PubMed] [Google Scholar]
- 166.Kim S.R., Lee S.-G., Kim S.H., Kim J.H., Choi E., Cho W., et al. SGLT2 inhibition modulates NLRP3 inflammasome activity via ketones and insulin in diabetes with cardiovascular disease. Nature Communications. 2020;11(1):2127. doi: 10.1038/s41467-020-15983-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deng Y., Xie M., Li Q., Xu X., Ou W., Zhang Y., et al. Targeting mitochondria-inflammation circuit by β-hydroxybutyrate mitigates HFpEF. Circulation Research. 2021;128(2):232–245. doi: 10.1161/CIRCRESAHA.120.317933. [DOI] [PubMed] [Google Scholar]
- 168.Tochiya M., Makino H., Tamanaha T., Matsuo M., Hishida A., Koezuka R., et al. Effect of tofogliflozin on cardiac and vascular endothelial function in patients with type 2 diabetes and heart diseases: a pilot study. Journal of Diabetes Investigation. 2020;11(2):400–404. doi: 10.1111/jdi.13122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vishvanath L., Gupta R.K. Contribution of adipogenesis to healthy adipose tissue expansion in obesity. The Journal of Clinical Investigation. 2019;129(10):4022–4031. doi: 10.1172/JCI129191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Oikonomou E.K., Antoniades C. The role of adipose tissue in cardiovascular health and disease. Nature Reviews Cardiology. 2019;16(2):83–99. doi: 10.1038/s41569-018-0097-6. [DOI] [PubMed] [Google Scholar]
- 171.Xia N., Li H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. British Journal of Pharmacology. 2017;174(20):3425–3442. doi: 10.1111/bph.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xia N., Horke S., Habermeier A., Closs E.I., Reifenberg G., Gericke A., et al. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2016;36(1):78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 173.Marchesi C., Ebrahimian T., Angulo O., Paradis P., Schiffrin E.L. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54(6):1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 174.Touyz R.M., Briones A.M. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertension Research: Official Journal of the Japanese Society of Hypertension. 2011;34(1):5–14. doi: 10.1038/hr.2010.201. [DOI] [PubMed] [Google Scholar]
- 175.Gao Y.-J., Takemori K., Su L.-Y., An W.-S., Lu C., Sharma A.M., et al. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovascular Research. 2006;71(2):363–373. doi: 10.1016/j.cardiores.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 176.Lee W.-J., Wu C.-S., Lin H., Lee I.-T., Wu C.-M., Tseng J.-J., et al. Visfatin-induced expression of inflammatory mediators in human endothelial cells through the NF-kappaB pathway. International Journal of Obesity. 2009;33(4):465–472. doi: 10.1038/ijo.2009.24. 2005. [DOI] [PubMed] [Google Scholar]
- 177.Kawanami D., Maemura K., Takeda N., Harada T., Nojiri T., Imai Y., et al. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine-endothelial cell interactions. Biochemical and Biophysical Research Communications. 2004;314(2):415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- 178.Ohyama K., Matsumoto Y., Takanami K., Ota H., Nishimiya K., Sugisawa J., et al. Coronary adventitial and perivascular adipose tissue inflammation in patients with vasospastic angina. Journal of the American College of Cardiology. 2018;71(4):414–425. doi: 10.1016/j.jacc.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 179.Mazzotta C., Basu S., Gower A.C., Karki S., Farb M.G., Sroczynski E., et al. Perivascular adipose tissue inflammation in ischemic heart disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2021;41(3):1239–1250. doi: 10.1161/ATVBAHA.120.315865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Iacobellis G. Local and systemic effects of the multifaceted epicardial adipose tissue depot. Nature Reviews Endocrinology. 2015;11(6):363–371. doi: 10.1038/nrendo.2015.58. [DOI] [PubMed] [Google Scholar]
- 181.Antonopoulos A.S., Margaritis M., Verheule S., Recalde A., Sanna F., Herdman L., et al. Mutual regulation of epicardial adipose tissue and myocardial redox state by PPAR-γ/adiponectin signalling. Circulation Research. 2016;118(5):842–855. doi: 10.1161/CIRCRESAHA.115.307856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Packer M. Epicardial adipose tissue may mediate deleterious effects of obesity and inflammation on the myocardium. Journal of the American College of Cardiology. 2018;71(20):2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 183.Ng A.C.T., Strudwick M., van der Geest R.J., Ng A.C.C., Gillinder L., Goo S.Y., et al. Impact of epicardial adipose tissue, left ventricular myocardial fat content, and interstitial fibrosis on myocardial contractile function. Circulation. Cardiovascular Imaging. 2018;11(8) doi: 10.1161/CIRCIMAGING.117.007372. [DOI] [PubMed] [Google Scholar]
- 184.Mahmoud I., Dykun I., Kärner L., Hendricks S., Totzeck M., Al-Rashid F., et al. Epicardial adipose tissue differentiates in patients with and without coronary microvascular dysfunction. International Journal of Obesity. 2021;45(9):2058–2063. doi: 10.1038/s41366-021-00875-6. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cheng K.-H., Chu C.-S., Lee K.-T., Lin T.-H., Hsieh C.-C., Chiu C.-C., et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with coronary artery disease. International Journal of Obesity. 2008;32(2):268–274. doi: 10.1038/sj.ijo.0803726. 2005. [DOI] [PubMed] [Google Scholar]
- 186.Nakanishi K., Fukuda S., Tanaka A., Otsuka K., Taguchi H., Shimada K. Relationships between periventricular epicardial adipose tissue accumulation, coronary microcirculation, and left ventricular diastolic dysfunction. Canadian Journal of Cardiology. 2017;33(11):1489–1497. doi: 10.1016/j.cjca.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 187.Natale F., Tedesco M.A., Mocerino R., de Simone V., Di Marco G.M., Aronne L., et al. Visceral adiposity and arterial stiffness: echocardiographic epicardial fat thickness reflects, better than waist circumference, carotid arterial stiffness in a large population of hypertensives. European Journal of Echocardiography: The Journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009;10(4):549–555. doi: 10.1093/ejechocard/jep002. [DOI] [PubMed] [Google Scholar]
- 188.Nerlekar N., Muthalaly R.G., Wong N., Thakur U., Wong D.T.L., Brown A.J., et al. Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. Journal of American Heart Association. 2018;7(23) doi: 10.1161/JAHA.118.009975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lee P.C., Ganguly S., Goh S.-Y. Weight loss associated with sodium-glucose cotransporter-2 inhibition: a review of evidence and underlying mechanisms. Obesity Reviews. 2018;19(12):1630–1641. doi: 10.1111/obr.12755. [DOI] [PubMed] [Google Scholar]
- 190.Zheng H., Liu M., Li S., Shi Q., Zhang S., Zhou Y., et al. Sodium-glucose Co-Transporter-2 inhibitors in non-diabetic adults with overweight or obesity: a systematic review and meta-analysis. Frontiers in Endocrinology. 2021;12 doi: 10.3389/fendo.2021.706914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Del Prato S., Nauck M., Durán-Garcia S., Maffei L., Rohwedder K., Theuerkauf A., et al. Long-term glycaemic response and tolerability of dapagliflozin versus a sulphonylurea as add-on therapy to metformin in patients with type 2 diabetes: 4-year data. Diabetes, Obesity and Metabolism. 2015;17(6):581–590. doi: 10.1111/dom.12459. [DOI] [PubMed] [Google Scholar]
- 192.McDowell K., Petrie M.C., Raihan N.A., Logue J. Effects of intentional weight loss in patients with obesity and heart failure: a systematic review. Obesity Reviews. 2018;19(9):1189–1204. doi: 10.1111/obr.12707. [DOI] [PubMed] [Google Scholar]
- 193.Koshizaka M., Ishikawa K., Ishibashi R., Maezawa Y., Sakamoto K., Uchida D., et al. Comparing the effects of ipragliflozin versus metformin on visceral fat reduction and metabolic dysfunction in Japanese patients with type 2 diabetes treated with sitagliptin: a prospective, multicentre, open-label, blinded-endpoint, randomized controlled study (PRIME-V study) Diabetes, Obesity and Metabolism. 2019;21(8):1990–1995. doi: 10.1111/dom.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Iacobellis G., Gra-Menendez S. Effects of dapagliflozin on epicardial fat thickness in patients with type 2 diabetes and obesity. Obesity. 2020;28(6):1068–1074. doi: 10.1002/oby.22798. [DOI] [PubMed] [Google Scholar]
- 195.Sato T., Aizawa Y., Yuasa S., Kishi S., Fuse K., Fujita S., et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovascular Diabetology. 2018;17(1):6. doi: 10.1186/s12933-017-0658-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bouchi R., Terashima M., Sasahara Y., Asakawa M., Fukuda T., Takeuchi T., et al. Luseogliflozin reduces epicardial fat accumulation in patients with type 2 diabetes: a pilot study. Cardiovascular Diabetology. 2017;16(1):32. doi: 10.1186/s12933-017-0516-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Braha A., Timar B., Diaconu L., Lupusoru R., Vasiluta L., Sima A., et al. Dynamics of epicardiac fat and heart function in type 2 diabetic patients initiated with SGLT-2 inhibitors. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2019;12:2559–2566. doi: 10.2147/DMSO.S223629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yagi S., Hirata Y., Ise T., Kusunose K., Yamada H., Fukuda D., et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetology & Metabolic Syndrome. 2017;9(1):78. doi: 10.1186/s13098-017-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Requena-Ibáñez J.A., Santos-Gallego C.G., Rodriguez-Cordero A., Vargas-Delgado A.P., Mancini D., Sartori S., et al. Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: from the EMPA-TROPISM study. JACC. Heart Failure. 2021;9(8):578–589. doi: 10.1016/j.jchf.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 200.Mullens W., Martens P. Empagliflozin-induced changes in epicardial fat: the centerpiece for myocardial protection? JACC. Heart Failure. 2021;9(8):590–593. doi: 10.1016/j.jchf.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 201.Gaborit B., Ancel P., Abdullah A.E., Maurice F., Abdesselam I., Calen A., et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: the EMPACEF study. Cardiovascular Diabetology. 2021;20:57. doi: 10.1186/s12933-021-01237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Masson W., Lavalle-Cobo A., Nogueira J.P. Effect of SGLT2-inhibitors on epicardial adipose tissue: a meta-analysis. Cells. 2021;10(8):2150. doi: 10.3390/cells10082150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Requena-Ibáñez J.A., Santos-Gallego C.G., Rodriguez Cordero A.J., Fardman B., Sartori S., Sanz J., et al. Not only how much, but also how to, when measuring epicardial adipose tissue. Magnetic Resonance Imaging. 2022;86:149–151. doi: 10.1016/j.mri.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 204.Díaz-Rodríguez E., Agra R.M., Fernández Á.L., Adrio B., García-Caballero T., González-Juanatey J.R., et al. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovascular Research. 2018;114(2):336–346. doi: 10.1093/cvr/cvx186. [DOI] [PubMed] [Google Scholar]
- 205.Mori Y., Terasaki M., Hiromura M., Saito T., Kushima H., Koshibu M., et al. Luseogliflozin attenuates neointimal hyperplasia after wire injury in high-fat diet-fed mice via inhibition of perivascular adipose tissue remodeling. Cardiovascular Diabetology. 2019;18:143. doi: 10.1186/s12933-019-0947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mori K., Tsuchiya K., Nakamura S., Miyachi Y., Shiba K., Ogawa Y., et al. Ipragliflozin-induced adipose expansion inhibits cuff-induced vascular remodeling in mice. Cardiovascular Diabetology. 2019;18(1):83. doi: 10.1186/s12933-019-0886-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kagota S., Maruyama-Fumoto K., McGuire J.J., Shinozuka K. A sodium glucose cotransporter 2 inhibitor fails to improve perivascular adipose tissue-mediated modulation of vasodilation and cardiac function in rats with metabolic syndrome. Journal of Cardiovascular Pharmacology and Therapeutics. 2021;26(5):480–489. doi: 10.1177/10742484211001853. [DOI] [PubMed] [Google Scholar]
- 208.Xu L., Nagata N., Nagashimada M., Zhuge F., Ni Y., Chen G., et al. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ferrannini E., Baldi S., Frascerra S., Astiarraga B., Heise T., Bizzotto R., et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 210.Hawley S.A., Ford R.J., Smith B.K., Gowans G.J., Mancini S.J., Pitt R.D., et al. The Na+/glucose co-transporter inhibitor canagliflozin activates AMP-activated protein kinase by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes. 2016;65(9):2784–2794. doi: 10.2337/db16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lowell B.B., Spiegelman B.M. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 212.Sawada Y., Izumida Y., Takeuchi Y., Aita Y., Wada N., Li E., et al. Effect of sodium-glucose cotransporter 2 (SGLT2) inhibition on weight loss is partly mediated by liver-brain-adipose neurocircuitry. Biochemical and Biophysical Research Communications. 2017;493(1):40–45. doi: 10.1016/j.bbrc.2017.09.081. [DOI] [PubMed] [Google Scholar]
- 213.Ferrario C.M., Strawn W.B. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. The American Journal of Cardiology. 2006;98(1):121–128. doi: 10.1016/j.amjcard.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 214.Marchesi C., Paradis P., Schiffrin E.L. Role of the renin-angiotensin system in vascular inflammation. Trends in Pharmacological Sciences. 2008;29(7):367–374. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 215.Weiss D., Kools J.J., Taylor W.R. Angiotensin II-induced hypertension accelerates the development of atherosclerosis in apoE-deficient mice. Circulation. 2001;103(3):448–454. doi: 10.1161/01.cir.103.3.448. [DOI] [PubMed] [Google Scholar]
- 216.Schieffer B., Schieffer E., Hilfiker-Kleiner D., Hilfiker A., Kovanen P.T., Kaartinen M., et al. Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation. 2000;101(12):1372–1378. doi: 10.1161/01.cir.101.12.1372. [DOI] [PubMed] [Google Scholar]
- 217.Vaughan D.E., Lazos S.A., Tong K. Angiotensin II regulates the expression of plasminogen activator inhibitor-1 in cultured endothelial cells. A potential link between the renin-angiotensin system and thrombosis. Journal of Clinical Investigation. 1995;95(3):995–1001. doi: 10.1172/JCI117809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rodríguez-Vita J., Sánchez-López E., Esteban V., Rupérez M., Egido J., Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111(19):2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 219.Nguyen Dinh Cat A., Montezano A.C., Burger D., Touyz R.M. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxidants and Redox Signaling. 2013;19(10):1110–1120. doi: 10.1089/ars.2012.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Berry C., Hamilton C.A., Brosnan M.J., Magill F.G., Berg G.A., McMurray J.J., et al. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101(18):2206–2212. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- 221.Doughan A.K., Harrison D.G., Dikalov S.I. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circulation Research. 2008;102(4):488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 222.Brasier A.R., Jamaluddin M., Han Y., Patterson C., Runge M.S. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Molecular and Cellular Biochemistry. 2000;212(1–2):155–169. [PubMed] [Google Scholar]
- 223.Dandona P., Dhindsa S., Ghanim H., Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. Journal of Human Hypertension. 2007;21(1):20–27. doi: 10.1038/sj.jhh.1002101. [DOI] [PubMed] [Google Scholar]
- 224.Fliser, D., Buchholz, K., Haller, H., EUropean Trial on Olmesartan and Pravastatin in Inflammation and Atherosclerosis (EUTOPIA) Investigators, 2004. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation 110(9): 1103–1107, Doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed]
- 225.Gullestad L., Aukrust P., Ueland T., Espevik T., Yee G., Vagelos R., et al. Effect of high- versus low-dose angiotensin converting enzyme inhibition on cytokine levels in chronic heart failure. Journal of the American College of Cardiology. 1999;34(7):2061–2067. doi: 10.1016/s0735-1097(99)00495-7. [DOI] [PubMed] [Google Scholar]
- 226.Yusuf S., Sleight P., Pogue J., Bosch J., Davies R., Dagenais G., Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. New England Journal of Medicine. 2000;342(3):145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 227.Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (Transcend) Investigators. Yusuf S., Teo K., Anderson C., et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008;372(9644):1174–1183. doi: 10.1016/S0140-6736(08)61242-8. [DOI] [PubMed] [Google Scholar]
- 228.Miyata K.N., Lo C.-S., Zhao S., Liao M.-C., Pang Y., Chang S.-Y., et al. Angiotensin II upregulates sodium-glucose Co-Transporter2 (SGLT2) expression and SGLT2 inhibitor attenuates ang II-induced hypertensive renal injury in mice. Clinical Science. 2021;135(7):943–961. doi: 10.1042/CS20210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Park S.-H., Belcastro E., Hasan H., Matsushita K., Marchandot B., Abbas M., et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovascular Diabetology. 2021;20:65. doi: 10.1186/s12933-021-01252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Schork A., Saynisch J., Vosseler A., Jaghutriz B.A., Heyne N., Peter A., et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin–angiotensin–aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovascular Diabetology. 2019;18:46. doi: 10.1186/s12933-019-0852-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Takeuchi T., Dohi K., Omori T., Moriwaki K., Sato Y., Nakamori S., et al. Diuretic effects of sodium-glucose cotransporter 2 inhibitor in patients with type 2 diabetes mellitus and heart failure. International Journal of Cardiology. 2015:1–3. doi: 10.1016/j.ijcard.2015.07.072. 201. [DOI] [PubMed] [Google Scholar]
- 232.Heerspink H.J.L., de Zeeuw D., Wie L., Leslie B., List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes, Obesity and Metabolism. 2013;15(9):853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Heise T., Jordan J., Wanner C., Heer M., Macha S., Mattheus M., et al. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clinical Therapeutics. 2016;38(10):2265–2276. doi: 10.1016/j.clinthera.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 234.Garcia D., Shaw R.J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Molecular Cell. 2017;66(6):789–800. doi: 10.1016/j.molcel.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee H.-M., Kim J.-J., Kim H.J., Shong M., Ku B.J., Jo E.-K. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62(1):194–204. doi: 10.2337/db12-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Galic S., Fullerton M.D., Schertzer J.D., Sikkema S., Marcinko K., Walkley C.R., et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. The Journal of Clinical Investigation. 2011;121(12):4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cacicedo J.M., Yagihashi N., Keaney J.F., Ruderman N.B., Ido Y. AMPK inhibits fatty acid-induced increases in NF-kappaB transactivation in cultured human umbilical vein endothelial cells. Biochemical and Biophysical Research Communications. 2004;324(4):1204–1209. doi: 10.1016/j.bbrc.2004.09.177. [DOI] [PubMed] [Google Scholar]
- 238.Ewart M.-A., Kohlhaas C.F., Salt I.P. Inhibition of tumor necrosis factor alpha-stimulated monocyte adhesion to human aortic endothelial cells by AMP-activated protein kinase. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(12):2255–2257. doi: 10.1161/ATVBAHA.108.175919. [DOI] [PubMed] [Google Scholar]
- 239.Bess E., Fisslthaler B., Frömel T., Fleming I. Nitric oxide-induced activation of the AMP-activated protein kinase α2 subunit attenuates IκB kinase activity and inflammatory responses in endothelial cells. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kim S.A., Choi H.C. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 2012;425(4):866–872. doi: 10.1016/j.bbrc.2012.07.165. [DOI] [PubMed] [Google Scholar]
- 241.Rutherford C., Speirs C., Williams J.J.L., Ewart M.-A., Mancini S.J., Hawley S.A., et al. Phosphorylation of Janus kinase 1 (JAK1) by AMP-activated protein kinase (AMPK) links energy sensing to anti-inflammatory signaling. Science Signaling. 2016;9(453):ra109. doi: 10.1126/scisignal.aaf8566. [DOI] [PubMed] [Google Scholar]
- 242.Tong D., Schiattarella G.G., Jiang N., Gillette T., Hill J.A. Impaired ampk signaling in hfpef-associated atrial fibrillation. Journal of the American College of Cardiology. 2020;75(11_Supplement_1):347. 347. [Google Scholar]
- 243.Zhang P., Hu X., Xu X., Fassett J., Zhu G., Viollet B., et al. AMPKα2 deficiency exacerbates pressure-overload induced left ventricular hypertrophy and dysfunction in mice. Hypertension. 2008;52(5):918–924. doi: 10.1161/HYPERTENSIONAHA.108.114702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Liao Y., Takashima S., Maeda N., Ouchi N., Komamura K., Shimomura I., et al. Exacerbation of heart failure in adiponectin-deficient mice due to impaired regulation of AMPK and glucose metabolism. Cardiovascular Research. 2005;67(4):705–713. doi: 10.1016/j.cardiores.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 245.Sung M.M., Zordoky B.N., Bujak A.L., Lally J.S.V., Fung D., Young M.E., et al. AMPK deficiency in cardiac muscle results in dilated cardiomyopathy in the absence of changes in energy metabolism. Cardiovascular Research. 2015;107(2):235–245. doi: 10.1093/cvr/cvv166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xu J., Kitada M., Ogura Y., Liu H., Koya D. Dapagliflozin restores impaired autophagy and suppresses inflammation in high glucose-treated HK-2 cells. Cells. 2021;10(6):1457. doi: 10.3390/cells10061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Birnbaum Y., Bajaj M., Yang H.-C., Ye Y. Combined SGLT2 and DPP4 inhibition reduces the activation of the Nlrp3/ASC inflammasome and attenuates the development of diabetic nephropathy in mice with type 2 diabetes. Cardiovascular Drugs and Therapy. 2018;32(2):135–145. doi: 10.1007/s10557-018-6778-x. [DOI] [PubMed] [Google Scholar]
- 248.Mancini S.J., Boyd D., Katwan O.J., Strembitska A., Almabrouk T.A., Kennedy S., et al. Canagliflozin inhibits interleukin-1β-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Scientific Reports. 2018;8:5276. doi: 10.1038/s41598-018-23420-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Koyani C.N., Plastira I., Sourij H., Hallström S., Schmidt A., Rainer P.P., et al. Empagliflozin protects heart from inflammation and energy depletion via AMPK activation. Pharmacological Research. 2020;158 doi: 10.1016/j.phrs.2020.104870. [DOI] [PubMed] [Google Scholar]
- 250.Tian J., Zhang M., Suo M., Liu D., Wang X., Liu M., et al. Dapagliflozin alleviates cardiac fibrosis through suppressing EndMT and fibroblast activation via AMPKα/TGF-β/Smad signalling in type 2 diabetic rats. Journal of Cellular and Molecular Medicine. 2021;25(16):7642–7659. doi: 10.1111/jcmm.16601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Glick D., Barth S., Macleod K.F. Autophagy: cellular and molecular mechanisms. The Journal of Pathology. 2010;221(1):3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Deretic V., Saitoh T., Akira S. Autophagy in infection, inflammation and immunity. Nature Reviews Immunology. 2013;13(10):722–737. doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Levine B., Mizushima N., Virgin H.W. Autophagy in immunity and inflammation. Nature. 2011;469(7330):323–335. doi: 10.1038/nature09782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Abdellatif M., Sedej S., Carmona-Gutierrez D., Madeo F., Kroemer G. Autophagy in cardiovascular aging. Circulation Research. 2018;123(7):803–824. doi: 10.1161/CIRCRESAHA.118.312208. [DOI] [PubMed] [Google Scholar]
- 255.Bravo-San Pedro J.M., Kroemer G., Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circulation Research. 2017;120(11):1812–1824. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 256.Kaludercic N., Maiuri M.C., Kaushik S., Fernández Á.F., de Bruijn J., Castoldi F., et al. Comprehensive autophagy evaluation in cardiac disease models. Cardiovascular Research. 2020;116(3):483–504. doi: 10.1093/cvr/cvz233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.He C., Klionsky D.J. Regulation mechanisms and signaling pathways of autophagy. Annual Review of Genetics. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Fan X., Wang J., Hou J., Lin C., Bensoussan A., Chang D., et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. Journal of Translational Medicine. 2015;13:92. doi: 10.1186/s12967-015-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ansari M.Y., Ahmad N., Haqqi T.M. Butein activates autophagy through AMPK/TSC2/ULK1/mTOR pathway to inhibit IL-6 expression in IL-1β stimulated human chondrocytes. Cellular Physiology and Biochemistry. 2018;49(3):932–946. doi: 10.1159/000493225. [DOI] [PubMed] [Google Scholar]
- 260.Nasiri-Ansari N., Nikolopoulou C., Papoutsi K., Kyrou I., Mantzoros C.S., Kyriakopoulos G., et al. Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE(-/-) mice by activating autophagy and reducing ER stress and apoptosis. International Journal of Molecular Sciences. 2021;22(2):818. doi: 10.3390/ijms22020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Meng Z., Liu X., Li T., Fang T., Cheng Y., Han L., et al. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. International Immunopharmacology. 2021;94 doi: 10.1016/j.intimp.2021.107492. [DOI] [PubMed] [Google Scholar]
- 262.Ren C., Sun K., Zhang Y., Hu Y., Hu B., Zhao J., et al. Sodium-glucose CoTransporter-2 inhibitor empagliflozin ameliorates sunitinib-induced cardiac dysfunction via regulation of AMPK-mTOR signaling pathway-mediated autophagy. Frontiers in Pharmacology. 2021;12 doi: 10.3389/fphar.2021.664181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yang Y., Wang H., Kouadir M., Song H., Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death & Disease. 2019;10(2):128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Swanson K.V., Deng M., Ting J.P.-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature Reviews Immunology. 2019;19(8):477–489. doi: 10.1038/s41577-019-0165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Abbate A., Toldo S., Marchetti C., Kron J., Van Tassell B.W., Dinarello C.A. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circulation Research. 2020;126(9):1260–1280. doi: 10.1161/CIRCRESAHA.120.315937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Toldo S., Mezzaroma E., Mauro A.G., Salloum F., Van Tassell B.W., Abbate A. The inflammasome in myocardial injury and cardiac remodeling. Antioxidants and Redox Signaling. 2015;22(13):1146–1161. doi: 10.1089/ars.2014.5989. [DOI] [PubMed] [Google Scholar]
- 267.Van Tassell B.W., Arena R.A., Toldo S., Mezzaroma E., Azam T., Seropian I.M., et al. Enhanced interleukin-1 activity contributes to exercise intolerance in patients with systolic heart failure. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Sauter K.A.D., Wood L.J., Wong J., Iordanov M., Magun B.E. Doxorubicin and daunorubicin induce processing and release of interleukin-1β through activation of the NLRP3 inflammasome. Cancer Biology & Therapy. 2011;11(12):1008–1016. doi: 10.4161/cbt.11.12.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Li R., Lu K., Wang Y., Chen M., Zhang F., Shen H., et al. Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochemical and Biophysical Research Communications. 2017;485(1):69–75. doi: 10.1016/j.bbrc.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 270.Duewell P., Kono H., Rayner K.J., Sirois C.M., Vladimer G., Bauernfeind F.G., et al. NLRP3 inflamasomes are required for atherogenesis and activated by cholesterol crystals that form early in disease. Nature. 2010;464(7293):1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Toldo S., Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nature Reviews Cardiology. 2018;15(4):203–214. doi: 10.1038/nrcardio.2017.161. [DOI] [PubMed] [Google Scholar]
- 272.Louwe M.C., Olsen M.B., Kaasbøll O.J., Yang K., Fosshaug L.E., Alfsnes K., et al. Absence of NLRP3 inflammasome in hematopoietic cells reduces adverse remodeling after experimental myocardial infarction. JACC. Basic to Translational Science. 2020;5(12):1210–1224. doi: 10.1016/j.jacbts.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Van Tassell B.W., Canada J., Carbone S., Trankle C., Buckley L., Oddi Erdle C., et al. Interleukin-1 blockade in recently decompensated systolic heart failure: results from REDHART (recently decompensated heart failure anakinra response trial) Circulation. Heart Failure. 2017;10(11) doi: 10.1161/CIRCHEARTFAILURE.117.004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Murakami T., Ockinger J., Yu J., Byles V., McColl A., Hofer A.M., et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11282–11287. doi: 10.1073/pnas.1117765109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jäger E., Murthy S., Schmidt C., Hahn M., Strobel S., Peters A., et al. Calcium-sensing receptor-mediated NLRP3 inflammasome response to calciprotein particles drives inflammation in rheumatoid arthritis. Nature Communications. 2020;11(1):4243. doi: 10.1038/s41467-020-17749-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Leblanc N., Hume J.R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- 277.Philippaert K., Kalyaanamoorthy S., Fatehi M., Long W., Soni S., Byrne N.J., et al. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation. 2021;143(22):2188–2204. doi: 10.1161/CIRCULATIONAHA.121.053350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Quagliariello V., De Laurentiis M., Rea D., Barbieri A., Monti M.G., Carbone A., et al. The SGLT-2 inhibitor empagliflozin improves myocardial strain, reduces cardiac fibrosis and pro-inflammatory cytokines in non-diabetic mice treated with doxorubicin. Cardiovascular Diabetology. 2021;20:150. doi: 10.1186/s12933-021-01346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. Journal of Immunology. 2000;164(12):6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 281.Liu G., Yang H. Modulation of macrophage activation and programming in immunity. Journal of Cellular Physiology. 2013;228(3):502–512. doi: 10.1002/jcp.24157. [DOI] [PubMed] [Google Scholar]
- 282.Oishi Y., Manabe I. Macrophages in inflammation, repair and regeneration. International Immunology. 2018;30(11):511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 283.Wan E., Yeap X.Y., Dehn S., Terry R., Novak M., Zhang S., et al. Enhanced efferocytosis of apoptotic cardiomyocytes through MER tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circulation Research. 2013;113(8) doi: 10.1161/CIRCRESAHA.113.301198. 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Wang Z., Huang S., Sheng Y., Peng X., Liu H., Jin N., et al. Topiramate modulates post-infarction inflammation primarily by targeting monocytes or macrophages. Cardiovascular Research. 2017;113(5):475–487. doi: 10.1093/cvr/cvx027. [DOI] [PubMed] [Google Scholar]
- 285.Courties G., Heidt T., Sebas M., Iwamoto Y., Jeon D., Truelove J., et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. Journal of the American College of Cardiology. 2014;63(15):1556–1566. doi: 10.1016/j.jacc.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Balzer M.S., Rong S., Nordlohne J., Zemtsovski J.D., Schmidt S., Stapel B., et al. SGLT2 inhibition by intraperitoneal dapagliflozin mitigates peritoneal fibrosis and ultrafiltration failure in a mouse model of chronic peritoneal exposure to high-glucose dialysate. Biomolecules. 2020;10(11):1573. doi: 10.3390/biom10111573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lin F., Song C., Zeng Y., Li Y., Li H., Liu B., et al. Canagliflozin alleviates LPS-induced acute lung injury by modulating alveolar macrophage polarization. International Immunopharmacology. 2020;88 doi: 10.1016/j.intimp.2020.106969. [DOI] [PubMed] [Google Scholar]
- 288.Hess D.A., Terenzi D.C., Trac J.Z., Quan A., Mason T., Al-Omran M., et al. SGLT2 inhibition with empagliflozin increases circulating provascular progenitor cells in people with type 2 diabetes mellitus. Cell Metabolism. 2019;30(4):609–613. doi: 10.1016/j.cmet.2019.08.015. [DOI] [PubMed] [Google Scholar]
- 289.Huh J.Y., Park Y.J., Ham M., Kim J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Molecules and Cells. 2014;37(5):365–371. doi: 10.14348/molcells.2014.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Toita R., Kawano T., Murata M., Kang J.-H. Anti-obesity and anti-inflammatory effects of macrophage-targeted interleukin-10-conjugated liposomes in obese mice. Biomaterials. 2016;110:81–88. doi: 10.1016/j.biomaterials.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 291.Wang Y., Tang B., Long L., Luo P., Xiang W., Li X., et al. Improvement of obesity-associated disorders by a small-molecule drug targeting mitochondria of adipose tissue macrophages. Nature Communications. 2021;12(1):102. doi: 10.1038/s41467-020-20315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Keiran N., Ceperuelo-Mallafré V., Calvo E., Hernández-Alvarez M.I., Ejarque M., Núñez-Roa C., et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nature Immunology. 2019;20(5):581–592. doi: 10.1038/s41590-019-0372-7. [DOI] [PubMed] [Google Scholar]
- 293.Xu L., Nagata N., Chen G., Nagashimada M., Zhuge F., Ni Y., et al. Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Research & Care. 2019;7(1) doi: 10.1136/bmjdrc-2019-000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Miyachi Y., Tsuchiya K., Shiba K., Mori K., Komiya C., Ogasawara N., et al. A reduced M1-like/M2-like ratio of macrophages in healthy adipose tissue expansion during SGLT2 inhibition. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-34305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.National Center for Biotechnology Information. PubChem compound summary for CID 11949646, empagliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Empagliflozin. [accessed March 17, 2022].
- 296.National Center for Biotechnology Information. PubChem compound summary for CID 9887712, dapagliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Dapagliflozin. [accessed March 17, 2022].
- 297.National Center for Biotechnology Information. PubChem compound summary for CID 24812758, canagliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Canagliflozin. [accessed March 17, 2022].
- 298.National Center for Biotechnology Information. PubChem compound summary for CID 44814423, ertugliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Ertugliflozin. [accessed March 17, 2022].
- 299.National Center for Biotechnology Information. PubChem compound summary for CID 24831714, sotagliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Sotagliflozin. [accessed March 17, 2022].
- 300.National Center for Biotechnology Information. PubChem compound summary for CID 10453870, ipragliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Ipragliflozin. [accessed March 17, 2022].
- 301.National Center for Biotechnology Information. PubChem compound summary for CID 11988953, luseogliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Luseogliflozin. [accessed March 17, 2022].
- 302.National Center for Biotechnology Information. PubChem compound summary for CID 46908929, tofogliflozin. https://pubchem.ncbi.nlm.nih.gov/compound/Tofogliflozin. [accessed March 17, 2022].
- 303.JARDIANCE® (empagliflozin) tablets. Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204629s008lbl.pdf. [accessed March 17, 2022].
- 304.Jardiance 10 mg film-coated tablets Jardiance 25 mg filmcoated tablets. Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002677/WC500168592.pdf. [accessed March 17, 2022].
- 305.FARXIGA (dapagliflozin) tablets. Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/202293s003lbl.pdf. [accessed March 17, 2022].
- 306.Forxiga 5 mg film-coated tablets Forxiga 10 mg film-coated tablets. Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/forxiga-epar-product-information_en.pdf. [accessed March 17, 2022].
- 307.INVOKANA (canagliflozin) tablets. Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/204042s036lbl.pdf. [accessed March 17, 2022].
- 308.Invokana 100 mg film-coated tablets Invokana 300 mg film-coated tablets. Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/invokana-epar-product-information_en.pdf. [accessed March 17, 2022].
- 309.STEGLATRO™ (ertugliflozin) table. Prescribing Information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209803s000lbl.pdf. [accessed March 17, 2022].
- 310.Steglatro 5 mg film-coated tablets Steglatro 15 mg film-coated tablets. Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/steglatro-epar-product-information_en.pdf. [accessed March 17, 2022].
- 311.Zynquista 200 mg film-coated tablets. Summary of Product Characteristics. https://www.ema.europa.eu/en/documents/product-information/zynquista-epar-product-information_en.pdf. [accessed March 17, 2022].
- 312.Alkabbani W., Gamble J.-M. Profile of ipragliflozin, an oral SGLT-2 inhibitor for the treatment of type 2 diabetes: the evidence to date. Drug Design, Development and Therapy. 2021;15:3057–3069. doi: 10.2147/DDDT.S281602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Markham A., Elkinson S. Luseogliflozin: first global approval. Drugs. 2014;74(8):945–950. doi: 10.1007/s40265-014-0230-8. [DOI] [PubMed] [Google Scholar]
- 314.Poole R.M., Prossler J.E. Tofogliflozin: first global approval. Drugs. 2014;74(8):939–944. doi: 10.1007/s40265-014-0229-1. [DOI] [PubMed] [Google Scholar]