Description
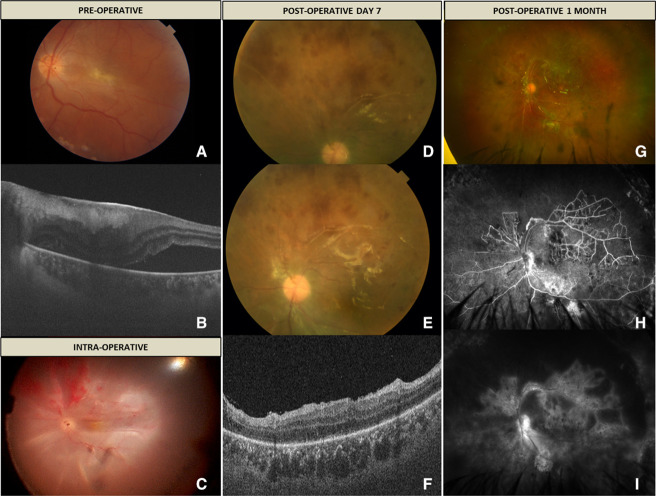
A young man in his mid-20s presented to our retina clinic with a diminution of vision in the left eye (OS) for the last 2 weeks. The best-corrected visual acuity (BCVA) was 6/60, and intraocular pressure was 14 mm Hg with Goldmann applanation tonometry. Anterior segment examination was within normal limits. Fundus examination revealed grade 2 epiretinal membrane (ERM) with the macula off inferior rhegmatogenous retinal detachment with inferotemporal breaks (figure 1A, B). The superior retina was attached and was within normal limits. The surgical intervention involved pars plana vitrectomy (PPV) with ERM peeling using heavy brilliant blue G (HBBG, diluted 2:1 with 10% dextrose) dye, subretinal fluid drainage using draining retinotomy, fluid air exchange, endolaser and finally, gas tamponade using 20% sulfur hexafluoride. The dye was used at a concentration of 0.025% with an exposure time of 2 min. The surgery went uneventful without any intraoperative complications. Postoperatively at 1 week, the gas fill was 40%, and the patient developed a total retinal redetachment. The likely cause was inadequate tamponade to the inferior retinotomy due to lack of prone positioning by the patient. Surprisingly, the superior retina showed signs of occlusive vasculitis with retinal haemorrhages. The patient was planned for retinal reattachment surgery and silicone oil tamponade. Figure 1C shows total retinal detachment and presence of occlusive vasculitis and retinal haemorrhages seen intraoperatively. Once the retina was attached under oil, the signs of retinal vasculitis could still be appreciated (figure 1D, E). Postoperative optical coherence tomography scan through the fovea showed attached retina with resolution of subretinal fluid and no evidence of any residual ERM (figure 1F). The BCVA of the patient improved to 6/36 at postoperative 1 month. Fundus examination revealed resolution of retinal haemorrhages and persistence of occluded vessels (figure 1G). Fundus fluorescein angiography revealed extensive peripheral ischaemia, collateral formation, and vascular and disc leakage in the late phase (figure 1H, I).
Figure 1.
(A) Fundus colour photo of the left eye showing presence of grade 2 epiretinal membrane (ERM) with retinal detachment. (B) Optical coherence tomography (OCT) through the macula shows the presence of ERM with subretinal fluid. (C) Intraoperative photo showing total retinal detachment and presence of occlusive vasculitis and retinal haemorrhages. (D and E) Postoperative colour photo showing signs of active retinal vasculitis in the superior retina. (F) OCT through the fovea of OS showing attached retina and no evidence of ERM. (G) Ultrawide field colour photo showing presence of occluded vessels and resolution of retinal haemorrhages. (H and I) Ultrawide field fundus fluorescein angiography showing extensive peripheral capillary non perfusion (CNP), collateral vessels, and disc and vascular leakage.
Presence of disc leakage along with vascular leakage and extensive peripheral ischaemia can be seen in idiopathic retinal vasculitis, aneurysms and neuroretinitis (IRVAN) syndrome.1 2 Lack of aneurysmal dilatations in our case goes against the diagnosis of IRVAN syndrome. Second, vitrectomy has been shown to improve vasculitis and stabilise the disease.3 Our case shows development of vasculitis post-PPV. Development of retinal vasculitis post-PPV is rarely reported. Retinal vasculitis, like the picture, has been described in patients undergoing vitrectomy for dropped lens matter.4 It is believed that decreased lens matter can induce focal inflammatory effects on the vessels. Besides this, Sahoo et al have described the development of occlusive retinal vasculitis postsilicone oil injection.5 Impurities in the silicone oil, direct immunogenicity of the silicone oil or secondary effects due to emulsification are some of the proposed mechanisms causing toxic posterior segment syndrome. Postoperative endophthalmitis following PPV can also present as retinal vasculitis.6 The use of contaminated instruments or products is reported to cause toxic anterior segment syndrome, though the development of occlusive retinal vasculitis is not registered.7 Various extrinsic agents like dyes and perfluorocarbon liquid are known to have toxic effects on the retina.8 9 Use of these agents have been associated with damage to retinal cells and retinal pigment epithelium. Studies have shown HBBG to be toxic to muller cells in a dose-dependent and time-dependent manner.10 Higher glucose concentrations cause acute toxicity or enhance the cytotoxic effects of other harmful substances. The addition of glucose makes the dye heavy and increases the contact time with the retinal surface. Muller cells are critical regulators of retinal vascular haemostasis.11 Damage to muller cells can induce vascular endothelium damage and cause vasculitis like the picture. The lack of standardisation for intraocular use of these dyes questions their use in clinical practice.
In this report, we highlight the occurrence of occlusive retinal vasculitis following an uneventful PPV. We suspect the use of HBBG dye as the cause of occlusive retinal vasculitis mediated through muller cell damage. Second, contamination of the dye per se or while reconstituting cannot be ruled out. Contaminated products can trigger an inflammatory reaction and thus develop occlusive retinal vasculitis.
Learning points.
The development of retinal vasculitis following pars plana vitrectomy is a rare event.
Among other reported causes, heavy brilliant blue G dye during surgery can present a vasculitis-like picture in the postoperative period.
Footnotes
Contributors: AM performed the surgery and prepared the manuscript. PS collected the images and helped in manuscript preparation. RS reviewed the manuscript and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Karasu B, Özkan D, Erdoğan G, et al. The fluorescein angiographic photodiagnosis of idiopathic retinal vasculitis, aneurysms, and neuroretinitis (IRVAN) syndrome: outcome of combined therapy. Photodiagnosis Photodyn Ther 2019;27:336–9. 10.1016/j.pdpdt.2019.06.013 [DOI] [PubMed] [Google Scholar]
- 2.Krishnan R, Shah P, Thomas D. Subacute idiopathic retinal vasculitis, aneurysms and neuroretinitis (IRVAN) in a child and review of paediatric cases of IRVAN revealing preserved capillary perfusion as a more common feature. Eye 2015;29:145–7. 10.1038/eye.2014.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin X, Meng Z, Deng A, et al. The multimodal imaging characteristics of IRVAN syndrome: a case report. Am J Transl Res 2021;13:9866–73. [PMC free article] [PubMed] [Google Scholar]
- 4.Ornek K, Onaran Z, Yimazbaş P. Retinal vasculitis following pars plana vitrectomy for retained lens material. Clin Exp Optom 2011;94:106–7. 10.1111/j.1444-0938.2010.00503.x [DOI] [PubMed] [Google Scholar]
- 5.Sahoo NK, Behera S, Narayanan R, et al. Toxic posterior segment syndrome presenting as occlusive retinal vasculitis following vitreoretinal surgery. J Curr Ophthalmol 2021;33:345–8. 10.4103/joco.joco_42_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subbiah S, McAvoy CE, Best JL. Retinal vasculitis as an early sign of bacterial post-operative endophthalmitis. Eye 2010;24:1410–1. 10.1038/eye.2010.18 [DOI] [PubMed] [Google Scholar]
- 7.Park CY, Lee JK, Chuck RS. Toxic anterior segment syndrome-an updated review. BMC Ophthalmol 2018;18:276. 10.1186/s12886-018-0939-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales M-C, Freire V, Asumendi A, et al. Comparative effects of six intraocular vital dyes on retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 2010;51:6018–29. 10.1167/iovs.09-4916 [DOI] [PubMed] [Google Scholar]
- 9.Méndez-Martínez S, Calvo P, Rodriguez-Marco NA, et al. Blindness related to presumed retinal toxicity after using perfluorocarbon liquid during vitreoretinal surgery. Retina 2018;38:1856–64. 10.1097/IAE.0000000000001783 [DOI] [PubMed] [Google Scholar]
- 10.Shen Y, Zhang L, Zhou H, et al. Comparative effects of commonly used intraocular dyes on the viability of human retina Müller cells. Biomed Pharmacother 2020;132:110790. 10.1016/j.biopha.2020.110790 [DOI] [PubMed] [Google Scholar]
- 11.Shen W, Fruttiger M, Zhu L, et al. Conditional Müllercell ablation causes independent neuronal and vascular pathologies in a novel transgenic model. J Neurosci 2012;32:15715–27. 10.1523/JNEUROSCI.2841-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]