Abstract
Introduction
It is unclear how internet-delivered cognitive-behavioural therapy for insomnia (CBT-I) can be integrated into healthcare systems, and little is known about the optimal level of therapist guidance. The aim of this study is to investigate three different versions of a stepped care model for insomnia (IG1, IG2, IG3) versus treatment as usual (TAU). IG1, IG2 and IG3 rely on treatment by general practitioners (GPs) in the entry level and differ in the amount of guidance by e-coaches in internet-delivered CBT-I.
Methods and analysis
In this randomised controlled trial, 4268 patients meeting International Classification of Diseases, Tenth Revision (ICD-10) criteria for insomnia will be recruited. The study will use cluster randomisation of GPs with an allocation ratio of 3:3:3:1 (IG1, IG2, IG3, TAU). In step 1 of the stepped care model, GPs will deliver psychoeducational treatment; in step 2, an internet-delivered CBT-I programme will be used; in step 3, GPs will refer patients to specialised treatment. Outcomes will be collected at baseline, and 4 weeks, 12 weeks and 6 months after baseline assessment. The primary outcome is insomnia severity at 6 months. An economic evaluation will be conducted and qualitative interviews will be used to explore barriers and facilitators of the stepped care model.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of the Medical Centre—University of Freiburg. The results of the study will be published irrespective of the outcome.
Trial registration number
DRKS00021503.
Keywords: SLEEP MEDICINE, PSYCHIATRY, Adult psychiatry
Strengths and limitations of this study.
This randomised controlled trial will recruit 4268 patients and will be the largest clinical trial on insomnia.
This trial will investigate three different versions of a stepped care model for insomnia which rely on treatment by general practitioners in the entry level and differ in the amount of guidance by e-coaches in internet-delivered cognitive behavioural therapy for insomnia.
The primary outcome is insomnia severity. An economic evaluation will be conducted and qualitative interviews will be used to explore barriers and facilitators of the stepped care model.
Patients with insomnia will not be blind to treatment allocation in this trial.
Introduction
Insomnia disorder is characterised by difficulties initiating and/or maintaining sleep resulting in significant daytime dysfunction.1 In Western industrialised countries, 5%–10% of the general population2 and 20% of primary care patients3 suffer from the disorder. Insomnia is associated with a reduced quality of life,4 and is a risk factor for other mental disorders, in particular depression and anxiety disorders,5 as well as for cardiovascular diseases.6 7
Clinical guidelines recommend cognitive-behavioural therapy for insomnia (CBT-I) as first-line treatment.8 9 CBT-I is a multicomponent intervention consisting of psychoeducation, relaxation therapy, sleep restriction therapy, stimulus control therapy and cognitive therapy. However, only a small proportion of patients with insomnia has access to this treatment. For example, data from BARMER, a large German public health insurance, indicate that around 1.6% of the insured persons received a diagnosis of insomnia in 2017, but only 10% of these patients received a psychotherapeutic treatment.10 Assuming a prevalence of insomnia of 5.7% in Germany,11 this suggests that only 2.8% of all insomnia patients in Germany receive psychotherapeutic treatment. Since CBT is not the only form of psychotherapy reimbursed by German health insurances and the focus of the psychotherapeutic treatment may, in many patients, be a comorbid disorder rather than insomnia, the assumption that 1% of all insomnia patients receive CBT-I might already be a very optimistic estimation. Instead, many insomnia patients are treated with benzodiazepine receptor agonists or sedating antidepressants on a long-term basis,12 which is potentially harmful and not recommended by clinical guidelines.8 9 This situation is unfortunate both from a clinical and from a health-economic perspective. Insomnia is associated with estimated annual costs of about €5900 per person in Germany due to absenteeism and presenteeism.13 Thus, given its prevalence, a reasonable estimate of the indirect costs of insomnia in Germany is €25 billion per year. This number is broadly in line with previously published socioeconomic data from the USA14 and Canada.15
The dissemination of CBT-I is a major healthcare challenge, and internet-delivered psychotherapy has been suggested as a possible mean to lower the treatment gap.16 Compared with face-to-face treatment, main advantages of internet-delivered CBT-I are convenience, increased accessibility and potentially lower costs. In particular, internet interventions are easily accessible anytime and anywhere. Patients do not incur travelling expenses; they can work at their own pace; they may provide more honest answers in the privacy of their own home; and barriers related to the stigma of mental disorders may be reduced.17 Hence, offering internet-delivered CBT-I might increase the utilisation of psychotherapy in undertreated populations. Meta-analyses suggest that internet-delivered CBT-I is highly effective in comparison to waitlist control conditions,18 19 and that the effects appear to be comparable in size to those of face-to-face CBT-I.20 In addition, follow-up data of up to 3 years demonstrate a high long-term effectiveness of online CBT-I.21 22
However, at least two questions with a high degree of healthcare relevance remain to be answered. First, it is unclear how internet-delivered CBT-I can be effectively integrated into existing healthcare systems that rely on general practitioners (GPs) to take the lead in coordinating patient care. Previous research has shown that the implementation of CBT-I techniques in primary care is challenging but promising.23 24 In line with a stepped care approach to the treatment of insomnia,25 GPs may serve as the entry level of a multistep model that offers more intense support for those with more complicated complaints in a cost-effective way. Although conceptually appealing, there are very few studies investigating such stepped care models for insomnia,26–28 and none of them included active treatment provided by GPs. Second, little is known about the optimal level of therapist guidance in the context of internet-delivered CBT-I. While it is generally thought that human support has positive effects on adherence and efficacy in online mental health interventions,29 many studies in the insomnia field have successfully implemented online interventions without any human support/guidance.16 22 30 31 One study has directly compared an online intervention for insomnia with and without guidance via email and found a superior efficacy in the guided group.32 However, there is limited knowledge about who needs and who does not need guidance and how this translates into cost-effectiveness estimates.
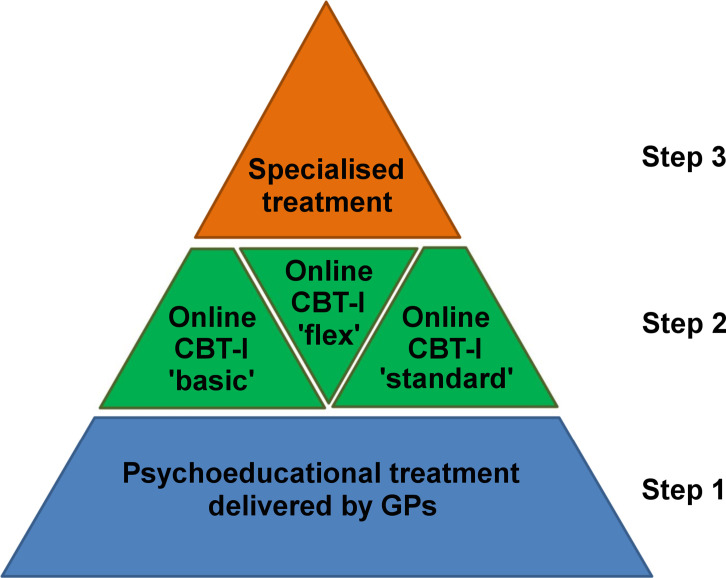
The central objective of this study is to improve the quality and efficiency of healthcare for patients with insomnia. In addition, it is intended to improve interdisciplinary and intersectoral cooperation between GPs, psychotherapists and medical specialists working in outpatient and inpatient settings. Three different versions of a stepped care model (intervention group 1, IG1; intervention group 2, IG2; intervention group 3, IG3) that differ in the amount of guidance that is provided by e-coaches in the internet-delivered intervention in step 2 will be compared with treatment as usual (TAU) in, to the best of our knowledge, the largest clinical trial to date on insomnia (see figure 1). At step 1, participating GPs will provide a brief psychoeducational treatment; at step 2, patients will receive an internet intervention based on CBT-I; and at step 3, patients will be referred to specialised medical face-to-face treatment. Patients who are unresponsive to the treatment at one step will proceed to the next step of the model. The primary research question is the effectiveness of the interventions. We will also investigate differential treatment outcomes in four subgroups of patients as follows: (1) insomnia without any comorbidity; (2) insomnia with mental comorbidity; (3) insomnia with somatic comorbidity; (4) insomnia with mental and somatic comorbidity. In addition, an economic evaluation will be carried out and qualitative interviews will be conducted to explore barriers and facilitators of the stepped care model. In case of a positive evaluation, it is intended to include the stepped care model in the guidelines of the Federal Joint Committee, the highest decision-making body of the joint self-government of physicians, dentists, hospitals, and health insurance funds in Germany.
Figure 1.
Stepped care model for insomnia that will be tested in the current trial. CBT-I, cognitive-behavioural treatment for insomnia; GPs, general practitioners.
Methods
Study design
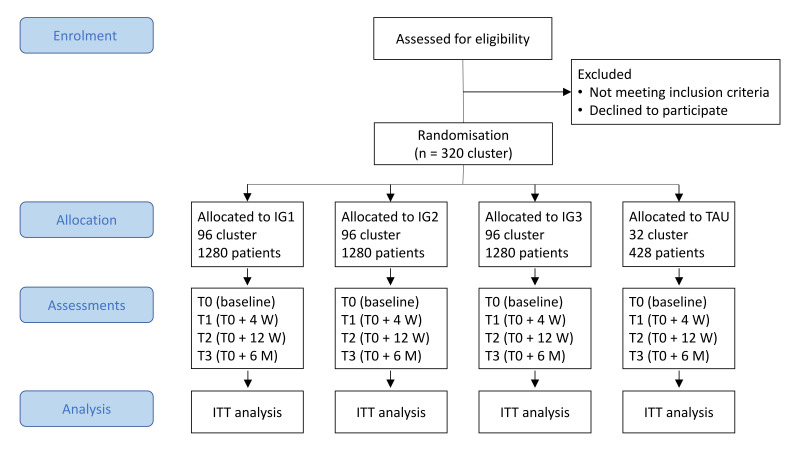
The study is a four-armed pragmatic parallel-group cluster-randomised controlled trial investigating three different versions of a stepped care model for insomnia versus TAU. The unit of randomisation will be the participating GPs to avoid treatment diffusion. Primary and secondary outcomes as well as moderating and mediating variables and intervention-related variables will be assessed online by patient self-report using LimeSurvey (https://www.limesurvey.org/). Online assessments will take place at baseline (T0) and after 4 (T1) and 12 (T2) weeks, as well as 6 months after baseline (T3; see figure 2 for trial design). Informed consent will also be given online. The trial might be continued with further annual follow-up assessments after 1–5 years in case of patients’ informed consent and dependent on follow-up assessment resources beyond the funded 6 months follow-up. The trial started recruitment of patients in October 2020 and will continue recruiting until September 2022.
Figure 2.
IG1, intervention group 1 (‘standard’ version of step 2 of the stepped care model); IG2, intervention group 2 (‘flex’ version of step 2 of the stepped care model); IG3, intervention group 3 (‘basic’ version of step 2 of the stepped care model); ITT, intention-to-treat; M, months; TAU, treatment-as-usual; W, weeks.
The study will be reported in accordance with the Consolidated Standards of Reporting Trials Statement 2010 and the extensions for reporting pragmatic trials, cluster randomised trials, multiarm parallel group trials and trials on psychological interventions.33–37 This trial protocol was created according to SPIRIT guidelines.38
Participants
Overall, 4268 patients are planned to be recruited. The inclusion criteria are: (1) age ≥18 years; and (2) International Classification of Diseases, Tenth Revision (ICD-10) diagnosis of non-organic insomnia (F51.0) or insomnia (G47.0). Exclusion criteria are: (1) untreated sleep apnoea syndrome (ICD-10: G47.3); (2) untreated restless legs syndrome or periodic leg movement disorder (ICD-10: G25.8); (3) untreated hyperthyroidism (ICD-10: E05.9); (4) ongoing psychotherapy for insomnia; (5) conditions that may be aggravated by CBT-I (bipolar disorder, ICD-10: F31.x; epilepsy, ICD-10: G40.x); (6) conditions that pose a serious threat to treatment adherence (eg, organic, including symptomatic, mental disorders (ICD-10: F00–F09); mental and behavioural disorders due to psychoactive substance use (ICD-10: F10-F19); schizophrenia, schizotypal and delusional disorders (ICD-10: F20–F29)); (f) acute suicidality.
Up to 320 GPs from Bavaria and Baden-Wuerttemberg, who participate in this study, will recruit eligible patients during consultations and check inclusion and exclusion criteria. In addition, online, print and broadcast media advertisements as well as postal mailings by the BARMER to potential patients will be used to recruit insomnia patients from all over Germany. These patients will be referred to a group of GPs that use telehealth consultations for checking inclusion and exclusion criteria, delivering step 1 of the stepped care model and guiding patients through the stepped care model. All GPs will receive remuneration for each participating patient (up to €158.25 depending on the number of consultations). In addition to receiving free access to the stepped care model or TAU, participants will receive payment after the completion of online assessments T1 (€15), T2 (€15) and T3 (€20) to increase adherence.
Randomisation and allocation concealment
This study will use cluster randomisation of GPs with an allocation ratio of 3:3:3:1 (IG1:IG2:IG3:TAU). Randomisation will be performed by authors MBa and MM (Ulm University) who are not otherwise involved in the trial and therefore blinded to all processes of the study. Population-density stratified permuted block randomisation (nine strata based on population density and average level of income, one stratum for GPs that exclusively employ telehealth consultations) will be employed with varying block sizes concealed to the investigators to minimise selection bias. GPs from community practices will be randomised into the same trial arm. The GPs are instructed to conceal group allocation until the baseline assessment is completed by the patient.
Blinding
Blinding of patients and healthcare providers is not feasible. However, screenings and baseline assessments will be performed before patients are informed about treatment assignment to avoid contamination with anticipated treatment effects. In case of non-completion of assessments participants will receive fully automated standardised reminders.
Intervention
The stepped care model that will be tested in the current study is presented in figure 1.
Step 1
In step 1 of the stepped care model, the responsible GP will deliver a brief standardised psychoeducational treatment after being trained by sleep medicine specialists of the Department of Psychiatry and Psychotherapy of the Medical Centre—University of Freiburg and by primary care physicians of the Department of Medicine, Division of General Practice, of the Medical Centre—University of Freiburg. The treatment includes the following psychoeducational recommendations: (1) avoid alcohol as a hypnotic; (2) avoid clock-watching at night; (3) avoid afternoon caffeine use; (4) exercise regularly. In addition, the following stimulus control instructions will be given by the GPs: (1) use the bed only for sleep and sexual activity; (2) get out of bed when unable to sleep; (3) do not nap during the day. Of note, the GPs do not use standardised leaflets that summarise the psychoeducational recommendations. GPs can also consult a psychiatrist of the Department of Psychiatry and Psychotherapy of the Medical Centre—University of Freiburg whenever they feel that discontinuation of hypnotic medication would be appropriate. After 4 weeks, all patients in the intervention groups will receive an email with a link providing the opportunity to access step 2 of the stepped care model without further consultation of the GPs. Importantly, for each patient, GPs can decide to skip step 1 of the stepped care model if they do not expect a substantial impact on insomnia severity.
Step 2
At step 2 of the stepped care model, the GET.ON Institut für Online Gesundheitstrainings (operating under the registered brand ‘HelloBetter’) will provide an internet intervention based on CBT-I with an accompanying mobile sleep diary app. The intervention was initially developed at Leuphana University Lüneburg by the team of author DL and was positively evaluated in three randomised controlled trials.39–41 Since the intervention was initially designed for workers, it has been adapted and technically updated for the current study by HelloBetter to meet the needs of all potential patients. Treatment content is based on CBT-I manuals and includes psychoeducation, relaxation therapy, sleep restriction therapy, stimulus control therapy, and cognitive interventions targeting rumination and worry. Delivery is structured into eight sessions, lasting approximately 45–60 min each. Participants are instructed to complete one session per week resulting in an overall duration of 8 weeks. However, participants are allowed to work through the sessions faster or slower accounting for interindividual differences in the therapeutic process.
Patients of the three IGs receive an initial and a final consultation (each about 20 min) with one of a team of e-coaches of HelloBetter, who are trained and supervised psychologists. The consultations will be conducted by telephone, or, if this is not possible, by in-platform messages. In addition to the initial and final consultation, patients randomised to the ‘standard’ version of the intervention (IG1) receive written feedback and support by the responsible e-coach after each session. E-coaches are instructed to spend, on average, 25 min per session for writing this feedback. Patients randomised to the ‘flex’ version of the intervention (IG2) receive written on-demand support by the responsible e-coach. Patients randomised to the ‘basic’ version of the intervention (IG3) do not receive additional human guidance.
The treatment platform operates according to the ISO 27000 and NEN 7510 standards. All data is securely stored on ISO 27000-certified servers and transmitted via Hypertext Transfer Protocol Secure (HTTPS) with Secure Sockets Layer (SSL) certificates (AES-256 and SHA-1, 2048-bit RSA). Industry-standard measures have been taken to ensure robust security for the platform.
Step 3
In step 3 of the stepped care model, non-responders will be referred by their GPs to specialised medical treatment. The decision about this referral lies with the responsible GP and is based on clinical judgement of response. However, the responsible e-coach of HelloBetter will send a report to the GP summarising step 2 treatment process and outcome. This includes a post-treatment Insomnia Severity Index (ISI) score based on an ISI that participants fill in on the treatment platform outside the research process, and a recommendation about whether and by whom the treatment should be continued after step 2. As a rule of thumb, GPs are recommended to refer patients with an ISI score ≥15 and a comorbid mental health syndrome to a psychiatrist and/or a psychotherapist in step 3, and all other patients with an ISI score ≥15 to a medical doctor that is a board-certified sleep medicine specialist.
Treatment as usual
In the TAU group, GPs are instructed to provide their routine clinical care for insomnia. This may or may not include non-pharmacological or pharmacological treatment by the GPs or referrals to specialised medical treatment. The GPs in the TAU group will not receive the specific training that is described in section 2.5.1. and will not be able to refer patients to the internet intervention described in section 2.5.2. All healthcare provisions in the TAU group will be retrospectively monitored with the Trimbos/iMTA questionnaire (TIC-P; see section 2.8.2.). Using these data, an accurate description of TAU can be provided.
Safety protocol
During the screening procedure, GPs exclude patients with acute suicidality. Suicidal ideation will also be screened by the e-coaches of HelloBetter at their initial consultations, and at T0, T1, T2 and T3 using 16-item Quick Inventory of Depressive Symptoms in the self-report (QIDS-SR16) and Negative Effects Questionnaire (NEQ) (see paragraph on measures for details). Reports of current suicidal ideation in the interview, a score ≥1 on the suicide item of the QIDS-SR16 (item 12; 0 = ‘I do not think of suicide or death’., 1 = ‘I feel that life is empty or wonder if it’s worth living’, 2 =’I think of suicide or death several times a week for several minutes’, 3 = ‘I think of suicide or death several times a day in some detail, or I have made specific plans for suicide or have actually tried to take my life’), or the answer ‘yes’ to item 10 of the NEQ (‘I got thoughts that it would be better if I did not exist anymore and that I should take my own life’) will result in a standardised safety protocol. In particular, participants will receive an information document with detailed information on available health services and the advice to consult their GP. The wording of the online information document is adapted in emphasis, depending on the severity of the indicated suicidality.
Measures
Table 1 presents an overview of measures that are assessed in this trial.
Table 1.
Overview of the assessments
| Activity/assessment | T-1 | T0 | T1 | T2 | T3 |
| Prestudy | Baseline (week 0) | 4 weeks after T0 | 12 weeks after T0 | 6 months after T0 | |
| Eligibility screen | X | ||||
| Informed consent | X | ||||
| Primary outcome | |||||
| Insomnia severity (ISI) | X | X | X | X* | |
| Secondary outcomes | |||||
| Sleep quality (PSQI) | X | X | X | X | |
| Quality of life (AQoL-8D) | X | X | X | X | |
| Depressive symptoms (QIDS-SR16) | X | X | X | X | |
| Anxiety symptoms (GAD-7) | X | X | X | X | |
| Somatic symptoms (SSS-8) | X | X | X | X | |
| Costs (TiC-P) | X | X | X | ||
| Potential treatment moderators and mediators | |||||
| DBAS-10 | X | X | X | X | |
| Pre-sleep arousal (PSAS) | X | X | X | X | |
| Fatigue (BFI) | X | X | X | X | |
| Stress (PSS) | X | X | X | X | |
| Sleep hygiene behaviour (SHI) | X | X | X | X | |
| Emotion regulation (CERQ-short) | X | X | X | X | |
| Intervention-related variables | |||||
| Alliance (WAI-I) | X† | ||||
| Technological alliance (TAI-OT) | X† | ||||
| Client satisfaction (CSQ-8) | X | ||||
| Adverse events and negative effects (NEQ, Questionnaire on adverse effects of CBT-I) | X | X | |||
| Dropout Questionnaire | X‡ | ||||
*The ISI at T3 (6 months after T0) is the primary outcome of this trial.
†Only in patients of the intervention groups (IG1, IG2, IG3) who entered step 2 of the stepped care model.
‡Only in patients of the intervention groups (IG1, IG2, IG3) not completing at least 80% of the internet-delivered intervention.
AQoL-8D, Assessment of Quality of Life instrument; BFI, Brief Fatigue Inventory; CERQ-short, Cognitive Emotion Regulation Questionnaire; CSQ-8, Client Satisfaction Questionnaire; DBAS-10, Dysfunctional Beliefs and Attitudes about Sleep Scale; GAD-7, General Anxiety Disorder 7 questionnaire; ISI, Insomnia Severity Index; NEQ, Negative Effects Questionnaire; PSAS, Pre-Sleep Arousal Scale; PSQI, Pittsburgh Sleep Quality Index; PSS, Perceived Stress Scale; QIDS-SR16, 16-item Quick Inventory of Depressive Symptoms in the self-report format; SHI, Sleep Hygiene Index; SSS-8, Somatic Symptom Scale 8; TAI-OT, Technological Alliance Inventory; TiC-P, Trimbos/iMTA questionnaire for costs associated with psychiatric illness; WAI-I, Working Alliance Inventory for guided Internet interventions.
Primary outcome measure
The primary outcome will be insomnia severity at T3, 6 months after the baseline assessment. Insomnia severity will be assessed with the ISI.42 The ISI is composed of seven 5-point Likert scale items (0–4 points; total score range: 0–28 points) probing perceived severity of insomnia symptoms during the preceding 2 weeks. Several studies have shown good internal consistency of the ISI with Cronbach’s Alpha ranging from 0.70 to 0.90.42–44
Secondary outcome measures
Sleep quality will be assessed using the Pittsburgh Sleep Quality Index (PSQI),45 a 19-item self-report measure covering different aspects of sleep quality. The total score of the PSQI ranges from 0 to 21, internal consistency was found to be 0.80.46 Quality of life will be assessed with the Assessment of Quality of Life 8-Dimension (AQoL-8D),47 an instrument composed of 35 items that measure eight dimensions (independent living, pain, senses, mental health, happiness, coping, relationships, self-worth). The AQoL-8D generates patient preference-based utilities on a scale of 0 (death) to 1 (perfect health), using the time-trade-off method,47 which will be used to estimate quality-adjusted life-years (QALYs) based on the area under the curve method. The AQoL-8D has been reported to have excellent internal consistency with a Cronbach’s Alpha of 0.96.47 Depressive symptoms will be measured using the QIDS-SR16.48 The total score of the QIDS-SR16 ranges from 0 to 27, internal consistency was reported to be good (Cronbach’s alpha=0.86).49 Incident depression will be assessed in patients without a depression diagnosis at T0 and defined using a cut-off score of ≥13 on the QIDS-SR16.50 Anxiety symptoms will be assessed with the 7-item General Anxiety Disorder 7 questionnaire51; total score 0–21; Cronbach’s alpha=0.89.52 Somatic symptoms will be measured using the 8-item Somatic Symptom Scale 853; (total score 0–32; Cronbach’s alpha=0.81). For the health economic evaluation, healthcare utilisation, patient and family expenditures and productivity losses due to absence from work or reduced efficiency during paid and unpaid work will be established with the Trimbos/iMTA questionnaire for costs associated with psychiatric illness (TiC-P), a retrospective self-report questionnaire covering the previous 3 months.54–56 A list of unit cost prices will be used to compute healthcare costs on a per-participant basis.57 Test–retest reliability has previously been shown to be satisfactory.55
Intervention-related variables
At T2 after 12 weeks, the 12-item Working Alliance Inventory for guided Internet interventions (WAI-I)58 and the 12-item Technological Alliance Inventory (TAI-OT) will be administered in all patients of the intervention groups (IG1, IG2, IG3) who entered step 2 of the stepped care model (internet-delivered CBT-I). The WAI-I and the TAI-OT will be used to assess the therapeutic alliance between patient and e-coach and the technological alliance between patient and e-coach and the technological alliance between client and the internet-based intervention, respectively. The WAI-I score ranges from 12 to 60 and the questionnaire has excellent internal consistency with a Cronbach’s alpha of 0.93.58 The TAI-OT is a new self-report questionnaire developed by Labpsitec at Jaume I University in Castellón, Spain (Labpsitec (http://www.labpsitec.uji.es/eng/index.php) and measures the degree to which the internet-based intervention is perceived as being helpful in achieving therapeutic goals. The TAI-OT score ranges from 12 to 84. Patients in all conditions will receive the 8-item Client Satisfaction Questionnaire;59 60 (total score 8–32), which is characterised by excellent internal consistency with a Cronbachs’s Alpha of 0.93.59 In addition, the 20-item NEQ61 will be used in all patients. The NEQ measures the frequency, with a total score ranging from 0 to 20, and impact, with a total score ranging from 0 to 80, of possible negative effects during treatment. Its internal consistency was found to be excellent with a Cronbach’s alpha of 0.95.61 Moreover, an additional self-developed 24-item Questionnaire on adverse effects of CBT-I will be used. For adverse events reported in the NEQ and the additional self-developed 24-item questionnaire, patients who entered step 2 of the stepped care model will be asked if they attribute the adverse events to the behavioural components of CBT-I. A self-developed Dropout Questionnaire based on the Health Action Process Approach62 will be used to identify dropout reasons in participants not completing at least 80% of the internet-delivered intervention. For a comprehensive evaluation of the implementation of the stepped care model a battery of self-developed self-report items will be used in all patients to assess the usage of and adherence to treatment components across the steps.
Potential treatment moderators and mediators
At T0, demographic variables (eg, age, gender), depressive and anxiety symptoms, as well as IT knowledge will be documented as potential moderators of treatment effectiveness. In addition, the baseline values of the following variables will be assessed as potential moderators: the 10-item Dysfunctional Beliefs and Attitudes about Sleep Scale (DBAS-10;63 64 Cronbach’s alpha=0.69), the 16-item Pre-Sleep Arousal Scale (PSAS;65 66 Cronbach’s alpha=0.80–0.94), the 10-item Brief Fatigue Inventory;67 (Cronbach’s alpha=0.96), the 10-item Perceived Stress Scale (PSS;68 Cronbach’s alpha=0.78), the 13-item Sleep Hygiene Index (SHI;69 Cronbach’s alpha=0.66), and the 18-item short version of the Cognitive Emotion Regulation Questionnaire (CERQ-short;70 Cronbach’s alpha=0.68–0.81). In addition, mediation analyses will be conducted using some of the constructs described in this section (DBAS-10, PSAS, PSS, SHI, CERQ) as well as two intervention-related variables described above (WAI-I, TAI-OT).
Other data
Medical record data (eg, ICD-10 diagnosis codes, treatment) will be provided by the GPs to enable allocation of patients to the four subgroups. In cases of missing medical record data, group allocation will be based on self-reported mental and somatic comorbidities. Sleep diary data will be assessed in step 2 of the stepped care model and will be used to evaluate treatment adherence, for example, adherence to personalised sleep restriction recommendations. Additionally, usage data from the treatment platform will be used to assess adherence to the internet-delivered intervention. Secondary data from BARMER will be used to assess the validity of the TiC-P. In addition, qualitative interviews will be conducted with a subgroup of patients, GPs and e-coaches to assess their experience of positive and negative aspects of the stepped care model. Trained interviewers will explore acceptance, perceived effectiveness, usage behaviour, barriers, facilitators, transferability into routine care as well as side effects of the stepped care model using semistandardised interview guides. The sample size and composition will be planned to consider the different intervention groups and gain sufficient theoretical data saturation. All subgroups will be represented in the interviews.
Sample size calculation
There is no universally accepted minimally important difference for the treatment of insomnia. Hence, this issue has been discussed among the clinicians involved in the current trial who are nationally and internationally leading experts in the field of insomnia research. Most clinicians agreed that 1.5 or more points on the ISI (exhibiting a common SD of 6.0 points) are a reasonable minimally important difference corresponding to a minimally important effect size of d=0.25. Based on previous research, it is assumed that all intervention groups (IG1, IG2, IG3) exhibit a considerably larger difference to the TAU group of at least d=0.50.24 Because of this, for ethical reasons and to reduce costs, the sample size calculation is based on the comparisons between the IGs, and the GPs are randomised with an allocation ratio of 3:3:3:1 (IG1, IG2, IG3, TAU), which ensures sufficient power for both differences between any IG and TAU (of at least d=0.35) as well for differences between IGs (of at least d=0.25). Based on 320 GPs with a median recruitment rate of n=9 ± 14 patients, an ICC of 0.02 (see Adams et al71 for comparison), a correlation of the outcome at T3 with the corresponding baseline assessment of r=0.5, d=0.25, an α of 0.05 and (1-β) of 80%, the required sample size is n=1067 for each of the four subgroups of patients (IGs: n=320 each; TAU: n=107). Thus, a total sample size of N=4268 (IGs: n=1280 each; TAU: n=428) is required. Sampling procedures for the qualitative interviews follow theoretical data saturation principles.72 73
Statistical and qualitative analysis
Effectiveness
Descriptive statistics of recruitment and drop-out as well as baseline characteristics for each group will be provided. The primary effectiveness analysis will be conducted according to the intention-to-treat principle based on all patients with their original treatment allocation. Additionally, per-protocol analyses based on the data of patients who completed a substantial proportion of the internet intervention (ie, 80% of the modules) will be conducted. Missing data will be handled via multiple imputation, using a multilevel imputation model to account for clustering. The effect of group allocation (IG1, IG2, IG3, TAU) on the primary endpoint ISI at T3 (6 months after baseline) will be tested using pairwise group comparisons based on linear mixed models with corresponding 95% CIs. These analyses will be conducted separately for each subgroup of patients. The alpha level will be adjusted using the Bonferroni-Holm procedure. All models will include the responsible GP as a clustering variable as well as baseline insomnia severity, age, and gender as covariates. Clinical significance will be determined using number needed to treat analyses.74 Additionally, reliable reduction in insomnia severity will be calculated with the Reliable Change Index (RCI) by Jacobson and Truax.75 For calculating the RCI, a prespecified Cronbach’s alpha of 0.92 will be used, based on a validation study in 410 primary care patients.44 Based on the RCI, participants will be categorised into responders and non-responders, and the proportion of responders will be compared between study groups (again accounting for clustering). Secondary outcomes will be analysed analogously to the primary outcome, using random effect regression models as appropriate for the respective type of data. Potential onset and remission of incident depression will be compared between study groups based on incidence rate ratios using multilevel Poisson regression. No interim analysis is planned for effectiveness or futility. Exploratory moderator analyses will be used to investigate whether pretreatment patient characteristics are associated with differential treatment effectiveness. Potential moderators include sociodemographic (eg, age) and clinical (eg, insomnia severity) variables. Exploratory mediator analyses will be employed to examine potential mechanisms of change. Among potential mediators are sleep-related (eg, DBAS) and intervention-related variables (eg, working alliance).
Economic evaluation
The economic evaluation will be performed from the societal and public healthcare perspective. Two multilevel models (MLMs) will be specified, one for costs and one for effects, which take into account the hierarchical structure of the data. MLMs will be combined with cluster bootstrapping, which is recommended for resampling clustered data.76 Across the four study groups, mean costs and QALYs will be compared with assess if any of the treatments are less effective and more expensive than the other treatments. If so, incremental cost-effectiveness ratios (ICERs) will not be estimated in relation to that treatment.77 Otherwise, ICERs will be estimated by calculating the difference in costs between two treatment options divided by the difference in effectiveness of these two treatment options. We will bootstrap seemingly unrelated regression equation models to generate 5000 simulations of cost and effect pairs while allowing for correlated residuals of the cost and effect equations and adjusting for potential confounders.78 The joint uncertainty surrounding costs and effects will be summarised using cost-effectiveness acceptability curves (CEACs) based on a net benefit regression framework.79 CEACs show the probability of an intervention being cost effective in comparison with the alternatives for a range of different willingness-to-pay thresholds. For patients insured by BARMER, the validity of the TiC-P will be assessed with secondary data from the health insurance.
Qualitative interviews
Qualitative interviews of patients, GPs and e-coaches will be used to assess barriers and supporting factors of the stepped care model. The sample size will be determined according to the principle of theoretical saturation. Thus, data collection will continue until no further insights can be gained from additional interviews.80 81 Following the principles of theoretical sampling, for the stakeholder group patients, cases will be deliberately selected based on the following criteria: remitters/non-remitters; male/female; lower/higher age; intervention-adherers (defined as completing more than 80% of the intervention within 12 weeks)/non-adherers. Additionally, care will be taken to include participants from all three IGs and the TAU group. Given the limited number of GPs and e-coaches, all participants of these stakeholder groups will be invited to the interviews.
For each stakeholder group, a semi-structured interview schedule will be prepared. The content of the interview schedules will be primarily based on the dimensions of the Hierarchical Model of Health Service Quality (ie, interpersonal quality, technical quality, environmental quality, administrative quality)82 and will be supplemented by other relevant dimensions (eg, therapeutic alliance, adverse effects). The questions aim at exploring acceptance, perceived effectiveness, usage behaviour, barriers, facilitators and transferability into routine care as well as adverse effects. Interviews will be 60–90 min long and will be conducted by trained and supervised psychologists. Recordings will be transcribed according to the rules for computer-assisted evaluation.83 Following the principles of qualitative content analysis by Kuckartz,83 text units will be systematised and classified following an inductive-deductive approach. The data analysis will be carried out using MAXQDA, a software for the analysis of qualitative data.84
Patient and public involvement
Representatives of patient groups were not formally involved in the design of this study but will be involved in the discussion and dissemination of results. In addition, patients were involved in user-experience and usability testing of the platform for the internet intervention in order to ensure that the interface is user-friendly and adaptive to factors related to age, gender and education. Public representatives approved the trial objectives and design as part of the application to the Innovationsfonds of the German Federal Joint Committee
Ethics and dissemination
The study has been registered in the German Clinical Trials Register (https://www.drks.de/drks_web/; DRKS00021503) and will be conducted in accordance with the Declaration of Helsinki. The study protocol was approved by both the Ethics Committee of the Medical Centre—University of Freiburg and the Ethics Committee of the State Chamber of Physicians (‘Landesärztekammer Baden-Württemberg’). In addition, the data protection officers of the Medical Centre—University of Freiburg and Ulm University have approved the formal data protection concept of this study. The results of the study will be published irrespective of the outcome.
Discussion
Insomnia is a common, costly and impairing sleep disorder. According to clinical guidelines, the first-line therapy is CBT-I, however, only few patients with insomnia have access to this treatment. Internet-delivered CBT-I has the potential to disseminate the recommended treatment to a larger number of patients. This study will determine whether a stepped care model for insomnia that includes psychoeducational treatment by GPs, internet-delivered CBT-I and specialised medical treatment, improves insomnia severity as well as psychological and physical well-being.
Supplementary Material
Footnotes
KS, HB, DR and DDE contributed equally.
Contributors: KS, DDE, HB and DR conceived the project, have overall responsibility for the trial design and treatment design and drafted the trial protocol. CB, MBa, EH, MK, AM and MM contributed to trial design. AA-K, MF, LF, EH, KH, DL and AM contributed to treatment design. HB, MBu, NB, LB, PD, A-MK, MM and LSi are responsible for statistical analysis. CB is responsible for economic analysis. All authors provided critical review on the trial protocol and approved the final manuscript.
Funding: The described work was supported by funding from the Innovationsfonds of the German Federal Joint Committee (Gemeinsamer Bundesausschuss/ G-BA; grant 01NVF18030). We also acknowledge support by the Open Access Publication Fund of the University of Freiburg.
Disclaimer: The funding source had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the trial protocol for publication.
Competing interests: HB received consultancy fees, reimbursement of congress attendance and travel costs as well as payments for lectures from Psychotherapy and Psychiatry Associations as well as Psychotherapy Training Institutes in the context of E-Mental-Health topics. He has been the beneficiary of study support (third-party funding) from several public funding organisations. MBa and MK are employees of BARMER. KD is a member of the Janssen Pharmaceuticals ‘Steering Committee Neuroscience’. MF, EH, DL and DDE are stakeholders of the GET.ON Institut für Online Gesundheitstrainings (operating under the registered brand ‘HelloBetter’), which aims to implement scientific findings related to digital health interventions into routine care. HelloBetter distributes the digital intervention for insomnia that is used in this study. CMM received research grant from Canopy Health, Eisai, Idorsia and Lallemand Health Solutions; he served as consultant to Eisai, Idorsia, Pear Therapeutics, Sunovion and Weight Watchers, and received royalties from Mapi Research Trust. DDE has served as a consultant to/on the scientific advisory boards of Sanofi, Novartis, Minddistrict, Lantern, Schoen Kliniken, Ideamed, German health insurance companies (BARMER, Techniker Krankenkasse) and a number of federal chambers for psychotherapy. None of the other authors declare any conflict of interest.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Morin CM, Drake CL, Harvey AG, et al. Insomnia disorder. Nat Rev Dis Primers 2015;1:15026. 10.1038/nrdp.2015.26 [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002;6:97–111. 10.1053/smrv.2002.0186 [DOI] [PubMed] [Google Scholar]
- 3.Shochat T, Umphress J, Israel AG, et al. Insomnia in primary care patients. Sleep 1999;22 Suppl 2:S359–65. [PubMed] [Google Scholar]
- 4.Kyle SD, Morgan K, Espie CA. Insomnia and health-related quality of life. Sleep Med Rev 2010;14:69–82. 10.1016/j.smrv.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 5.Hertenstein E, Feige B, Gmeiner T, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev 2019;43:96–105. 10.1016/j.smrv.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 6.Lane JM, Jones SE, Dashti HS, et al. Biological and clinical insights from genetics of insomnia symptoms. Nat Genet 2019;51:387–93. 10.1038/s41588-019-0361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Zhang X-W, Hou W-S, et al. Insomnia and risk of cardiovascular disease: a meta-analysis of cohort studies. Int J Cardiol 2014;176:1044–7. 10.1016/j.ijcard.2014.07.284 [DOI] [PubMed] [Google Scholar]
- 8.Qaseem A, Kansagara D, Forciea MA, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of physicians. Ann Intern Med 2016;165:125–33. 10.7326/M15-2175 [DOI] [PubMed] [Google Scholar]
- 9.Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26:675–700. 10.1111/jsr.12594 [DOI] [PubMed] [Google Scholar]
- 10.Grobe TG, Steinmann S, Gerr J. Gesundheitsreport 2019 – Schlafstörungen. Schriftenreihe Zur Gesundheitsanalyse – band 17. 2019. BARMER, 2019. [Google Scholar]
- 11.Schlack R, Hapke U, Maske U, et al. [Frequency and distribution of sleep problems and insomnia in the adult population in Germany: results of the German Health Interview and Examination Survey for Adults (DEGS1)]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013;56:740–8. 10.1007/s00103-013-1689-2 [DOI] [PubMed] [Google Scholar]
- 12.Buth S, Holzbach R, Martens M-S, et al. Problematic Medication With Benzodiazepines, "Z-drugs", and Opioid Analgesics. Dtsch Arztebl Int 2019;116:607–15. 10.3238/arztebl.2019.0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thiart H, Ebert DD, Lehr D, et al. Internet-based cognitive behavioral therapy for insomnia: a health economic evaluation. Sleep 2016;39:1769–78. 10.5665/sleep.6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev 2010;14:379–89. 10.1016/j.smrv.2010.01.003 [DOI] [PubMed] [Google Scholar]
- 15.Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009;32:55–64. [PMC free article] [PubMed] [Google Scholar]
- 16.Ritterband LM, Thorndike FP, Gonder-Frederick LA, et al. Efficacy of an Internet-based behavioral intervention for adults with insomnia. Arch Gen Psychiatry 2009;66:692–8. 10.1001/archgenpsychiatry.2009.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebert DD, Van Daele T, Nordgreen T, et al. Internet- and Mobile-Based psychological interventions: applications, efficacy, and potential for improving mental health. Eur Psychol 2018;23:167–87. 10.1027/1016-9040/a000318 [DOI] [Google Scholar]
- 18.Zachariae R, Lyby MS, Ritterband LM, et al. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia - A systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2016;30:1–10. 10.1016/j.smrv.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Soh HL, Ho RC, Ho CS, et al. Efficacy of digital cognitive behavioural therapy for insomnia: a meta-analysis of randomised controlled trials. Sleep Med 2020;75:315–25. 10.1016/j.sleep.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 20.van Straten A, van der Zweerde T, Kleiboer A, et al. Cognitive and behavioral therapies in the treatment of insomnia: a meta-analysis. Sleep Med Rev 2018;38:3–16. 10.1016/j.smrv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 21.Blom K, Jernelöv S, Rück C, et al. Three-year follow-up of insomnia and hypnotics after controlled Internet treatment for insomnia. Sleep 2016;39:1267–74. 10.5665/sleep.5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a web-based cognitive behavior therapy for insomnia intervention with 1-year follow-up: a randomized clinical trial. JAMA Psychiatry 2017;74:68–75. 10.1001/jamapsychiatry.2016.3249 [DOI] [PubMed] [Google Scholar]
- 23.Cheung JMY, Jarrin DC, Ballot O, et al. A systematic review of cognitive behavioral therapy for insomnia implemented in primary care and community settings. Sleep Med Rev 2019;44:23–36. 10.1016/j.smrv.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 24.Van der Zweerde T, Lancee J, Slottje P, et al. Nurse-guided internet-delivered cognitive behavioral therapy for insomnia in general practice: results from a pragmatic randomized clinical trial. Psychother Psychosom 2020;89:174–84. 10.1159/000505600 [DOI] [PubMed] [Google Scholar]
- 25.Espie CA. "Stepped care": a health technology solution for delivering cognitive behavioral therapy as a first line insomnia treatment. Sleep 2009;32:1549–58. 10.1093/sleep/32.12.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomonsson S, Santoft F, Lindsäter E, et al. Stepped care in primary care - guided self-help and face-to-face cognitive behavioural therapy for common mental disorders: a randomized controlled trial. Psychol Med 2018;48:1644–54. 10.1017/S0033291717003129 [DOI] [PubMed] [Google Scholar]
- 27.Forsell E, Jernelöv S, Blom K, et al. Proof of concept for an adaptive treatment strategy to prevent failures in internet-delivered CBT: a single-blind randomized clinical trial with insomnia patients. Am J Psychiatry 2019;176:315–23. 10.1176/appi.ajp.2018.18060699 [DOI] [PubMed] [Google Scholar]
- 28.Zhou ES, Michaud AL, Recklitis CJ. Developing efficient and effective behavioral treatment for insomnia in cancer survivors: results of a stepped care trial. Cancer 2020;126:165–73. 10.1002/cncr.32509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumeister H, Reichler L, Munzinger M, et al. The impact of guidance on Internet-based mental health interventions — a systematic review. Internet Interv 2014;1:205–15. 10.1016/j.invent.2014.08.003 [DOI] [Google Scholar]
- 30.Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep 2012;35:769–81. 10.5665/sleep.1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espie CA, Emsley R, Kyle SD, et al. Effect of digital cognitive behavioral therapy for insomnia on health, psychological well-being, and sleep-related quality of life: a randomized clinical trial. JAMA Psychiatry 2019;76:21–30. 10.1001/jamapsychiatry.2018.2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancee J, van den Bout J, Sorbi MJ, et al. Motivational support provided via email improves the effectiveness of internet-delivered self-help treatment for insomnia: a randomized trial. Behav Res Ther 2013;51:797–805. 10.1016/j.brat.2013.09.004 [DOI] [PubMed] [Google Scholar]
- 33.Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schulz KF, Altman DG, Moher D, et al. Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 2010;7:e1000251. 10.1371/journal.pmed.1000251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell MK, Piaggio G, Elbourne DR, et al. Consort 2010 statement: extension to cluster randomised trials. BMJ 2012;345:e5661. 10.1136/bmj.e5661 [DOI] [PubMed] [Google Scholar]
- 36.Montgomery P, Grant S, Mayo-Wilson E, et al. Reporting randomised trials of social and psychological interventions: the CONSORT-SPI 2018 extension. Trials 2018;19:407. 10.1186/s13063-018-2733-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juszczak E, Altman DG, Hopewell S, et al. Reporting of multi-arm parallel-group randomized trials: extension of the CONSORT 2010 statement. JAMA 2019;321:1610–20. 10.1001/jama.2019.3087 [DOI] [PubMed] [Google Scholar]
- 38.Chan A-W, Tetzlaff JM, Altman DG, et al. Spirit 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiart H, Lehr D, Ebert DD, et al. Log in and breathe out: internet-based recovery training for sleepless employees with work-related strain - results of a randomized controlled trial. Scand J Work Environ Health 2015;41:164–74. 10.5271/sjweh.3478 [DOI] [PubMed] [Google Scholar]
- 40.Ebert DD, Berking M, Thiart H, et al. Restoring depleted resources: efficacy and mechanisms of change of an Internet-based unguided recovery training for better sleep and psychological detachment from work. Health Psychol 2015;34S:1240–51. 10.1037/hea0000277 [DOI] [PubMed] [Google Scholar]
- 41.Behrendt D, Ebert DD, Spiegelhalder K, et al. Efficacy of a self-help web-based recovery training in improving sleep in workers: randomized controlled trial in the general working population. J Med Internet Res 2020;22:e13346. 10.2196/13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med 2001;2:297–307. 10.1016/s1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- 43.Morin CM, Belleville G, Bélanger L, et al. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8. 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gagnon C, Bélanger L, Ivers H, et al. Validation of the insomnia severity index in primary care. J Am Board Fam Med 2013;26:701–10. 10.3122/jabfm.2013.06.130064 [DOI] [PubMed] [Google Scholar]
- 45.Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 46.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh sleep quality index. J Psychosom Res 1998;45:5–13. 10.1016/s0022-3999(97)00298-5 [DOI] [PubMed] [Google Scholar]
- 47.Richardson J, Iezzi A, Khan MA, et al. Validity and reliability of the Assessment of Quality of Life (AQoL)-8D multi-attribute utility instrument. Patient 2014;7:85–96. 10.1007/s40271-013-0036-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003;54:573–83. 10.1016/s0006-3223(02)01866-8 [DOI] [PubMed] [Google Scholar]
- 49.Trujols J, de Diego-Adeliño J, Feliu-Soler A, et al. Looking into the effect of multi-item symptom domains on psychometric characteristics of the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR16). Psychiatry Res 2018;267:126–30. 10.1016/j.psychres.2018.05.076 [DOI] [PubMed] [Google Scholar]
- 50.Lamoureux BE, Linardatos E, Fresco DM, et al. Using the QIDS-SR16 to identify major depressive disorder in primary care medical patients. Behav Ther 2010;41:423–31. 10.1016/j.beth.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 51.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 52.Löwe B, Decker O, Müller S, et al. Validation and standardization of the generalized anxiety disorder screener (GAD-7) in the general population. Med Care 2008;46:266–74. 10.1097/MLR.0b013e318160d093 [DOI] [PubMed] [Google Scholar]
- 53.Gierk B, Kohlmann S, Kroenke K, et al. The somatic symptom scale-8 (SSS-8): a brief measure of somatic symptom burden. JAMA Intern Med 2014;174:399–407. 10.1001/jamainternmed.2013.12179 [DOI] [PubMed] [Google Scholar]
- 54.Hakkaart-van Roijen L, van Straten A, Donker M, et al. Manual Trimbos/iMTA questionnaire for costs associated with psychiatric illness (TiC-P). Erasmus University, 2002. [Google Scholar]
- 55.Bouwmans C, De Jong K, Timman R, et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P). BMC Health Serv Res 2013;13:217. 10.1186/1472-6963-13-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buntrock C, Lehr D, Smit F, et al. Guided Internet-based cognitive behavioral therapy for insomnia: health-economic evaluation from the societal and public health care perspective alongside a randomized controlled trial. J Med Internet Res 2021;23:e25609. 10.2196/25609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bock JO, Brettschneider C, Seidl H. Ermittlung standardisierter Bewertungssätze AUS gesellschaftlicher Perspektive für die gesundheitsökonomische evaluation. Gesundheitswesen 2015;77:53–61. 10.1055/s-0034-1374621 [DOI] [PubMed] [Google Scholar]
- 58.Gómez Penedo JM, Berger T, Grosse Holtforth M, et al. The working alliance inventory for guided Internet interventions (WAI-I). J Clin Psychol 2020;76:973–86. 10.1002/jclp.22823 [DOI] [PubMed] [Google Scholar]
- 59.Attkisson CC, Zwick R. The client satisfaction questionnaire. psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann 1982;5:233–7. 10.1016/0149-7189(82)90074-x [DOI] [PubMed] [Google Scholar]
- 60.Boß L, Lehr D, Reis D, et al. Reliability and validity of assessing user satisfaction with web-based health interventions. J Med Internet Res 2016;18:e234. 10.2196/jmir.5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rozental A, Kottorp A, Forsström D, et al. The negative effects questionnaire: psychometric properties of an instrument for assessing negative effects in psychological treatments. Behav Cogn Psychother 2019;47:559–72. 10.1017/S1352465819000018 [DOI] [PubMed] [Google Scholar]
- 62.Self-efficacy SR. Thought control of action. New York: Taylor & Francis, 1992. [Google Scholar]
- 63.Morin CM, Stone J, Trinkle D, et al. Dysfunctional beliefs and attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging 1993;8:463–7. 10.1037//0882-7974.8.3.463 [DOI] [PubMed] [Google Scholar]
- 64.Espie CA, Inglis SJ, Harvey L, et al. Insomniacs' attributions. psychometric properties of the dysfunctional beliefs and attitudes about sleep scale and the sleep disturbance questionnaire. J Psychosom Res 2000;48:141–8. 10.1016/s0022-3999(99)00090-2 [DOI] [PubMed] [Google Scholar]
- 65.Nicassio PM, Mendlowitz DR, Fussell JJ, et al. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther 1985;23:263–71. 10.1016/0005-7967(85)90004-x [DOI] [PubMed] [Google Scholar]
- 66.Gieselmann A, de Jong-Meyer R, Pietrowsky R. Kognitive und körperliche Erregung in Der phase VOR dem Einschlafen. die Deutsche version Der pre-sleep arousal scale (PSAS). Z Klin Psychol Psychother 2012;41:73–80. 10.1026/1616-3443/A000134 [DOI] [Google Scholar]
- 67.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer 1999;85:1186–96. [DOI] [PubMed] [Google Scholar]
- 68.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- 69.Mastin DF, Bryson J, Corwyn R. Assessment of sleep hygiene using the sleep hygiene index. J Behav Med 2006;29:223–7. 10.1007/s10865-006-9047-6 [DOI] [PubMed] [Google Scholar]
- 70.Garnefski N, Kraaij V. Cognitive emotion regulation questionnaire – development of a short 18-item version (CERQ-short). Pers Individ Dif 2006;41:1045–53. 10.1016/j.paid.2006.04.010 [DOI] [Google Scholar]
- 71.Adams G, Gulliford MC, Ukoumunne OC, et al. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol 2004;57:785–94. 10.1016/j.jclinepi.2003.12.013 [DOI] [PubMed] [Google Scholar]
- 72.Aldiabat KM, Le Navenec CL. Data saturation: the mysterious step in grounded theory methodology. Qual Rep 2018;23:245–61. 10.46743/2160-3715/2018.2994 [DOI] [Google Scholar]
- 73.Francis JJ, Johnston M, Robertson C, et al. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol Health 2010;25:1229–45. 10.1080/08870440903194015 [DOI] [PubMed] [Google Scholar]
- 74.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 1988;318:1728–33. 10.1056/NEJM198806303182605 [DOI] [PubMed] [Google Scholar]
- 75.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–19. 10.1037//0022-006x.59.1.12 [DOI] [PubMed] [Google Scholar]
- 76.Ren S, Lai H, Tong W, et al. Nonparametric bootstrapping for hierarchical data. J Appl Stat 2010;37:1487–98. 10.1080/02664760903046102 [DOI] [Google Scholar]
- 77.Glick HA, Doshi JA, Sonnad SS, et al. Economic evaluation in clinical trials. Oxford University Press: Oxford, 2007. [Google Scholar]
- 78.Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461–75. 10.1002/hec.843 [DOI] [PubMed] [Google Scholar]
- 79.Hoch JS, Rockx MA, Krahn AD. Using the net benefit regression framework to construct cost-effectiveness acceptability curves: an example using data from a trial of external loop recorders versus Holter monitoring for ambulatory monitoring of "community acquired" syncope. BMC Health Serv Res 2006;6:68. 10.1186/1472-6963-6-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patton MQ. Qualitative Research & Evaluation Methods. 3rd ed. Thousand Oaks: Sage, 2002. [Google Scholar]
- 81.Sandelowski M. Sample size in qualitative research. Res Nurs Health 1995;18:179–83. 10.1002/nur.4770180211 [DOI] [PubMed] [Google Scholar]
- 82.Dagger TS, Sweeney JC, Johnson LW. A hierarchical model of health service quality: scale development and investigation of an integrated model. J Serv Res 2007;10:123–42. 10.1177/1094670507309594 [DOI] [Google Scholar]
- 83.Kuckartz U. Qualitative Inhaltsanalyse. Methoden, praxis, Computerunterstützung. Beltz Juventa, 2018. [Google Scholar]
- 84.Rädiker S, Kuckartz U. Analyse qualitativer Daten mit MAXQDA. In: Text, audio und video. Springer: VS, 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.