Abstract
Objective
Studies, mainly from high-income countries, suggest that there are ethnic and racial variations in prevalence of uterine fibroids (UF). However, there have been few studies of the epidemiology of UF in sub-Saharan Africa (SSA). We reviewed published articles on the epidemiology of UF in SSA.
Design
This was a scoping review of literature.
Settings
We searched three databases (PubMed, African Wide Information (EBSCO) and African Journals OnLine (AJOL)). The search for eligible articles was conducted between December 2019 and January 2021.
Primary and secondary outcome measures
To describe the reported prevalence/incidence of, and risk factors for UF in SSA.
Results
Of the 1052 articles retrieved, 9 met the inclusion criteria for review. The articles were from Nigeria (4/9), Ghana (2/9), Cameroon (1/9), Kenya (1/9) and South Africa (1/9). Two studies from pathology departments and three studies from radiology departments reported prevalence of UF. We did not find any study on the incidence or genomics of UF in SSA. Of the three studies that reported on the risk factors of UF, only one case–control study that was conducted using retrospective data of attendees at a gynaecological clinic conducted multivariable analysis.
Conclusion
There is lack of robust epidemiological studies of the prevalence, incidence and risk factors of UF in SSA. There is urgent need to study epidemiological and genomics risk factors of UF in SSA because UF is the most common gynaecological neoplasm in this population where it is associated with significant morbidity and occasional, usually perioperative, mortality.
Keywords: PUBLIC HEALTH, GYNAECOLOGY, EPIDEMIOLOGY
Strengths and limitations of this study.
We comprehensively reviewed all publications on uterine fibroids (UF) in sub-Saharan African (SSA) women, and found dearth of robust epidemiological studies and no genomic studies despite UF being the most common neoplasm in this population.
We were careful to correctly interpret the results of the publications we reviewed.
Because there were few high quality studies, we were unable to conduct a systematic review and to combine effect estimators to generate summary statistics.
While unlikely, we may have omitted eligible articles that were not in the three major research databases we searched (PubMed, African Wide Information (EBSCO) and African Journals OnLine) because many SSA journals are not indexed.
The interpretation of this review is limited to published information in the manuscript we reviewed, and we assumed that missing information were not collected.
Introduction
Uterine fibroids or uterine leiomyomas (UF) are the most common neoplasms affecting women.1 They are typically composed of disordered fascicles of smooth-muscle cells, vascular smooth-muscle cells, fibroblasts, leiomyoma-associated fibroblasts and an excess of acellular extracellular matrix.2 They tend to be multiple and may be found in any part of the uterus however, they are the most common in the muscular wall of the uterus (the myometrium).
The incidence and prevalence of UF reported in the literature varies significantly by study design, methods of diagnosis, ethnic composition and age distribution of study participants.1 3 The cumulative incidence of UFs by the age of 50 years in women in developed countries is 70%–80%.1 4
Variations in the incidence and prevalence of UF by race and ethnic groups have been widely reported. Studies show that the incidence and prevalence of UF in women of African ancestry is higher than that in other races.4–6 For example, a large longitudinal study (Nurses’ Health Study II) in the USA showed that the incidence of UF confirmed by pelvic examination, ultrasound (USS) or hysterectomy per 1000 woman-years was 37.9 in African American, 14.5 in Hispanic, 12.5 in white and 10.4 in Asian women.5 In another longitudinal study conducted in UK, the crude incidence of UF based on primary care physicians’ diagnosis with USS, hysteroscopy, laparoscopy or pelvic examination was 5.8 per 1000 woman-years.7
There are several epidemiological risk factors for UF. These include advanced age, race, age at menarche, low or nulliparity, family history, obesity, diet, physical activity, smoking, oral contraceptives, hormone replacement therapy, environmental exposure to high levels of oestrogen and progesterone and vitamin D deficiency.3 8–10 Age is consistently associated with the incidence and prevalence of UF irrespective of ethnicity, race and other risk factors. In general, the risk of UF is about 4–11 times higher in women aged 40–60 years compared with 20–30 years old women and women older than 60 years.1 3 Several studies show that early age at menarche is associated with higher risk UF.3 11 12 Multiparity is linearly associated with reduced risk of UF.3 13 The risk reduction among multiparous women ranges from 20% to 50% compared with nulliparous women.1
Overweight and obesity are independent risk factors for UF.14 A meta-analysis of 325 899 women among whom 19 593 had UF showed association with obesity.14 The association was present whether obesity was assessed using waist-to-hip ratio, waist circumference, weight change from age 18 years, or body mass index (BMI).14 Some studies found a dose response relationship between obesity and UF while other studies did not find such relationship.3 14–16
While few studies reported no associations between dietary intakes and UF, other studies showed a reduced risk with consumption of vegetables and fruits, and increased risk with intakes of food additives, sweeteners, soya milk and dietary fats.1 14 17–19 Most studies found low level of serum vitamin D to be associated with increased risk of UF while a few reported no effect.20 21 The association between vitamin D and UF was stronger in black compared with white women. Exposure to sunlight for more than an hour a day was also associated with reduced risk of UF.20 Smoking was associated with reduced risk of UF, especially in women with low BMI.1 Most studies reported an inverse relationship between regular physical activities and risk of UF.3 19 Oral and injectable contraceptives use were associated with reduced risk of UF, however a few studies found increased or no risk in women using oral contraceptives.1 3 Hormone replacement therapy or exposure to exogenous hormones, particularly among postmenopausal women was associated with increased risk of UF in some studies.3
Genetic and epigenetic factors have been associated with risk of UF. Positive family history is associated with increased risk of UF and higher risk was reported among sisters.1 22–26 The estimates of heritability for UF were 26%–69% in twin studies while data from genome-wide association studies (GWAS) reported heritability risk of 13%.27 28 The risk of UF is 2.5-fold among first degree relatives compared with the general population.28 The concordance rate of UF among monozygotic twins is twice that of dizygotic twins of the same sex, and a lot higher than in first-degree relatives.28 29 Recently, GWAS identified several candidate loci for UF in chromosome regions among African American—22q13.1 (CYTH4); Caucasian—11p15.5 (BETIL), 17q25.3 (FASN, CCDC57 and SLC16A3), 22q13.1 (TNRC6B); and Asian—10q24.33 (OBFC1), 11p15.5 (BET1L) and 22q13.1 (TNRC6B) populations.30–33
UF is associated with significant morbidity and substantial socioeconomic costs.34–36 Data from a global systematic review of the cost of UF showed that the total direct and indirect cost after diagnosis or from surgical care ranged from US$11 717 to US$25 023 per patient per year.37 In USA, the annual cost of UF to the economy was estimated to be between US$5.9 and US$34.4 billion with obstetrical complications contributing the highest fraction of the economic burden.38
Consistent with the high incidence and prevalence of UF in African populations in developed countries, case reports and clinical evidence suggest high prevalence of UF in black women living in Africa. However, in contrast to developed countries, there have been very few, adequately powered, systematic epidemiological studies of UF in Africa. In this scoping review of current publications on the epidemiology of UF in Africa, we aim to establish the state of the evidence and their limitations, the burden of UF and priorities for research on UF in black women living in sub-Saharan Africa (SSA).
Methods
In this review, we used the Joanna Briggs Institute guidelines for the conduct of systematic scoping review which was earlier described by Arksey and O’Malley.39 40 Briefly, we base this review on five frameworks: (a) identifying the research question, (b) identifying the relevant studies (search strategy), (c) selecting the eligible studies, (d) charting the data and (e) collating, summarising and reporting the results with or without consultation with experts on the specific field.40
Research question
The research questions for this scoping review are: What are the prevalence and incidence of UF among black women in SSA? What are the risk factors for UF among SSA women?
Information sources and search strategy
We conducted a systematic search of three online databases for records in English: PubMed, African Wide Information (EBSCO) and African Journal OnLine (AJOL). We used the following keywords to search the databases to retrieve published articles on the incidence, prevalence and risk factors of UF; uterine fibroids or fibroids or leiomyoma or myoma; prevalence, incidence, risk factors or causes and SSA (using subregions within SSA (West Africa OR East Africa OR Central Africa OR Southern Africa), and by specific country names) (online supplemental table 1—Search Term Strategy). We used Boolean terms AND/OR to separate the keywords during the search. We included Medical Subject Headings (MeSH) terms in the search terms. We also manually searched references and bibliography of relevant articles on this subject. The search was conducted between December 2019 and 27 January 2021.
bmjopen-2021-052053supp001.pdf (269.7KB, pdf)
Eligibility criteria
We used the PICO format (population, intervention, comparator and outcome) to design the eligibility criteria for the studies that were included in this review. These are (a) published peer-reviewed article with observational or experimental design that reported on the aetiology or risk factors or incidence or prevalence or proportion of women with UFs and (b) data must have been collected in SSA among Indigenous black women population. We excluded case reports, letter to editors or expert opinion without primary data on UFs in SSA as well as studies that only reported the outcome of treatment.
Study selection process
All titles retrieved from searches were compiled and reviewed with EndNote X8.0 (Thomson Reuters). We removed all duplicates using the EndNote automated system and manually. We screened abstracts in accordance with our inclusion and exclusion criteria. Next, we screened the full texts of abstracts that were eligible for further consideration. Only articles that met the inclusion criteria during full-text screening were finally selected for data charting in this review.
Charting data
We entered our data into a prepared Microsoft Excel sheet using the following data charting fields: authors, date, country, study design, aim/objectives, sample size, recruitment strategy (probability or non-probability sampling), study settings (health facility/community/online), outcome measured (prevalence/incidence/proportion), analysis (descriptive/test of association/multivariable analysis) and summary of key findings.
Collating, summarising and reporting the results
We present a descriptive summary of eligible studies and we created a Preferred Reporting Items for Systematic Reviews and Meta-Analyses-extension for Scoping Reviews flow chart to summarise the process and number of articles that were finally selected for data charting (online supplemental table 2).41 The chart shows the overall number of studies included, study designs and settings, publication years, the characteristics of the study populations, the outcomes reported and the countries where the studies were conducted. In line with scoping reviews’ methodology, we did not perform an assessment of the quality of the included studies.
Patient and public involvement
It was not possible to describe patient and public involvement in this research.
Results
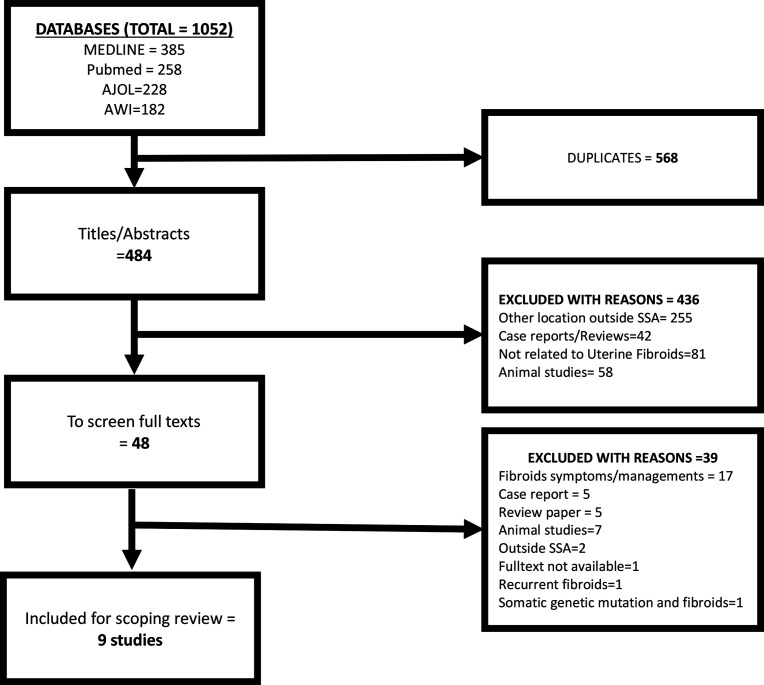
We retrieved 1052 studies from the three databases (figure 1). After removal of duplicate publications, we screened 484 titles and abstracts and found only 48 articles were eligible for full-text screening. We excluded 39 of the 48 full-text articles because 17 of them were on symptoms/management of UF, 7 were animal studies, 5 each were case reports and reviews, 2 were from outside SSA, 1 each were on recurrent UF after treatment, full texts not available and on somatic genetic mutation in UF. Of the 9 studies that met the inclusion criteria, 4 were from Nigeria,42–45 2 from Ghana46 47 and 1 study each from Cameroon,48 Kenya49 and South Africa.50
Figure 1.
The PRISMA flow chart for the scoping review. AJOL, African Journals OnLine; AWI, African Wide Information; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis; SSA, sub-Saharan Africa.
Incidence or prevalence of UF
Five of the nine studies screened described the prevalence of UF (table 1).42 44 46 48 50
Table 1.
Descriptive analysis of studies included in the scoping review
| Author; year | Reference | Research focus | Study design | Sampling methods | Sample size | Outcome measured | Age of study participants | Summary of key findings |
| Tiltman et al (South Africa) |
50 | Pathology | Case series | Non-probability | 661 | Proportion of UF within hysterectomy specimen | 12.0–84.0 | The proportion of UF was 427/661 (64.6%). |
| Wango et al (Kenya) |
49 | Pathology | Case series | Not clearly described | 20 | Evaluation of oestradiol, progesterone and their receptors | Range 31.0–42.0 | The UF tissue contained significantly higher levels of oestrogen receptor (28.2±1.6 vs 19.1±0.4 fm/mg protein) and progesterone receptor (16.8±0.7 vs 9.4±0.2 fm/mg protein) compared with normal myometrial tissue, a relatively significant higher levels of oestrogen (1117.6±20.9 vs 616.9±19.8 pm/mg protein) and progesterone (7.7±0.25 vs 3.2±0.34 nm/mg protein) in the myometrium than in the leiomyomata. |
| Mohammed et al (Nigeria) | 42 | Pathology | Case series | Non-probability | 209 | Proportion of UF pathological specimen and degenerative changes | Range 25.0–50.0 | The proportion of myometrial UF was 2.2% of all surgical specimen over 5 years. |
| Eze et al (Nigeria) |
43 | Radiology | Case control | Non-probability | 200 (100 cases vs 100 controls) | Frequency and growth rate of uterine fibroids in pregnancy | Cases (31.6±4.5 year); controls (29.1±5.5 year) | The frequency of UFs in pregnancy was 12.3%; the most common type was subserous fibroids (27.5%). The mean size of UFs measured on ultrasound was lowest during third scan. |
| Oluwole et al (Nigeria) |
44 | Clinical | Case control | Non-probability | 580 | Proportion of UF and risk factor analysis | 35.5±5.8 | The proportion of women with UFs was 31% (178/580). Presence of UFs was associated with 40–49 years (OR=4.9%; 95% CI 1.8% to 31.1%); lower parity (OR=0.6; 95% CI 0.2 to 0.9); family history of UFs (OR=1.9; 95% CI 1.9 to 4.8); and history of infertility (OR=5.0; 95% CI 0.9 to 25.9). |
| Awowole et al (Nigeria) |
45 | Pathology | Cross-sectional | Non-probability | 60 | To measure expression of oestrogen receptor α (ERα) and progesterone receptor (PR) in myometrium and UF | 26.0–53.0 | UF had a higher mean expression of ERα (H-score 193.4±64.6 vs 153.3±69.1; p=0.01) and PR (214.9±66.6 vs 171.5±63.5; p<0.001) than in myometrial tissues. The tumour diameter correlated negatively with the immunoscores of both receptors irrespective of age, parity and body mass index, but this was only significant for PR (p=–0.44; p<0.001). |
| Sarkodie et al (Ghana) |
46 | Radiology | Cross-sectional | Non-probability | 244 | Prevalence of UF and risk factors analysis | 14.0–54.0 | In this study, 23% (38/168) of women <35 had prevalent fibroids, compared with 67% (36/54) of women 35–44 and 73% (16/22) of women at 45 or above years. Factors that associated significantly with UF in Ghanaian women included obesity (X2=17.3, p value=0.001), participant’s age range (X2=47.4, p=0.001), parity (X2=−10.2, p=0.001) and age at last delivery (X2=34.6, p=0.001). |
| Sarkodie et al (Ghana) |
47 | Radiology | Cross-sectional | Non-probability | 244 | Assessment of sonographic characteristics of UF | 14.0–54.0 | The prevalence of UF was 36.9% (90/244). The majority of the UFs were intramural (57.8 %) with only 4.4% noted as submucosal. Most (55.6 %) of the UFs were located in more than one part of the uterus. |
| Egbe et al (Cameroon) |
48 | Radiology and clinical | Cross-sectional | Non-probability | 226 | Proportion of UF and risk factors analysis | ≥21.0 | The prevalence of UF in pregnancy was 16.7% (38/226). Respondents with UF were older than those without (p<0.001) and of low parity (p=0.02). |
UF, uterine fibroids.
Two of these studies, one each from pathology departments in single institutions in South Africa and Nigeria, examined the proportion of UF in surgical specimens.42 50 In Northern Nigeria, UF accounted for 2.2% of all surgical specimen at a single facility over a 5-year period.42 The South African study reported that the proportion of UF among all hysterectomy specimens in a single institution over a 6-month period was 64.6%.50
A cross-sectional study of pregnant women undergoing abdominal USS examination in two regional hospitals in Cameroon reported that 16.8% (38/226) had UF.48 Another cross-sectional study in Ghana among 244 non-pregnant women referred for abdominal USS showed that 36.9% had UF and the proportion of women with UF increased with age.46 A 2-year retrospective review of attendees at the gynaecology clinic of a public tertiary health institution in Nigeria showed that 30.7% (178/580) of all patients had a diagnosis of UF.44 Another study of pregnant women referred for prenatal abdominal USS at a tertiary hospital in eastern Nigeria showed that the prevalence of UF was 12.3% during pregnancy.43
Role of oestrogen, progesterone and their receptors
A study in Kenya reported on cytosolic quantification of oestrogen and progesterone and their receptors in UF tissue measured using radioimmunoassay.49 The study showed that UF contained lower levels of oestrogen and progesterone but higher levels of receptors for these hormones compared with normal uterine tissue.49 In a more recent Nigerian study using immunohistochemistry, the level of oestrogen and progesterone receptors in UF was higher than in uterine tissue.45 The Nigerian study further showed a significant negative correlation between UF size and the progesterone receptors levels only (table 1).45
Risk factors for UF
Three studies presented data on risk factors of UF (tables 1 and 2).44 46 48
Table 2.
Summary of reported risk factors associated with UF in SSA
| Risk factors | Pregnant women | Non-pregnant women | |
| Egbe et al 2018 (Cross-sectional study from Cameroon) |
Sarkodie et al 2016a (Cross-sectional study from Ghana) |
Oluwole et al 2015 (Case–control study from Nigeria) |
|
| Advanced age | ↑ | ↑ | ↑ |
| Family history | Not considered | Not considered | ↑ |
| Obesity | Not considered | ↑ | ↓ |
| Nulliparity | Not considered | ↑ | Not considered |
| Gravidity | ↑ | Not considered | Not considered |
| Advanced age at delivery | Not considered | ↑ | Not considered |
| At least primiparity | Not considered | Not considered | ↓ |
↑ - increased risk, ↓ - decreased risk, not considered as a risk factor in the study.
SSA, sub-Saharan Africa; UF, uterine fibroid.
In a Nigerian case–control study of gynaecology clinic attendees, advanced age (OR=4.90; 95% CI 1.80 to 31.1) and positive family history (OR=3.0; 95% CI 1.90 to 4.80) were associated with higher risk while obesity (OR=0.4; 95% CI 0.10 to 0.90) and primiparity (OR=0.60; 95% CI 0.20 to 0.90) were associated with lower risk of UF.44 A cross-sectional study of 244 women referred for abdominal USS at three centres in Ghana found that women with UF tended to be older (p=0.001), obese (0.001), older at last pregnancy and delivery (p=0.001) and have lower parity (p=0.001).46 In another cross-sectional study of factors associated with UF in pregnancy in Cameroon, women with UF were older (p<0.001) and had higher gravidity (p=0.02).48
Discussion
In this review, we mapped published epidemiological studies on incidence, prevalence and risk factors for UF in indigenous African women. Our results confirmed the paucity of systematic epidemiological study of UF among black women in Africa. Only few studies have some information on prevalence/proportion of, and risk factors for UF.42 44 46 48 50 The five studies that reported the prevalence of UF used different populations, denominators and study designs.42 44 46 48 50 Two studies from pathology departments in Nigeria and South Africa used different reporting periods and denominators to calculate the proportions of UF.42 50 We also observed variations in the reporting of the prevalence of UF in pregnancy in the two studies from radiology departments in Nigeria and Cameroon.43 48 They both used convenience sampling technique and were silent on the gestational ages of participants. The only Nigerian study that presented data on the prevalence of UF among non-pregnant women was a retrospective review of case records that used all other attendees at a gynaecological clinic as controls.44 There was no study in this review that has information on the incidence of UF in pregnant or non-pregnant women.
Two studies were on the role of oestrogen and progesterone and their receptors. The two hormonal studies used different diagnostic techniques (radioimmunoassay vs immunochemistry), laboratory estimation of cut-off levels for oestrogen and progesterone and comparator groups (UF and normal myometrial tissue from same patient versus UF and normal myometrial tissue from different patients as cases and control).45 49 The observed differences in the methodology of the two studies make it difficult to compare and interpret their findings. We observed that the sample sizes of these three studies were too small to allow for rigorous multivariable analysis for confounders. In addition, the three studies were conducted with specimen from women who had treatment in specific health facilities.
Three studies described risk factors for UF among black African women, but they all used different research designs and data analysis techniques.44 46 48 All the studies were conducted within single facilities, two were cross-sectional and one was a retrospective case–control study. The risk factors identified in the three studies were similar to those reported in studies conducted in USA, Europe and Asia.5 12 51 Briefly, advancing age was the only risk factors that was common to all three studies and low parity was reported in two studies.44 46 48 The only other risk factor reported among non-pregnant women was self-report of family history of UF.44 Obesity was reported as a protective factor in non-pregnant Nigerian women and as a risk factor in pregnant women in Ghana.44 48 The tests for association in these studies were not well described in the methods sections of their manuscripts.44 46 48 The studies from Cameroon and Ghana used bivariate tests and did not adjust for age in their analyses.46 48 The only Nigerian study that used multivariable analysis to adjust for confounders, used data collected from a retrospective review of cases managed in a tertiary public health facility and assigned other attendees as controls.44
Although, we did not assess the risk of bias in studies that we reviewed because that is outside the objective of scoping review generally, we observed that the majority of the studies used data collected from case series or cross-sectional studies (6/9) while two (3/9) were case–control studies.42–50 None of the nine studies we reviewed used probability sampling technique to select their subjects and only one study reported on sample size and power calculation.
We found several gaps in the epidemiology of UF in SSA. There was no genomic epidemiology study of UF in SSA. Studies from high-income countries have shown that only 20.0%–40.0% of women with symptomatic UF seek medical treatment, suggesting that a significant number of women with UF are not captured by facilities-based studies.52 We did not find any published population-based study with adequate statistical power and sampling strategy which can generate generalisable information on incidence, prevalence and risk factors of UF among Indigenous black African women. There are many epidemiological risk factors of UF that are yet to be investigated in SSA. These factors include reproductive factors (age at menarche and menopause, birth interval or inter pregnancy interval, contraceptives and hormone replacement therapy), diets including vitamin D, trace elements and heavy metals, lifestyle and physical activity, reproductive tract infections, microbiome and pollution.3 8 12 53 54 Lack of information on these risk factors prevent development of preventive and therapeutic interventions. This is a serious gap in knowledge considering the morbidity, mortality and economic costs of UF in SSA.
The interpretation of findings from this scoping review may be limited for the following reasons. We searched published articles from online databases only. We may have missed papers published in journals that are not indexed in these online databases. We excluded one article that we could not retrieve the full texts, but the abstract shows that this was on the association between UF and BMI. Despite these limitations, this scoping review confirmed the dearth of studies on the epidemiology of UF among SSA women and argues for urgent remediation of this situation.
Conclusions
Our results show that there is limited information on the epidemiology of UF and identified gaps in knowledge of UF among women in SSA despite its high prevalence, morbidity and economic costs. We recommend urgent implementation of well-designed and adequately powered studies to address this gap.
Supplementary Material
Footnotes
Twitter: @adebamowo
Contributors: CAA conceived and designed the study. He conducted literature review, reviewed, revised and approved the manuscript. IOM-B designed, conducted literature search, screening of articles and data charting. He wrote the first draft, revised and approved the manuscript. CAA is responsible for the overall content as the guarantor.
Funding: Support for CAA was provided by the African Collaborative Center for Microbiome and Genomics Research (ACCME) Grant (1U54HG006947) and African Female Breast Cancer Epidemiology (AFBRECANE) Grant (U01HG009784) from the Office Of The Director, National Institutes Of Health (OD) and the National Human Genome Research Institute (NHGRI); funds through the Maryland Department of Health's Cigarette Restitution Fund Program (CH-649-CRF); and the University of Maryland Greenebaum Cancer Center Support Grant (P30CA134274). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Maryland Department of Health.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. The data will be made available upon request from the corresponding author.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Stewart EA, Cookson CL, Gandolfo RA, et al. Epidemiology of uterine fibroids: a systematic review. BJOG 2017;124:1501–12. 10.1111/1471-0528.14640 [DOI] [PubMed] [Google Scholar]
- 2.Holdsworth-Carson SJ, Zaitseva M, Vollenhoven BJ, et al. Clonality of smooth muscle and fibroblast cell populations isolated from human fibroid and myometrial tissues. Mol Hum Reprod 2014;20:250–9. 10.1093/molehr/gat083 [DOI] [PubMed] [Google Scholar]
- 3.Wise LA, Laughlin-Tommaso SK. Epidemiology of uterine fibroids: from menarche to menopause. Clin Obstet Gynecol 2016;59:2–24. 10.1097/GRF.0000000000000164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day Baird D, Dunson DB, Hill MC, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188:100–7. 10.1067/mob.2003.99 [DOI] [PubMed] [Google Scholar]
- 5.Marshall L, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 1997;90:967–73. 10.1016/S0029-7844(97)00534-6 [DOI] [PubMed] [Google Scholar]
- 6.Wise LA, Palmer JR, Stewart EA, et al. Age-specific incidence rates for self-reported uterine leiomyomata in the black womenʼs health study. Obstetrics & Gynecology 2005;105:563–8. 10.1097/01.AOG.0000154161.03418.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martín-Merino E, Wallander M-A, Andersson S, et al. The reporting and diagnosis of uterine fibroids in the UK: an observational study. BMC Womens Health 2016;16:45. 10.1186/s12905-016-0320-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brakta S, Diamond JS, Al-Hendy A, et al. Role of vitamin D in uterine fibroid biology. Fertil Steril 2015;104:698–706. 10.1016/j.fertnstert.2015.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird DD, Harmon QE, Upson K, et al. A prospective, ultrasound-based study to evaluate risk factors for uterine fibroid incidence and growth: methods and results of recruitment. J Womens Health 2015;24:907–15. 10.1089/jwh.2015.5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wise LA, Palmer JR, Rosenberg L, et al. FASN, dietary fat intake, and risk of uterine leiomyomata in the black women’s health study. Fertil Steril 2016;106:1136–41. 10.1016/j.fertnstert.2016.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Styer AK, Rueda BR. The epidemiology and genetics of uterine leiomyoma. Best Pract Res Clin Obstet Gynaecol 2016;34:3–12. 10.1016/j.bpobgyn.2015.11.018 [DOI] [PubMed] [Google Scholar]
- 12.Sparic R, Mirkovic L, Malvasi A, et al. Epidemiology of uterine Myomas: a review. Int J Fertil Steril 2016;9:424–35. 10.22074/ijfs.2015.4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day Baird D, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology 2003;14:247–50. 10.1097/01.EDE.0000054360.61254.27 [DOI] [PubMed] [Google Scholar]
- 14.Qin H, Lin Z, Vásquez E, et al. Association between obesity and the risk of uterine fibroids: a systematic review and meta-analysis. J Epidemiol Community Health 2021;75:197–204. 10.1136/jech-2019-213364 [DOI] [PubMed] [Google Scholar]
- 15.Lee J-E, Song S, Cho E, et al. Weight change and risk of uterine leiomyomas: Korea nurses’ health study. Curr Med Res Opin 2018;34:1913–9. 10.1080/03007995.2018.1462783 [DOI] [PubMed] [Google Scholar]
- 16.Sato F, Nishi M, Kudo R, et al. Body fat distribution and uterine leiomyomas. Journal of Epidemiology 1998;8:176–80. 10.2188/jea.8.176 [DOI] [PubMed] [Google Scholar]
- 17.Shen Y, Xu Q, Xu J, et al. Environmental exposure and risk of uterine leiomyoma: an epidemiologic survey. Eur Rev Med Pharmacol Sci 2013;17:3249–56. [PubMed] [Google Scholar]
- 18.Wise LA, Radin RG, Kumanyika SK, et al. Prospective study of dietary fat and risk of uterine leiomyomata. Am J Clin Nutr 2014;99:1105–16. 10.3945/ajcn.113.073635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Zeng Q, Dong S, et al. Associations between uterine fibroids and lifestyles including diet, physical activity and stress: a case-control study in China. Asia Pac J Clin Nutr 2013;22:109–17. 10.6133/apjcn.2013.22.1.07 [DOI] [PubMed] [Google Scholar]
- 20.Baird DD, Hill MC, Schectman JM, et al. Vitamin D and the risk of uterine fibroids. Epidemiology 2013;24:447–53. 10.1097/EDE.0b013e31828acca0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciebiera M, Włodarczyk M, Ciebiera M. Vitamin D and uterine fibroids—review of the literature and novel concepts. Int J Mol Sci 2018;2051. 10.3390/ijms19072051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Aloisio AA, Baird DD, DeRoo LA, et al. Early-life exposures and early-onset uterine leiomyomata in black women in the sister study. Environ Health Perspect 2012;120:406–12. 10.1289/ehp.1103620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mu X, Du X, Yao K, et al. Association between HSD17B1 rs605059 polymorphisms and the risk of uterine diseases: a systemic review and meta-analysis. Int J Clin Exp Pathol 2015;8:6012–8. [PMC free article] [PubMed] [Google Scholar]
- 24.Bideau VS, Alleyne AT. Leu/Val SNP polymorphism of CYP1B1 and risk of uterine leiomyoma in a black population. Tumor Biol. 2016;37:4035–40. 10.1007/s13277-015-4239-8 [DOI] [PubMed] [Google Scholar]
- 25.Gallagher C, Morton C. Genetic association studies in uterine fibroids: risk alleles presage the path to personalized therapies. Semin Reprod Med 2016;34:235–41. 10.1055/s-0036-1585401 [DOI] [PubMed] [Google Scholar]
- 26.Aissani B, Zhang K, Wiener H. Genetic determinants of uterine fibroid size in the multiethnic NIEHS uterine fibroid study. Int J Mol Epidemiol Genet 2015;6:9–19. [PMC free article] [PubMed] [Google Scholar]
- 27.Ponomarenko I, Reshetnikov E, Polonikov A, et al. Candidate genes for age at menarche are associated with uterine leiomyoma. Front Genet 2020;11:512940. 10.3389/fgene.2020.512940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luoto R, Kaprio J, Rutanen E-M, et al. Heritability and risk factors of uterine fibroids — the finnish twin cohort study. Maturitas 2000;37:15–26. 10.1016/S0378-5122(00)00160-2 [DOI] [PubMed] [Google Scholar]
- 29.Vikhlyaeva EM, Khodzhaeva ZS, Fantschenko ND. Familial predisposition to uterine leiomyomas. Int J Gynaecol Obstet 1995;51:127–31. 10.1016/0020-7292(95)02533-I [DOI] [PubMed] [Google Scholar]
- 30.Hellwege JN, Jeff JM, Wise LA, et al. A multi-stage genome-wide association study of uterine fibroids in African Americans. Hum Genet 2017;136:1363–73. 10.1007/s00439-017-1836-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edwards TL, Michels KA, Hartmann KE, et al. BET1L and TNRC6B associate with uterine fibroid risk among European Americans. Hum Genet 2013;132:943–53. 10.1007/s00439-013-1306-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha P-C, Takahashi A, Hosono N, et al. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat Genet 2011;43:447–50. 10.1038/ng.805 [DOI] [PubMed] [Google Scholar]
- 33.Eggert SL, Huyck KL, Somasundaram P, et al. Genome-wide linkage and association analyses implicate FASN in predisposition to uterine leiomyomata. The American Journal of Human Genetics 2012;91:621–8. 10.1016/j.ajhg.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agboola AD, Bello OO, Olayemi OO. A clinical audit of the patterns of presentations and complications of abdominal myomectomy at the University College Hospital, Ibadan, Nigeria. J Obstet Gynaecol 2021;41:1145–50. 10.1080/01443615.2020.1845632 [DOI] [PubMed] [Google Scholar]
- 35.Yorgancı A, Meydanlı MM, Kadıoğlu N, et al. Incidence and outcome of occult uterine sarcoma: a multi-centre study of 18604 operations performed for presumed uterine leiomyoma. J Gynecol Obstet Hum Reprod 2020;49:101631. 10.1016/j.jogoh.2019.101631 [DOI] [PubMed] [Google Scholar]
- 36.Foth D, Röhl F-W, Friedrich C, et al. Symptoms of uterine myomas: data of an epidemiological study in Germany. Arch Gynecol Obstet 2017;295:415–26. 10.1007/s00404-016-4239-y [DOI] [PubMed] [Google Scholar]
- 37.Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: a systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol 2015;213:141–60. 10.1016/j.ajog.2015.03.019 [DOI] [PubMed] [Google Scholar]
- 38.Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol 2012;206:211.e1–211.e9. 10.1016/j.ajog.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters MDJ, Godfrey C, McInerney P, et al. Chapter 11: Scoping Reviews (2020 version). In: Aromataris E, Munn Z, eds. JBI manual for evidence synthesis. 2020. JBI, 2020. https://synthesismanual.jbi.global [Google Scholar]
- 40.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol 2005;8:19–32. 10.1080/1364557032000119616 [DOI] [Google Scholar]
- 41.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 42.Mohammed A, Shehu S, Ahmed S. Uterine leiomyomata: a five year clinicopathological review in Zaria, Nigeria. Niger J Surg Res 2005;7:206–8. [Google Scholar]
- 43.Eze CU, Odumeru EA, Ochie K, et al. Sonographic assessment of pregnancy co-existing with uterine leiomyoma in Owerri, Nigeria. Afr Health Sci 2013;13:453–60. 10.4314/ahs.v13i2.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oluwole A, Owie E, Babah O. Epidemiology of uterine leiomyomata at the Lagos university teaching hospital, Idi-Araba, Lagos. Nig Hosp Pract 2015;15:14–20. [Google Scholar]
- 45.Awowole IO, Makinde ON, Badejoko OO, et al. Clinical correlates of leiomyoma estrogen and progesterone receptors among nigerian women. Int J Gynaecol Obstet 2016;135:314–8. 10.1016/j.ijgo.2016.06.019 [DOI] [PubMed] [Google Scholar]
- 46.Sarkodie BD, Botwe BO, Adjei DN, et al. Factors associated with uterine fibroid in ghanaian women undergoing pelvic scans with suspected uterine fibroid. Fertility Research and Practice 2016;2:1–7. 10.1186/s40738-016-0022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarkodie BD, Botwe BO, Ofori EK. Uterine fibroid characteristics and sonographic pattern among ghanaian females undergoing pelvic ultrasound scan: a study at 3-major centres. BMC Womens Health 2016;16:1–6. 10.1186/s12905-016-0288-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Egbe TO, Badjang TG, Tchounzou R, et al. Uterine fibroids in pregnancy: prevalence, clinical presentation, associated factors and outcomes at the limbe and buea regional hospitals, cameroon: a cross-sectional study. BMC Res Notes 2018;11:1–6. 10.1186/s13104-018-4007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wango EO, Tabifor HN, Muchiri LW, et al. Progesterone, estradiol and their receptors in leiomyomata and the adjacent normal myometria of black Kenyan women. Afr J Health Sci 2002;9:123–8. 10.4314/ajhs.v9i2.30765 [DOI] [PubMed] [Google Scholar]
- 50.Tiltman AJ. Leiomyomas of the uterine cervix: a study of frequency. Int J Gynecol Pathol 1998;17:231–4. 10.1097/00004347-199807000-00006 [DOI] [PubMed] [Google Scholar]
- 51.Wise LA, Palmer JR, Harlow BL, et al. Reproductive factors, hormonal contraception, and risk of uterine leiomyomata in African-American women: a prospective study. Am J Epidemiol 2004;159:113–23. 10.1093/aje/kwh016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsh EE, Ekpo GE, Cardozo ER, et al. Racial differences in fibroid prevalence and ultrasound findings in asymptomatic young women (18–30 years old): a pilot study. Fertil Steril 2013;99:1951–7. 10.1016/j.fertnstert.2013.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hansen JG, Tang W, Hootman KC, et al. Genetic and environmental factors are associated with serum 25-hydroxyvitamin D concentrations in older African Americans. J Nutr 2015;145:799–805. 10.3945/jn.114.202093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moore KR, Cole SR, Dittmer DP, et al. Self-reported reproductive tract infections and ultrasound diagnosed uterine fibroids in African-American women. J Womens Health 2015;24:489–95. 10.1089/jwh.2014.5051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-052053supp001.pdf (269.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. The data will be made available upon request from the corresponding author.