Abstract
Background
Inconsistent and unequal access to medical care is an issue that predates the COVID19 pandemic, which only worsened the problem. Limited access to care from asthma specialists and other specialists treating comorbid diseases may adversely affect asthma.
Objective
The purpose of this review is to identify health disparities associated with access to care for asthma, and cost-effectiveness of therapies and interventions addressing this health disparity.
Methods
A narrative systematic review was undertaken using MeSH searches of English language articles published in CINAHL, Scopus, or PubMed.
Results
A total of 725 articles were identified. Barriers recognized from the literature included access to diagnostic spirometry, access to specialists, medication formulary restrictions, and issues leading to medical nonadherence. Telemedicine, school-based health care interventions, digital applications, and non–office-based digital spirometry could be used to address these gaps in access to asthma care while potentially being cost-effective.
Conclusion
With the widespread adoption of telemedicine because of the pandemic, and adoption of other mobile services, we now have potential tools that can increase access to asthma care, which can help address this health care inequity. Evidence is limited, but favorable, that some of these tools may be cost-effective.
Key Words: COVID-19, Allergy, Asthma, Access to care, Telemedicine, Asthma cost-effectiveness, Asthma biologics, Health care disparities, Health equity
What is already known about this topic? Asthma costs are substantial. Greater and more equitable patient access to specialist care could lower the costs. Newer health care modes evolved and accelerated during the COVID pandemic may be useful.
What does this article add to our knowledge? We review that new health care modes evolved and accelerated during the COVID pandemic may be useful in closing health care disparities.
How does this study impact current management guidelines? Further research is needed to determine if these new health care tools are cost-effective.
The COVID-19 pandemic has forced health care to innovate including the expansion of telemedicine.1 The management model of allergic disease needs to evolve to allow access to critically necessary therapeutics, to ensure that patient care remains minimally disrupted.2 Before the pandemic, the field of health disparities in other allergic and immunologic diseases was an emerging field, whereas there were already well-established data regarding asthma health disparities and the impact this has on disease diagnosis, management, and outcomes. However, the pandemic has further exposed the morbidity and mortality disparities among poor and minority populations, as recent reviews have highlighted the growing disparities in asthma, including access to care.3 , 4 In this article, we review the role of access to care, adherence with care, and cost/cost burden as major health disparities affecting our ability to provide optimal asthma care, with a focus on the cost-effectiveness of interventions that can increase access in managing asthma.
Methods
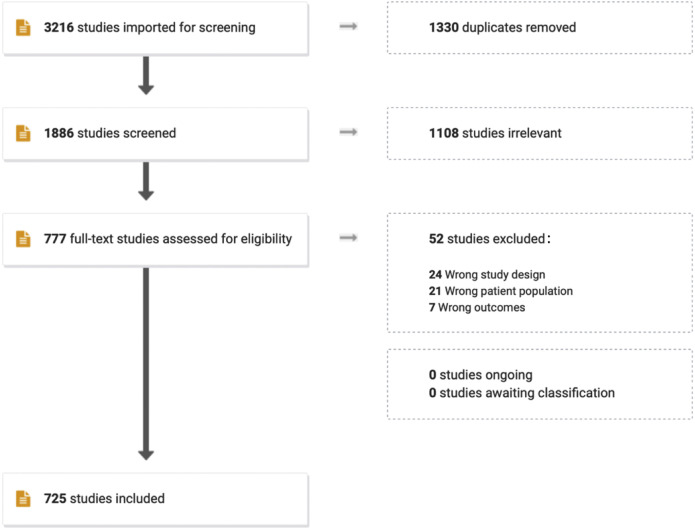
Our objective was to perform a narrative systematic review of the relevant literature regarding health care access and equity for asthma. We undertook a literature search for English language articles published in CINAHL, Scopus, or PubMed. We used the MESH terms (“Asthma”[Mesh] OR asthma∗[tiab]) AND (“Health Services Accessibility”[Mesh] OR “Vulnerable Populations”[Mesh] OR “Healthcare Disparities”[Mesh] OR “health access”[tiab] OR “healthcare access”[tiab] OR “health care access”[tiab] OR “health services access”[tiab] OR “health accessibility”[tiab] OR “healthcare accessibility”[tiab] OR “health care accessibility”[tiab] OR “health services accessibility”[tiab] OR “access to care”[tiab] OR “access to health care”[tiab] OR “access to healthcare”[tiab] OR “access to health services”[tiab] OR “healthcare availability”[tiab] OR “health care availability”[tiab] OR “health services availability”[tiab] OR “health equity”[tiab] OR “healthcare equity”[tiab] OR “health care equity”[tiab] OR “vulnerable population”[tiab] OR “vulnerable populations”[tiab] OR “underserved population”[tiab] OR “underserved populations”[tiab] OR “under-served population”[tiab] OR “under-served populations”[tiab] OR disparit∗[tiab]) AND (“Internet-Based Intervention”[Mesh] OR interven∗[tiab] OR increas∗[tiab] OR expan∗[tiab] OR broaden∗[tiab]). A total of 1886 nonduplicate studies were identified from the 3 databases and downloaded into Covidence (Melbourne, Australia) for review. Reviewers screened the titles and abstracts, and if relevant, were included in a more careful review of the titles and full text. A total of 725 relevant articles were selected, along with additional articles relevant to the topic and cost-effectiveness. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram of the literature search can be found in Figure 1 . No institutional review board approval was needed as reviews are not considered the human subject’s research.
Figure 1.
PRISMA flow diagram of the study selection process.5PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results
The economic burden of asthma
Asthma is a disease associated with significant direct and indirect costs, which have steadily increased over time. A 1992 study calculated the direct cost at $6.2 billion, and a 2011 study estimated the national cost at $56 billion just 20 years later.6 , 7 At an individual level, this direct cost has been estimated to be between $1907 and $3856.8 A more recent estimate, incorporating both direct and indirect costs, of individuals with at least 1 case of treated asthma (eg, 1 medical encounter or pharmaceutical claim associated with asthma in a 12-month period) from Medical Expenditure Panel Survey data between 2008 and 2013 calculated this cost to be $3226 per person and a societal cost of $81.9 billion (in 2015 dollars).9 For the individual burden, there were $1830 in prescription medication costs, $640 in ambulatory visits, $529 in hospitalizations, $176 in hospital-based outpatient visits, and $105 in emergency department (ED) visits. The individual cost burden was not experienced equally. Uninsured persons had direct costs of $2145, and persons living below the poverty line had costs of $3581. Overall, the total direct burden of all medical costs was $50.3 billion, with an additional $29 billion resulting from asthma-related mortality and $3 billion from lost work/school days. The issue is not just the staggering direct and indirect cost expenditures, but the rapid increase in these costs within a decade or two. As noted by Nurmagambetov,9 the more recent estimates accounted for approximately 1 in 3 individuals with current asthma had no asthma-related encounter with a medical provider or pharmacy in 2013. As their analysis was restricted to persons who had a claim/encounter in the prior 12 months of analysis, these cost approximations might be underestimates.9 Table I details how these societal and individual level costs fluctuated during the study period on a year-to-year basis. One of the limitations of this analysis is, again, that this only accounts for persons with access to health care and having a diagnosis code for asthma.
Table I.
Annual prevalence and total medical costs of asthma—United States, 2008-2013
| Year | Prevalence of asthma (%) | No. of people with asthma | Per-person incremental medical cost of asthma ($) | Total medical cost of asthma (billion $) | 95% confidence interval ($) |
|---|---|---|---|---|---|
| Pooled sample | 5.0 | 15,406,570 | 3266 | 50.3 | 32.0-68.7 |
| 2008 | 4.8 | 14,549,170 | 2698 | 39.3 | 21.8-56.7 |
| 2009 | 4.8 | 14,750,374 | 3657 | 53.9 | 25.4-82.5 |
| 2010 | 5.1 | 15,798,988 | 3027 | 47.8 | 27.3-68.4 |
| 2011 | 5.2 | 16,054,089 | 4022 | 64.6 | 46.6-82.5 |
| 2012 | 5.0 | 15,674,493 | 4304 | 67.5 | 40.9-94.1 |
| 2013 | 4.9 | 15,533,522 | 3728 | 57.9 | 28.3-87.6 |
Monetary values are given in 2015 US dollars. The numbers of people with asthma were estimated by the use of personal weights provided in the 2008-2013 Medical Expenditure Panel Survey samples. Confidence intervals were estimated assuming that prevalence and per-person medical cost are independent random variables.
Reprinted with permission of the American Thoracic Society. Copyright © 2021 American Thoracic Society. All rights reserved.
Controlling the sources of the independent variables that are associated with these increased costs (eg, sex, income level, health insurance category, etc), as well as controlling the overall future costs to patients and society, may prove more difficult. One large source of variation is the cost of uncontrolled asthma relative to controlled asthma. A recently published forward-looking cost model explores projected future costs of the burden of uncontrolled asthma over a 20-year horizon (2019-2038).10 Their model incorporates 6 sources to estimate the excess financial burden of uncontrolled asthma over controlled asthma and paints a rather bleak outlook that cost containment will be feasible, mainly attributable to suboptimal disease management. They calculate the direct costs of uncontrolled asthma in the United States increasing modestly from $14.6 billion in 2019 to $15.23 billion in 2038 (increase of 4%), the indirect cost increasing from $32.2 billion in 2019 to $33.5 billion in 2038 (also a 4% increase), and total quality-adjusted life years lost (QALY, the value of a healthy year of life in someone with a medical condition) in this time frame to be 15.46 million QALY (eg, 15.46 healthy years of life lost) from uncontrolled asthma. Altogether, they estimate that poorly controlled asthma will be associated with $963 billion in direct and indirect costs including lost work productivity in the next 20 years, with this cost per patient ranging from $2209 to $6132 depending on where in the United States one lives. Their model further estimated that over this 20-year period, 52% of the 175.3 million patient years with asthma will be associated with uncontrolled asthma, and that 20% of the costs could be saved with more optimal asthma control.10
There are now 6 biologic agents approved for severe asthma to improve asthma control. Biologics for asthma are expensive medications. The cost-effectiveness of these medicines will be predicated by 2 main levers: (1) low wholesale acquisition costs (which may not be realistic to change) and (2) producing a favorable outcome in a short period of time and being able to discontinue therapy quickly, which enhances the value (and cost-effectiveness) of such a product. High-value care could be achieved through the sensible selection of what outcomes to evaluate and the selection of specific populations to access and use these therapies. Knowing when to stop a therapy is also essential when there is marginal to no clear benefit to the outcome of interest in a targeted population. Under certain circumstances, particular choices could become more cost-effective than others. There is a significant knowledge gap in understanding what type of patient will benefit the most from the addition of a specific biologic, which agent to choose from, and how clinicians should evaluate “optimal” response. This is the aim of starting a specific biologic for specific patient endotype or phenotype and following a biomarker. One potential strategy would be to develop cost-effectiveness planes based on patient endotypes (eg, eosinophilic asthma), so that the choice of a therapy such as a biologic has a higher probability of potential response, and hence more value for that choice.11 , 12
Adherence with asthma guidelines
Medication adherence
The 2007 Expert Panel Report 3 (EPR-3) guidelines and the 2020 Focused Update emphasized adherence before stepping up pharmacotherapy.13 , 14 Treatment nonadherence compromises treatment effectiveness and drives up health care costs. The cost of nonadherence was estimated to be $300 billion per year US dollars in 2002.15 Nonadherence results in increased asthma-related urgent care and hospitalization.16 Although changing behavior to improve adherence is difficult, it is not impossible.17 A recent systematic review of medication adherence–enhancing interventions evaluated different strategies.18 The strategies evaluated in the review included educational programs, telephone-aided programs, simplified dosing regimens (eg, once a day compared with twice a day), and programs with a mixture of these strategies. All these strategies were cost-effective after comparing costs and consequences between interventions including increased adherence rate and improved clinical effectiveness.
Another source of increased expenditure is escalation of medication to more expensive options to achieve control in patients struggling with their asthma management. Nonadherent patients are more likely to have poor control, with this poor control making them “eligible” for needing more novel/expensive therapies, such as biologics, leading to an increase in direct and indirect costs.19 Data regarding outcomes with the use of asthma biologics are not reassuring regarding their cost-effectiveness. Anderson and Szefler20 recently reviewed data specifically pertaining to the cost-effectiveness of asthma biologic therapy. They note analysis by the Institute for Clinical and Economic Review for anti-IgE, multiple anti-IL-5 agents, and anti-IL-4 receptor α therapy, which showed that these drugs currently are not cost-effective at current prices, and cite additional studies of omalizumab, reslizumab, and mepolizumab that reached similar conclusions.20 The cost-effectiveness of all the biological agents used in allergic disorders, not just asthma, was also recently reviewed.21
Access and equity to spirometry
The importance of spirometry in the initial diagnosis of asthma was emphasized in box 3-2 of the EPR-3 guidelines, reiterated in the 2020 focused update and in the 2021 Global Initiative of Asthma.13 , 14 , 22 The underuse of spirometry was shown in a 1999 cross-sectional survey of primary care physicians in the Chicago area; only 54.6% of primary care physicians used spirometry as part of initial evaluation of asthma.23 As part of a diagnostic algorithm in the cohort model of 10,000 patients, spirometry and/or methacholine challenge testing was estimated to remove the diagnosis of asthma in 3366 subjects.24 In those whose asthma diagnosis was removed, spirometry and/or methacholine challenge testing could potentially save $36.26 million. In this model, the diagnostic algorithm was the dominant strategy (less costly and better outcomes) in 99% of simulations. Not only is spirometry underused, but it is also not equally used across at-risk groups. Among children 7 years or older with newly diagnosed asthma, the children in lower socioeconomic status quintile were less likely to have spirometry testing than those in the highest socioeconomic status quintile.25 This underuse of spirometry can lead to misdiagnosis of asthma. In a group of adults with frequent severe exacerbations of physician-diagnosed asthma or physician-diagnosed chronic obstructive pulmonary disease (COPD), spirometry was significantly underused among those with physician-diagnosed asthma compared with those with physician-diagnosed COPD (28% compared with 41%; P < .001).26 In the final multivariable model, spirometry underuse was an independent risk factor for misdiagnosis of physician-diagnosed asthma and physician-diagnosed COPD. Together these studies highlight the importance of adhering to guidelines, especially for individuals of lower socioeconomic status, and use of an objective measure of lung function to accurately diagnose asthma that can then reduce prescription of unnecessary medications.
Given these present and future projected costs, there is urgency for interventions that can reduce costs while effectively managing disease. One such method would be, as previously noted, a validated algorithm for asthma diagnosis, to reduce the costs associated with a false-positive asthma diagnosis. Yaghoubi et al24 have proposed an algorithm to diagnose asthma in adults (patients ≥15 years old) using objective testing methods (stepwise use of spirometry with a bronchodilator and methacholine challenge if there is less than 12% change in forced expiratory volume in 1 second [FEV1]). Using a simulated cohort of 10,000 adult patients with self-reported asthma who underwent stepwise evaluation using this algorithm (vs the current standard of care), use of the algorithm approach removed the diagnosis of asthma in one-third of these patients and was associated with an estimated cost savings of $36.26 million in direct costs over a 20-year horizon and a gain of 4049 QALY (eg, healthy years of life), concluding that even more savings could be actualized if this model were extrapolated to the US adult population.24
Given the potential cost savings of such an algorithm, there are additional interventions that can increase the use of spirometry, the first step of the cost-saving algorithm. Bender et al27 used an asthma toolkit bootcamp which provided intensive training to rural clinicians on the NHLBI asthma guidelines for children, including a focus on increasing use of spirometry, action plans, use of the asthma control test, and a team-based approach in clinic. Each participating practice was given a spirometer and training. This intervention, performed in rural Colorado (USA), increased spirometry rates 4-fold and doubled the rate of both severity assessments and use of asthma action plans. Moreover, this resulted in a 10% decrease in ED visits, a 35% decrease in asthma-related hospitalizations, and a 29% decrease in oral glucocorticoid prescriptions using Medicaid claims data as the main utilization source. Nonparticipating clinics showed no reduction in any asthma-related health care utilization.27 Unfortunately, economic modeling was not performed to demonstrate the cost-effectiveness of this program.
Access and equity to specialists
When one considers care of asthmatic patients, beyond need for access to asthma specialists, there is a need to consider access to other medical subspecialties. The American Thoracic Society (ATS)-European Respiratory Society (ERS) guidelines for severe asthma emphasize the evaluation and treatment of the comorbid illness such as sinusitis, gastroesophageal reflux disease, sleep-disordered breathing, and vocal cord dysfunction.28 Appropriate management of asthma comorbidities is important in both children and adults.29 , 30 Unfortunately, not all patients have easy access to these specialists. There may be geographic impediments to being evaluated by any of the specialists of treating these comorbid conditions. In Taiwan, rural patients in need of evaluation for gastrointestinal disorders had lower access to specialists compared with urban patients.31 This geographic disparity is not unique to gastroenterologists or Taiwan, but also access to otolaryngologist in the United States. A recent population study investigated the distribution of otolaryngologists within the State of Illinois.32 In a state where there are 276 otolaryngologists registered with the American Academy of Otolaryngology, 151 were located within a single county (Cook County), a large central metropolitan county, equating to 2.91 otolaryngologists per 100,000 population. In contrast, there were only 11 otolaryngologists practicing among the 62 nonmetropolitan counties, equating to 0.72 otolaryngologists per 100,000 population. These disparities to access other medical specialists are in addition to the access to asthma specialists, including allergists. Access to an asthma specialist was significantly associated with severe pediatric asthma ED visits.33 In addition, urban minority children are more likely to have no access to an asthma specialist.34 Clearly, there is much work to be done on improving access to specialties who treat comorbidities of asthma and allergies, including allergists and immunologists.
Restricted and changing formularies
Access to specialists is just one aspect of improving care. Accessing medications, especially those medications that are preferred by the individual patient, is another aspect. Switching a patient’s preferred inhaled controller to a less-expensive formulary inhaler is associated with an impaired inhaler technique, reduced asthma control, and reduced quality of life, whereas it is associated with increased use of health care resources and greater use of unsuccessful treatment.35 A retrospective chart review of a group of Medicaid children 6 to 18 years old before and after a formulary change demonstrated that payor-initiated inhaled corticosteroids switching led to a decrease in percent predicted FEV1 and peak expiratory flow rate among children.36 Among adult asthma and/or COPD patients, enrolled in Medicare Part D, who were receiving budesonide/formoterol but were later switched to another controller, there was on average a 4-month gap in care. In addition, of those patients who experienced a gap in care, 47% of patients filled a prescription for either an oral systemic corticosteroid or an antibiotic, indicating reduced control of their disease.37 High-deductible health plans often use preventive drug lists that patients may receive at low, or no cost, before meeting their deductible. When preventive drug lists were used as part of high-deductible health plans, members reported difficulty affording nonmedication costs, including ED visits, hospitalizations, diagnostic testing (eg, spirometry), and access to specialists.38 These findings suggest that the cost associated with therapeutic substitutions can impact adherence and the affordability of health care.
Available and emerging solutions
One of the ways we could possibly improve health care equity is by improving health care access. Alternative delivery systems can be avenues to improve access.
Digital platforms
Telemedicine
One of the consequences of the COVID-19 pandemic was that it brought telemedicine services to many more people, and this is a vehicle to improve health care access. Telemedicine and its role since the onset of COVID-19 pandemic has been thoroughly evaluated, including some of the benefits and cautions (eg, coding, licensing, liability) in a recent American Academy of Allergy, Asthma & Immunology work group report.39 A recent systematic review of pediatric randomized controlled trials across a range of specialties concluded that telemedicine may be able to deliver comparable, or better, services compared with in-person visits.40 Telemedicine demonstrated the ability to reduce no-show rates even for mobile asthma vans.41 In a quality-improvement project of a mobile asthma van that evaluates underserved pediatric asthmatics at their school, before implementation of telehealth, the mean no-show rate was 36%. After implementation of video-conference capability that allowed off-site parent input to talk to the clinical team in addition to on-site patient examination, the no-show rate decreased to 7.9% to 18%. The ability to increase access without any reduced quality indicates that telemedicine could be a useful tool.
School-based interventions
In-person school-based interventions
Not only does the study by Van Houten41 highlight the role of telemedicine, but it also highlights the utility of having a clinician’s presence in the pediatric asthmatic’s school. A study by Weber42 investigated how the presence of a school-based health center (SBHC) in inner-city Bronx affected asthma outcomes. Asthmatic children attending a school without an SBHC were more likely to have been hospitalized for asthma (17.1%) compared with asthmatic children attending elementary school with an SBHC (10.5%) (relative risk: 1.6; 95% confidence interval: 1.2-2.3). In addition, asthmatic children attending a school without an SBHC had 3 more absentee days compared with children attending a school with an SBHC (21.3 [15.4] to 18.2 [13], mean [standard deviation]; P = .02). These SBHCs have potential to do much more. In a systematic review, Knopf et al43 found that these SBHCs can improve educational outcomes (eg, reducing rates of suspension and noncompletion while also improving grade point averages and grade promotion) and asthma health care outcomes (eg, reducing asthma symptoms, asthma-related ED visits, and hospitalizations).42, 44, 45, 43 Not only can these SBHCs help reduce health care disparities, they can also be cost-beneficial.46 In a study of schools with or without SBHCs in Greater Cincinnati, asthmatic students from schools with SBHCs had a lower risk of hospitalization and ED visits compared with students attending a school without an SBHC. The potential cost savings for hospitalization was estimated at $970 per student with asthma. With greater primary care access, the SBHCs were able to reduce health care disparities among Black patients and those with disabilities overall. Thus, SBHC could be a way to improve overall health care access, reduce asthma disparities, and reduce costs.
A potentially cost-effective intervention is the expansion of the asthma “Breathmobile,” a mobile asthma clinic aimed to provide care in underserved areas/populations. Bollinger et al47 investigated the cost saving of the use of the Breathmobile program over a 5-year period in Baltimore, MD (USA), whereby children were evaluated at school every 4 to 12 weeks depending on their asthma severity. Use of the Breathmobile was associated with a significant increase in symptom-free days, resulting in a mean increase in 44 symptom-free days in a single year, at a cost savings of $79/symptom-free day. The cost savings per symptom-free day was $116 among the 5- to 11-year-old population and $126 among those with intermittent asthma. Morphew et al48 performed a retrospective study of the use of 4 Breathmobile programs in Southern California (USA) over a single-year period, encompassing 15,986 patients and 88,865 visits in 2008-2009. This program provided an overall return on investment of $6.73 per $1 spent, and $24,381,000 for the total QALYs saved (343.3 QALY saved at $70,000 per healthy year of life).
Telemedicine school-based Interventions
The combination of telemedicine within a school setting could also be a powerful partnership. A school-based telemedicine-enhanced asthma management program in an elementary school in Rochester, New York, increased the number of symptom-free days and reduced asthma-related health care utilization.49 Although this result in an urban setting is encouraging, in a rural setting, a school-based asthma education program delivered by telemedicine did not yield significant differences in symptom-free days.50 A review of telemedicine school-based interventions noted the difference in design, population, and lack of primary care involvement for the conflicting results. Perry and Turner concluded that future, larger scale randomized trials with primary care and specialists are needed to accurately assess the efficacy of these programs.51
Mobile digital applications
Digital applications
With the impact of the COVID-19 pandemic ongoing and the shift toward increasing use of mobile health systems and telehealth, digital applications are increasingly valuable health care delivery tools. However, evidence is limited study as to the potential value and cost savings of these digital applications, though that may change given the shifts in delivery in 2020-2021. In 2015, de la Torre-Dietz et al52 performed a systematic review of cost-effectiveness studies of electronic/mobile health and telemedicine, noting 35 relevant studies between 1998 and 2013, the majority of which focused on the cost-effectiveness of telemedicine for various disease states and reported that outpatient pulmonary subspecialty telehealth for rural populations was a cost-effective alternative. However, there were no specific asthma-focused studies identified, and overall there were few studies identified investigating this issue.52 A recent American Academy of Allergy, Asthma & Immunology workgroup report scoping review by Mosnaim et al53 regarding digital health technology in asthma identified 121 articles detailing digital asthma interventions, but this did not investigate the cost-effectiveness of these interventions, though their review gives hope that there are enough interventions to potentially evaluate such cost-effectiveness.
Digital applications may have role in the post-COVID allergy clinic for disease management. Here the evidence for the role is limited but evolving. A digital application for asthma management, called AsthmaCare, which sends reminders to patients to take controller medications and records if they used their controller or had symptoms requiring rescue medication. While the AsthmaCare application improved asthma-related urgent care visits, it did not improve healthcare asthma-related emergency department visits, hospitalizations or total healthcare utilization compared to online asthma information from tertiary university.54 The authors note that this study may have been underpowered to see any change in total health care utilization that approached statistical significance (P = .07). As discussed in a recent review, although there is potential, more research is needed for mobile digital applications to demonstrate effectiveness.55 , 56
Poor asthma control is a potentially modifiable source of high costs, as poor asthma control leads to the use of more expensive medications as well as ED and hospital visit utilization. In a single-arm observational study sponsored by a digital health technology company, Merchant et al57 observed the effect of patients enrolled in a digital health intervention, consisting of an electronic medication monitor tracking rescue and controller use as markers of asthma-related health care utilization. They found that patients enrolled in this intervention had a significant decrease in asthma-related ED visits, hospitalizations, use of short-acting β–agonists (SABA), and an improvement of controller to total medication (number of controller medication puffs recorded divided by the total number of puffs of SABA plus controller medications, with lower numbers reflecting lower rescue medication utilization, a surrogate for better control).57 The authors concluded that such an intervention could help with medication adherence, leading to lower costs through reduced overall expenditures.
Mobile spirometry
Digital applications can serve to extend diagnostic tests that are commonly performed within the clinic. This need for the out-of-office diagnostics was highlighted during the COVID-19 pandemic. Some have suggested that the pandemic has focused on alternatives to office-based spirometry that may not just replace but also improve open existing tests.58 For example, a smartphone-based spirometer has been developed that meets the ATS-ERS recommendations for spirometry performance.59 , 60 As more of these diagnostic tools are developed, clinicians can expand access to these diagnostic tests. A recent study, however, does bring this into question, demonstrating poor agreement between home FEV1 trough measurements in comparison to the trough FEV1 measured in the clinic setting.61 Further research is needed, but likely such home testing will have greater uptake in the future.
Conclusions
Access to medical care is not a temporary problem, but a chronic problem for many patients. The barrier to medical care is not uniform. As can be seen in the example of asthma, impeded access to care, both to asthma specialists and physicians specializing in potential comorbid diseases that may be impacting the asthma, translates to escalation in health care utilization in an already strapped system. Decreased utilization from poor access can lead to uncontrolled asthma, escalating the economic burden of disease, and may necessitate use of more expensive management tools, which results in a vicious cycle of ineffective and expensive provision of care. Ample and broad access to cost-effective management strategies is a way to break this cycle. Recognizing the relationship between how poor access influences high costs and necessitates more expensive management strategies is paramount in understanding how cost of care presents an enormous health disparity and one that can leverage access to care, an equally problematic disparity. With the widespread adoption of telemedicine and digital applications, we now have tools that can increase access to care when in-office visits are not possible (eg, during a pandemic), or in underserved areas and populations. These may be very valuable tools to help close the access and equity gap, and control costs through better management of asthma. The cost-effectiveness of these interventions and therapies will need to be determined, as will more stable regulations regarding reimbursement and licensure, but such tools could be game changers for high-risk populations. Although there are not many positive examples of how the pandemic helped evolve health care, innovative models of health care delivery, including telemedicine, may be the rare example of a silver lining to emerge that can address barriers to an equitable and accessible health care system.
Footnotes
No funding was received for this work.
Conflicts of interest: M. Greenhawt is a consultant for Aquestive; is a member of physician/medical advisory boards for DBV Technologies, Sanofi/Regeneron, Genentech, Nutricia, Novartis, Acquestive, Allergy Therapeutics, Pfizer, US World Meds, Allergenis, Aravax, and Prota, all unrelated to vaccines/vaccine development; is a member of the scientific advisory council for the National Peanut Board; is an associate editor for the Annals of Allergy, Asthma, and Immunology; and is a member of the Joint Taskforce on Allergy Practice Parameters. He has received honorarium for lectures from ImSci and MedLearningGroup. J. Oppenheimer reports serving in the research/adjudication for AstraZeneca (unrelated to vaccines/vaccine development), GSK, Sanofi, and Novartis; is a consultant for GSK, AstraZeneca (unrelated to vaccines/vaccine development), and Sanofi; is a consultant for Aquestive and Aimmune; is an associate editor for the Annals of Allergy Asthma Immunology and AllergyWatch; is a section editor for the Current Opinion of Allergy; receives royalties from Up to Date; and is Board Liaison American Board of Internal Medicine for the American Board of Allergy and Immunology. C. D. Codispoti declares that he has no relevant conflicts of interest.
References
- 1.Mann D.M., Chen J., Chunara R., Testa P.A., Nov O. COVID-19 transforms health care through telemedicine: evidence from the field. J Am Med Inform Assoc. 2020;27:1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malipiero G., Heffler E., Pelaia C., Puggioni F., Racca F., Ferri S., et al. Allergy clinics in times of the SARS-CoV-2 pandemic: an integrated model. Clin Transl Allergy. 2020;10:23. doi: 10.1186/s13601-020-00333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogbogu P.U., Capers Q., 4th, Apter A.J. Disparities in asthma and allergy care: what can we do? J Allergy Clin Immunol Pract. 2021;9:663–669. doi: 10.1016/j.jaip.2020.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Davis C.M., Apter A.J., Casillas A., Foggs M.B., Louisias M., Morris E.C., et al. Health disparities in allergic and immunologic conditions in racial and ethnic underserved populations: a work group report of the AAAAI committee on the underserved. J Allergy Clin Immunol. 2021;147:1579–1593. doi: 10.1016/j.jaci.2021.02.034. [DOI] [PubMed] [Google Scholar]
- 5.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss K.B., Gergen P.J., Hodgson T.A. An economic evaluation of asthma in the United States. N Engl J Med. 1992;326:862–866. doi: 10.1056/NEJM199203263261304. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan P.W., Ghushchyan V.H., Slejko J.F., Belozeroff V., Globe D.R., Lin S.L. The burden of adult asthma in the United States: evidence from the Medical Expenditure Panel Survey. J Allergy Clin Immunol. 2011;127:363–369.e1-3. doi: 10.1016/j.jaci.2010.10.042. [DOI] [PubMed] [Google Scholar]
- 8.Barnett S.B., Nurmagambetov T.A. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol. 2011;127:145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Nurmagambetov T., Kuwahara R., Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15:348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 10.Yaghoubi M., Adibi A., Safari A., FitzGerald J.M., Sadatsafavi M. The projected economic and health burden of uncontrolled asthma in the United States. Am J Respir Crit Care Med. 2019;200:1102–1112. doi: 10.1164/rccm.201901-0016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Draikiwicz S., Oppenheimer J. Use of biological agents in asthma: pharmacoeconomic lessons learned from omalizumab. Chest. 2017;151:249–251. doi: 10.1016/j.chest.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Barnes P.J. COUNTERPOINT: Will new anti-eosinophilic drugs be useful in asthma management? No. Chest. 2017;151:17–20. doi: 10.1016/j.chest.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 13.National Asthma Education and Prevention Program Expert Panel Report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Expert Panel Working Group of the National Heart, Lung, and Blood Institute (NHLBI) administered and coordinated National Asthma Education and Prevention Program Coordinating Committee (NAEPPCC) Cloutier M.M., Baptist A.P., Blake K.V., Brooks E.G., Bryant-Stephens T. 2020 focused updates to the asthma management guidelines: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. J Allergy Clin Immunol. 2020;146:1217–1270. doi: 10.1016/j.jaci.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DiMatteo M.R. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 16.Bender B.G., Rand C. Medication non-adherence and asthma treatment cost. Curr Opin Allergy Clin Immunol. 2004;4:191–195. doi: 10.1097/00130832-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Normansell R., Kew K.M., Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database Syst Rev. 2017;4:CD012226. doi: 10.1002/14651858.CD012226.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaw S.M., Li S.C., Mohd Tahir N.A. A systematic review of the cost-effectiveness of medication adherence-enhancing intervention for asthma. J Asthma. 2022;59:697–711. doi: 10.1080/02770903.2021.1875483. [DOI] [PubMed] [Google Scholar]
- 19.Lee J., Tay T.R., Radhakrishna N., Hore-Lacy F., Mackay A., Hoy R., et al. Nonadherence in the era of severe asthma biologics and thermoplasty. Eur Respir J. 2018;51:1701836. doi: 10.1183/13993003.01836-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson W.C., 3rd, Szefler S.J. Cost-effectiveness and comparative effectiveness of biologic therapy for asthma: to biologic or not to biologic? Ann Allergy Asthma Immunol. 2019;122:367–372. doi: 10.1016/j.anai.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Wu A.C., Fuhlbrigge A.L., Robayo M.A., Shaker M. Cost-effectiveness of biologics for allergic diseases. J Allergy Clin Immunol Pract. 2021;9:1107–1117.e2. doi: 10.1016/j.jaip.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Global Initiative for Asthma. Global strategy for asthma management and prevention. Accessed January 2, 2022. https://www.ginaasthma.org/
- 23.Grant E.N., Moy J.N., Turner-Roan K., Daugherty S.R., Weiss K.B. Asthma care practices, perceptions, and beliefs of Chicago-area primary-care physicians. Chicago Asthma Surveillance Initiative Project Team. Chest. 1999;116(Suppl 1):145S–1454S. doi: 10.1378/chest.116.suppl_2.145s. [DOI] [PubMed] [Google Scholar]
- 24.Yaghoubi M., Adibi A., Zafari Z., FitzGerald J.M., Aaron S.D., Johnson K.M., et al. Cost-effectiveness of implementing objective diagnostic verification of asthma in the United States. J Allergy Clin Immunol. 2020;145:1367–1377.e4. doi: 10.1016/j.jaci.2019.11.038. [DOI] [PubMed] [Google Scholar]
- 25.Gershon A.S., Victor J.C., Guan J., Aaron S.D., To T. Pulmonary function testing in the diagnosis of asthma: a population study. Chest. 2012;141:1190–1196. doi: 10.1378/chest.11-0831. [DOI] [PubMed] [Google Scholar]
- 26.Jain V.V., Allison D.R., Andrews S., Mejia J., Mills P.K., Peterson M.W. Misdiagnosis among frequent exacerbators of clinically diagnosed asthma and COPD in absence of confirmation of airflow obstruction. Lung. 2015;193:505–512. doi: 10.1007/s00408-015-9734-6. [DOI] [PubMed] [Google Scholar]
- 27.Bender B.G., Simmons B., Konkoly N., Liu A.H. The Asthma Toolkit Bootcamp to improve rural primary care for pediatric asthma. J Allergy Clin Immunol Pract. 2021;9:3091–3097.e1. doi: 10.1016/j.jaip.2021.03.058. [DOI] [PubMed] [Google Scholar]
- 28.Chung K.F., Wenzel S.E., Brozek J.L., Bush A., Castro M., Sterk P.J., et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 29.Kercsmar C.M., Shipp C. Management/comorbidities of school-aged children with asthma. Immunol Allergy Clin North Am. 2019;39:191–204. doi: 10.1016/j.iac.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Rogliani P., Sforza M., Calzetta L. The impact of comorbidities on severe asthma. Curr Opin Pulm Med. 2020;26:47–55. doi: 10.1097/MCP.0000000000000640. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y.H., Tseng Y.H., Chen Y.C., Lin M.H., Chou L.F., Chen T.J., et al. The rural-urban divide in ambulatory care of gastrointestinal diseases in Taiwan. BMC Int Health Hum Rights. 2013;13:15. doi: 10.1186/1472-698X-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urban M.J., Wojcik C., Eggerstedt M., Jagasia A.J. Rural-urban disparities in otolaryngology: the State of Illinois. Laryngoscope. 2021;131:E70–E75. doi: 10.1002/lary.28652. [DOI] [PubMed] [Google Scholar]
- 33.Garcia E., Serban N., Swann J., Fitzpatrick A. The effect of geographic access on severe health outcomes for pediatric asthma. J Allergy Clin Immunol. 2015;136:610–618. doi: 10.1016/j.jaci.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Flores G., Snowden-Bridon C., Torres S., Perez R., Walter T., Brotanek J., et al. Urban minority children with asthma: substantial morbidity, compromised quality and access to specialists, and the importance of poverty and specialty care. J Asthma. 2009;46:392–398. doi: 10.1080/02770900802712971. [DOI] [PubMed] [Google Scholar]
- 35.Bjermer L. The importance of continuity in inhaler device choice for asthma and chronic obstructive pulmonary disease. Respiration. 2014;88:346–352. doi: 10.1159/000363771. [DOI] [PubMed] [Google Scholar]
- 36.Bickel S., Morton R., O’Hagan A., Canal C., Sayat J., Eid N. Impact of payor-initiated switching of inhaled corticosteroids on lung function. J Pediatr. 2021;234:128–133.e1. doi: 10.1016/j.jpeds.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert I., Aslam Mahmood A., Devane K., Tan L. Association of nonmedical switches in inhaled respiratory medications with disruptions in care: a retrospective prescription claims database analysis. Pulm Ther. 2021;7:189–201. doi: 10.1007/s41030-021-00147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilkey M.B., Cripps L.A., Gruver R.S., Washington D.V., Galbraith A.A. Preventive drug lists as tools for managing asthma medication costs. Am J Manag Care. 2020;26:75–79. doi: 10.37765/ajmc.2020.42396. [DOI] [PubMed] [Google Scholar]
- 39.Hare N., Bansal P., Bajowala S.S., Abramson S.L., Chervinskiy S., Corriel R., et al. Work group report: COVID-19: unmasking telemedicine. J Allergy Clin Immunol Pract. 2020;8:2461–2473.e3. doi: 10.1016/j.jaip.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah A.C., Badawy S.M. Telemedicine in pediatrics: systematic review of randomized controlled trials. JMIR Pediatr Parent. 2021;4 doi: 10.2196/22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Houten L., Deegan K., Siemer M., Walsh S. A telehealth initiative to decrease no-show rates in a pediatric asthma mobile clinic. J Pediatr Nurs. 2021;59:143–150. doi: 10.1016/j.pedn.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Webber M.P., Carpiniello K.E., Oruwariye T., Lo Y., Burton W.B., Appel D.K. Burden of asthma in inner-city elementary schoolchildren: do school-based health centers make a difference? Arch Pediatr Adolesc Med. 2003;157:125–129. doi: 10.1001/archpedi.157.2.125. [DOI] [PubMed] [Google Scholar]
- 43.Knopf J.A., Finnie R.K., Peng Y., Hahn R.A., Truman B.I., Vernon-Smiley M., et al. School-based health centers to advance health equity: a community guide systematic review. Am J Prev Med. 2016;51:114–126. doi: 10.1016/j.amepre.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lurie N., Bauer E.J., Brady C. Asthma outcomes at an inner-city school-based health center. J Sch Health. 2001;71:9–16. doi: 10.1111/j.1746-1561.2001.tb06481.x. [DOI] [PubMed] [Google Scholar]
- 45.Guo J.J., Jang R., Keller K.N., McCracken A.L., Pan W., Cluxton R.J. Impact of school-based health centers on children with asthma. J Adolesc Health. 2005;37:266–274. doi: 10.1016/j.jadohealth.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Guo J.J., Wade T.J., Pan W., Keller K.N. School-based health centers: cost-benefit analysis and impact on health care disparities. Am J Public Health. 2010;100:1617–1623. doi: 10.2105/AJPH.2009.185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bollinger M.E., Morphew T., Mullins C.D. The Breathmobile program: a good investment for underserved children with asthma. Ann Allergy Asthma Immunol. 2010;105:274–281. doi: 10.1016/j.anai.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 48.Morphew T., Altamirano W., Bassin S.L., Galant S.P. The Breathmobile improves the asthma medication ratio and decreases emergency department utilization. Am J Manag Care. 2017;23 [PubMed] [Google Scholar]
- 49.Halterman J.S., Fagnano M., Tajon R.S., Tremblay P., Wang H., Butz A., et al. Effect of the school-based telemedicine enhanced asthma management (SB-TEAM) program on asthma morbidity: a randomized clinical trial. JAMA Pediatr. 2018;172 doi: 10.1001/jamapediatrics.2017.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perry T.T., Halterman J.S., Brown R.H., Luo C., Randle S.M., Hunter C.R., et al. Results of an asthma education program delivered via telemedicine in rural schools. Ann Allergy Asthma Immunol. 2018;120:401–408. doi: 10.1016/j.anai.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry T.T., Turner J.H. School-based telemedicine for asthma management. J Allergy Clin Immunol Pract. 2019;7:2524–2532. doi: 10.1016/j.jaip.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 52.de la Torre-Diez I., Lopez-Coronado M., Vaca C., Aguado J.S., de Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. 2015;21:81–85. doi: 10.1089/tmj.2014.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosnaim G., Safioti G., Brown R., DePietro M., Szefler S.J., Lang D.M., et al. Digital health technology in asthma: a comprehensive scoping review. J Allergy Clin Immunol Pract. 2021;9:2377–2398. doi: 10.1016/j.jaip.2021.02.028. [DOI] [PubMed] [Google Scholar]
- 54.Stukus D.R., Farooqui N., Strothman K., Ryan K., Zhao S., Stevens J.H., et al. Real-world evaluation of a mobile health application in children with asthma. Ann Allergy Asthma Immunol. 2018;120:395–400.e1. doi: 10.1016/j.anai.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 55.Himes B.E., Leszinsky L., Walsh R., Hepner H., Wu A.C. Mobile health and inhaler-based monitoring devices for asthma management. J Allergy Clin Immunol Pract. 2019;7:2535–2543. doi: 10.1016/j.jaip.2019.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Licari A., Ferrante G., Marseglia Md G.L., Corsello Md G., La Grutta S. What is the impact of innovative electronic health interventions in improving treatment adherence in asthma? The pediatric perspective. J Allergy Clin Immunol Pract. 2019;7:2574–2579. doi: 10.1016/j.jaip.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Merchant R., Szefler S.J., Bender B.G., Tuffli M., Barrett M.A., Gondalia R., et al. Impact of a digital health intervention on asthma resource utilization. World Allergy Organ J. 2018;11:28. doi: 10.1186/s40413-018-0209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kouri A., Gupta S., Yadollahi A., Ryan C.M., Gershon A.S., To T., et al. Addressing reduced laboratory-based pulmonary function testing during a pandemic. Chest. 2020;158:2502–2510. doi: 10.1016/j.chest.2020.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou P., Yang L., Huang Y.X. A smart phone based handheld wireless spirometer with functions and precision comparable to laboratory spirometers. Sensors (Basel) 2019;19:2487. doi: 10.3390/s19112487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller M.R., Hankinson J., Brusasco V., Burgos F., Casaburi R., Coates A., et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 61.Gardiner F., Pizzichini E., Bailes Z., Peachey G., Zarakaite A., Chaudhuri R., et al. A comparison of clinic versus home spirometry in the CAPTAIN study. Am J Respir Crit Care Med. 2021;203:A1348. [Google Scholar]