Abstract
Objectives:
Increased inflammation and myocardial injury can be observed in the absence of myocardial infarction or obstructive coronary artery disease (CAD). We determined whether biomarkers of inflammation - interleukin 6 (IL-6) and myocardial injury - high-sensitivity troponin (hs-cTn) were associated with the presence and extent of CAD and were independent predictors of major adverse cardiovascular events (MACE) in stable chest pain.
Methods:
Using participants from the PROMISE trial, we measured hs-cTn I and IL-6 concentrations and analyzed CTA images in the core laboratory for CAD characteristics: significant stenosis (≥70%), high-risk plaque (HRP), CAD-RADS categories, segment involvement score (SIS), and coronary artery calcium (CAC) score. The primary endpoint was a composite MACE (death, myocardial infarction, or unstable angina).
Results:
We included 1796 participants (age 60.2±8.0 years, men 47.5%, median follow-up 25 months). In multivariable linear regression adjusted for atherosclerotic cardiovascular disease risk (ASCVD), hs-cTn was associated with HRP, stenosis, CAD-RADS, and SIS. IL-6 was only associated with stenosis and CAD-RADS. Hs-cTn above median (1.5 ng/l) was associated with MACE in univariable analysis (HR 2.1, 95%CI 1.3–3.6, p=0.006), but not in multivariable analysis adjusted for ASCVD and CAD. IL-6 above median (1.8 ng/l) was associated with MACE in multivariable analysis adjusted for ASCVD and HRP (HR 1.9, 95%CI 1.1–3.3, p=0.03), CAC (HR 1.9, 95%CI 1.0–3.4, p=0.04), and SIS (HR 1.8, 95%CI 1.0–3.2, p=0.04), but not for stenosis or CAD-RADS. In participants with non-obstructive CAD (stenosis 1–69%), the presence of both hs-cTn and IL-6 above median was strongly associated with MACE (HR 2.5–2.7 after adjustment for CAD characteristics).
Conclusions:
Concentrations of hs-cTn and IL-6 were associated with CAD characteristics and MACE indicating that myocardial injury and inflammation may each contribute to pathways in CAD pathophysiology. This association was most pronounced among participants with non-obstructive CAD representing an opportunity to tailor treatment in this at-risk group.
Keywords: coronary atherosclerotic plaque, biomarkers, high-sensitivity cardiac troponin, interleukin-6, computed tomography angiography
Introduction
Evaluation of patients with symptoms suggestive of obstructive coronary artery disease (CAD) is one of the most common clinical scenarios in outpatient practices. In the United States, new stable angina is diagnosed in 565,000 patients annually.(1) Non-invasive evaluation with functional stress testing and coronary computed tomography angiography (CTA) are cornerstones for the diagnosis of obstructive CAD and risk stratification for future major adverse cardiovascular events (MACE).(2,3) In addition to detection of ischemia and obstructive CAD, coronary plaque characteristics and CAD burden can refine prediction of MACE when evaluated by coronary CTA.(4–7) During follow-up, the majority of MACE occurs in patients with non-obstructive CAD.(7,8) Further risk stratification in this subgroup of patients is therefore of utmost importance.
Circulating biomarkers of myocardial injury have been traditionally used in the evaluation of patients with suspected acute coronary syndrome.(9) More recently, circulating biomarkers of myocardial injury (e.g. high-sensitivity troponin – hs-cTn) and inflammation (e.g. interleukin 6 – IL-6) have been associated with CAD and MACE in large epidemiologic cohorts and symptomatic patients.(10–21) However, the association of these biomarkers with detailed assessment of coronary atherosclerosis, including high-risk plaque and various measures of CAD burden, has not been reported. Furthermore, prior studies have not explored whether the predictive value of circulating biomarkers is independent of atherosclerotic cardiovascular disease (ASCVD) risk score and CAD characteristics, which might indicate different mechanisms of pathophysiologic involvement. Therefore, we determined whether hs-cTn and IL-6 were independent predictors of MACE and how they were associated with the presence and extent of CAD in stable chest pain population in the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial.
Methods
Study design and population
PROMISE was a pragmatic comparative effectiveness trial that enrolled 10,003 stable symptomatic outpatients without known CAD who required noninvasive cardiovascular testing at 193 sites in North America between July 2010 and September 2013.(2) We randomly assigned patients who provided written informed consent to either the CTA or the functional testing. We documented patient demographics, cardiovascular risk factors and atherosclerotic cardiovascular disease (ASCVD) risk scores at the time of enrollment. Patients were followed up for a minimum of 1 year after randomization. Local or central institutional review boards approved the study.
In our cohort study nested in the original PROMISE trial, we included all patients who were randomized to the CTA arm, received the initial diagnostic test as randomized, and had blood samples collected for the measurements of circulating biomarkers. We excluded subjects who received other tests as their first test, did not undergo any diagnostic test, received non-contrast CT only, for whom coronary CTA datasets were not available or were of non-diagnostic quality (Figure 1).
Figure 1. Patient Inclusions and Exclusions.
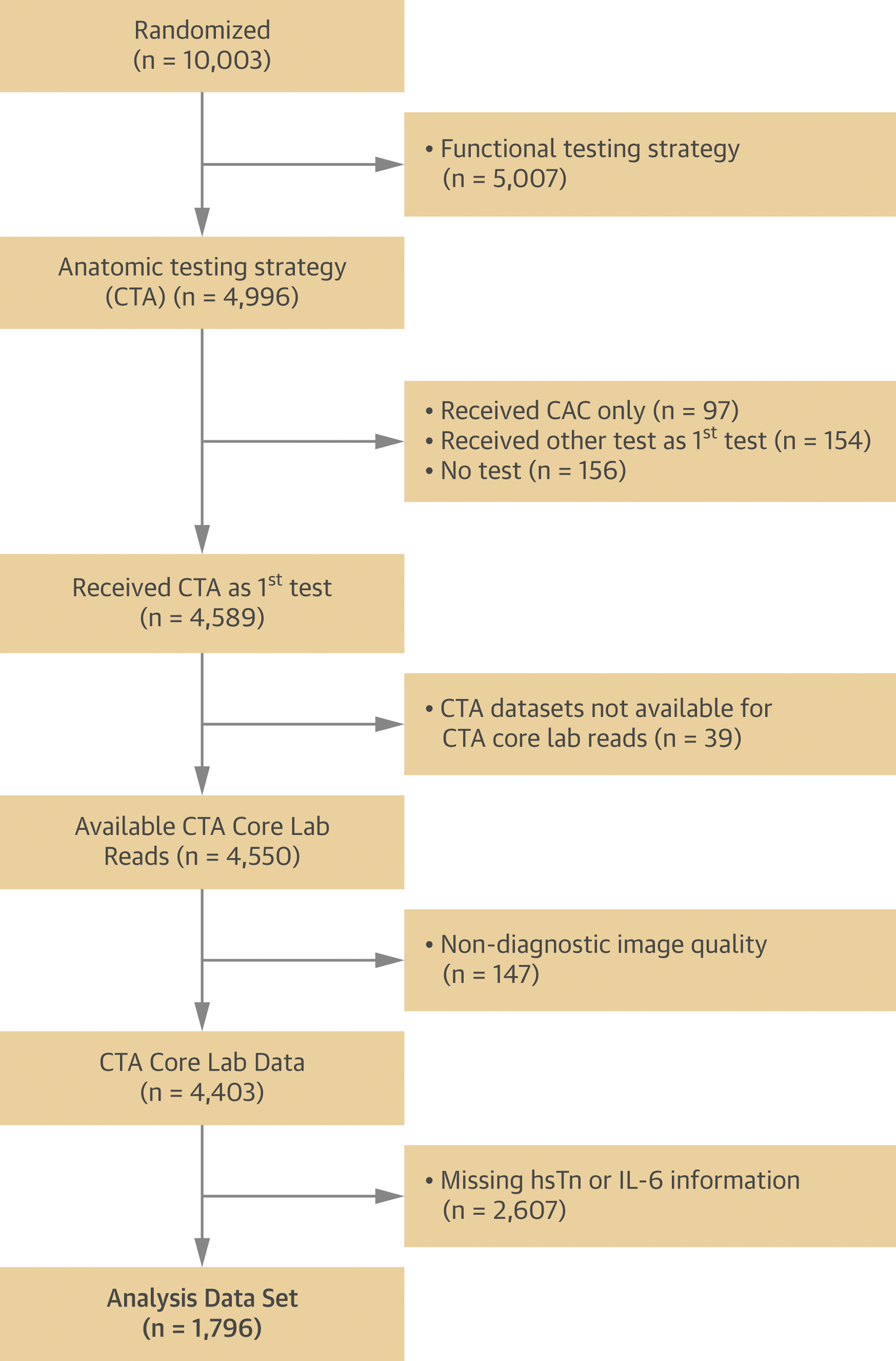
The flow of patients from the PROMISE trial included and excluded from the study.
Circulating biomarker analysis
Concentrations of hs-cTn I and IL-6 were quantified using single-molecule counting methods (Singulex, Alameda, California) on an Erenna platform as described previously.(11,12,21,22) Hs-cTn I assay had a limit of detection of 0.5 ng/l and a 99th percentile reference limit of 6 ng/l in apparently healthy individuals.(11,12,23) IL-6 assay had a limit of quantification of 0.4 ng/l.(21,24)
Coronary CTA analysis
Coronary CTA images were acquired using either retrospectively electrocardiogram-gated or prospectively electrocardiogram-triggered protocols according to guidelines and local protocols.(7) The images were transferred to the core laboratory for the analysis on a cardiac workstation (TeraRecon, Foster City, California). Six readers with level III training in coronary CTA analyzed randomly assigned datasets. The coronary CTA analysis was performed per coronary segment in accordance with the guidelines from the Society of Cardiovascular Computed Tomography.(25)
Each evaluable coronary segment was assessed for the presence of stenosis. The severity of stenosis was quantified by visual estimation into 5 categories: 0%, 1–29%, 30–49%, 50–69%, or ≥70% stenosis.(25) We defined significant stenosis as the presence of ≥70% stenosis in any vessel or ≥50% stenosis in the left main coronary artery. We performed a sensitivity analysis using the definition of ≥50% stenosis in any coronary artery. Coronary Artery Disease Reporting and Data System (CAD-RADs) category was assigned on a per-patient basis.(26)
For each evaluable coronary segment, we noted the presence of plaque (calcified, non-calcified, or partially calcified).(27) Each coronary segment with plaque was evaluated for the presence of high-risk plaque. High-risk plaque features were defined as positive remodeling (remodeling index >1.1), low CT attenuation (mean CT number <30 HU), or napkin-ring sign (ring-like peripheral higher attenuation with central low CT attenuation).(7) The patient was classified as having high-risk plaque if at least one high-risk plaque feature was present.
We use three measures for the assessment of coronary plaque burden. On non-contrast CT scan, we calculated total coronary calcium score using Agatston method.(28) Segment involvement score was calculated by counting the total number of coronary segments exhibiting plaque (minimum 0; maximum 16).(29) CT-adapted Leaman score was calculated using 3 sets of weighting factors: localization of coronary plaques; type of plaque, and degree of stenosis.(30)
Study outcomes
The primary endpoint was a composite of time to MACE including death from any cause, myocardial infarction, or hospitalization for unstable angina. An independent clinical events committee adjudicated all endpoint events in a blinded fashion on the basis of standard, prospectively determined definitions.(2)
Statistical analysis
Continuous variables are presented as mean±standard deviation or median (25th-75th percentile). Categorical variables are presented as frequencies and percentages. Comparisons between groups were performed with the use of a 2-sample Student t-test or Wilcoxon rank-sum test for continuous variables and Fisher exact test for categorical variables. Multivariable linear regression analysis was performed with the dependent variables of hs-cTn or IL-6 (both log-transformed) and multivariable logistic regression analysis was performed with the dependent variable of high-risk (patients with both hs-cTn and IL-6 above the median). Multivariable Cox proportional hazards models were used to assess the relationship of the hs-cTn above median, IL-6 above median, or high-risk group (patients with both hs-cTn and IL-6 above median) and time to the first clinical event (or censoring) for the composite endpoint. All multivariable regression models (i.e. linear, logistic, and Cox) used the same set of pre-specified measures of coronary atherosclerosis and stenosis as independent variables (any coronary plaque, any high-risk plaque, positive remodeling, low CT attenuation plaque, napkin-ring sign, significant stenosis, ≥50% stenosis, CAD-RADS categories, log-transformed CAC score, SIS and Leaman score; each in a separate model) with adjustment for age, gender and ASCVD risk score. For all regression analyses (i.e. linear, logistic, and Cox) robust standard errors were used to calculate p-values. Cumulative event rates based on test results were computed using the Kaplan–Meier method and compared using the log-rank test. All p-values are 2-sided, and were considered significant at the nominal 0.05 level. All statistical analyses were performed using Stata 14.2 (StataCorp LP, College Station, Texas).
Results
Study population
The baseline characteristics of 1796 patients included in the study stratified by median concentration of hs-cTn (1.5 ng/l) and IL-6 (1.8 ng/l) are summarized in Table 1. Age, proportion of men and burden of cardiovascular risk factors (number of risk factors and ASCVD risk score) were higher in patients with hs-cTn above median. There were more women in the group of patients with IL-6 above median. Age and risk factor burden were higher in patients with IL-6 above median. We performed similar comparison in patients with hs-cTn and IL-6 in the 4th vs. 1st quartile and the results were similar (Supplemental Table S1).
Table 1.
Baseline characteristics of patients included in the study stratified by the median values of high-sensitivity cardiac troponin and interleukin 6.
| Variables | All patients (n=1,796) | ≤Median hs-cTn (≤1.5 ng/l) (n=904) | >Median hs-cTn (>1.5 ng/l) (n=892) | p value | ≤Median IL-6 (≤1.8 ng/l) (n=954) | >Median IL-6 (>1.8 ng/l) (n=842) | p value |
|---|---|---|---|---|---|---|---|
| Age – mean ± SD | 60.2 ± 8.0 | 59.0 ± 7.3 | 61.4 ± 8.5 | <0.001 | 59.4 ± 7.9 | 61.1 ± 8.1 | <0.001 |
| Male sex – n (%) | 853 (47.5) | 356 (39.4) | 497 (55.7) | <0.001 | 504 (52.8) | 349 (41.5) | <0.001 |
| Cardiovascular risk factors | |||||||
| Hypertension – n (%) | 1,160 (64.6) | 543 (60.1) | 617 (69.2) | <0.001 | 566 (59.3) | 594 (70.6) | <0.001 |
| Diabetes mellitus – n (%) | 364 (20.3) | 172 (19.0) | 192 (21.5) | 0.197 | 160 (16.8) | 204 (24.2) | <0.001 |
| Dyslipidemia – n (%) | 1,201 (66.9) | 628 (69.5) | 573 (64.2) | 0.021 | 644 (67.5) | 557 (66.2) | 0.547 |
| Family history CAD – n (%) | 612/1,792 (34.2) | 329/903 (36.4) | 283/889 (31.8) | 0.041 | 306/952 (32.1) | 306/840 (36.4) | 0.058 |
| Current/former smoker – n (%) | 943 (52.5) | 462 (51.1) | 481 (53.9) | 0.238 | 463 (48.5) | 480 (57.0) | <0.001 |
| BMI – mean ± SD (kg/m2) | 30.6 ± 5.9 | 30.2 ± 5.9 | 30.9 ± 5.9 | 0.019 | 29.0 ± 5.0 | 32.4 ± 6.3 | <0.001 |
| Risk burden | |||||||
| No risk factors – n (%) | 49 (2.7) | 22 (2.4) | 27 (3.0) | 0.471 | 40 (4.2) | 9 (1.1) | <0.001 |
| Mean number of risk factors – mean ± SD | 2.4 ± 1.1 | 2.4 ± 1.1 | 2.4 ± 1.1 | 0.373 | 2.2 ± 1.1 | 2.5 ± 1.1 | <0.001 |
| ASCVD Risk – median (IQR) | 10.6 (6.018.6) | 8.5 (4.9–14.1) | 12.9 (7.8–22.3) | <0.001 | 9.6 (5.7–16.0) | 12.2 (6.6 21.2) | <0.001 |
| Baseline medications – n (%) | |||||||
| Beta-blocker | 439/1,734 (25.3) | 188/879 (21.4) | 251/855 (29.4) | <0.001 | 212/919 (23.1) | 228/815 (27.9) | 0.023 |
| ACE inhibitor or ARB | 733/1,734 (42.3) | 338/879 (38.5) | 395/855 (46.2) | 0.001 | 361/919 (39.3) | 372/815 (45.6) | 0.009 |
| Statin | 781/1,734 (45.0) | 407/879 (46.3) | 374/855 (43.7) | 0.289 | 430/919 (46.8) | 351/815 (43.1) | 0.122 |
| Aspirin | 817/1,734 (47.1) | 387/879 (44.0) | 430/855 (50.3) | 0.009 | 435/919 (47.3) | 382/815 (46.9) | 0.885 |
| Primary symptom – n (%) | |||||||
| Chest pain | 1,307/1,794 (72.9) | 674/903 (74.6) | 633/891 (71.0) | 0.090 | 723/952 (76.0) | 584/842 (69.4) | 0.002 |
| Dyspnea on exertion | 269/1,794 (15.0) | 118/903 (13.1) | 151/891 (17.0) | 0.024 | 127/952 (13.3) | 142/842 (16.9) | 0.040 |
| Other | 218/1,794 (12.2) | 111/903 (12.3) | 107/891 (12.0) | 0.885 | 102/952 (10.7) | 116/842 (13.8) | 0.051 |
| Type of angina – n | 0.561 | 0.543 | |||||
| Typical | 224 (12.5) | 109 (12.1) | 115 (12.9) | 113 (11.8) | 111 (13.2) | ||
| Atypical | 1,411 (78.6) | 708 (78.3) | 703 (78.8) | 759 (79.6) | 652 (77.4) | ||
| Nonanginal pain | 161 (9.0) | 87 (9.6) | 74 (8.3) | 82 (8.6) | 79 (9.4) | ||
| MACE – n (%) | 60 (3.3) | 20 (2.2) | 40 (4.5) | 0.005 | 22 (2.3) | 38 (4.5) | 0.013 |
Abbreviations: SD standard deviation; IQR interquartile range (25th to 75th percentile); hs-cTn high-sensitivity cardiac troponin; IL-6 interleukin 6; BMI body mass index; ACE, angiotensin-converting enzyme; ARB angiotensin receptor blocker; ASCVD atherosclerotic cardiovascular disease; CAD coronary artery disease; MACE major adverse cardiovascular events.
We defined a high-risk subgroup of 459 patients in whom both biomarkers of myocardial injury (hs-cTn) and inflammation (IL-6) were above the median value (Supplemental Table S2). High-risk patients were older and had higher burden of cardiovascular risk factors. We also performed a subgroup analysis in patients with non-obstructive CAD (stenosis 1–69%, n=1,059). As shown in Supplemental Table S3, patients with higher concentrations of either hs-cTn and IL-6 were older and had higher ASCVD risk score. We performed similar comparison in patients with hs-cTn and IL-6 in the 4th vs. 1st quartile and the results were similar (Supplemental Table S4).
Association of circulating biomarkers with coronary atherosclerosis
Coronary plaque (any coronary plaque, any high-risk plaque, positive remodeling, low CT attenuation plaque) and obstructive CAD (presence of stenosis and higher CAD-RADS categories) were more prevalent in patients with higher concentrations of hs-cTn (Table 2). Burden of coronary atherosclerosis measured as CAC score, SIS and Leaman score were also higher in patients with hs-cTn above the median. In the analysis comparing 4th vs. 1st quartile of hs-cTn, we found similar differences with higher prevalence of plaque and stenosis, and higher plaque burden among those with higher hs-cTn (Supplemental Table S5). Higher concentrations of IL-6 were associated with the presence of stenosis and CAD-RADS categories, but not with the presence of plaque (any coronary plaque, high-risk plaque) and other measures of coronary atherosclerosis burden (CAC score, SIS and Leaman score; Table 2). In the analysis comparing 4th vs. 1st quartile of IL-6, we found association of low CT attenuation plaque, obstructive CAD (presence of stenosis and higher CAD-RADS categories), CAC score, SIS with higher IL-6 (Supplemental Table S5).
Table 2:
The results of the core lab assessment of coronary atherosclerosis by coronary computed tomography angiography stratified by the median values of high-sensitivity cardiac troponin and interleukin 6.
| Variables | All patients (n=1,796) | ≤Median hs-cTn (≤1.5 ng/l) (n=904) | >Median hs-cTn (>1.5 ng/l) (n=892) | p value | ≤Median IL-6 (≤1.8 ng/l) (n=954) | >Median IL-6 (>1.8 ng/l) (n=842) | p value |
|---|---|---|---|---|---|---|---|
| Coronary plaque – n (%) | |||||||
| Any plaque | 1,177 (65.5) | 543 (60.1) | 634 (71.1) | <0.001 | 617 (64.7) | 560 (66.5) | 0.426 |
| Non-Ca or partially Ca plaque | 1,032 (57.5) | 477 (52.8) | 555 (62.2) | <0.001 | 541 (56.7) | 491 (58.3) | 0.503 |
| Ca plaque | 145 (8.1) | 66 (7.3) | 79 (8.9) | 0.260 | 76 (8.0) | 69 (8.2) | 0.863 |
| High-risk plaque | |||||||
| Any high-risk plaque | 277 (15.4) | 110 (12.2) | 167 (18.7) | <0.001 | 142 (14.9) | 135 (16.0) | 0.513 |
| Positive remodeling | 256 (14.3) | 100 (11.1) | 156 (17.5) | <0.001 | 135 (14.2) | 121 (14.4) | 0.893 |
| Low CT attenuation | 83 (4.6) | 26 (2.9) | 57 (6.4) | <0.001 | 34 (3.6) | 49 (5.8) | 0.024 |
| Napkin ring sign | 60 (3.3) | 26 (2.9) | 34 (3.8) | 0.295 | 33 (3.5) | 27 (3.2) | 0.794 |
| Coronary stenosis – n (%) | |||||||
| No stenosis | 619 (34.5) | 361 (39.9) | 258 (28.9) | <0.001 | 337 (35.3) | 282 (33.5) | 0.007 |
| 1–29% stenosis | 549 (30.6) | 267 (29.5) | 282 (31.6) | 315 (33.0) | 234 (27.8) | ||
| 30–49% stenosis | 380 (21.2) | 188 (20.8) | 192 (21.5) | 189 (19.8) | 191 (22.7) | ||
| 50–69% stenosis | 132 (7.4) | 56 (6.2) | 76 (8.5) | 66 (6.9) | 66 (7.8) | ||
| 70–100% stenosis | 116 (6.5) | 32 (3.5) | 84 (9.4) | 47 (4.9) | 69 (8.2) | ||
| Any stenosis ≥70% or LM ≥50% | 118 (6.6) | 34 (3.8) | 84 (9.4) | <0.001 | 47 (4.9) | 71 (8.4) | 0.003 |
| Any stenosis ≥50% – n (%) | 248 (13.8) | 88 (9.7) | 160 (17.9) | <0.001 | 113 (11.8) | 135 (16.0) | 0.011 |
| CAD-RADS categories | <0.001 | 0.006 | |||||
| CAD-RADS 0 | 569 (31.7) | 330 (36.5) | 239 (26.8) | 313 (32.8) | 256 (30.4) | ||
| CAD-RADS 1 | 599 (33.4) | 298 (33.0) | 301 (33.7) | 339 (35.5) | 260 (30.9) | ||
| CAD-RADS 2 | 380 (21.2) | 188 (20.8) | 192 (21.5) | 189 (19.8) | 191 (22.7) | ||
| CAD-RADS 3 | 130 (7.2) | 54 (6.0) | 76 (8.5) | 66 (6.9) | 64 (7.6) | ||
| CAD-RADS 4a | 98 (5.5) | 27 (3.0) | 71 (8.0) | 43 (4.5) | 55 (6.5) | ||
| CAD-RADS 4b and 5 | 20 (1.1) | 7 (0.8) | 13 (1.5) | 4 (0.4) | 16 (1.9) | ||
| Coronary plaque burden | |||||||
| Coronary calcium score | |||||||
| AS Median (IQR) | 20.7 (0.0–175.9) | 9.1 (0.0–107.7) | 35.5 (0.0–249.3) | <0.001 | 19.3 (0.0–154.9) | 24.7 (0.0–214.7) | 0.142 |
| Mean ± SD | 190.8 ± 507.8 | 133.8 ± 395.5 | 249.6 ± 596.7 | 185.9 ± 521.0 | 196.3 ± 492.5 | ||
| AS 0 – n (%) | 559/1,575 (35.5) | 319/800 (39.9) | 240/775 (31.0) | <0.001 | 308 (36.7) | 251 (34.1) | 0.128 |
| AS 1–100 – n (%) | 512/1,575 (32.5) | 276/800 (34.5) | 236/775 (30.5) | 275 (32.8) | 237 (32.2) | ||
| AS 101–300 – n (%) | 229/1,575 (14.5) | 103/800 (12.9) | 126/775 (16.3) | 127 (15.1) | 102 (13.9) | ||
| AS >300 – n (%) | 275/1,575 | 102/800 | 173/775 (22.3) | 129 (15.4) | 146 (19.8) | ||
| Segment involvement score | |||||||
| Median (IQR) | 2 (0–5) | 1 (0–4) | 2 (0–5) | <0.001 | 2 (0–4) | 2 (0–5) | 0.187 |
| Mean ± SD | 2.8 ± 3.0 | 2.3 ± 2.7 | 3.2 ± 3.2 | 2.6 ± 2.9 | 2.9 ± 3.1 | ||
| Leaman score | |||||||
| Median (IQR) | 3.7 (0–8.5) | 3.2 (0–7.4) | 4.8 (0–9.6) | <0.001 | 3.4 (0–8.0) | 4.0 (0–9.0) | 0.117 |
| Mean ± SD | 5.0 ± 5.1 | 4.2 ± 4.7 | 5.8 ± 5.3 | 4.8 ± 4.9 | 5.2 ± 5.3 |
Abbreviations: SD standard deviation; IQR interquartile range (25th to 75th percentile); Ca calcified; LM left main coronary artery; AS Agatston score
Coronary atherosclerotic plaque (any coronary plaque, high-risk plaque), obstructive CAD (presence of stenosis and higher CAD-RADS categories) and other measures of coronary atherosclerosis burden (CAC score, SIS and Leaman score) were more prevalent and higher in high-risk group of patients with both hs-cTn and IL-6 above the median (Supplemental Table S6).
In multivariable models adjusted for age, gender and ASCVD risk score, presence of any high-risk plaque, positive remodeling, low CT attenuation plaque, significant stenosis, >50% stenosis and CAD-RADS categories were associated with log-transformed hs-cTn concentrations (Table 3). Similarly, measures of plaque and stenosis burden were associated with log hs-cTn (log-transformed CAC score, SIS, Leaman score). Only low CT attenuation plaque, significant stenosis and CAD-RADS categories were found to be significantly associated with log-transformed IL-6 after controlling for age, gender and ASCVD risk score.
Table 3.
Association between coronary atherosclerosis/coronary artery disease characteristics and high-sensitivity cardiac troponin and interleukin 6 in multivariable linear regression analyses.
| Independent Variable* (each row shows one model) | Hs-cTn (log-transformed) | IL-6 (log-transformed) | ||||
|---|---|---|---|---|---|---|
| ß coefficient | 95%CI | P value | ß coefficient | 95%CI | P value | |
| Any coronary plaque | 0.04 | −0.02, 0.09 | 0.173 | 0.02 | −0.04, 0.07 | 0.572 |
| Any high-risk plaque | 0.09 | 0.01, 0.18 | 0.034 | 0.03 | −0.01, 0.00 | 0.420 |
| Positive remodeling | 0.11 | 0.02, 0.20 | 0.016 | 0.01 | −0–06, 0.08 | 0.750 |
| Low CT attenuation | 0.18 | 0.02, 0.34 | 0.025 | 0.13 | 0.00, 0.25 | 0.043 |
| Napkin-ring sign | 0.08 | −0.10, 0.26 | 0.373 | −0.08 | −0.18, 0.01 | 0.085 |
| Any stenosis ≥70% or LM ≥50% | 0.22 | 0.08, 0.36 | 0.002 | 0.17 | 0.05, 0.29 | 0.005 |
| Any stenosis ≥50% | 0.13 | 0.04, 0.22 | 0.006 | 0.07 | 0.00, 0.14 | 0.062 |
| CADRADS categories | 0.04 | 0.01, 0.06 | 0.004 | 0.04 | 0.01, 0.06 | 0.001 |
| CAC score (log-transformed) | 0.01 | 0.00, 0.03 | 0.050 | 0.01 | 0.00, 0.02 | 0.132 |
| SIS | 0.02 | 0.00, 0.03 | 0.008 | 0.01 | 0.00, 0.02 | 0.083 |
| Leaman Score | 0.01 | 0.00, 0.01 | 0.019 | 0.00 | 0.00 ,0.01 | 0.205 |
Adjusted for age, gender, ASCVD risk score (coefficients not shown)
The presence of high-risk plaque, positive remodeling, low CT attenuation plaque, significant stenosis, >50% stenosis, CAD-RADS categories, and SIS were also associated with high-risk of both hs-cTn and IL-6 above median (Supplemental Table S7). The other measures of coronary atherosclerosis were not significantly associated with high-risk.
Association of circulating biomarkers with major adverse cardiovascular events
The primary outcome of composite MACE occurred in 60 of 1796 patients (total incidence 3.3%) during a median (25th-75th percentile) follow-up of 24.7 (17.7–32.9) months. Hs-cTn above median was associated with MACE in univariable analysis (HR 2.14, 95%CI 1.25–3.64, p=0.005; Figure 2). However, hs-cTn was not an independent predictor of MACE in multivariable analysis adjusted for age, gender and ASCVD risk score (HR 1.76, 95%CI 0.97–3.20, p=0.06), nor after additional adjustment for any coronary plaque (HR 1.71, 95%CI 0.95–3.08, p=0.07), any high-risk plaque (HR 1.73, 95%CI 0.96–3.12, p=0.07), positive remodeling (HR 1.73, 95%CI 0.96–3.12, p=0.07), low CT attenuation plaque (HR 1.73, 95%CI 0.96–3.14, p=0.07), napkin-ring sign (HR 1.76, 95%CI 0.97–3.22, p=0.06), significant stenosis (HR 1.64, 95%CI 0.89–3.01, p=0.11), >50% stenosis (HR 1.63, 95%CI 0.89–2.98, p=0.11), CAD-RADS categories (HR 1.62, 95%CI 0.90–2.94, p=0.11), log-transformed CAC score (HR 1.68, 95%CI 0.89–3.19, p=0.11), SIS score (HR 1.64, 95%CI 0.90–2.99, p=0.11), or Leaman score (HR 1.62, 95%CI 0.89–2.96, p=0.11).
Figure 2. Kaplan–Meier Estimates of the Composite Primary Endpoint as a Function of Time after Randomization.
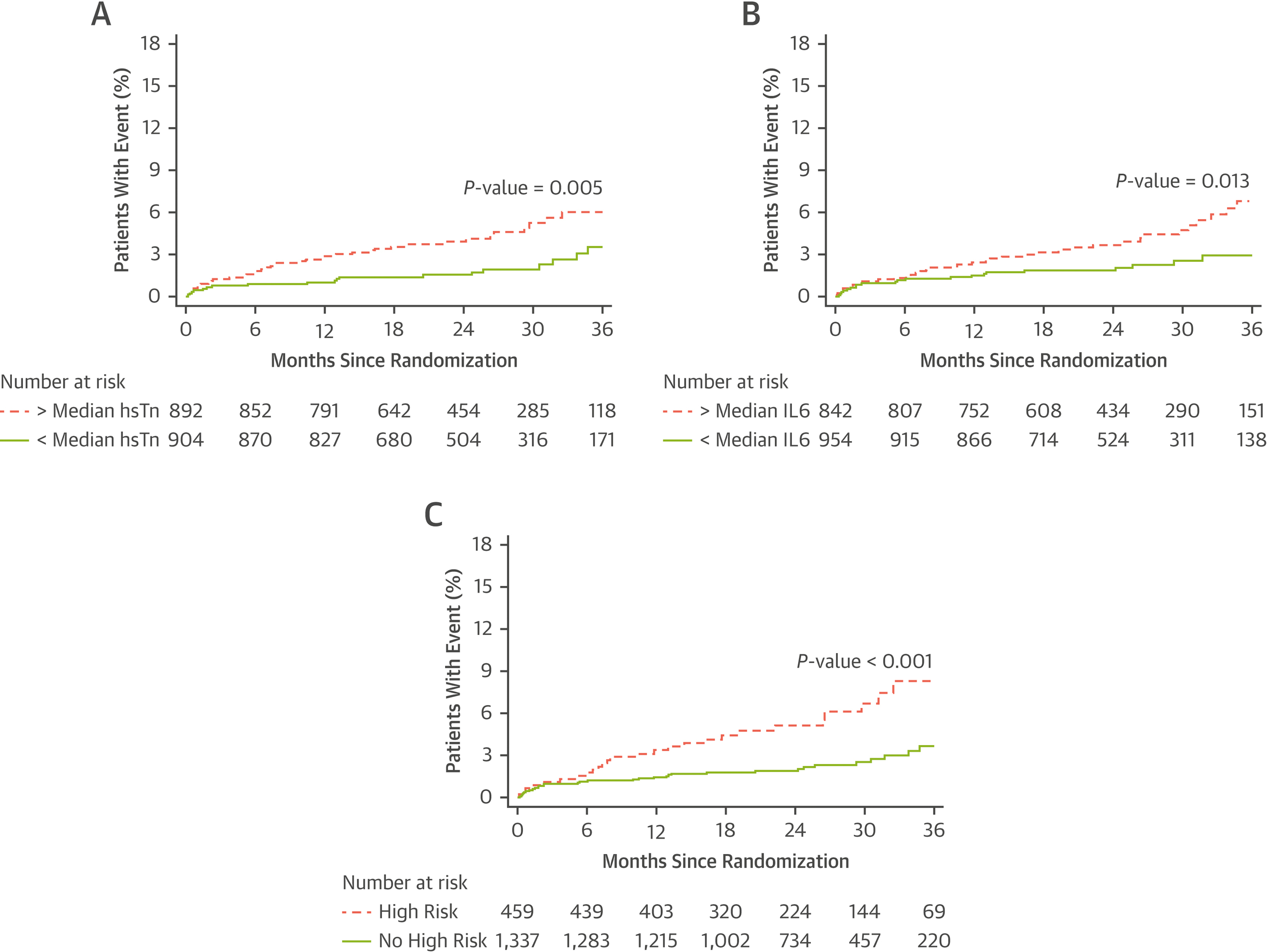
Panel A shows Kaplan-Meier estimates stratified by the median high-sensitivity troponin values. Panel B shows Kaplan-Meier estimates stratified by the median interleukin-6 values. Panel C shows Kaplan-Meier estimates stratified by the presence of high-risk vs. no high-risk.
IL-6 above median was associated with MACE in univariable analysis (HR 1.93, 95%CI 1.14–3.26, p=0.01; Figure 2) and in multivariable analysis adjusted for age, gender and ASCVD risk score (HR 1.92, 95%CI 1.09–3.39, p=0.03). The association of IL-6 concentration above the median with MACE persisted after additional adjustment for any coronary plaque (HR 1.93, 95%CI 1.10–3.41, p=0.02), any high-risk plaque (HR 1.88, 95%CI 1.06–3.35, p=0.03), positive remodeling (HR 1.91, 95%CI 1.08–3.38, p=0.03), low CT attenuation plaque (HR 1.87, 95%CI 1.06–3.32, p=0.03), napkin-ring sign (HR 1.92 95%CI 1.08–3.39, p=0.03), >50% stenosis (HR 1.79, 95%CI 1.02–3.15, p=0.04), log-transformed CAC score (HR 1.89, 95%CI 1.05–3.41, p=0.034), SIS score (HR 1.83, 95%CI 1.04–3.22, p=0.04), or Leaman score (HR 1.79, 95%CI 1.02–3.16, p=0.04) but not for significant stenosis (HR 1.77, 95%CI 0.99–3.15, p=0.05) or CAD-RADS categories (HR 1.71, 95%CI 0.98–3.00, p=0.06).
High-risk biomarker subgroup was associated with MACE in univariable analysis (HR 2.47, 95%CI 1.49–4.11, p<0.001; Figure 2) and in multivariable analysis adjusted for age, gender and ASCVD risk score (HR 2.22, 95%CI 1.25–3.92, p=0.01). The association of high-risk with MACE persisted after additional adjustment for any coronary plaque (HR 2.22, 95%CI 1.26–3.91, p=0.01), any high-risk plaque (HR 2.15, 95%CI 1.21–3.81, p=0.01), positive remodeling (HR 2.16, 95%CI 1.22–3.83, p=0.01), low CT attenuation plaque (HR 2.16, 95%CI 1.22–3.83, p=0.01), napkin-ring sign (HR 2.22 95%CI 1.25–3.93, p=0.01), significant stenosis (HR 2.00, 95%CI 1.11–3.60, p=0.02), >50% stenosis (HR 2.01, 95%CI 1.13–3.56, p=0.02), CAD-RADS categories (HR 1.94, 95%CI 1.10–3.43, p=0.02), log-transformed CAC score (HR 1.98, 95%CI 1.09–3.59, p=0.03), SIS score (HR 2.04, 95%CI 1.16–3.60, p=0.01), and Leaman score (HR 2.02, 95%CI 1.13–3.59, p=0.02).
We performed additional analysis in patients with non-obstructive CAD (stenosis 1–69%) and found that hs-cTn was not a predictor of MACE after adjustment (Supplemental Table S8, Supplemental Figure S1). We found stronger association with MACE for IL-6 and high-risk group of patients, which persisted after adjustment for the presence of plaque, any high-risk plaque, positive remodeling, low CT attenuation plaque, napkin-ring sign, stenosis or plaque burden measures.
Finally, we repeated analyses exploring the association of hs-cTn, IL-6, and high-risk with MACE after adjustment for age, gender, individual coronary atherosclerosis and stenosis characteristics and included the interaction term of serum biomarkers and individual coronary atherosclerosis and stenosis characteristics. For all analyses, the interaction term was non-significant (p>0.05) suggesting that the outcome of MACE was not significantly driven by the interaction between serum biomarkers and coronary atherosclerosis and stenosis characteristics.
Discussion
We found that circulating biomarkers of myocardial injury (hs-cTn) and inflammation (IL-6) were associated with the presence and extent of coronary atherosclerosis in the population of outpatients evaluated for suspected CAD by coronary CTA (Central Illustration). High-risk coronary plaque, obstructive CAD (significant stenosis, CAD-RADS) and CAD burden (CAC score, SIS, Leaman score) were associated with hs-cTn concentrations. Obstructive CAD (significant stenosis, CAD-RADS), but not other measures of coronary atherosclerosis, was associated with IL-6 concentrations. The high-risk group of patients with both hs-cTn and Il-6 above median had the highest burden of CAD.
Central Illustration. Association of biomarkers of myocardial injury and inflammation with coronary artery disease and major cardiovascular events.
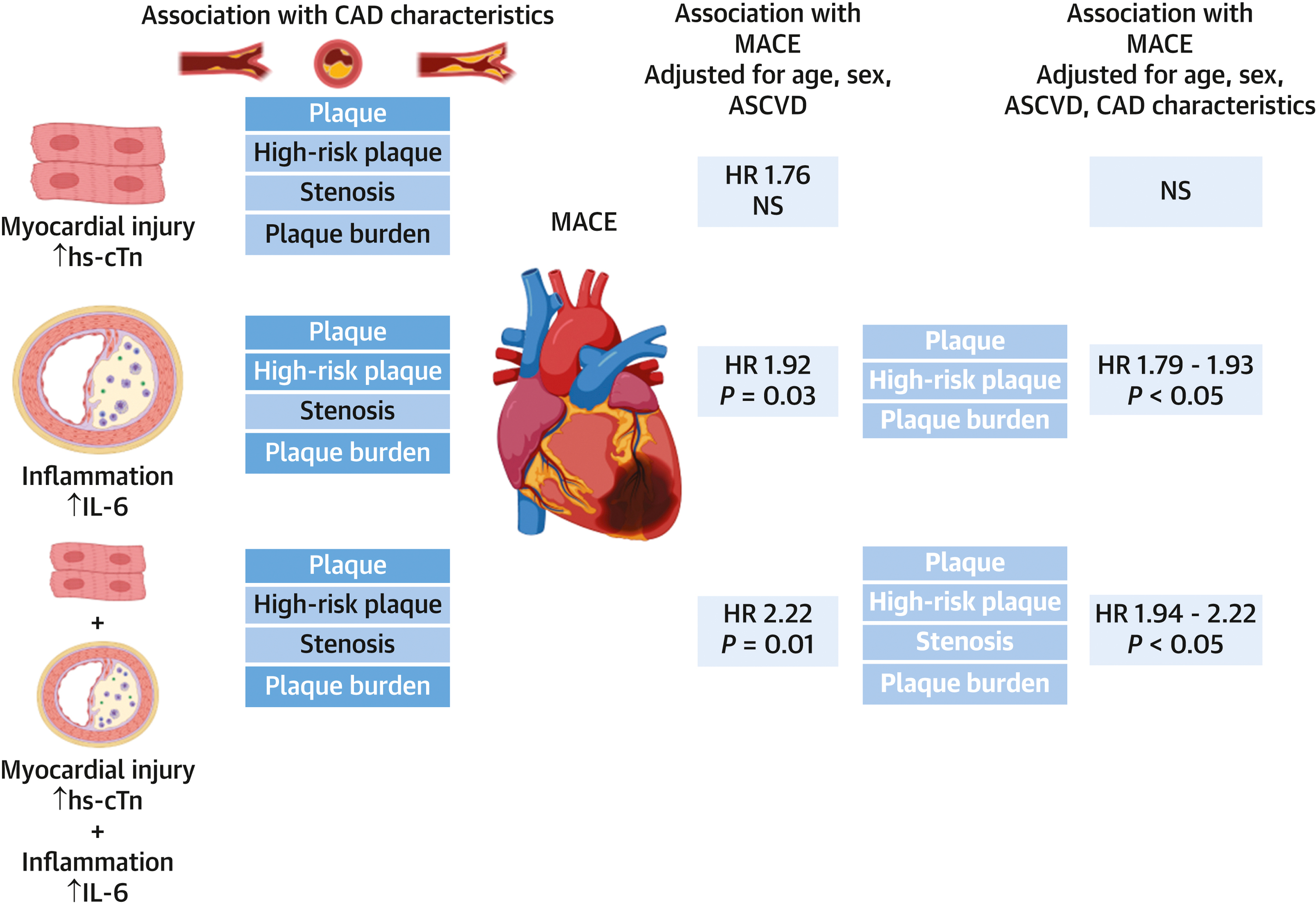
The depiction of the associations between circulating biomarkers of myocardial injury (high-sensitivity cardiac troponin – hs-cTn) and inflammation (interleukin 6 – IL-6) and coronary artery disease (CAD) characteristics and major adverse cardiovascular events. The statistically significant (p<0.05) associations between biomarkers and CAD characteristics in bold white text. The associations between hs-cTn and high-risk (patients both hs-cTn and IL-6 above median) was significant after adjustments for age, gender, ASCVD risk score and individual CAD characteristics (bold white text).
Further, we observed that hs-cTn predicted MACE, but not after adjusting for cardiovascular risk factors and CAD characteristics (Central Illustration). In contrast, IL-6 predicted MACE even after adjustment for the presence of coronary plaque, high-risk plaque and plaque burden (CAC score, SIS, Leaman score). The association between biomarkers and MACE was the strongest in high-risk patients with both elevated markers of myocardial injury and inflammation and in subgroup of patients with non-obstructive CAD. High-risk was associated with approximately 2 to 2.5-fold increased risk of MACE after multivariable adjustment for cardiovascular risk factors and CAD characteristics in patients with non-obstructive CAD.
Hs-cTn in the assessment of CAD
The association between increased risk of future cardiovascular events in normal population of patients with low concentrations of hs-cTn has been well established. Hs-cTn concentrations predict structural heart abnormalities and cardiovascular events.(10–12) Elevated concentrations of hs-cTn within normal range values have been also predictors of functionally significant CAD on stress test and obstructive CAD on coronary CTA.(12–14) In the analysis from the Scottish Computed Tomography of the HEART (SCOT-HEART) trial, higher concentrations of hs-cTn were associated with obstructive CAD and improved the discrimination and calibration of the CAD Consortium model for identifying obstructive CAD and improved classification of ACC/AHA pretest probability risk categories.(15) Our prior analyses in the PROMISE trial showed that higher concentrations of hs-cTn within the normal range were associated with heightened near-term risk of MACE.(11) The results of the present analysis in the same cohort extend prior knowledge by demonstrating a significant association between hs-cTn measurements within the normal range and detailed characteristics of coronary atherosclerosis, specifically high-risk plaque, stenosis and CAD burden. Specifically, we observed that higher burden of CAD and obstructive CAD probably explain some of the mild elevations of hs-cTn within what is considered normal range. The association between hs-cTn and MACE was attenuated by cardiovascular risk factors and CAD characteristics, suggesting that significant portion of the risk from elevated hs-cTn as mediated by the presence of CAD.
IL-6 in the assessment of CAD
Prior studies have demonstrated discordant results with regard to the association of IL-6 concentrations with coronary atherosclerosis and CAD. High IL-6 was an independent predictor of coronary CTA-derived atherosclerotic risk score integrating plaque type, location and stenosis in a small population of symptomatic chest pain patients.(16) In another small study, IL-6 predicted CAD defined as >30% stenosis on invasive coronary angiography.(17) IL-6 was also a predictor of carotid atherosclerotic plaque in the Tromso study.(18) Furthermore, IL-6 across increasing tertiles was associated with higher coronary atherosclerotic burden and obstructive CAD and this association persisted in groups of patients with both non-obstructive and obstructive CAD.(19) However, the review of available evidence on the association between inflammatory markers, including IL-6, and severity and extent of CAD on invasive coronary angiography concluded that the association was overall weak and mostly explained by concomitant burden of cardiovascular risk factors.(20) Similarly, patients with functionally significant CAD as detected by myocardial perfusion imaging had higher IL-6 concentrations in univariable analysis, but not in fully adjusted multivariable analysis.(21)
A possible explanation of the differences observed in prior studies is that IL-6 is prone to fluctuations over time. IL-6 can be produced by T-cells, endothelial cells, macrophages, cardiomyocytes, and fibroblasts. It is thought that T-cell cytokines generated during plaque development stimulate the production of quantifiable amounts of circulating IL-6 downstream and may reflect the dynamic nature of atherosclerosis development and progression.(31) Nevertheless, circulating IL-6 concentrations were associated with coronary heart disease and higher hazard ratio for events when long-term concentrations were considered.(32) Indeed, IL-6 was a strong predictor of CV events in multiple studies and a meta-analysis.(21,33,34)
Our study builds on previous experiences demonstrating that IL-6 was associated the presence of obstructive CAD (significant stenosis and CAD-RADS categories). Interestingly, IL-6 was predictor of MACE in the fully adjusted model for ASCVD risk score and coronary plaque, high-risk plaque and CAD burden, but not when adjusted for stenosis parameters. This suggests that IL6 may influence MACE through pathways independent of CTA-based CAD measures and also raises possibility of the stronger predictive value of IL-6 in patients with non-obstructive CAD.
Biomarkers in patients with non-obstructive CAD
Indeed, in the analysis of patients with non-obstructive CAD (1–69% stenosis) in our study, IL-6, but not hs-cTn, was a predictor of MACE in multivariable analysis. The concentrations of IL-6 above median were associated with higher, almost 3-fold, increase in MACE among patient with non-obstructive CAD. These findings suggest that the measurements of increased inflammation have increased predictive value in patients with non-obstructive CAD. Anti-inflammatory treatment with canakinumab, a human anti-IL-1β monoclonal antibody, reduced the rate of myocardial infarction and coronary revascularization in the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) trial.(35) We may speculate that patients with non-obstructive CAD and elevated inflammation (e.g. IL-6) may be a target populations for the most efficient use of anti-inflammatory therapies. Further studies will be needed to confirm this hypothesis.
High-risk group with elevated biomarkers of myocardial injury and inflammation
In our final analysis, we found that high-risk patients with both increased hs-cTn and IL-6 had more plaque, high-risk plaque, stenosis and CAD burden compared to those with elevated concentrations of only one or neither biomarker. High-risk was a predictor of MACE after adjusting for ASCVD risk score and CAD characteristics. The results persisted in the subgroup of patients with non-obstructive CAD. As we have shown previously in the PROMISE trial, the majority of MACE occurs in patients with non-obstructive CAD, despite the lower relative risk compared to obstructive CAD given the large size of this population.(8) In addition to high-risk plaque (7), circulating biomarkers may provide an additional risk assessment tool in this subgroup.
Values of hs-cTn and IL-6
In our study, we performed primary analyses of the association between biomarkers, CAD characteristics, and MACE using stratification of biomarkers by the median value. There are no pre-defined cut-off values of hs-cTn and IL-6 in the range of values that basically represent normal distribution in the population. The analyses using median cut-off provided the best power. The use of categorized biomarker values (i.e., above vs. below median) also permitted easy conceptualization of the results for the readers providing simple hazard ratios (e.g., approximately two-fold increased risk of MACE in those with higher biomarker levels).
Limitations
Biomarkers were available only in a subgroup of patients who consented for the participation in the biorepository. However, the baseline characteristics of patients with and without biomarkers were not significantly different, suggesting that there was no systematic bias.(12) The study was performed among symptomatic patients with suspected CAD who were scheduled for a non-invasive testing. Our results may not be applicable in general asymptomatic population with lower risk or in higher risk patients diagnosed with acute coronary syndrome. The hs-cTn I method that was used in our study is considerably more sensitive than conventionally used assays and does not have regulatory approval for this indication. Thus, our results cannot be necessarily extrapolated to other hs-cTn tests. Blood samples were obtained during the baseline visit in the trial. The sites were encouraged to process and store the samples immediately; however, delays in processing might have occurred and affected the values of IL-6. The details on the possible delays in the sample processing were not available in the trial. We do not have serial biomarker measurement, which may be reasonable to refine risk prediction further. We did not determine thresholds for the biomarkers to be considered “abnormal”. Measurements of high-sensitivity C-reactive protein, which are more commonly used in clinical practice for the assessment of increased inflammation, were not available in our study.
Conclusions
Circulating biomarkers of myocardial injury (hs-cTn) and inflammation (IL-6) are associated with the presence and severity of CAD and IL-6 predicts future MACE in patients with stable chest pain undergoing coronary CTA for the diagnostic evaluation. The predictive value of IL-6 is stronger in the subgroup of patients with non-obstructive CAD, suggesting upregulated inflammatory pathways may play role in this subgroup and can be a target of anti-inflammatory therapeutic interventions. High-risk patients with elevated biomarkers of both myocardial injury and inflammation are at especially high risk for MACE compared to all others even after adjustment for CAD characteristics. This was especially true in the absence of obstructive CAD suggesting that future trials are needed to better determine whether additional interventions (e.g. more potent lipid-lowering or anti-inflammatory therapies) can decrease their cardiovascular risk.
Supplementary Material
Perspectives:
Clinical Competencies – Competency in Clinical Knowledge
In patients evaluated for suspected stable coronary artery disease by coronary computed tomography, circulating biomarkers of myocardial injury (hs-cTn) and inflammation (IL-6) are associated with the presence and severity of coronary atherosclerosis and IL-6 predicts future major adverse cardiovascular events.
Translational Outlook
Future trials are needed to better determine whether additional interventions (e.g. more potent lipid-lowering or anti-inflammatory therapies) can decrease cardiovascular risk in patients with increased biomarkers of myocardial injury (hs-cTn) and inflammation (IL-6), especially in subgroup of patients with non-obstructive coronary artery disease.
Acknowledgments
Funding:
The PROMISE trial was funded by grants R01HL098237, R01HL098236, R01HL98305, and R01HL098235 from the National Heart, Lung, and Blood Institute (NHLBI).
Role of the Funder:
The funding source had no role in the design and conduct of this study, study analyses and interpretation of the data, the drafting and editing of the manuscript and its final contents, approval of the manuscript, and the decision to submit the manuscript for publication.
Disclosures:
Dr. Ferencik reports receiving a grant from the American Heart Association, National Institutes of Health and consulting fees from Biograph, Inc.
Dr. Bittner was supported by grant from NIH/NHLBI 5K24HL113128.
Dr. Lu reports receiving grant support from the American Roentgen Ray Society Scholarship during the conduct of the study and personal fees from PQBypass outside the submitted work.
Dr. Meyersohn was supported by NIH/NHLBI T32 HL076136.
Dr. Douglas reports receiving grant support from HeartFlow and service on a data and safety monitoring board for GE HealthCare outside the submitted work.
Dr. Hoffmann reports receiving grants from the American College of Radiology Imaging Network and HeartFlow during the conduct of the study, and from Siemens Healthcare outside the submitted work.
The other authors report no potential conflicts of interest.
Abbreviations:
- CAD
coronary artery disease
- hs-cTn
high-sensitivity cardiac troponin
- IL-6
interleukin 6
- CTA
computed tomography angiograph
- HRP
high-risk plaque
- CAD-RADS
Coronary Artery Disease Reporting & Data System
- SIS
segment involvement score
- CAC
coronary artery calcium
- ASCVD
atherosclerotic cardiovascular disease
- HR
hazard ratio
- CI
confidence interval
- MACE
major adverse cardiovascular events
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: The views expressed in this article do not necessarily represent the official views of NHLBI. This article was prepared while Geoffrey S. Ginsburg was employed at Duke University. The opinions expressed in this article are the author’s own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Trial Registration:
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019;139(10):e56–28. Doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372(14):1291–300. Doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SCOT-HEART investigators, Newby DE, Adamson PD, et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med 2018;379(10):924–33. Doi: 10.1056/NEJMoa1805971. [DOI] [PubMed] [Google Scholar]
- 4.Williams MC, Kwiecinski J, Doris M, et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020;141(18):1452–62. Doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams MC, Moss AJ, Dweck M, et al. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J Am Coll Cardiol 2019;73(3):291–301. Doi: 10.1016/j.jacc.2018.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bittner DO, Mayrhofer T, Budoff M, et al. Prognostic Value of Coronary CTA in Stable Chest Pain: CAD-RADS, CAC, and Cardiovascular Events in PROMISE. JACC Cardiovasc Imaging 2019. Doi: 10.1016/j.jcmg.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferencik M, Mayrhofer T, Bittner DO, et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol 2018;3(2):144–52. Doi: 10.1001/jamacardio.2017.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients with Stable Chest Pain: Insights from the PROMISE Trial. Circulation 2017;135(24):2320–32. Doi: 10.1161/CIRCULATIONAHA.116.024360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sandoval Y, Thygesen K, Jaffe AS The Universal Definition of Myocardial Infarction: Present and Future. Circulation 2020;141(18):1434–6. Doi: 10.1161/CIRCULATIONAHA.120.045708. [DOI] [PubMed] [Google Scholar]
- 10.Everett BM, Brooks MM, Vlachos HEA, et al. Troponin and Cardiac Events in Stable Ischemic Heart Disease and Diabetes. N Engl J Med 2015;373(7):610–20. Doi: 10.1056/NEJMoa1415921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Januzzi JL, Suchindran S, Hoffmann U, et al. Single-Molecule hsTnI and Short-Term Risk in Stable Patients With Chest Pain. J Am Coll Cardiol 2019;73(3):251–60. Doi: 10.1016/j.jacc.2018.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Januzzi JL, Suchindran S, Coles A, et al. High-Sensitivity Troponin I and Coronary Computed Tomography in Symptomatic Outpatients With Suspected Coronary Artery Disease: Insights From the PROMISE Trial. JACC Cardiovasc Imaging 2018. Doi: 10.1016/j.jcmg.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee G, Twerenbold R, Tanglay Y, et al. Clinical benefit of high-sensitivity cardiac troponin I in the detection of exercise-induced myocardial ischemia. Am Heart J 2016;173:8–17. Doi: 10.1016/j.ahj.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Walter JE, Honegger U, Puelacher C, et al. Prospective Validation of a Biomarker-Based Rule Out Strategy for Functionally Relevant Coronary Artery Disease. Clin Chem 2018;64(2):386–95. Doi: 10.1373/clinchem.2017.277210. [DOI] [PubMed] [Google Scholar]
- 15.Adamson PD, Hunter A, Madsen DM, et al. High-Sensitivity Cardiac Troponin I and the Diagnosis of Coronary Artery Disease in Patients With Suspected Angina Pectoris. Circ Cardiovasc Qual Outcomes 2018;11(2):e004227. Doi: 10.1161/CIRCOUTCOMES.117.004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caselli C, de Graaf MA, Lorenzoni V, et al. HDL cholesterol, leptin and interleukin-6 predict high risk coronary anatomy assessed by CT angiography in patients with stable chest pain. Atherosclerosis 2015;241(1):55–61. Doi: 10.1016/j.atherosclerosis.2015.04.811. [DOI] [PubMed] [Google Scholar]
- 17.Wainstein MV, Mossmann M, Araujo GN, et al. Elevated serum interleukin-6 is predictive of coronary artery disease in intermediate risk overweight patients referred for coronary angiography. Diabetol Metab Syndr 2017;9(1):67. Doi: 10.1186/s13098-017-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltoft A, Arntzen KA, Wilsgaard T, Mathiesen EB, Johnsen SH Interleukin-6 is an independent predictor of progressive atherosclerosis in the carotid artery: The Tromsø Study. Atherosclerosis 2018;271:1–8. Doi: 10.1016/j.atherosclerosis.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Wang X, Yang Y Association between interleukin-6 and the risk of cardiac events measured by coronary computed tomography angiography. Int J Cardiovasc Imaging 2017;33(8):1237–44. Doi: 10.1007/s10554-017-1098-y. [DOI] [PubMed] [Google Scholar]
- 20.Drakopoulou M, Toutouzas K, Stefanadi E, Tsiamis E, Tousoulis D, Stefanadis C Association of inflammatory markers with angiographic severity and extent of coronary artery disease. Atherosclerosis 2009;206(2):335–9. Doi: 10.1016/j.atherosclerosis.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Walter J, Tanglay Y, Fay de Lavallaz du J, et al. Clinical utility of circulating interleukin-6 concentrations in the detection of functionally relevant coronary artery disease. Int J Cardiol 2019;275:20–5. Doi: 10.1016/j.ijcard.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Todd J, Freese B, Lu A, et al. Ultrasensitive flow-based immunoassays using single-molecule counting. Clin Chem 2007;53(11):1990–5. Doi: 10.1373/clinchem.2007.091181. [DOI] [PubMed] [Google Scholar]
- 23.Estis J, Wu AHB, Todd J, Bishop J, Sandlund J, Kavsak PA Comprehensive Age and Sex 99th Percentiles for a High-Sensitivity Cardiac Troponin I Assay. Clin Chem 2018;64(2):398–9. Doi: 10.1373/clinchem.2017.276972. [DOI] [PubMed] [Google Scholar]
- 24.Todd J, Simpson P, Estis J, Torres V, Wub AHB Reference range and short- and long-term biological variation of interleukin (IL)-6, IL-17A and tissue necrosis factor-alpha using high sensitivity assays. Cytokine 2013;64(3):660–5. Doi: 10.1016/j.cyto.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016:435–49. Doi: 10.1016/j.jcct.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Cury RC, Abbara S, Achenbach S, et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10(4):269–81. Doi: 10.1016/j.jcct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 2004;109(1):14–7. Doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 28.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15(4):827–32. [DOI] [PubMed] [Google Scholar]
- 29.Min JK, Shaw LJ, Devereux RB, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol 2007;50(12):1161–70. Doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 30.de Araújo Gonçalves P, Garcia-Garcia HM, Dores H, et al. Coronary computed tomography angiography-adapted Leaman score as a tool to noninvasively quantify total coronary atherosclerotic burden. Int J Cardiovasc Imaging 2013;29(7):1575–84. Doi: 10.1007/s10554-013-0232-8. [DOI] [PubMed] [Google Scholar]
- 31.Hansson GK Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005;352(16):1685–95. Doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 32.Danesh J, Kaptoge S, Mann AG, et al. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. Plos Med 2008;5(4):e78. Doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cesari M, Penninx BWJH, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation 2003;108(19):2317–22. Doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 34.Kaptoge S, Seshasai SRK, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014;35(9):578–89. Doi: 10.1093/eurheartj/eht367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017:NEJMoa1707914–13. Doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


