Abstract
Background:
The Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) is a new iteration of prior National Heart, Lung, and Blood Institute (NHLBI) REDS programs that focus on improving transfusion recipient outcomes across the lifespan as well as the safety and availability of the blood supply.
Study Design and Methods:
The US program includes blood centers and hospitals (22 including 6 free-standing Children’s hospitals) in four geographic regions. The Brazilian program has 5 participating hemocenters. A Center for Transfusion Laboratory Studies (CTLS) and a Data Coordinating Center (DCC) support synergistic studies and activities over the 7-year REDS-IV-P program.
Results:
The US is building a centralized, vein-to-vein (V2V) database, linking information collected from blood donors, their donations, the resulting manufactured components, and data extracts from hospital electronic medical records of transfused and non-transfused patients. Simultaneously, the Brazilian program is building a donor, donation, and component database. The databases will serve as the backbone for retrospective and prospective observational studies in transfusion epidemiology, transfusion recipient outcomes, blood component quality, and emerging blood safety issues. Special focus will be on preterm infants, patients with sickle cell disease, thalassemia or cancer, and the effect of donor biologic variability and component manufacturing on recipient outcomes. A rapid response capability to emerging safety threats has resulted in timely studies related to Severe Acute Respiratory Syndrome Corona Virus-2 (SARS-CoV-2).
Conclusions:
The REDS-IV-P program endeavors to improve donor-recipient-linked research with a focus on children and special populations while also maintaining the flexibility to address emerging blood safety issues.
Keywords: adult transfusions, linked blood-donor-recipient studies, pediatric transfusion, transfusion safety and availability
1 |. INTRODUCTION
Over the past 30 years, successful iterations of the National Heart, Lung, Blood Institute (NHLBI)-initiated REDS programs have generated data to respond to emerging blood safety threats, helping to ensure a safer blood supply when REDS research findings were acted upon by blood center and hospital transfusion services nationally and internationally. Simultaneously the programs were advancing research on effective and optimal transfusion strategies in recipients. The programs evolved from the Retrovirus Epidemiology Donor Study (REDS), 1989–20011 and REDS-II, 2004–2012,2 primarily focused on improving blood availability and safeguarding the blood supply (with attention to infectious risks), to the programs of today encompassing epidemiological research on the entire transfusion chain, from donors to recipients. These linked donor-donation-recipient centered programs include the Recipient Epidemiology and Donor Evaluation Study - III (REDS-III), 2011–2020,3 and the current phase of the program, the Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P), launched in 2019 by NHLBI in collaboration with National Institute of Child Health and Human Development (NICHD). The REDS programs have produced results that have informed blood donor and donation screening policies to help maintain a safe and available blood supply both nationally and internationally. The shift to linking information collected on blood donors, the components made from their donations, and transfusion recipients through data extractions from blood centers, hospital transfusion services, and hospital/clinic patient electronic medical records was catalyzed by the understanding that blood donor characteristics and the biologic composition and characteristics of blood components may play an integral role in both individual and population differential responses to transfusion.
While REDS-III established a large vein-to-vein (V2V) research database that permitted linked donor-recipient analyses at 12 community and tertiary hospitals, the vast majority of the transfused patient populations evaluated at these hospitals were adults and specific study protocols within REDS-III concentrated on adult recipient populations.3 The REDS-IV-P program has expanded its scope to address research questions across the lifespan, with at least half of its research portfolio dedicated to neonatal and pediatric populations that receive blood transfusions. The rationale behind the REDS-IV-P program stemmed from recommendations provided at the 2015 NHLBI State of the Science in Transfusion Medicine symposium4 and the 2016 NHLBI Scientific Priorities in Pediatric Transfusion Medicine Working Group.5 These recommendations highlighted the need for research in transfused populations <18-years-old because of the unique physiologic differences and disease states in neonates, infants, and children as compared to adults, coupled with the paucity of data to determine the best neonatal and pediatric transfusion practices. Furthermore, a panoply of unanswered questions pertaining to pediatric transfusion arose from these meetings providing a major impetus for REDS-IV-P.4,5 As a result, the program has established an infrastructure enabling the conduct of epidemiologic and laboratory studies to identify, characterize, and evaluate determinants of transfusion safety and effectiveness in neonates, infants, and children. Acquisition of these data will aid in the support and design of new randomized clinical trials that are necessary to shape evidence-based neonatal and pediatric transfusion practices. In addition to studies in pediatrics, the program is also dedicated to evaluating and improving the safety and effectiveness of transfusion therapies across the adult lifespan including in special populations (e.g., pregnant women, the elderly) and specific diseases such as sickle cell disease (SCD), thalassemia, and malignancies.
Similar to its predecessors, the REDS-IV-P program is designed to be versatile, dynamic, and capable of reacting to current blood safety and availability issues, such as emerging infectious risks (e.g., Severe Acute Respiratory Syndrome Corona Virus-2 [SARS-CoV-2] and arboviral infections), or potential donor nucleic acid testing (NAT) and/or antibody screening detection issues (e.g., the ability to detect human immunodeficiency virus (HIV) infection if a blood donor is taking antiretroviral or pre-exposure prophylaxis (PrEP) therapies6,7). To enable these sophisticated capabilities, cutting-edge technologies (i.e., novel testing assays, multiple Omics platforms, and genetic arrays) are being developed within the framework of the program and utilized to execute REDS-IV-P studies. In an effort to exponentially grow the REDS-IV-P investment, another major objective is to leverage collaborations with blood collection organizations (e.g., American Red Cross [ARC], Bloodworks Northwest, New York Blood Center (NYBC), Versiti, Vitalant), industry (e.g., Thermo Fisher Scientific, Grifols Diagnostics Solutions), international partners (e.g., Brazil Program, SCANDAT, Blood transfusion Genomics Consortium [BGC]), US government agencies (e.g., other NIH Institutes, CDC, FDA), and non-profit networks such as the Vermont Oxford Network (VON) for Infants.8 Investigators who are not part of REDS-IV-P are encouraged to collaborate with the program. Examples of already established collaborations that access REDS-IV-P data and biospecimen resources include investigations into SARS-CoV-2 variants, severity score modeling for SCD, and metabolomics characterization of linked blood donation and recipient biospecimens.9,10
Finally, the REDS-IV-P program is committed to mentoring the next generation of transfusion medicine investigators. A testimony to this important mission is that many of the mentors in REDS-IV-P were once mentees in previous iterations of REDS (Figure 1).
FIGURE 1.
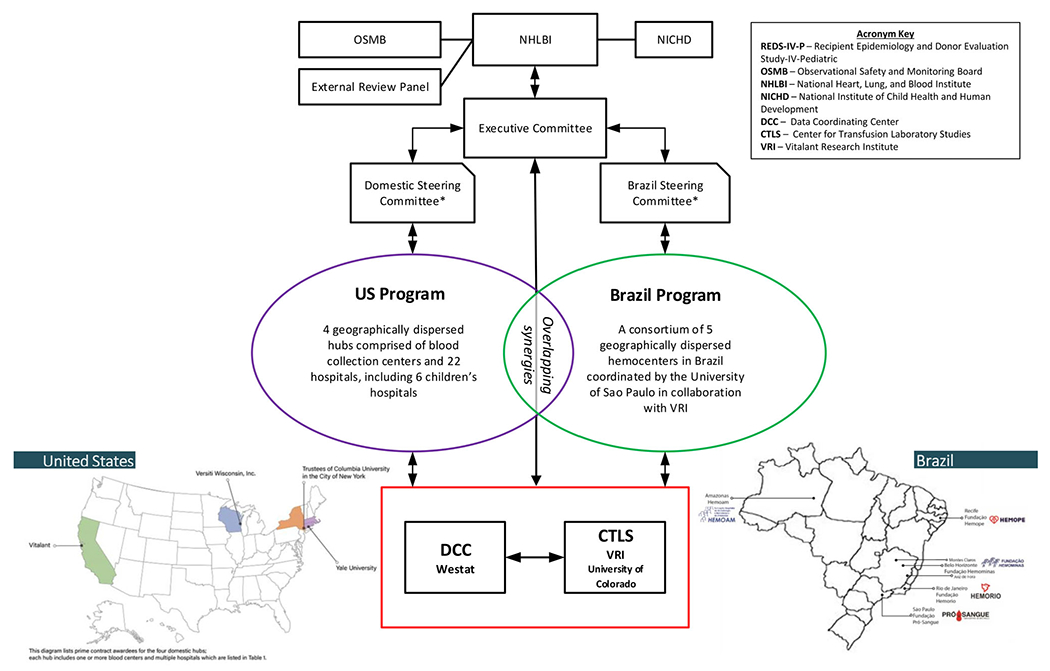
The relationships between committees and institutions participating in the REDS-IV-P program. *The Domestic Steering Committee and Brazil Steering Committee govern the activities of the domestic and Brazil programs, respectively. These committees are responsible for developing and implementing the study portfolio and for ensuring the scientific quality and productivity of the programs. All program investigators may participate in committee meetings, and specified voting members provide input and direction as needed
2 |. INFRASTRUCTURE OF THE REDS-IV-P PROGRAM
The 7-year multi-center program (April 2019 to March 2026), will conduct epidemiologic and laboratory studies on blood donors and transfusion recipients in the US and Brazil, and ultimately collect 5 total years of comprehensive donor/donation, component manufacturing, and recipient data from 2019 to 2023. The US program is comprised of 4 hubs (western–California; mid-west–Wisconsin; eastern New York; and New England – Connecticut, Rhode Island, Massachusetts), each including blood center(s) and multiple participating hospitals. There are 22 affiliated hospitals (including 6 children’s hospitals) transfusing blood components from participating blood centers. The Brazilian program has 5 participating hemocenters, which are both blood collection agencies and treatment centers for persons with hemoglobinopathies and other conditions requiring transfusion. REDS-IV-P includes a Center for Transfusion Laboratory Studies (CTLS) and a Data Coordinating Center (DCC) (Table 1). The NHLBI, NICHD, REDS-IV-P Executive Committee, US and Brazil Steering Committees, Observational Study Monitoring Board, External Review Panel, and REDS-IV-P committees (e.g., Maternal/In-utero/Neonatal/Kids [MINeKids], Publications, and Mentoring committees) all help either to guide or monitor the studies and activities of REDS-IV-P.
TABLE 1.
REDS-IV-P domestic and Brazil Transfusion Safety Research Programs, Central Laboratory, and Data Coordinating Center
| Domestic transfusion safety research program: Four participating Hubs | Annual blood collections (units) by Typea |
|---|---|
| Versiti Wisconsin, Inc., Milwaukee, WI | |
| Participating blood center: Versiti Wisconsin | RBC: 137, 151 Platelets: 16,336 Plasma: 8683 Total: 162,170 |
| Partner hospitals: Froedtert Hospital at the Medical College of Wisconsin (tertiary care hospital), the Marshfield Clinic Health System (rural hospital), Community Memorial Hospital, St. Joseph’s Hospital, and Children’s Medical College of Wisconsin | |
|
| |
| New York Presbyterian Hospital (NYPH), New York, NY | |
| Participating blood center: New York Blood Center | RBC: 364,473 Platelets: 41,976 Plasma: 13,402 Total: 419,851 |
| Partner hospitals: Columbia University Irving Medical Center (tertiary care hospital), The Joan & Sanford I. Weill Cornell Medical College of Cornell University (tertiary care hospital), Allen Hospital (community hospital), Lower Manhattan Hospital (community hospital), Children’s Hospital of New York (children’s hospital), and Komansky Children’s Hospital (children’s hospital) | |
|
| |
| Vitalant, San Francisco, CA | |
| Participating blood center: Vitalant, Vitalant Research Institute, San Francisco with participation from Kaiser Permanente Northern California, Oakland, CA and the Karolinska Institutet, Sweden | RBC: 274,720 Platelets: 120,650 Plasma: 91,006 Total: 411,994b |
| Partner research organizations: Kaiser Permanente Northern California, Oakland, CA and the Karolinska Institutet, Stockholm, Sweden | |
| Partner hospitals: UCSF Medical Centers (Moffitt and Long Hospitals at Parnassus, Mount Zion, Mission Bay and Benioff Children’s Hospitals in Oakland and San Francisco), Zuckerberg San Francisco General Hospital and Trauma Center (public safety-net hospital), Betty Irene Moore Women’s Hospital and Bakar Cancer Hospital | |
|
| |
| Yale University School of Medicine, New Haven, CT | |
| Participating blood centers: American Red Cross (ARC)—Connecticut Region, New York Blood Center Enterprises including Rhode Island Blood Center, and The Children’s Hospital Corporation of Boston Blood Center | RBC: 125,619 Platelets: 19,538 Plasma: 95,006 Total: 240,163 |
| Partner hospitals: Yale-New Haven Hospital Health system on behalf of Yale-New Haven Hospital (tertiary care hospital) and Bridgeport Hospital (community hospital), and Boston Children’s Hospital | |
|
| |
| The Brazil transfusion safety research program: A US - Brazil Collaboration | |
|
| |
| Vitalant Research Institute and the Fundaçao Faculdade de Medicine and Hospital das Clinicas of the Medical School of the University of São Paulo with participation of these blood centers and clinical care organizations: Fundação Hemominas (Belo Horizonte, Juiz de Fora, and Montes Claros, Minas Gerais), Fundação Hemope (Recife, Pernambuco), Fundação Hemorio (Rio de Janeiro), Fundação Hemoam (Manaus, Amazonas), and Fundação Pro-Sangue and Hospital das Clinicas (Sao Paulo) | Total: 422,470c |
|
| |
| Center for Transfusion Laboratory Studies | |
|
| |
| Vitalant Research Institute, San Francisco, CA and Denver, CO with participation from Creative Testing Solutions, Temple, AZ, the University of Colorado, Auroras, CO, and Kaiser Permanente Northern California, Oakland, CA | |
|
| |
| Data Coordinating Center | |
|
| |
| Westat Inc., Rockville, MD with participation from DARTNet Institute, Aurora, CO and Children’s National Medical Center, Washington, D.C. | |
Abbreviation: RBC, red blood cells.
The annual blood collection numbers are estimates based the past 1–5 fiscal years, as defined by each hub. These collections are comprised of RBC (whole blood, apheresis, and/or double red cell), platelets, and plasma.
The total number of collections at the participating San Francisco sites are less than the sum of the three component types because multiple components can be collected and manufactured from whole blood and various apheresis procedures.
The Brazil blood centers were unable to provide a break down of the collections by type. Most are whole blood collections.
The US and Brazil programs each have established robust research portfolios which have multiple areas of synergy. Figures 2 and 3 depict these activities and approved studies. The portfolios feature a diverse array of subject areas including transfusion epidemiology, transfusion recipient outcomes, rapid response to new blood safety issues, and blood component quality, which are detailed in Table 2.
FIGURE 2.
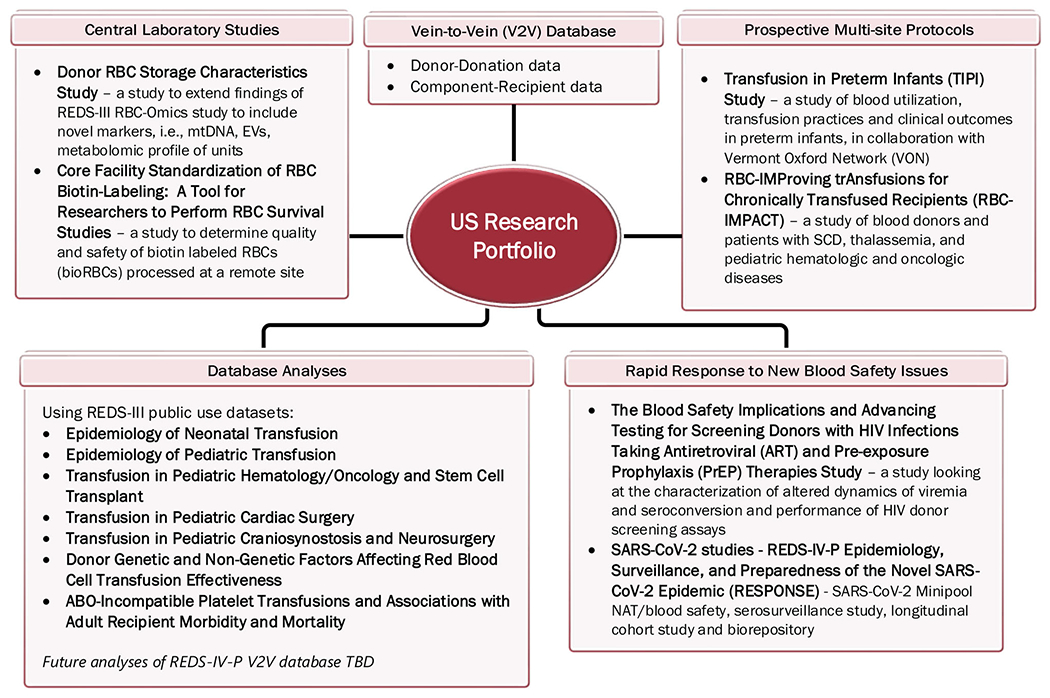
A depiction of ongoing activities of the U.S. program by category
FIGURE 3.
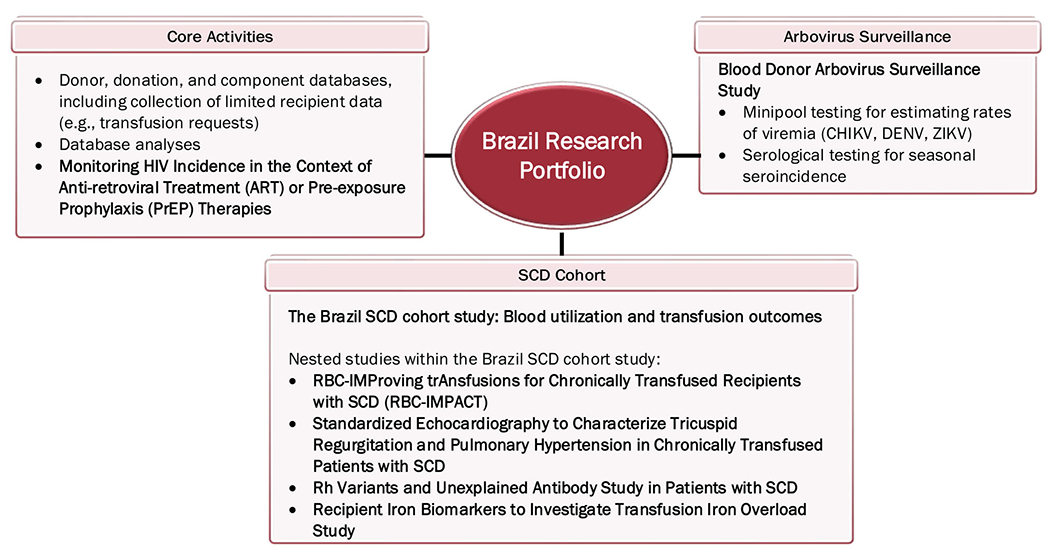
A depiction of ongoing activities of the Brazil program
TABLE 2.
REDS-IV-P Study Portfolio and Study Categories
| Study category | Patient population | Study title | Linked donor-recipient study | Analysis of existing database(s) | Database queried |
|---|---|---|---|---|---|
| Transfusion epidemiology | Pediatrics | Epidemiology of Neonatal Transfusion | No | Yes | REDS-III patient database (7 hospitals, 2013–20169) |
| Epidemiology of Pediatric Transfusion | No | Yes | REDS-III patient database (11 hospitals, 2013–201610) | ||
| Transfusion in Pediatric Hematology/Oncology and Stem Cell Transplant | No | Yes | REDS-III patient database (11 hospitals, 2013–201611) | ||
| Transfusion in Pediatric Cardiac Surgery | No | Yes | REDS-III patient database (3 hospitals, 2013–201612) | ||
| Transfusion in Pediatric Craniosynostosis and Neurosurgery | No | Yes | REDS-III patient database (4 hospitals, 2013–2016) | ||
|
| |||||
| Transfusion recipient outcomes | Pediatrics | Transfusion in Preterm infants (TIPI) Studyc | Yes | Yes | REDS-IV-P-V2V database and VON database (8 hospitals, 2019–2022) |
| Adults | Donor Genetic and Non-Genetic Factors Affecting Red Blood Cell Transfusion Effectiveness | Yes | Yes | REDS-III-RBC-Omics and REDS-III V2V (4 Blood Centers 2013–201613) | |
| ABO-Incompatible Platelet Transfusions and Associations with Adult Recipient Morbidity and Mortality | Yes | Yes | REDS-III patient database (12 hospitals, 2013–2016) | ||
| Adults and Pediatrics | RBC-IMProving trAnsfusions for Chronically Transfused Recipients with SCD (RBC-IMPACT) (Domestic)† | Yes | No | ||
| The Brazil SCD Cohort Study: Blood Utilization and Patient outcomes | Yes | No | |||
| RBC-IMProving trAnsfusions for Chronically Transfused Recipients with SCD (RBC-IMPACT) – Nested study within the Brazil SCD cohort study | Yes | ||||
| Standardized Echocardiography to Characterize Tricuspid Regulation and Pulmonary Hypertension in Chronically Transfused Patients with SCD - Nested study within the Brazil SCD cohort study | No | ||||
| Rh variants and Unexplained Antibody Study in Patients with SCD - Nested study within the Brazil SCD cohort study | Yes | ||||
| Recipient iron Biomarkers to investigate Transfusion Iron Overload Study - Nested study within the Brazil SCD cohort study | Yes | ||||
|
| |||||
| Rapid response to new blood safety issues | Adults | SARS-CoV-2 studies - REDS-IV-P Epidemiology, Surveillance, and Preparedness of the Novel SARS-CoV-2 Epidemic (RESPONSE) (Domestic)a | No | No | |
| Blood Donor Arbovirus Surveillance Study (Brazil)a | No | ||||
| REDS-IV-P HIV Studies - Monitoring HIV Incidence in the Context of Anti-retroviral Treatment (ART) or Pre-exposure Prophylaxis (PrEP) Therapies (Brazil)a | No | ||||
| The Blood Safety Implications and Advancing Testing for Screening Donors with HIV Infections Taking Antiretroviral (ART) and Pre-exposure Prophylaxis (PrEP) Therapies Studya | No | ||||
|
| |||||
| Blood component quality | Adults | Donor RBC Storage Characteristics Study | Yesd | Yesb | REDS-III RBC-Omics and REDS-III V2V (4 Blood Banks, 12 hospitals, 2013–2016) |
| Core Facility Standardization of RBC Biotin-Labeling: A Tool for Researchers to Perform RBC Survival Studies | Yes | No | |||
Blood donor study only.
New data added to enrich existing database(s).
Databases in collaboration with Non- REDS-IV-P investigators - TIPI_Response_PTMA.
The conduct of the linked aspect of this study is contingent upon ongoing donation-based laboratory studies.
3 |. US PROGRAM AND THE V2V DATABASE
The backbone of the US program is the V2V database which encompasses information from blood donors and their donations, the resulting manufactured blood components, and Electronic Health Record (EHR) data for patients at participating hospitals where the components were distributed for use. Longitudinal databases for donor/donation, components, and patients/transfusion recipients are linked together so that blood products can be tracked from donation to transfusion. These three databases collectively form the V2V database which can be queried to extract the information needed for a particular REDS-IV-P study or analysis and/or to characterize or evaluate trends in blood donor demographics or transfusion practices in particular patient populations. Each hub is expected per the request for proposals (RFP No. HHSN26819HB00003R) from the NHLBI for REDS-IV-P acceptance to have at least a total of 20,000 RBC transfusion exposures occur per year for each hospital/outpatient clinic participating. Further, it was requested that each hub admit, at a minimum, the following number of patients each year:
adult intensive care units: 1000 cross-matched adult patients,
adult cardiac surgery: 1000 cross-matched adults;
adult orthopedic surgery: 500 cross-matched adults;
adult oncology: 1000 cross-matched adults;
adult trauma service: 1000 cross-matched adults or level 1 trauma program;
obstetrical service: 2000 deliveries;
neonatal intensive care unit(s): 1000 neonates admitted;
pediatric intensive care unit(s): 1000 children admitted;
pediatric oncology: 100 pediatric patients with a new cancer diagnosis;
pediatric trauma service: trauma patients or level 1 pediatric trauma program.
The V2V database can also be queried to evaluate whether donor, donation or component characteristics affect the safety and effectiveness of transfused blood products in different patient populations. The inclusion of non-transfused patients in the database allows for comparative effectiveness studies designed to improve transfusion practices. See Table 1 for details on donor unit numbers collected per hub.
The donor, donation, component, and patient data are extracted and transferred to the DCC’s Amazon Web Services Virtual Private Cloud using secure data transfer (sFTP) keys. Submissions are tracked in real-time using dashboards (e.g., dates, records, ongoing status, QC results).
Rooted in principles and best practices of data science, the REDS-IV-P V2V database architecture is based on the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) v.6, which allows for the transformation of data contained within disparate observational databases into a common format and a common representation with minimal data loss (Figure 4, Database Architecture Diagram). The OMOP database tables contain all available information within each data domain (e.g., condition occurrence includes all diagnoses associated with each healthcare encounter and measurements includes all laboratory, body measure, vital sign, and other measurements recorded at the encounter). Data extracted from blood center and hospital systems are transformed into the OMOP CDM for analyses. The source data are retained allowing for potential new analyses or investigations without the need for additional data extractions. The OMOP CDM is scalable and is compatible with other data sources and easily transformed or mapped into other database ontologies to allow for analytics across various data sources. Studies within the REDS-IV-P program will rely on this newly constructed REDS-IV-P V2V database which will include data on a sufficient number of pediatric and obstetrical patients to allow for robust statistical analyses. A waiver of informed consent was requested and granted by the single IRB for both hospital patients and blood donors.
FIGURE 4.
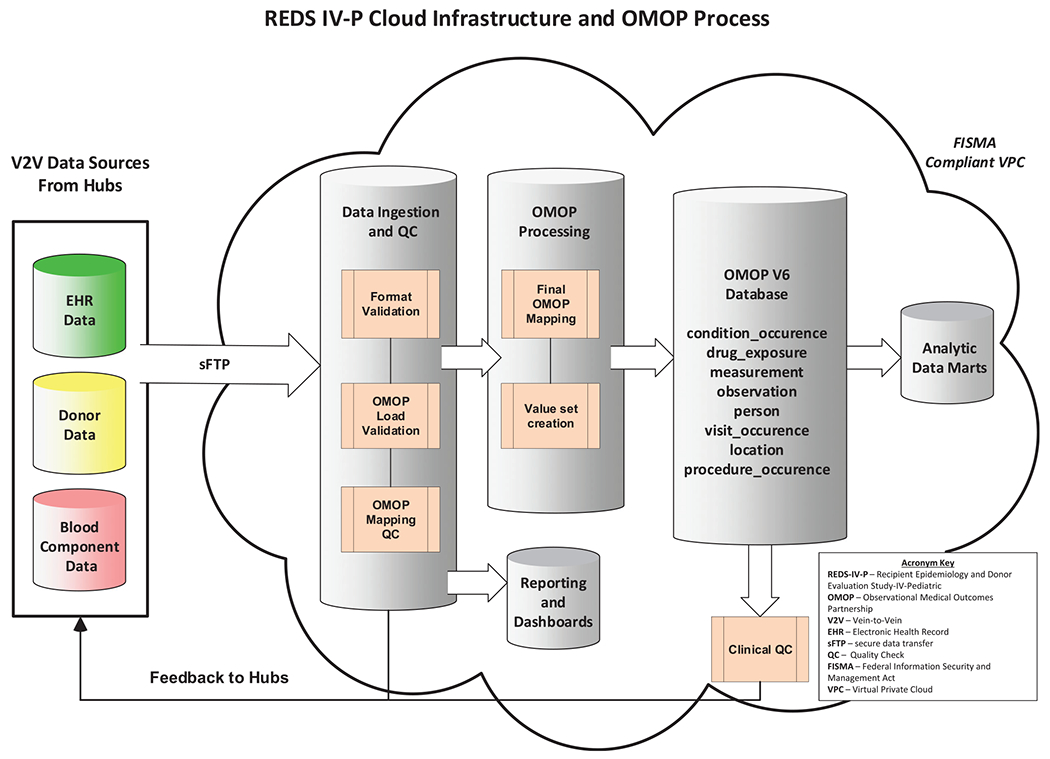
The flow of data for the REDS-IV-P program. Data from the hubs are transferred via sFTP which then goes through multiple QC steps. Feedback is submitted to the hubs at initial intake QC, and after processing stages
4 |. TRANSFUSION EPIDEMIOLOGY
As part of the REDS-IV-P programmatic goals, exploration of the public-use version of the REDS-III V2V database (accessed through BioLINCC at https://www.biolincc.nhlbi.nih.gov.),has enabled investigators to publish multiple pediatric database analyses over the past year.11,12,14,15 The information gleaned for neonatal and pediatric patients was primarily descriptive, yielding insights into blood component utilization and transfusion thresholds in preterm infants, hospitalized children, children undergoing cardiopulmonary by-pass surgery, and those treated for cancer.
5 |. TRANSFUSION RECIPIENT OUTCOMES
As described below, this category of studies includes pediatric only (<18 years of age), adult only, and combined populations.
5.1 |. Transfusion in preterm infants (TIPI)
TIPI endeavors to evaluate transfusion practices, donor characteristics, and blood component manufacturing methods and their association with serious morbidity and mortality among a birth cohort study of transfused very low birthweight (VLBW) infants. The primary hypothesis is that modifiable variation in transfused products, transfusion thresholds, and donor characteristics are associated with serious adverse outcomes in VLBW infants. The study will run from April 1, 2019 to March 31, 2023 an expected enrollment of approximately 2500–3300 VLBW infants, 50% of which will have received at least one transfusion.
Studies involving multiple hospitals, with variations within standard-of-care practices, are required to identify practice differences associated with fewer complications following red blood cell (RBC) or platelet transfusions among a generalizable cohort of infants. Eight birth hospitals participating in REDS-IV-P (Children’s Wisconsin and Marshfield, Yale and Bridgeport, Weill Cornell Medical Center and Morgan Stanley Children’s Hospital of NY/Columbia University Irving Medical Center, University of California San Francisco (UCSF) Medical Center Mission Bay, and Zuckerberg San Francisco General Hospital) are part of the VON and contribute data to VON on VLBW infants. The VON is a voluntary organization dedicated to improving the quality, safety and value of care through a coordinated program of data-driven quality improvement, education, and research of newborns across the world.8 In TIPI, the robust clinical nature of the data inputted into the VON will be leveraged and combined with the donor-component transfusion recipient data collection strengths of REDS-IV-P, creating a novel combined database.
Approximately 50% of VLBW infants (<1500 g) receive a transfusion. Although transfusion can be lifesaving in these very preterm infants, transfusion exposure has been associated with an increased risk of multiple complications, including necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD, a type of chronic lung disease), retinopathy of prematurity (ROP), or death.16
NEC is the most studied with multiple publications describing an association with RBC transfusion17–24 and one meta-analysis identifying RBC transfusion as a significant risk factor for NEC.25 However, at least one prospective study and one meta-analysis did not find such an association,26,27 and a recent GRADE review28 noted the low quality and uncertainty of the overall evidence. A recent multi-center birth cohort study of VLBW infants identified the severity of anemia, defined as hemoglobin ≤8 g/dl, prior to RBC transfusion as being an independent risk factor for NEC.29 Further, a sub-group analysis of a 2017 case-crossover study found that infants with anemia were at greater risk of NEC than infants who were not anemic.30
Respiratory complications are the most common morbidity in VLBW infants, given most infants receive positive-airway pressure respiratory support after birth. The effect of transfusion and specific aspects of transfusion (volume, rate, component characteristics, etc.) on adverse respiratory events, such as the need for an increase in support immediately following transfusion, has been largely unstudied to date.
In this protocol, all VLBW infants will be followed from the time of birth until death or hospital discharge. The primary outcome is defined as at least one of the following: NEC (Bell stage 2 or higher), ROP (stage 3 or higher), moderate or severe BPD (oxygen requirement at 36 weeks postmenstrual age), late-onset sepsis (after 3 days of life), intraventricular hemorrhage (grade 3 or higher), or death. The lack of any of these events will classify an infant as having “survival without morbidity.” Each morbidity that comprises the primary outcome will be analyzed individually as secondary outcomes.
5.2 |. Donor genetic and non-genetic factors affecting RBC transfusion effectiveness in adults
RBC transfusion effectiveness varies due to donor, manufacturing, and recipient factors. Prior studies have identified characteristics accounting for variation in hemoglobin increments post transfusion.31 This study endeavored to extend these observations, interrogating donor genetic and non-genetic factors that may explain the differential RBC transfusion effectiveness in adults. A linked donor-recipient analysis was performed using the public use REDS-III databases. Transfusion effectiveness, defined as the hemoglobin or bilirubin increment following a single RBC unit transfusion was analyzed. Models incorporating a subset of donors with data on single nucleotide polymorphisms associated with in vitro osmotic and oxidative hemolysis are being performed with the goal of identifying additional predictors of transfusion effectiveness.
This multicenter retrospective study investigated 46,705 patients and 102,043 evaluable RBC transfusions from 2013 to 2016 across 12 hospitals. The study found that donor genetics and other factors, such as RBC storage duration, affect transfusion effectiveness.32
5.3 |. ABO-non-identical platelet transfusions and associations with adult recipient morbidity and mortality
Prior studies suggest that transfusion of ABO non-identical plasma and platelets may have adverse clinical effects.33,34 In the case of a major ABO incompatibility, transfused platelets express ABO antigens that are recognized by antibodies in recipient plasma. Such platelets become coated with recipient antibodies and are cleared more quickly and have altered functioning; also, there is transfusion of soluble ABO glycoproteins that are bound by recipient antibodies resulting in the formation of circulating immune complexes that can bind to receptors on cells including platelets.35 In the case of minor ABO incompatibility, the transfused plasma in the platelet unit contains anti-ABO antibodies that recognize recipient ABO glycoproteins which could lead to the formation of soluble and cell surface immune complexes that can have deleterious clinical effects.
This study will access the public use REDS-III linked database to determine whether ABO non-identical platelet transfusions, (i.e., major or minor mismatched) are associated with increased mortality when compared with ABO identical transfusions. The sample size estimate required to achieve 90 percent power for a hazard ratio of 1.15, and R2 of 0–0.75 would be is between 479 and 1913 transfusions.
5.4 |. Red blood cell - IMProving trAnsfusions for chronically transfused pediatric and adult recipients (RBC – IMPACT) - US and Brazilian studies
RBC-IMPACT will be conducted in children and adults in both the US (in patients with SCD, thalassemia, or malignancies) and Brazil (in patients with SCD). SCD and thalassemia are genetic disorders inducing chronic anemia of differing pathophysiology. A primary therapy for preventing certain SCD complications (e.g., stroke) and for thalassemia major is regular RBC transfusion, coupled with iron chelation to prevent the complications of transfusion-induced iron overload. For pediatric hematology-oncology patients with chemotherapy-induced aplasia, RBC transfusion is also common, but the degree of transfusion-induced iron overload and its implications for these patients is incompletely understood.
The objective of this observational cohort study is to assess how blood donor, component, and recipient factors contribute to RBC effectiveness in these chronically and episodically transfused patients, focusing on how specific genetic factors in donors and recipients may impact post-transfusion RBC survival. Aim 1 is to assess markers of RBC survival in recipients receiving simple transfusion therapy using data obtained from a single nucleotide polymorphism (SNP) and copy number polymorphism array typing of donors and recipients, component/manufacturing data, and clinical care data.36,37 Effectiveness will be defined by the calculated change in hemoglobin or hemoglobin variants per day between transfusion visits.38,39
Iron-related tissue toxicity is a major cause of morbidity and mortality in regularly transfused patients. Another study objective is to optimize RBC unit characteristics that patients with SCD and thalassemia receive (beyond RBC phenotype matching for Rh C, E and K antigens), so as to minimize iron loading and iron toxicity.40 Thus, aim 2 will measure markers of hemolysis and iron parameters before and two hours after single-unit transfusion in simply transfused patients with SCD (in both the US and Brazil), thalassemia (US only), or in pediatric patients with a hematologic/oncologic diagnosis and a hypo-proliferative bone marrow (US only). Since anemia in these patient populations is due to differing pathophysiology, it is hypothesized that the relationship between blood donor, manufacturing, and recipient factors and change in serum iron after transfusion will differ by disease. In total, for both the US and Brazil programs the target enrollment for both aims is 500 participants (ClinicalTrials.gov Identifier: NCT05255445).
A residual blood sample from an anticipated 500 blood donors for performing selected, non-actionable gene variant testing, using a ~50,000 feature precision transfusion medicine array (PTMA) developed for the study will be obtained. Survey and other data relating to donor, RBC manufacturing process, and recipient characteristics will be incorporated as well. The study will collect clinical and laboratory data, and extra blood samples pre and post-transfusion from adult and pediatric subjects who consent/assent to participate. Figure 5 depicts the study schema for RBC donors (Figure 5A), recipients in Aim 1 (Figure 5B), and recipients in Aim 2 (Figure 5C).
FIGURE 5.
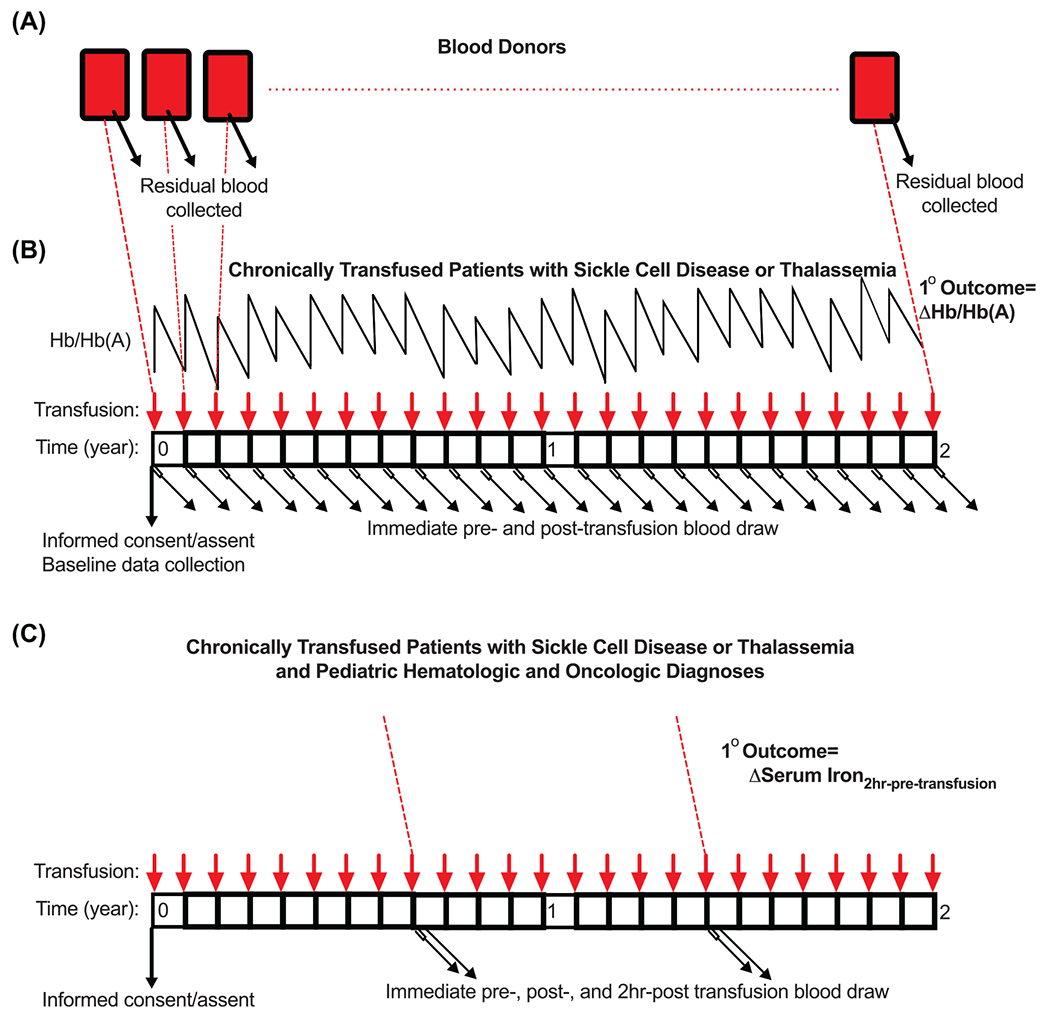
The study schema for donors (A), Aim 1 recipients (B), and Aim 2 recipients (C) in the RBC-MPACT study. (A) Residual blood will be obtained from standard blood donation for determining genetic variant status. (B) In Aim 1, chronically and simply transfused patients with SCD or thalassemia will be consented to obtain clinical data and additional blood samples immediately pre- and post-transfusion from every transfusion episode over a 2-year study period. (C) In Aim 2, subjects from Aim 1 and an additional cohort of pediatric hematology/oncology subjects with hypo-proliferative bone marrow will be recruited to obtain the blood samples collected in Aim 1 plus an additional blood draw 2-hours post-transfusion. Only single RBC unit transfusion events will be enrolled and participation in Aim 2 will be optional for SCD and thalassemia patients at each scheduled transfusion
5.5 |. Brazil sickle cell disease cohort study: Blood utilization and patient outcomes
During REDS-III, a cohort of patients with SCD was established at six participating sites in the Brazilian cities of Sao Paulo, Belo Horizonte, Rio de Janeiro, Recife, Montes Claros, and Juiz de fora.38 The objective in forming this cohort was to characterize clinical and transfusion outcomes in the Brazilian SCD population. This cohort is one of the largest SCD cohorts worldwide with a breadth of well-characterized phenotypic and genotypic data. SNP typing using the ~880,000 feature REDS-III Transfusion Medicine discovery array37 and whole-genome sequencing (WGS) as part of the NHLBI Trans-Omics for Precision Medicine (TOPMed) initiative were also performed to create the infrastructure to perform genotype–phenotype association studies.36 From 2013 to 2015, almost 2800 participants were enrolled with follow up visits conducted through September 2018 as part of REDS-III. Key analyses focused on transfusion-transmitted infections, alloimmunization, blood utilization, and other SCD health outcomes.41–44
In REDS-IV-P, the overall objectives are (1) the continued follow-up and enrichment of this cohort in order to characterize transfusion outcomes in participants, (2) the creation of the infrastructure to successfully execute additional targeted studies, and (3) support of future translational studies relevant to SCD pathogenesis and transfusion complications. Cohort follow-up is critical to characterize long-term outcomes and correlate clinical outcomes with SNP and WGS data. REDS-IV-P will attempt to recruit all children and adults who were consented to the REDS-III cohort and will be expanded by recruiting additional children at the existing REDS-III sites and by adding two new sites, with the goal of bringing the total cohort to 3400 members. Data collection consists of further phenotyping of existing and new participants with SCD, including the collection of standardized quality of life data and additional clinical and transfusion data as well as blood samples to support translational studies relevant to SCD pathogenesis and transfusion complications. New cohort members will be genotyped using the same Transfusion Medicine array used during REDS-III. In addition to multiple analyses on the entire cohort, targeted studies, such as the Recipient Iron biomarkers to investigate iron overload; Rh Variants and Unexplained Antibody Study; and Standardized Echocardiography to Characterize Tricuspid Regurgitation and Pulmonary Hypertension in Chronically Transfused Patients are nested within the Brazil SCD cohort.45
6 |. RAPID RESPONSE TO NEW BLOOD SAFETY ISSUES
6.1 |. Blood donor arbovirus surveillance study in Brazil
Potential transfusion-transmission (TT) of arboviruses has been a key blood safety topic throughout the Americas, especially after the West Nile virus (WNV) US epidemics in the early 2000s46,47 and Zika virus (ZIKV) re-emergence in late 2015/early 2016 in South and Central America, resulting in large outbreaks in Brazil. Direct monitoring of arbovirus infections (ZIKV, Chikungunya virus [CHIKV] and dengue virus [DENV]) in blood donors and other populations is not routinely performed in Brazil. In a continuation of a longitudinal surveillance study launched in REDS-III, the REDS-IV-P CTLS will use a triplex NAT assay developed by Grifols to determine the incidence of these agents in donors based on RNA detection in plasma through time. Minipools (MP) containing pooled plasma samples from 18 blood donors at each of the REDS-IV-P hemocenters will be prepared monthly from July 2019 to March 2024. The project will also retrieve stored individual donor plasma/serum samples twice per year (June and November) for research. In regions where NAT testing indicates large arbovirus outbreaks, these individual samples flanking the MP-NAT identified outbreak will be tested for antibodies to the corresponding arbovirus to establish seasonal sero-incidence of ZIKV, CHIKV, or other arboviruses.48,49
6.2 |. SARS-CoV-2 studies - REDS-IV-P epidemiology, surveillance and preparedness of the novel SARS-CoV-2 epidemic (RESPONSE) - US
The RESPONSE project, funded by NHLBI and in part by the National Institute of Allergy and Infectious Diseases, is led by the REDS-IV-P CTLS and the coordination, data management, and analysis are being performed by the DCC. This project consists of several aims. Firstly, RESPONSE evaluated whether SARS-CoV-2 is a potentially significant TT-pathogen by determining the rate of detection of SARS-CoV-2 RNA in asymptomatic blood donations using very sensitive NAT assays.50 Screening for SARS-CoV-2 RNAemia was performed on retained blood donor minipool samples; approximately 18,000 minipools representing 257,809 individual donations were tested from collaborating blood centers spanning much of the US (Bloodworks Northwest in Seattle, New York Blood Center, Vitalant San Francisco Bay Area, and selected ARC regions (Los Angeles, Boston, and Minneapolis).51 Secondly, in 2020, RESPONSE conducted sero-surveillance of approximately 50,000 donations from these six US regions to document accruing sero-incidence in the blood donor population. This study, using optimized assays/algorithms to monitor seroreactivity in blood donor populations over time,51 served as the precursor of the CDC-funded National Blood donor seroprevalence study (MASS-BD).52 RESPONSE is also studying donors who report post-donation information (PDI) consistent with COVID-19 and determining these rates through December 2021 in approximately 2600 PDI plasma donations. Symptoms of COVID 19 infection are being collected and available plasma from these PDI donations has been tested for SARS-CoV-2 RNA by NAT. In a related study, those PDI donors who were diagnosed with COVID-19 based on reported positive swab tests or whose plasma from their PDI donation tested positive by SARS-CoV-NAT have been offered enrollment into a 12-month longitudinal follow-up to answer fundamental questions on the evolution of RNAemia, early immune responses, and waning of immunity through the collection and testing of multiple sample types (including plasma, gingival swabs, and saliva). Study enrollment from donors reporting PDI and from acutely infected community (i.e., non-blood donor) enrollees totaling 109 as of February 28, 2022. RESPONSE is establishing a sharable biorepository that includes multiple specimen types from these enrolled study subjects.
6.3 |. REDS-IV-P HIV studies: Monitoring HIV incidence in the context of anti-retroviral treatment (ART) or PrEP therapies
As in REDS III, the Brazil program will continue to monitor the incidence and prevalence of HIV infection in blood donors. HIV incidence measurement is important to identify current transmission dynamics, assess risk factors for infection, monitor changes in HIV infection patterns in donors, and evaluate the impact of risk mitigation interventions. In Brazil, ongoing monitoring is highly relevant given the Brazil Supreme Court Decision requiring the removal of all questions related to sexual orientation from the donation selection process.53 REDS-IV-P will identify incident HIV cases using the limiting antigen avidity assay to distinguish between recent and long-term HIV-1 infections in donors who have seroconverted for HIV. This testing allows for the estimation of HIV-1 incidence in cross-sectional studies54 in both repeat and first-time donors.55,56
Recent studies of blood donors from several countries including the US have documented that persons with non-disclosed HIV infections, who are taking antiretroviral therapy (ART) and donors on PrEP, are increasingly donating blood despite intake questions that should have been deferred them from donation.6,7 To further evaluate this new potential threat to blood safety, (1) the REDS-IV-P program will conduct studies to evaluate whether some blood donors in Brazil donate while taking HIV treatment and prevention therapies and (2) the potential impact of ART (approximately 200 donors; 1458 samples) and PrEP (approximately 1100 donors; 1500 samples) on HIV detection by blood donor screening assays using samples sourced from the US, Brazil, South Africa, and Thailand (below).
6.4 |. The blood safety implications and advancing testing for screening donors with HIV infections taking ART and PrEP therapies study
ART and PrEP suppress viremia and immune responses, potentially hampering detection of HIV infection with assays that are widely employed for diagnostics and likely also those used for blood donor screening.57,58 Furthermore, studies of highly exposed persons on PrEP have documented HIV-specific immune responses indicating that abortive or latent infections may be occurring.59,60 Suppressed plasma viremia and delayed seroconversion pose challenges in diagnosing PrEP breakthrough infections, potentially resulting in missed detection of HIV RNA and antibodies by NAT and serological assays. This could result in transfusion of units that would not be detected by routine blood donor screening, but which could transmit HIV.
The objective of this international study led by the REDS-IV-P CTLS is to characterize the altered dynamics of HIV viremia and seroconversion and the performance of donor screening assays in persons taking ART and PrEP. Panels will be constructed using archived longitudinal plasma samples from participants in HIV early treatment studies conducted in San Francisco, South Africa, and Thailand. For PrEP, panels of longitudinal plasma from the iPrEx and Partners PrEP studies will be constructed, including from participants diagnosed as infected when initiating, while on, or shortly after stopping PrEP and from the highest risk participants who were not determined to be infected based on the study diagnostic testing assays/algorithms. Panels will be tested by currently employed blood screening NAT and serology assays and by next-generation HIV molecular and serology assays developed by Roche, Ortho, and Abbott. The study will then evaluate whether testing whole blood by NAT and viral load (VL) assays/platforms enables the detection of HIV infections not detectable by current plasma-based testing algorithms.
7 |. BLOOD COMPONENT QUALITY
7.1 |. Donor RBC storage characteristics
Prior work in the REDS-III RBC-Omics study demonstrated that genetic variation in blood donors underlies predisposition to in vitro hemolysis in RBC components. Osmotic and oxidative hemolysis were highly donor-dependent, while spontaneous hemolysis showed more variability within donors from one donation (screening phase) to the next (recall phase). Spontaneous and oxidative hemolysis showed large increases throughout 42 days of RBC component storage, while osmotic hemolysis showed only a modest increase with storage.61,62
This study is designed to extend these previous findings62 by measuring two potential soluble inflammatory mediators - mitochondrial DNA (mtDNA) and extracellular vesicles (EVs) - as well as by assessing the metabolomic profile of stored RBC units. These analytes will be measured using samples collected at three-time points from stored leukoreduced packed RBC units from donors enrolled in the recall phase of the REDS-III RBC-Omics study which involves approximately 652 donors and 1956 samples from storage time point days, 10, 23, and 42). Testing of samples collected at end of storage (~42 days) from leukoreduced packed RBCs transfer bags from the same donor at two different donations (screening phase and recall phase) will allow an assessment of the continued presence of these markers. These measurements will be correlated with the existing REDS-III in vitro spontaneous, oxidative, and osmotic hemolysis data and with donor genetic data in order to identify whether these novel markers associate with RBC hemolysis.
If one or more of these newly measured parameters are found to be stable in donors across donations, these findings will be correlated with transfusion effectiveness in patients who received RBC from the evaluated donors using the public-use version of the REDS-III V2V database. Outcome measures will include posttransfusion Hgb increment and change in bilirubin as an indirect measure of post-transfusion hemolysis.
7.2 |. Core facility standardization of RBC Biotin-Labeling: A tool for researchers to perform RBC survival studies
The classic method for measuring the survival of transfused RBCs relies on Cr52 labeling of RBCs prior to transfusion. A newer alternative method is biotin labeling of RBCs (BioRBCs) which has several advantages. BioRBCs avoid exposure to radioactivity, allow detection of differentially labeled populations of RBCs, allow detection of a lower proportion of labeled RBCs for a longer duration than Cr52 labeling, and avoid the problem of the very limited, availability of the radioactive chromium reagent.13
The first step in wider use of BioRBCs is to document the quality and safety of biotinylated RBCs that are produced at a site that is remote from the site of BioRBC transfusion. To our knowledge, no previous studies in the US have evaluated the feasibility of using a remote central lab for RBC biotinylation and analyses services (all previous studies used local facilities). Therefore, this REDS-IV-P study is needed to facilitate future post - investigations that would employ a standardized multi-center protocol for the quantification of RBC post-transfusion recovery.
With the successful development of this technique, the REDS-IV-P program intends to compare post-transfusion RBC survival from donors whose RBCs possess genetic variants associated with altered in-vitro hemolysis susceptibility to RBCs from donors who lack these variants.63 This will inform whether findings from in-vitro and genetic studies can be translated to clinical transfusion scenarios.
8 |. SUMMARY AND CONCLUSIONS
The NHLBI and NICHD sponsored multi-center REDS-IV-P program is designed to improve transfusion recipient outcomes across the lifespan with a special emphasis on pediatrics while simultaneously evaluating how to assure a safe and available blood supply for all recipients. The program is poised to launch new hypothesis-driven and hypothesis-generating studies. The infrastructure of REDS-IV-P supports the linkage of donor and recipient data, creating the capability to address key blood banking and transfusion safety concerns. The newly established OMOP structured REDS-IV-P V2V database in combination with the synergistic collaborations between senior and junior REDS-IV-P investigators and multiple outside networks and government agencies provides a recipe for moving towards improved patient outcomes using optimal precision transfusion medicine practices.
ACKNOWLEDGMENTS
The NHLBI Recipient Epidemiology Donor Evaluation Study – IV – Pediatric (REDS-IV-P) domestic program is the responsibility of the following persons:
Hubs
A.E. Mast and L. Baumann Kreuziger, Versiti Wisconsin, Milwaukee, WI.
E.A. Hod, Columbia University Medical Center, New York, NY and B.S. Sachais, New York Blood Center, New York, NY.
B.S. Custer, Vitalant Research Institute, San Francisco, CA and E.P. Vichinsky, Benioff Children’s Hospital Oakland, Oakland, CA.
J.E. Hendrickson, Yale University School of Medicine, New Haven, CT and B.R. Spencer, American Red Cross, Dedham, MA.
Data Coordinating Center
S.M. Mathew, Westat, Rockville, MD.
N.L. Luban, Children’s National Medical Center, Washington, D.C.
Central Laboratory
P.J. Norris and M. Stone, Vitalant Research Institute, San Francisco, CA.
Publications Committee Chairman
P.M. Ness, Johns Hopkins University, Baltimore, MD.
Steering Committee Chairpersons
C.D. Josephson, Emory University, Atlanta, GA.
S.H. Kleinman, University of British Columbia, Victoria, BC, Canada.
National Institute of Child Health and Human Development (NICHD)
R. Tamburro.
National Heart, Lung, and Blood Institute, National Institutes of Health
S. A. Glynn, B. Hailu, and K. Malkin.
The NHLBI Recipient Epidemiology Donor Evaluation Study – IV – P (REDS-IV-P) Brazil Program, is the responsibility of the following persons:
B.S. Custer, U.S. Principal Investigator, Vitalant Research Institute, San Francisco, CA.
S. Kelly, REDS-IV-P U.S. Co-Principal Investigator, UCSF Benioff Children’s Hospital, Oakland, CA.
E.C. Sabino, Brazil Principal Investigator, Faculdade de Medicina da Universidade de Sao Paulo, Brazil.
A.B. Carneiro-Proietti, Brazil Co-Principal Investigator, Hemocentro de Belo Horizonte Hemominas, Minas Gerais, Brazil.
Funding information
The REDS-IV-P program is supported by research contracts from the NHLBI (Contracts 75N92019D00032, 75N92019D00033, 75N92019D00034, 75N92019D00035, 75N92019D00036, 75N92019D00037 and 75N92019D00038). Additional funding was provided by the National Institute of Child Health and Human Development (NICHD) to support some of the pediatric research activities, and by the National Institute of Allergy and Infectious Diseases to support SARS-CoV-2 research within the program.
CONFLICT OF INTEREST
Sonia Bakkour: Financial - Laboratory support from Grifols. Michael P. Busch: Professional - AABB (TTID Committee); ISBT (president elect). Cassandra D. Josephson: Financial - Immucor, LLC (Consultant); Octapharma (Consultant); Professional - Meditronics (Unrestricted Grant). Alan Mast: Professional - Novo Nordisk (Research Funding; honoraria for Advisory Boarch; honorariua for educational seminars); Vega Therapeutics (Honoraria for Advisory Board). All other authors have disclosed no conflicts of interest.
Abbreviations:
- ABO
ABO blood type
- ARC
american red cross
- ART
anti-retroviral treatment
- AZ
Arizona
- BC
British Columbia
- BGC
blood transfusion genomics consortium
- BioLINCC
biologic specimen and data repository information coordinating center
- bioRBCs
biotin labeled RBCs
- BPD
bronchopulmonary dysplasia
- CA
California
- CDC
centers for disease control and prevention
- CDM
common data model
- CHIKV
chikungunya virus
- CO
Colorado
- COVID-19
Coronavirus disease 2019
- Cr52
chromium 52 Isotope
- CT
connecticut
- CTLS
center for transfusion laboratory studies
- D.C.
district of Columbia
- DCC
data coordinating center
- DENV
dengue virus
- EHR
electronic health record
- EVs
extracellular vesicles
- FDA
US food and drug administration
- FISMA
federal information security and management act
- g
grams
- g/dl
grams per deciliter
- GA
georgia
- GRADE
grading of recommendations assessment, development and evaluation
- h
hours
- Hb
hemoglobin
- Hb(A)
hemoglobin A (adult hemoglobin)
- HIV
human immunodeficiency virus
- Inc.
incorporated
- iPrEx
pre-exposure prophylaxis initiative
- IRB
institutional review board
- MA
Massachusetts
- MASS-BD
multistate assessment of SARS-CoV-2 seroprevalence in blood donors
- MD
Maryland
- MINeKids
maternal/in-utero/neonatal/kids
- MP
Minipools
- MP-NAT
Minipools-nucleic acid testing
- mtDNA
mitochondrial deoxyribonucleic acid
- NAT
nucleic acid testing
- NE
northeast
- NEC
necrotizing enterocolitis
- NHLBI
national heart, lung, and blood institute
- NICHD
national institute of child health and human development
- NIH
national institutes of health
- no.
number
- NY
New York
- NYBC
New York Blood Center
- NYPH
New York Presbyterian Hospital
- OMOP
observational medical outcomes partnership
- OSMB
observational study monitoring board
- PDI
post-donation information
- PrEP
pre-exposure prophylaxis
- PTMA
precision transfusion medicine array
- QC
quality check
- RBC
red blood cell
- RBC-IMPACT
red blood cell-improving transfusions for chronically transfused recipients
- RBC-Omics
red blood cell-omics study
- REDS
retrovirus epidemiology donor study
- REDS-II
recipient epidemiology and donor evaluation study - II
- REDS-III
recipient epidemiology and donor evaluation study - III
- REDS-IV-P
recipient epidemiology and donor evaluation study-IV-Pediatric
- RESPONSE
REDS-IV-P epidemiology, surveillance, and preparedness of the novel SARS-CoV-2 epidemic
- RFP
request for proposal
- Rh
Rhesus
- RNA
ribonucleic acid
- ROP
retinopathy of prematurity
- SARS-CoV-2
severe acute respiratory syndrome corona virus-2
- SCANDAT
scandinavian donations and transfusions
- SCD
sickle cell disease
- sFTP
secure file transfer protocol
- SNP
single nucleotide polymorphism
- St.
saint
- TBD
to be determined
- TIPI
transfusion in preterm infants
- TOPMed
trans-omics for precision medicine
- TT
transfusion-transmission
- US
United States
- USA
United States of America
- USCF
University of California San Francisco
- v.6
version 6
- V2V
vein-to-vein
- VL
viral load
- VLBW
very low birthweight
- VON
vermont oxford network
- VPC
virtual private cloud
- VRI
vitalant research institute
- WNV
West nile virus
- WGS
whole-genome sequencing
- WI
Wisconsin
- ZIKV
zika virus
REFERENCES
- 1.Zuck TF, Thomson RA, Schreiber GB, Glicher RO, Kleinman SH, Murphy EL, et al. The Retrovirus Epidemiology Donor Study (REDS): rationale and methods. Transfusion. 1995;35:944–51. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman S, King MR, Busch MP, El M, Glynn SA, National Heart Lung Blood Institute Retrovirus Epidemiology Donor Study; Retrovirus Epidemiology Donor Study-II. The National Heart, Lung, and Blood Institute retrovirus epidemiology donor studies (Retrovirus Epidemiology Donor Study and Retrovirus Epidemiology Donor Study-II): twenty years of research to advance blood product safety and availability. Transfus Med Rev. 2012;26:281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleinman S, Busch MP, Murphy EL, Shan H, Ness P, Glynn SA. The National Heart, Lung and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion. 2014;54:942–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spitalnik SL, Triulzi D, Devine DV, Dzik WH, Eder AF, Gernsheimer T, et al. 2015 proceedings of the National Heart, Lung, and Blood Institute’s State of the Science in Transfusion Medicine symposium. Transfusion. 2015;55:2282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cure P, Bembea M, Chou S, Doctor A, Eder A, Hendrickson J, et al. 2016 proceedings of the National Heart, Lung, and Blood Institute’s scientific priorities in pediatric transfusion medicine. Transfusion. 2017;57:1568–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sykes W, Van den Berg K, Jacobs G, Jauregui A, Roubinian N, Wiesner L, et al. Discovery of false elite controllers: HIV antibody-positive RNA-negative blood donors found to be on antiretroviral therapy. J Infect Dis. 2019;220:643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Custer B, Quiner C, Haaland R, Martin A, Stone M, Reik R, et al. HIV antiretroviral therapy and prevention use in US blood donors: a new blood safety concern. Blood. 2020;136:1351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards EM, Ehret DEY, Soll RF, Horbar JD. Vermont Oxford Network: a worldwide learning community. Transl Pediatr. 2019;8:182–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stone M, Di Germanio C, Wright DJ, Sulaeman H, Dave H, Fink RV, et al. Use of U.S. blood donors for national serosurveillance of SARS-CoV-2 antibodies: basis for an Expanded National Donor Serosurveillance Program. Clin Infect Dis. 2021;74(5):871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326(14):1400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nellis ME, Goel R, Hendrickson JE, Birch R, Patel RM, Karafin MS, et al. Transfusion practices in a large cohort of hospitalized children. Transfusion. 2021;61:2042–53. [DOI] [PubMed] [Google Scholar]
- 12.Patel RM, Hendrickson JE, Nellis ME, Birch R, Goel R, Karem O, et al. Variation in neonatal transfusion practice. J Pediatr. 2021;235:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg AD, Kanias T, Triulzi DJ, Dennis CJ, Moore LR, Meyer EM, et al. Current good manufacturing practices compliant manufacture and measurement of biotin-labeled red blood cells. Cytotherapy. 2019;21:793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanson SJ, Karam O, Birch R, Goel R, Patel RM, Sola-Visner M, et al. Transfusion practices in pediatric cardiac surgery requiring cardiopulmonary bypass: a secondary analysis of a clinical database. Pediatr Crit Care Med. 2021;22:978–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goel R, Nellis ME, Karam O, Hansen SJ, Tormey CA, Patel RM, et al. Transfusion practices for pediatric oncology and hematopoietic stem cell transplantation patients: data from the National Heart Lung and Blood Institute Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). Transfusion. 2021;61:2589–600. [DOI] [PubMed] [Google Scholar]
- 16.Wang YC, Chan OW, Chiang MC, Yang PH, Chu SM, Hsu JF, et al. Red blood cell transfusion and clinical outcomes in extremely low birth weight preterm infants. Pediatr Neonatol. 2017;58:216–22. [DOI] [PubMed] [Google Scholar]
- 17.Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011;158:403–9. [DOI] [PubMed] [Google Scholar]
- 18.Christensen RD, Lambert DK, Henry E, Wiedmeirer SE, Snow GL, Baer VL, et al. Is “transfusion-associated necrotizing enterocolitis” an authentic pathogenic entity? Transfusion. 2010;50:1106–12. [DOI] [PubMed] [Google Scholar]
- 19.Christensen RD, Wiedmeier SE, Baer VL, Henry E, Gerday E, Lambert DK, et al. Antecedents of Bell stage III necrotizing enterocolitis. J Perinatol. 2010;30:54–7. [DOI] [PubMed] [Google Scholar]
- 20.Derienzo C, Smith PB, Tanaka D, Bandarenko N, Campbell ML, Herman A, et al. Feeding practices and other risk factors for developing transfusion-associated necrotizing enterocolitis. Early Hum Dev. 2014;90:237–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Dib M, Narang S, Lee E, Massaro AN, Aly H. Red blood cell transfusion, feeding and necrotizing enterocolitis in preterm infants. J Perinatol. 2011;31:183–7. [DOI] [PubMed] [Google Scholar]
- 22.Josephson CD, Wesolowski A, Bao G, Sola-Visner M, Dudell G, Castillejo M, et al. Do red cell transfusions increase the risk of necrotizing enterocolitis in premature infants? J Pediatr. 2010; 157(6):972–978.e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin T, Strickland OL. Transfusion-related necrotizing enterocolitis: a conceptual framework. Adv Neonatal Care: Off. J Natl Assoc Neonatal Nurses. 2013;13:166–74. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Visintainer PF, Frantz ID 3rd, Shah BL, Meyer KM, Favila SA, et al. Association of necrotizing enterocolitis with anemia and packed red blood cell transfusions in preterm infants. J Perinatol. 2011;31:176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed A, Shah PS. Transfusion associated necrotizing enterocolitis: a meta-analysis of observational data. Pediatrics. 2012;129:529–40. [DOI] [PubMed] [Google Scholar]
- 26.Sharma R, Kraemer DF, Torrazza RM, Mai V, Neu J, Shuster JJ, et al. Packed red blood cell transfusion is not associated with increased risk of necrotizing enterocolitis in premature infants. J Perinatol. 2014;34:858–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg P, Pinotti R, Lal CV, Salas AA. Transfusion-associated necrotizing enterocolitis in preterm infants: an updated meta-analysis of observational data. J Perinat Med. 2018;46:677–85. [DOI] [PubMed] [Google Scholar]
- 28.Hay S, Zupancic JA, Flannery DD, Kirpalani H, Dukhovny D. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Semin Perinatol. 2017;41:80–91. [DOI] [PubMed] [Google Scholar]
- 29.Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. 2016;315:889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le VT, Klebanoff MA, Talavera MM, Slaughter JL. Transient effects of transfusion and feeding advances (volumetric and caloric) on necrotizing enterocolitis development: a case-crossover study. PLoS One. 2017;12:e0179724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roubinian NH, Jennifer C, Woo P, Lee C, Bruhn R, Liu VX, et al. Effect of donor, component, and recipient characteristics on hemoglobin increments following red blood cell transfusion. Blood. 2019;134(13):1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roubinian NH, Reese SE, Qiao H, Plimier C, Fang F, Page GP, et al. Donor genetic and non-genetic factors affecting RBC transfusion effectiveness. JCI Insight. 2022;7(1):e152598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanwell A, Andersson TM, Rostgaard K, Edgren G, Hjalgrim H, Norda R, et al. Post-transfusion mortality among recipients of ABO-compatible but non-identical plasma. Vox Sang. 2009;96:316–23. [DOI] [PubMed] [Google Scholar]
- 34.Magid-Bernstein J, Beaman CB, Carvalho Poyraz F, Boehme A, Hod EA, Francis RO, et al. Impacts of ABO incompatible platelet transfusions on platelet recovery and outcomes after intracerebral hemorrhage. Blood. 2021;137:2699–703. 10.1182/blood.2020008381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heal JM, Masel D, Rowe JM, Blumberg N. Circulating immune complexes involving the ABO system after platelet transfusion. Br J Haematol. 1993;85:566–72. [DOI] [PubMed] [Google Scholar]
- 36.Carneiro-Proietti ABF, Kelly S, Miranda Teixeira C, Sabino EC, Alencar CS, Capuani L, et al. Clinical and genetic ancestry profile of a large multi-centre sickle cell disease cohort in Brazil. Br J Haematol. 2018;182:895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Y, Busch MP, Seielstad M, Endres-Dighe S, Westhoff CM, Keating B, et al. Development and evaluation of a transfusion medicine genome wide genotyping array. Transfusion. 2019;59:101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sagiv E, Fasano RM, Luban NLC, Josephson CD, Stowell SR, Roback JD, et al. Glucose-6-phosphate-dehydrogenase deficient red blood cell units are associated with decreased posttransfusion red blood cell survival in children with sickle cell disease. Am J Hematol. 2018;93:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Alessandro A, Fu X, Kanias T, Reisz JA, Culp-Hill R, Guo Y, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2021;106(5):1290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dinardo CL, Oliveira TGM, Kelly S, Ashley-Koch A, Telen M, Schmidt LC, et al. Diversity of variant alleles encoding Kidd, Duffy, and Kell antigens in individuals with sickle cell disease using whole genome sequencing data from the NHLBI TOPMed Program. Transfusion. 2021;61:603–16. [DOI] [PubMed] [Google Scholar]
- 41.Blatyta PF, Kelly S, Sabino E, Preiss L, Mendes F, Carneiro-Proietti AB, et al. Prevalence of serologic markers of transfusion and sexually transmitted infections and their correlation with clinical features in a large cohort of Brazilian patients with sickle cell disease. Transfusion. 2020;60:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belisario AR, Carneiro-Proietti AB, Sabino EC, Araujo A, Loureiro P, Maximo C, et al. Hb S/β-thalassemia in the REDS-III Brazil sickle cell disease cohort: clinical, laboratory and molecular characteristics. Hemoglobin. 2020;44:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelly S, Belisário AR, Werneck Rodrigues DO, Carneiro-Proietti ABF, Gonçalez TT, Loureiro P, et al. Blood utilization and characteristics of patients treated with chronic transfusion therapy in a large cohort of Brazilian patients with sickle cell disease. Transfusion. 2020;60:1713–22. [DOI] [PubMed] [Google Scholar]
- 44.Nunes K, Aguiar VRC, Silva M, Sena AC, de Oliveira DCM, Dinardo CL, et al. How ancestry influences the chances of finding unrelated donors: an investigation in admixed Brazilians. Front Immunol. 2020;11:584950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinardo CL, Kelly S, Dezan MR, Ribeiro IH, Castilho SL, Schimidt LC, et al. Diversity of RH and transfusion support in Brazilian sickle cell disease patients with unexplained Rh antibodies. Transfusion. 2019;59:3228–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bianco C. Dengue and Chikungunya viruses in blood donations: risks to the blood supply? Transfusion. 2008;48:1279–81. [DOI] [PubMed] [Google Scholar]
- 47.Petersen LR, Busch MP. Transfusion-transmitted arboviruses. Vox Sang. 2010;98:495–503. [DOI] [PubMed] [Google Scholar]
- 48.Simmons G, Bres V, Lu K, Liss NM, Brambilla DJ, Ryff KR, et al. High incidence of Chikungunya virus and frequency of viremic blood donations during epidemic, Puerto Rico, USA, 2014. Emerg Infect Dis. 2016;22(7):1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williamson PC, Custer B, Biggerstaff BJ, Lanciotti RS, Sayers MH, Eason SJ, et al. Incidence of West Nile virus infection in the Dallas-Fort Worth metropolitan area during the 2012 epidemic. Epidemiol Infect. 2017;145(12):2536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakkour S, Saá P, Groves JA, Montalvo L, Germanio CD, Best SM, et al. Minipool testing for SARS-CoV-2 RNA in United States blood donors. Transfusion. 2021;61:2384–91. [DOI] [PubMed] [Google Scholar]
- 51.Stone M, Germanio CD, Wright DJ, Sulaeman H, Dave H, Fink RV, et al. Use of U.S. blood donors for national serosurveillance of SARS-CoV-2 antibodies: basis for an Expanded National Donor Serosurveillance Program. Clin Infect Dis. 2022;74:871–81. 10.1093/cid/ciab537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326(14):1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Federal Court of Justice. Prohibition of blood donation by homosexual men is unconstitutional, decides STF. 2020. Available from: http://portal.stf.jus.br/noticias/verNoticiaDetalhe.asp?idConteudo=443015&ori=1. Accessed 1 July.
- 54.Murphy G, Pilcher CD, Keating SM, Kassanjee R, Facente SN, Welte A, et al. Moving towards a reliable HIV incidence test – current status, resources available, future directions and challenges ahead. Epidemiol Infect. 2017;145:925–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grebe E, Busch MP, Notari EP, Bruhn R, Quiner C, Hindes D, et al. HIV incidence in US first-time blood donors and transfusion risk with a 12-month deferral for men who have sex with men. Blood. 2020;136(11):1359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vermeulen M, Chowdhury D, Swanevelder R, Grebe E, Bambrilla D, Jentsch U, et al. HIV incidence in South African blood donors from 2012 to 2016: a comparison of estimation methods. Vox Sang. 2021;116:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donnell D, Ramos E, Celum C, Balten J, Dragavon J, Tappero J, et al. The effect of oral preexposure prophylaxis on the progression of HIV-1 seroconversion. AIDS. 2017;31:2007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manak MM, Jagodzinski LL, Shutt A, Malia JA, Leos M, Ouellette J, et al. Decreased seroreactivity in individuals initiating antiretroviral therapy during acute HIV infection. J Clin Microbiol. 2019;57:e00757–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuebler PJ, Mehrotra ML, McConnell JJ, Holditch SJ, Shaw BI, Tarosso LF, et al. Cellular immune correlates analysis of an HIV-1 preexposure prophylaxis trial. Proc Natl Acad Sci U S A. 2015;112:8379–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pattacini L, Baeten JM, Thomas KK, Fluharty TR, Murnane PM, Donnell D, et al. Regulatory T-cell activity but not conventional HIV-specific T-cell responses are associated with protection from HIV-1 infection. J Acquir Immune Defic Syndr. 2016;72:119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lanteri MC, Kanias T, Keating S, Stone M, Guo Y, Page GP, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: results of recall phase of the REDS-III RBC-Omics study. Transfusion. 2019;59:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page GP, Kanias T, Guo YJ, Lanteri MC, Zhang X, Mast AE, et al. Multiple-ancestry genome-wide association study identifies 27 loci associated with measures of hemolysis following blood storage. J Clin Invest. 2021;131:e146077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Endres-Dighe SM, Guo Y, Kanias T, Lanteri M, Stone M, Spencer B, et al. Blood, sweat, and tears: Red Blood Cell-Omics study objectives, design, and recruitment activities. Transfusion. 2019;59:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]


