Abstract
The purpose of this retrospective study was to assess the efficacy of antimicrobial therapy for bovine acute Klebsiella pneumoniae mastitis. We evaluated data from cattle in Ehime, Japan, with naturally occurring acute mastitis due to K. pneumoniae (n=208) or Escherichia coli (n=201). Survival was significantly shorter in cattle with acute K. pneumoniae mastitis (median, 76 days) compared with the disease caused by E. coli (median 464 days). In 2004–2008, because both species were highly susceptible to cefazolin, cases of K. pneumoniae and E. coli mastitis were treated solely with cefazolin, yielding clinical cure rates of 52.8% for K. pneumoniae and 86.0% for E. coli. However, since 2009, the efficacy of treatment of K. pneumoniae mastitis with cefazolin alone has decreased. When cefazolin administered on the first disease day led to clinical improvement, treatment with cefazolin was continued. However, when cefazolin administered on the first disease day failed to yield clinical improvement, the antibiotic was switched to a fluoroquinolone on the second day, resulting in cure rates of 76.7% for K. pneumoniae and 80.0% for E. coli. These findings suggest that, when the first-line drug (e.g., cefazolin) is ineffective, promptly changing to a second-line drug (e.g., a fluoroquinolone) increases the cure rate for bovine K. pneumoniae mastitis.
Keywords: antimicrobial therapy, bovine acute mastitis, Klebsiella pneumoniae
Mastitis remains an important disease among dairy herds worldwide despite widespread control programs that include techniques for teat disinfection, antimicrobial therapy for dry cows, and culling [4]. Although the implementation of these effective control measures significantly reduces the incidence of subclinical mastitis caused by contagious pathogens, including Staphylococcus aureus and Streptococcus agalactiae, these programs alone are generally ineffective for preventing inframammary infections caused by gram-negative pathogens [1, 20]. Furthermore, the rates of coliform-induced inframammary infections are increasing worldwide compared with those from contagious pathogens such as S. aureus and S. agalactiae [13, 21, 28].
Coliform pathogens typically account for the majority of cases of acute clinical mastitis in a dairy herd, and Escherichia coli and Klebsiella pneumoniae are the organisms isolated most frequently [9]. Moreover, Klebsiella spp. account for 39.4% of gram-negative bacterial inframammary infections [27].
The clinical signs of coliform mastitis are more severe than those caused by Streptococcus spp. or Staphylococcus spp. [29], and the severity of clinical disease is positively correlated with peak coliform counts in mammary secretions [9, 29]. Depending on the number of infectious bacteria, cases vary from mild, with local signs only (e.g. swelling of the infected quarter, secretion of abnormal milk) to severe, with remarkable systemic signs (e.g. fever or hypothermia, anorexia, dehydration, diarrhea, weakness) [18]. When clinical signs are extreme, infected cows often die or are culled [18].
When a diseased cow survives, the severe inflammatory reactions in the mammary gland and necrosis of diverse tissues [3, 30] remarkably decrease lactation that cannot recover to preinfection levels [5]. For this reason, severe mastitis causes huge economic losses, including costs associated with treatment [8, 12], the disposal of drug-contaminated milk, and decreased milk yield because of reduced or terminated milking [7]. In particular, acute mastitis caused by K. pneumoniae is associated with more severe clinical symptoms than those from E. coli, leading to further decreased milk yields, death, or culling [3, 5]. Moreover, compared with cow with acute E. coli mastitis, cows infected with K. pneumoniae tend to respond less robustly to antibiotics and vaccines [3, 9, 19], and acute K. pneumoniae is often associated with prolonged inframammary infection [27].
Aggressive systemic administration of antimicrobials is recommended for the treatment of severe coliform mastitis to reduce or eliminate bacteria from the infected quarter [23]. However, the efficacy of antimicrobial treatment for acute coliform mastitis is debated, as are various factors that might influence therapeutic effects, such as antimicrobial selection, administration route, and schedule, and disease severity [29]. Furthermore, most investigations of these factors focus on acute E. coli mastitis; few surveys of K. pneumoniae mastitis are available.
To fill these gaps in our knowledge, we retrospectively analyzed clinical data to assess the efficacy of antimicrobial treatment of acute K. pneumoniae mastitis, particularly cases with severe clinical symptoms.
MATERIALS AND METHODS
Cows
We assessed naturally occurring cases of acute mastitis caused by K. pneumoniae (n=208) and E. coli (n=201) in Ehime, Japan, from April 2004 through August 2014. All cows were examined according to livestock medical treatment guidelines. Mastitis was treated in accordance with the disease benefit program established by the Ministry of Agriculture, Forestry and Fisheries of Japan.
Sampling and laboratory analysis
On the first visit, the teat ends of the affected quarters were disinfected with 70% ethanol before sample collection. Samples were collected in sterile vials before treatment by the veterinarian. Samples were stored on ice for transport to the laboratory and processed on the day of collection. Approximately 0.01 ml of milk from each affected quarter was cultured on trypticase soy agar containing 5% sheep blood agar (Becton Dickinson and Co., Japan, Tokyo, Japan) or CHROM agar Orientation/blood agar (Kanto Chemical Co., Inc., Tokyo, Japan). The plates were incubated aerobically at 37°C for 24 to 48 hr. After colony morphology and hemolytic patterns were noted, isolates were examined by using Gram staining and assays for catalase and oxidase activity. The diagnosis of K. pneumoniae or E. coli infections was confirmed by using the EB-20 test (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). Other organisms were processed using routine methods for the isolation and identification of pathogens that cause mastitis [15].
The antimicrobial susceptibilities of K. pneumoniae and E. coli isolates were evaluated by using trypticase soy agar containing 5% sheep blood agar (Becton Dickinson) or sensitivity test agar (Pearlcore, Eiken Chemistry Co., Ltd., Tokyo, Japan), and tested by using the Kirby-Bauer method and antimicrobial disks (BD Sensi-Disk, Becton Dickinson). We tested penicillin, ampicillin, cefazolin, kanamycin, oxytetracycline, enrofloxacin and orbifloxacin.
Survival analysis
We performed a survival analysis to compare the severities of acute mastitis caused by K. pneumoniae and E. coli. We defined the first medical examination as the day when a dairy farmer detected clinical signs of acute mastitis; the day of onset was defined as the day when the dairy farmer asked a veterinarian to administer medical treatment. We assigned the survival or death of each cow according to a common earmark attached to the records of all domestic cows, which were retrieved from the National Administrative Institution, Japan. The date of the survival analysis was that of final confirmation (September 4, 2014). The length of survival of cattle that had died by the final confirmation date was defined as the number of days from the first medical examination to the date of death. The survival of cows alive on the final confirmation date was defined as the number of days from the first examination to the final confirmation date.
Antimicrobial therapy
To investigate antibiotic-driven differences in cure rates cows were classified into the following groups.
From 2004 through 2008, all affected cattle received intravenous cefazolin (Fujita Pharmaceutical Co., Ltd., Tokyo, Japan) at 5.0 mg/kg in 1 l of saline infused into the jugular vein over 1 hr once every 24 hr from the first visit to the final examination. (K. pneumoniae, n=53; E. coli, n=50).
From 2009 through 2014, the first-line drug was cefazolin, which was administered to all cows at the first visit as described for 2004 through 2008. In addition, clinical symptoms present at the first visit were recorded according to the evaluation method of Wenz et al. (Table 1). On the second day of disease, clinical scoring was repeated, and the results of drug sensitivity testing were noted [28]. When the causative organism was susceptible to cefazolin and the clinical score had improved from that on the previous day cefazolin was considered effective, and treatment with cefazolin was continued until the final examination (K. pneumoniae, n=60; E. coli, n=118).
Table 1. Scoring system based on systemic disease signs for classifying the severity of acute coliform mastitis in dairy cows.
| Variable | Score |
||
|---|---|---|---|
| 0 | 1 | 2 | |
| Rectal temperature (°C) | 37.8–39.2 | 39.3–39.8 | >39.8 or <37.8 |
| Hydration status | None | Mild | Marked |
| Rumen contraction (no. per minute) | ≥2 | 1 | 0 |
| Behavioral depression | None | Mild | Marked |
However, when the clinical score was unchanged or worse on the second disease day than on the previous day, cefazolin was considered ineffective, and was replaced with a fluoroquinolone (either enrofloxacin [Elanco Japan Co., Ltd., Tokyo, Japan] at 5 mg/kg IV onece daily or orbifloxacin [DS Pharma Animal Health Co., Ltd., Osaka, Japan] at 5 mg/kg IM onece daily) when the causative bacterium was susceptible to this class of antibiotics (K. pneumoniae, n=30, E. coli, n=10).
We did not control for treatments other than systemic administration of antimicrobials, steroids or nonsteroidal anti-inflammatory drugs, and oxytocin. Fluid therapy was performed according to the judgment of the attending veterinarian.
Clinical cure in dairy cows was defined as the return of appetite, fever, and other systemic symptoms to the pre-disease state, with no abnormalities in the milk or udder.
Statistical analysis
We compared the clinical cure rates of cows with bovine mastitis caused by K. pneumoniae or E. coli by using Fisher’s exact test. Survival analysis was performed according to the Kaplan-Meier method and by using the log-rank test (R software version 2.8.1, R Foundation for Statistical Computing, Vienna, Austria). A P value less than 0.05 was considered to indicate a significant difference.
RESULTS
Frequency of bovine acute mastitis caused by K. pneumoniae
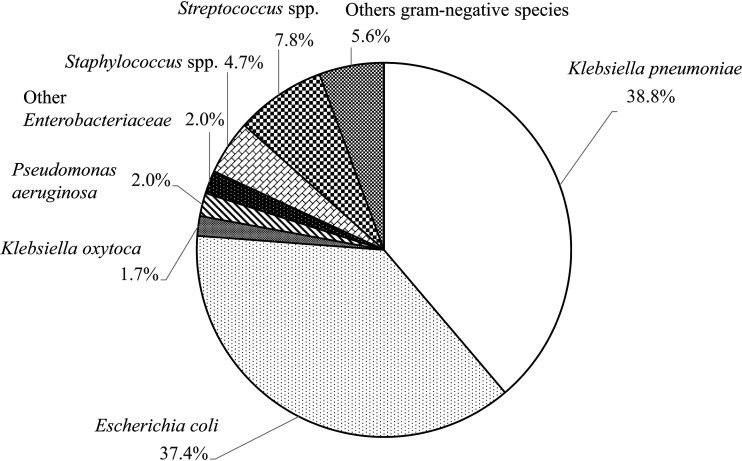
K. pneumoniae and E. coli accounted for 38.8% and 37.4%, respectively, of the 537 natural cases of acute bovine mastitis that occurred in Ehime, Japan, from April 2004 through August 2014. In addition, other gram-negative bacteria were isolated from 5.8% of cases of acute bovine mastitis, Streptococcus spp. from 7.8%, and Staphylococcus spp. from 4.7%. Overall, approximately 75% of cases of acute bovine mastitis were caused by coliforms, most of which were K. pneumoniae or E. coli (Fig. 1).
Fig. 1.
Rates of various bacterial species isolated from cases of acute bovine mastitis in Ehime, Japan (2004–2014, n=537). Klebsiella pneumoniae and Escherichia coli accounted for 38.8% and 37.4%, respectively, of cases of acute mastitis among dairy cows in Ehime.
Survival analysis of cows with acute bovine mastitis
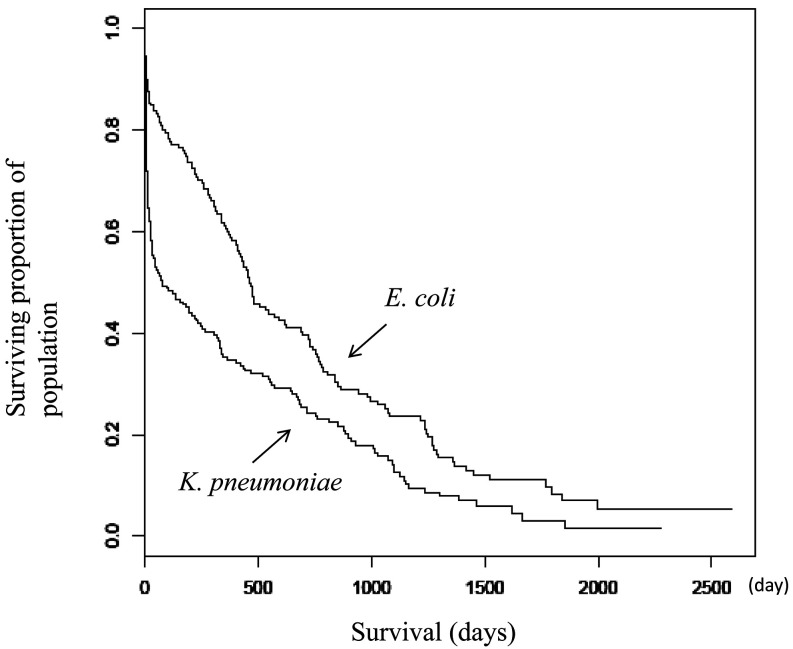
Survival was significantly shorter in dairy cows with acute mastitis caused by K. pneumoniae compared with E. coli (P=0.000148; K. pneumoniae: median survival, 76 days; 95% lower confidence limit, 34 days; 95% upper confidence limit, 230 days; E. coli: median, 464 days; 95% lower confidence limit, 409 days; 95% upper confidence limit, 618 days; Fig. 2). In contrast to that for E. coli, the slope of the survival curve of cows with acute K. pneumoniae mastitis immediately descended steeply indicating that infected cattle died soon after mastitis onset (Fig. 2). Cow age at onset of mastitis (mean ± 1 standard deviation) did not differ between K. pneumoniae (64.4 ± 27.0 d) and E. coli (63.4 ± 25.7 d).
Fig. 2.
Survival curves for acute mastitis caused by Klebsiella pneumoniae and Escherichia coli. Survival was significantly shorter in dairy cows with acute mastitis caused by K. pneumoniae compared with E. coli (P=0.000148; K. pneumoniae: median survival, 76 days; 95% lower confidence limit, 34 days; 95% upper confidence limit, 230 days; E. coli, median survival, 464 days; 95% lower confidence limit, 409 days; 95% upper confidence limit, 618 days.
Sensitivities of K. pneumoniae and E. coli isolates to various antibiotics
During 2004 to 2008, more than 80% of the K. pneumoniae and E.coli strains isolated from dairy cows with acute mastitis were highly sensitive to cefazolin, kanamycin and orbifloxacin (Table 2). Likewise, during 2009 through 2014, similar proportions of isolates from cows that remained on cefazolin throughout the treatment period showed high sensitivity to cefazolin, kanamycin, orbifloxacin, and enrofloxacin. However, only about 70% of K. pneumoniae and E. coli isolates from cows that did not respond to treatment with cefazolin on the first day of illness were sensitive to that antibiotic (Table 2).
Table 2. Sensitivities of Klebsiella pneumoniae and Escherichia coli isolates to various antibiotics.
| Year | 2004–2008 |
2009–2014 |
||||
|---|---|---|---|---|---|---|
| Antibiotic therapy | Cefazolin throughout treatment period |
Cefazolin throughout treatment period |
Cefazolin replaced with a fluoroquinolone on day 2 |
|||
| Causative agent | K. pneumoniae | E. coli | K. pneumoniae | E. coli | K. pneumoniae | E. coli |
| (n=53) | (n=50) | (n=60) | (n=118) | (n=30) | (n=10) | |
| PC | 0/47 (0%)* | 0/47 (0%) | 0/58 (0%) | 0/92 (0%) | 0/30 (0%) | 0/9 (0%) |
| ABPC | 6/46 (13.0%) | 36/47 (76.6%) | 3/58 (5.2%) | 56/88 (63.6%) | 2/28 (7.1%) | 3/9 (33.3%) |
| CEZ | 42/47 (89.4%) | 45/47 (95.7%) | 48/58 (82.8%) | 115/115 (100%) | 22/30 (73.3%) | 7/10 (70.0%) |
| OTC | 34/46 (73.9%) | 34/47 (72.3%) | 41/58 (70.7%) | 81/96 (84.4%) | 21/30 (70.0%) | 3/9 (33.3%) |
| KM | 46/47 (97.9%) | 38/47 (80.9%) | 52/58 (89.7%) | 91/97 (93.8%) | 20/30 (66.7%) | 9/10 (90.0%) |
| OBFX | 11/11 (100%) | 3/3 (100%) | 44/44 (100%) | 88/88 (100%) | 25/25 (100%) | 8/8 (100%) |
| ERFX | Not tested | 1/1 (100%) | 17/17 (100%) | 31/31 (100%) | 6/6 (100%) | 4/4 (100%) |
*: Number of susceptible isolates. PC: penicillin, ABPC: ampicillin, CEZ: cefazolin, OTC: oxytetracycline, KM: kanamycin, OBFX: orbifloxacin and ERFX: enrofloxacin. Data are given as the number of susceptible isolates / the total number of isolates tested (percentage).
Comparison of clinical cure rates
During 2004 through 2008, when all cows received cefazolin throughout the treatment period, clinical cure rates of acute bovine mastitis differed significantly (P<0.01) between K. pneumoniae (52.8%) and E. coli (86.0%) (Table 3). From 2009 onwards, the efficacy of cefazolin monotherapy for acute bovine mastitis decreased. As a result, when cefazolin was administered on the first day of disease and symptoms on day 2 were improved compared with day 1, treatment with cefazolin was continued and yielded a cure rate of 68.3% (41 of 60 cases) for K. pneumoniae (P=0.248 compared with rate during 2004–2008) and 91.5% (108 of 118) for E. coli (P=0.363). In contrast, when cefazolin failed to improve symptoms on day 2, a fluoroquinolone was substituted for cefazolin as a second-line antimicrobial, resulting in a cure rate of 76.7% (23 of 30 cases) for K. pneumoniae and of 80.0% (8 of 10) for E. coli; these cure rates were similar to those for cefazolin monotherapy during 2004 through 2008.
Table 3. Comparison of clinical cure rate of an acute mastitis caused by Klebsiella pneumoniae and Escherichia coli.
| Year | 2004–2008 |
2009–2014 |
||||
|---|---|---|---|---|---|---|
| Antibiotic therapy | Cefazolin throughout treatment period |
Cefazolin throughout treatment period |
Cefazolin replaced with a fluoroquinolone on day 2 |
|||
| Causative agent | K. pneumoniae | E. coli | K. pneumoniae | E. coli | K. pneumoniae | E. coli |
| Cows | 53 | 50 | 60 | 118 | 30 | 10 |
| Cure | 28 | 43 | 41 | 108 | 23 | 8 |
| Death or culling | 25 | 7 | 19 | 10 | 7 | 2 |
| Cure rate | 52.8%a | 86.0%b | 68.3%c | 91.5%d | 76.7%e | 80.0%f |
Statistical analysis showed that neither a and c (P=0.248), b and d (P=0.363), c and d (P=0.197), nor a and e (P=0.843) differed significantly from each other.
DISCUSSION
In Ehime, Japan, during 2004 through 2014, acute mastitis in dairy cows was more frequently caused by E. coli (37.5%) than K. pneumoniae (35.4%). Coliform-associated mastitis represented 76.9% of samples in our current study, which is a higher rate than those reported for the state of Ohio in the United States (E. coli, 28.8%; K. pneumoniae, 28.1% [27]; coliforms, 58.9% [8]). This difference may be explained by the use of sawdust as bedding as well as the hotter and more humid climate of Ehime Prefecture in southwestern Japan compared with midwestern Ohio.
We are unaware of any previous reports that compared the clinical cure rates after naturally occurring acute bovine mastitis due to K. pneumoniae or E. coli. Here we show that systemic administration of cefazolin achieved significantly lower cure rates for acute mastitis caused by K. pneumoniae compared with E. coli. Bovine acute K. pneumoniae mastitis is characterized by a three-time higher maximum hazard ratio of culling at the time of onset of mastitis compared with that caused by E. coli [6]. Similarly, in the present study, significantly more cattle with acute K. pneumoniae mastitis died or were culled compared with cattle with acute E. coli mastitis. Moreover, our survival analysis revealed that dairy cows with acute K. pneumoniae mastitis died or were culled soon after the onset of acute mastitis. Therefore, our current findings suggest that acute K. pneumoniae mastitis was more severe and its associated symptoms progressed faster than the E. coli-driven disease.
The administration of antimicrobial agents to treat acute gram-negative bacterial mastitis is a matter of debate. Because it is effective in eliminating or reducing the causative organism [17], many advocate prompt thorough antimicrobial therapy for the treatment of bovine mastitis [16]. However, others argue that antibiotics should not be given because they lead to the release of endotoxin (lipopolysaccharide) [24], which is considered a health risk to humans [2]. In addition, some consider that antibiotic treatment is insufficient for bovine acute mastitis caused by K. pneumoniae [9, 10, 13].
Regarding acute E. coli mastitis, the only antimicrobials for which there is scientific evidence of beneficial effects are the fluoroquinolones and third- and fourth-generation cephalosporins; and systemic antimicrobial administration of these drugs is suggested for severe mastitis [25]. However, third- and fourth-generation cephalosporins are not approved for bovine mastitis in Japan. Although insufficient data are available for antimicrobial efficacy, suggested alternatives for treating E. coli mastitis include glucocorticoids, nonsteroidal anti-inflammatory agents, frequent milking using oxytocin, and fluid therapy [26].
Here we used cefazolin, a first-generation bactericidal cephalosporin, as a first-line drug, because it is safe and effective and causes few adverse effects [9]. In addition, because cefazolin is a time-dependent antibiotic, its effective use requires maintaining a concentration higher than its minimum inhibitory concentration. Furthermore, the 50% effective dose of cefazolin for K. pneumoniae is approximately 45 times higher than that for E. coli [11]. In the current study, the cure rate for acute mastitis caused by K. pneumoniae during 2004 through 2008 was significantly lower than that caused by E. coli when cefazolin was used throughout the treatment course. This finding suggests that differences in clinical cure rates between pathogens are explained by differences in pathogenicity or antibiotic efficacy.
In a parallel study, we found a correlation between the number of bacteria at the first visit and the severity of clinical symptoms of K. pneumoniae mastitis. The prognosis was poor when the number of bacteria in the milk at the first visit exceeded 2.2 × 105 CFU/ml (data not shown). In support of these findings, Nagasawa et al. reported that the coliform bacterial load in milk is associated with the clinical severity score in bovine mastitis [14]. For this reason, we consider that using cefazolin to treat acute bovine mastitis caused by K. pneumoniae is suboptimal.
Beginning in 2009, when cefazolin failed to achieve a confirmed improvement in clinical symptoms on the second day of disease, cefazolin was discontinued and the administration of a fluoroquinolone was started. When cefazolin was used throughout the treatment period for acute mastitis caused by K. pneumoniae, the cure rate was 68.3%, but when a fluoroquinolone was administered, the cure rate increased to 76.7%. Like other fluoroquinolones, enrofloxacin exerts concentration-dependent bactericidal effects, which persist after a brief exposure (i.e., postantibiotic effect). We surmise that this effect compensated for the disadvantage of the time-dependent effects of cefazolin. Accordingly, we suggest that selecting antibiotics in consideration of the clinical symptoms and drug susceptibility on the second day of disease will increase the clinical cure rate in acute severe K. pneumoniae mastitis.
However, although cephalosporins and fluoroquinolones are recommended for the treatment of severe coliform mastitis, these are critically important drugs, and their use to treat animals used or destined for food production must be rigorously regulated according to bacteriological diagnoses [25]. Therefore, these antimicrobials should only be used to treat severe infections for which their efficacy has been established [26]. Several countries regulate the use of fluoroquinolones in food animals. In Japan, for example, the only cephalosporin approved to treat mastitis in agricultural animals is cefazolin, and enrofloxacin and marbofloxacin are fluoroquinolones. Furthermore, the use of enrofloxacin is limited to cases in which the causative bacteria are those that cause acute mastitis such as E. coli and K. pneumoniae, and when the first-line drug is ineffective.
Given these constraints, we used fluoroquinolones only when the first-line drug, cefazolin, was ineffective. In particular, considering that K. pneumoniae is more pathogenic than E. coli, it is especially important to reduce the number of bacteria promptly. Schukken et al. report that reducing the number of bacteria in milk increases the bacteriological cure rate [22].
Our findings provide convincing support for establishing a simple method for early diagnosis of acute K. pneumoniae mastitis, which will guide the selection of an optimal treatment method. The treatment protocols we described here applied information regarding the antibiotic sensitivities of causal organism on the second day of disease, even when we identified the suspected pathogen earlier. Therefore, early diagnosis including identification of the causative pathogen on the first examination date, will facilitate prompt appropriate, and curative treatment of acute bovine mastitis.
In summary, bovine acute mastitis due to K. pneumoniae is more severe than that caused by E. coli, resulting in a lower clinical cure rate and shorter survival. Therefore, when K. pneumoniae is identified as the causative agent and the first-line antibiotic (e.g., cefazolin) is ineffective, we recommend the prompt substitution with a fluoroquinolones to improve the likelihood of clinical cure.
CONFLICT OF INTEREST
The authors have nothing to disclose.
Acknowledgments
We thank our colleagues in other laboratories for the collection of milk samples from cows with acute bovine mastitis and their help throughout the study.
REFELENCES
- 1.Bannerman D. D., Paape M. J., Hare W. R., Hope J. C.2004. Characterization of the bovine innate immune response to intramammary infection with Klebsiella pneumoniae. J. Dairy Sci. 87: 2420–2432. doi: 10.3168/jds.S0022-0302(04)73365-2 [DOI] [PubMed] [Google Scholar]
- 2.Duda K. A., Lindner B., Brade H., Leimbach A., Brzuszkiewicz E., Dobrindt U., Holst O.2011. The lipopolysaccharide of the mastitis isolate Escherichia coli strain 1303 comprises a novel O-antigen and the rare K-12 core type. Microbiology (Reading) 157: 1750–1760. doi: 10.1099/mic.0.046912-0 [DOI] [PubMed] [Google Scholar]
- 3.Erskine R. J., Bartlett P. C., VanLente J. L., Phipps C. R.2002. Efficacy of systemic ceftiofur as a therapy for severe clinical mastitis in dairy cattle. J. Dairy Sci. 85: 2571–2575. doi: 10.3168/jds.S0022-0302(02)74340-3 [DOI] [PubMed] [Google Scholar]
- 4.Esslemont R. J., Kossaibati M. A.1997. Culling in 50 dairy herds in England. Vet. Rec. 140: 36–39. doi: 10.1136/vr.140.2.36 [DOI] [PubMed] [Google Scholar]
- 5.Gröhn Y. T., Wilson D. J., González R. N., Hertl J. A., Schulte H., Bennett G., Schukken Y. H.2004. Effect of pathogen-specific clinical mastitis on milk yield in dairy cows. J. Dairy Sci. 87: 3358–3374. doi: 10.3168/jds.S0022-0302(04)73472-4 [DOI] [PubMed] [Google Scholar]
- 6.Gröhn Y. T., González R. N., Wilson D. J., Hertl J. A., Bennett G., Schulte H., Schukken Y. H.2005. Effect of pathogen-specific clinical mastitis on herd life in two New York State dairy herds. Prev. Vet. Med. 71: 105–125. doi: 10.1016/j.prevetmed.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Hansen P. J., Soto P., Natzke R. P.2004. Mastitis and fertility in cattle - possible involvement of inflammation or immune activation in embryonic mortality. Am. J. Reprod. Immunol. 51: 294–301. doi: 10.1111/j.1600-0897.2004.00160.x [DOI] [PubMed] [Google Scholar]
- 8.Hoblet K. H., Schnitkey G. D., Arbaugh D., Hogan J. S., Smith K. L., Schoenberger P. S., Todhunter D. A., Hueston W. D., Pritchard D. E., Bowman G. L., Heider L. E., Brockett B. L., Conrad H. R.1991. Costs associated with selected preventive practices and with episodes of clinical mastitis in nine herds with low somatic cell counts. J. Am. Vet. Med. Assoc. 199: 190–196. [PubMed] [Google Scholar]
- 9.Hogan J., Larry Smith K.2003. Coliform mastitis. Vet. Res. 34: 507–519. doi: 10.1051/vetres:2003022 [DOI] [PubMed] [Google Scholar]
- 10.Kitazaki K., Koga S., Nagatoshi K., Kuwano K., Zendo T., Nakayama J., Sonomoto K., Ano H., Katamoto H.2017. In vitro synergistic activities of cefazolin and nisin A against mastitis pathogens. J. Vet. Med. Sci. 79: 1472–1479. doi: 10.1292/jvms.17-0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mastumoto K.1991. Cephems (in Japanese), Iyaku Journal, Osaka. [Google Scholar]
- 12.Miller G. Y., Bartlett P. C., Lance S. E., Anderson J., Heider L. E.1993. Costs of clinical mastitis and mastitis prevention in dairy herds. J. Am. Vet. Med. Assoc. 202: 1230–1236. [PubMed] [Google Scholar]
- 13.Munoz M. A., Welcome F. L., Schukken Y. H., Zadoks R. N.2007. Molecular epidemiology of two Klebsiella pneumoniae mastitis outbreaks on a dairy farm in New York State. J. Clin. Microbiol. 45: 3964–3971. doi: 10.1128/JCM.00795-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagasawa Y., Kiku Y., Sugawara K., Yabusaki T., Oono K., Fujii K., Suzuki T., Maehana K., Hayashi T.2019. The bacterial load in milk is associated with clinical severity in cases of bovine coliform mastitis. J. Vet. Med. Sci. 81: 107–112. doi: 10.1292/jvms.18-0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Mastitis Council. Laboratory and Field Handbook on Bovine Mastitis. 1999. National Mastitis Council, Madison. [Google Scholar]
- 16.Philip M. S., David J. W.2003. Mastitis. Vet Clin North Am Food Anim Pract, WB Saunders Co., Philadelphia. [Google Scholar]
- 17.Rantala M., Kaartinen L., Välimäki E., Stryrman M., Hiekkaranta M., Niemi A., Saari L., Pyörälä S.2002. Efficacy and pharmacokinetics of enrofloxacin and flunixin meglumine for treatment of cows with experimentally induced Escherichia coli mastitis. J. Vet. Pharmacol. Ther. 25: 251–258. doi: 10.1046/j.1365-2885.2002.00411.x [DOI] [PubMed] [Google Scholar]
- 18.Ribeiro M. G., Motta R. G., Paes A. C., Allendorf S. D., Salerno T., Siqueira A. K., Fernandes M. C., Lara G. H. B.2008. Peracute bovine mastitis caused by Klebsiella pneumoniae. Arq. Bras. Med. Vet. Zootec. 60: 485–488. doi: 10.1590/S0102-09352008000200031 [DOI] [Google Scholar]
- 19.Roberson J. R., Warnick L. D., Moore G.2004. Mild to moderate clinical mastitis: efficacy of intramammary amoxicillin, frequent milk-out, a combined intramammary amoxicillin, and frequent milk-out treatment versus no treatment. J. Dairy Sci. 87: 583–592. doi: 10.3168/jds.S0022-0302(04)73200-2 [DOI] [PubMed] [Google Scholar]
- 20.Schukken Y., Chuff M., Moroni P., Gurjar A., Santisteban C., Welcome F., Zadoks R.2012. The “other” gram-negative bacteria in mastitis: Klebsiella, serratia, and more. Vet. Clin. North Am. Food Anim. Pract. 28: 239–256. doi: 10.1016/j.cvfa.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Schukken Y. H., Grommers F. J., van de Geer D., Brand A.1989. Incidence of clinical mastitis on farms with low somatic cell counts in bulk milk. Vet. Rec. 125: 60–63. doi: 10.1136/vr.125.3.60 [DOI] [PubMed] [Google Scholar]
- 22.Schukken Y. H., Bennett G. J., Zurakowski M. J., Sharkey H. L., Rauch B. J., Thomas M. J., Ceglowski B., Saltman R. L., Belomestnykh N., Zadoks R. N.2011. Randomized clinical trial to evaluate the efficacy of a 5-day ceftiofur hydrochloride intramammary treatment on nonsevere gram-negative clinical mastitis. J. Dairy Sci. 94: 6203–6215. doi: 10.3168/jds.2011-4290 [DOI] [PubMed] [Google Scholar]
- 23.Shpigel N. Y., Levin D., Winkler M., Saran A., Ziv G., Böttner A.1997. Efficacy of cefquinome for treatment of cows with mastitis experimentally induced using Escherichia coli. J. Dairy Sci. 80: 318–323. doi: 10.3168/jds.S0022-0302(97)75941-1 [DOI] [PubMed] [Google Scholar]
- 24.Shinozuka Y., Uematsu K., Takagi M., Taura Y.2008. Comparison of the amounts of endotoxin released from Escherichia coli after exposure to antibiotics and ozone: an in vitro evaluation. J. Vet. Med. Sci. 70: 419–422. doi: 10.1292/jvms.70.419 [DOI] [PubMed] [Google Scholar]
- 25.Suojala L., Kaartinen L., Pyörälä S.2013. Treatment for bovine Escherichia coli mastitis-an evidence-based approach. J. Vet. Pharmacol. Ther. 36: 521–531. doi: 10.1111/jvp.12057 [DOI] [PubMed] [Google Scholar]
- 26.Suojala L., Simojoki H., Mustonen K., Kaartinen L., Pyörälä S.2010. Efficacy of enrofloxacin in the treatment of naturally occurring acute clinical Escherichia coli mastitis. J. Dairy Sci. 93: 1960–1969. doi: 10.3168/jds.2009-2462 [DOI] [PubMed] [Google Scholar]
- 27.Todhunter D. A., Smith K. L., Hogan J. S., Schoenberger P. S.1991. Gram-negative bacterial infections of the mammary gland in cows. Am. J. Vet. Res. 52: 184–188. [PubMed] [Google Scholar]
- 28.Wenz J. R., Barrington G. M., Garry F. B., Dinsmore R. P., Callan R. J.2001. Use of systemic disease signs to assess disease severity in dairy cows with acute coliform mastitis. J. Am. Vet. Med. Assoc. 218: 567–572. doi: 10.2460/javma.2001.218.567 [DOI] [PubMed] [Google Scholar]
- 29.Wenz J. R., Barrington G. M., Garry F. B., McSweeney K. D., Dinsmore R. P., Goodell G., Callan R. J.2001. Bacteremia associated with naturally occuring acute coliform mastitis in dairy cows. J. Am. Vet. Med. Assoc. 219: 976–981. doi: 10.2460/javma.2001.219.976 [DOI] [PubMed] [Google Scholar]
- 30.Zhao X., Lacasse P.2008. Mammary tissue damage during bovine mastitis: causes and control. J. Anim. Sci. 86 Suppl: 57–65. doi: 10.2527/jas.2007-0302 [DOI] [PubMed] [Google Scholar]