Abstract
Trichodectes pinguis, referred to commonly as the bear-biting louse, has been reported in several bear species. However, graphical (blurred or coarse) and genetic information on the louse is limited. In this study, we identified T. pinguis collected from Japanese black bears in the Aomori Prefecture, Japan. We confirmed 12S rDNA sequences derived from the collected T. pinguis and performed molecular phylogenetic analysis based on 12S rDNA. The analysis revealed the parasitic louse to be T. pinguis. Interestingly, the body size of T. pinguis found in this study was smaller than the previous recorded body size of them in Japan and Turkey. To better understand the biting louse infesting bears, morphometric and genetic information from other bear hosts needs to be accumulated.
Keywords: morphological identification, phylogenetical analysis, Trichodectes pinguis, 12S rDNA, Ursus thibetanus japonicus
Japanese black bears (Ursus thibetanus japonicus) inhabit wide regions of Honshu Island and Shikoku Island in Japan [7]. Bears tend to inhabit broad-leaved deciduous forests that support their diet, consisting of grasses, berries, and nuts [8]. However, due to the decrease in food resources as results of climate change and other factors, their distribution has expanded over recent years [9, 17]. Thus, conflicts between bears and humans on farms and in villages are more likely. Indeed, the number of Japanese black bears hunted in Japan as a result of harmful wildlife control has increased from 2008 to 2020 (from 1,019 to 6,002 bears) [16]. In Towada City, Aomori Prefecture, several Japanese black bears have been hunted as harmful wildlife control. This made it possible to survey parasitic infections in those bears. So far, ticks and biting louse are the only ectoparasites reported from Japanese black bears [11, 21]. Ticks could serve as a vector of some protozoans such as Babesia sp. and Hepatozoon ursi, which have been detected from the bear blood in the previous studies [10, 12]. Though the disease risk caused by biting louse on Japanese black bears is not known, a previous study reported a case of alopecia and hyperpigmentation caused by biting louse on a Scandinavian brown bear [4].
From July to August 2020, we observed ten Japanese black bears hunted by Aomori Hunting Association (Table 1). The heads or a whole body from the hunted Japanese black bears were kept refrigerated or frozen for 3 to 12 hr, and then, the bear specimens were brought to room temperature prior to the physical examination. During the physical examination, we discovered some ectoparasites on bears, and the ectoparasite samples were collected in 70% ethanol after washing by 0.7% NaCl solution and kept at −30°C until use (Fig. 1). The samples preserved in 70% ethanol were observed under a digital microscope (KH-8700, Hirox, Tokyo, Japan). In previous studies, parasitic lice that use bears as hosts, specifically American black bears [4, 15, 18], European brown bears [2, 3], Japanese brown bear, and Japanese black bear [11, 21], have been identified as Trichodectes pinguis [1]. The morphological features of T. pinguis are described as follows [1, 3] (Fig. 1):
Table 1. Data on the hunted Japanese black bears and the biting louse collected in Towada, Aomori.
|
Ursus thibetanus japonica
|
Trichodectes pinguis |
||||||
|---|---|---|---|---|---|---|---|
| Hunting date | Sex | Age | BW | BL | Physical examination | Female | Male |
| (year) | (kg) | (cm) | |||||
| 18-Jul-20 | Male | 7.5 | 88 | 140 | Head | ||
| 5-Aug-20 | Male | 8.5 | 74 | 150 | Head | ||
| 5-Aug-20 | Male | 2.5 | 45 | 115 | Head | ||
| 16-Aug-20 | Male | 6.5 | 60 | 130 | Head | ||
| 17-Aug-20 | Male | 3.5 | 56 | 134 | Head | 1 | |
| 18-Aug-20 | Male | 3.5 | 55 | 135 | Head | ||
| 18-Aug-20 | Male | 1.5 | 38 | 110 | Head | ||
| 19-Aug-20 | Male | 8.5 | 90 | 145 | Head | 9 | 4 |
| 20-Aug-20 | Female | 1.5 | 30 | 100 | Head | ||
| 25-Aug-20 | Female | 2.5 | 27 | 100 | Whole body | 1 | 2 |
The “Age” of the bears was estimated using the cementum layer method. BW, body weight; BL, body length. Physical examination refers to the body part of the bear reviewed on physical examination.
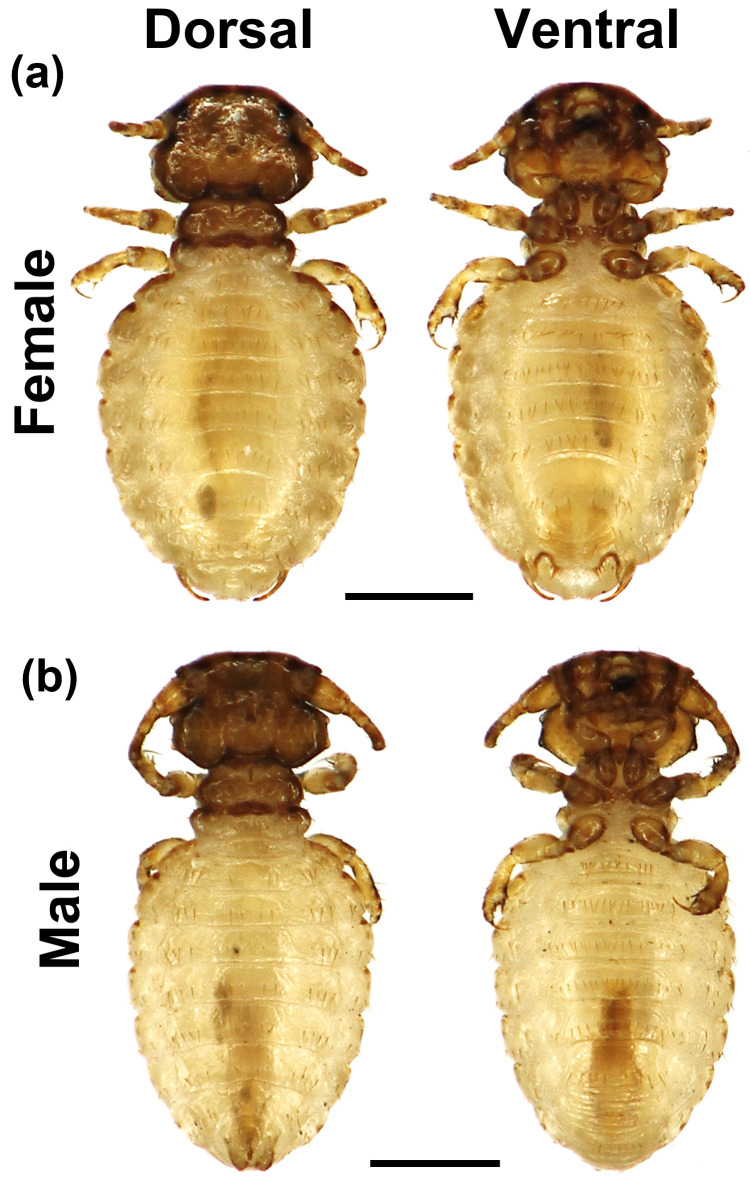
Fig. 1.
Photographs of the biting lice collected from a Japanese black bear. (a) and (b) are female and male specimen, respectively. The right side is dorsal view and the left side is ventral view. The bars indicate 0.5 mm.
“Female [Fig. 1(a)]: Head is relatively wider than the length (Length=0.42 ± 0.07 / Width=0.61 ± 0.1) and originally smooth in anterior, but slightly rounded on the slide-mounted specimen. The anterior part of the head is heavily pigmented. The antenna has three segments, the first of which is slightly wider than the others. The mandible is well developed; the eyes are prominent and have a short seta. The thorax is shorter and narrower than the head (Thorax: Length=0.24 ± 0.04, Width=0.44 ± 0.08 / Head: Length=0.42 ± 0.07, Width=0.61 ± 0.1). The prothorax is trapezoidal and anteriorly narrow. The pterothorax is narrower than the prothorax, lengthwise. The legs are thin, short, and have a fine claw. The abdomen is relatively long and wide (Length=1.19 ± 0.22 / Width=0.97 ± 0.16), and there is only one seta order on the sternits. It has one stout and a sharp speculum on each side of the posterior end.
Male [Fig. 1(b)]: It resembles the female morphology. Normally, the head is slightly convex; however, on the mounted specimen, it is concave anteriorly. The antenna is well-developed; the first segment is clearly larger than the other segments. Male genitalia are observed, as in Fig. 1(b), male, abdomen”.
The morphological features of the biting louse obtained in this study were similar to those of T. pinguis in the Burmeister study [1]. We measured the length and width of each segment (head, thorax, and abdomen) of the collected specimens under a digital microscope (KH-8700, Hirox). The measurements revealed that the body size of the bear-biting louse obtained in this study was smaller than that of the previously reported biting louse (Table 2).
Table 2. The length and width of body parts of Trichodectes pinguis in comparison with other reports.
| Data sources Host bear |
This study Japanese black bear |
Dik & Kılınç, 2015. [2] European brown bear |
Kadosaki et al., 1990. [11] Japanese brown bear |
||||
|---|---|---|---|---|---|---|---|
| (Hunted location) | (Towada, Aomori) | (Turkey) | (Hokkaido, Japan) | ||||
| [Body length (cm)] |
[110–140*] |
[170–220**] |
[160–200*] |
||||
| Body parts | Female | Male | Female | Male | Female | Male | |
| (mm) | n=11 | n=6 | n=1 | n=1 | n=9 | n=5 | |
| Head | Length | 0.42 ± 0.07 | 0.42 ± 0.01 | 0.61 | 0.55 | ND | ND |
| (0.22–0.47) | (0.41–0.43) | ||||||
| Width | 0.61 ± 0.1 | 0.59 ± 0.02 | 0.79 | 0.74 | 0.86 | 0.74 | |
| (0.31–0.66) | (0.56–0.61) | (0.70–0.80) | (0.68–0.85) | ||||
| Thorax | Length | 0.24 ± 0.04 | 0.28 ± 0.03 | 0.42 | 0.43 | ND | ND |
| (0.12–0.29) | (0.25–0.31) | ||||||
| Width | 0.44 ± 0.08 | 0.45 ± 0.02 | 0.64 | 0.62 | ND | ND | |
| (0.22–0.49) | (0.43–0.47) | ||||||
| Abdomen | Length | 1.19 ± 0.22 | 1.14 ± 0.16 | 1.59 | 1.56 | ND | ND |
| (0.58–1.38) | (0.94–1.33) | ||||||
| Width | 0.97 ± 0.16 | 0.88 ± 0.06 | 1.31 | 1.14 | 1.06 | 0.98 | |
| (0.50–1.07) | (0.80–0.94) | (0.91–1.17) | (0.92–1.03) | ||||
| Total length | 1.85 ± 0.32 | 1.84 ± 0.18 | 2.59 | 2.55 | 2.18 | 2.19 | |
| (0.92–2.04) | (1.61–2.04) | (2.00–2.36) | (2.12–2.36) | ||||
Data are presented as mean ± standard deviation (SD). The units of all data are mm. The numbers in parentheses indicate the minimum and maximum values for each entry. In first line, “[Body length (cm)]” indicates the body length range of host bears. In “Body parts (mm)” line, “n=” indicates the biting louse specimens’ measured numbers of each line, respectively. ND, no data. *: The information was derived from Gifu University Research of Japanese brown bear (Higumano-kenkyu) (https://www1.gifu-u.ac.jp/~rcwm/bear_research.html). **: The information was derived from Brief fact sheet European bear (https://www.euronatur.org/en/what-we-do/endangered-species/bear/fact-sheet-brown-bear/).
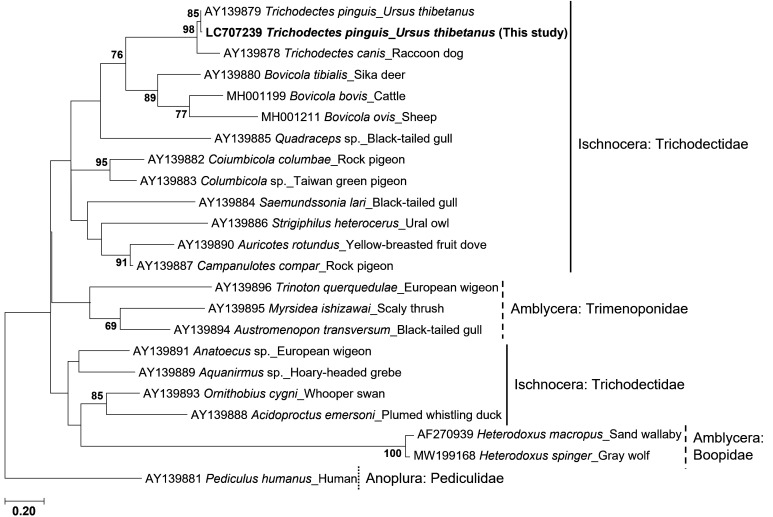
Although we morphologically identified the louse biting the Towanda City bear samples as T. pinguis, our findings showing a body size smaller than that of previous reports propelled us to further confirm the species by a molecular method. In accordance with a previous report, the 12S rDNA sequence of T. pinguis derived from a Japanese black bear was registered in GenBank [22]. Genomic DNA from each biting lice specimen (11 Females and 6 Males) was extracted using a Quick-gDNATM Miniprep Kit (Zymo Research, Irvine, CA, USA), according to the manufacturer’s protocol. A 383-bp fragment of the 12S rDNA gene was amplified by polymerase chain reaction (PCR) using primers 12Sai (5′- AAACTAGGATTAGATACCCTATTAT-3′) and 12Sbi (5′- AAGAGCGACGGGCGATGTGT-3′) [19]. PCR amplification consisted of denaturation at 98°C for 3 min, followed by 40 cycles of denaturation at 94°C for 30 sec, annealing at 45°C for 45 sec, and extension at 72°C for 1 min. Finally, the PCR products were sequenced directly using the Fasmac sequencing service (FASMAC, Atsugi, Japan), using either 12Sai or 12Sbi. The sequence analysis revealed that all PCR products were the identical 12S rDNA sequences. A phylogenetic tree was constructed from 12S rDNA sequences of biting louse derived from several host species using maximum likelihood, as implemented in MEGA7 (https://www.megasoftware.net/) [13]. The evolutionary history was inferred using the maximum likelihood method based on the Tamura-Nei model [20]. A bootstrap method, performed with 1,000 replicates, was used to assess the stability of the phylogenetic trees. Sequence analysis revealed that the same 12S rDNA sequence previously reported (AY139879) was detected in T. pinguis specimens from Japanese black bears in this study. Furthermore, molecular phylogenetic analysis demonstrated that T. pinguis 12S rDNA (Accession number: LC707239) derived from this study was classified into a clade of the biting louse derived from mammalian hosts (Fig. 2). These molecular findings strongly support that the biting louse from Japanese black bears in Towada, Aomori, was T. pinguis.
Fig. 2.
The molecular phylogenetic tree of the biting louse based on the dataset of 12S rDNA sequences. The published sequences of 12S rDNA were used [22]. A partial sequence of Pediculus humanus 12S rDNA (AY139881) was used as a potential outgroup. Branch lengths are drawn to scale, with the scale bar indicating the number of nucleotide substitutions. The boot strap values lower than 60 are not shown.
Sequence and molecular phylogenetic analyses based on 12S rDNA revealed that the biting louse derived from Japanese black bears in Towada, Aomori, were T. pinguis, but their average body size was smaller than that reported in previous studies. According to a previous report comparing the body sizes of avian hosts and their parasites, there was a positive correlation between body size of the host birds and biting louse [6]. In addition, the relationship between the average body length of fleas and the average physique of their rodent hosts across Mongolia was reported as a strong positive correlation [14]. This type of relationship is known as Harrison’s rule [5]. It is an observation of evolutionary biology that parasite body size and the body size of its host are positively correlated. Together with our results and the previous reports, the relationship of body lengths between the biting louse (This study, Female=1.85 ± 0.32 mm / Male=1.84 ± 0.18 mm; Dik & Kilinç, 2015: Female=2.59 mm / Male=2.55 mm, Kadosaki et al., 1990; Female=2.18 mm / Male=2.19 mm) and their host bears (This study, Japanese black bears=110–140 cm; Dik & Kilinç, 2015: European brown bears=170–220 cm, Kadosaki et al., 1990; mainly Japanese brown bears=160–200 cm) seemed to be correlated. These results and previous reports suggest that Harrison’s rule could be applied to T. pinguis and their host bears. However, T. pinguis 12S rDNA in this study was not compared with that derived from other bears; thus, further analysis is needed.
POTENTIAL CONFLICTS OF INTEREST
The authors have nothing to disclose.
Acknowledgments
We would like to thank the two anonymous reviewers for their suggestions and comments, which helped us improve our manuscript. We express our thanks to Aomori Hunting Association for providing Japanese black bear samples.
REFERENCES
- 1.Burmeister H.1838. Malloghaga Nitzsch. Handbuch der Entomologie, 2, Berlin 2: 418–443 (in German). [Google Scholar]
- 2.Dik B., Orunç Kılınç Ö.2015. First case of Trichodectes pinguis (Phthiraptera: Ischnocera: Trichodectidae) on a Bear (Ursus arctos) in Turkey. Turkiye Parazitol. Derg. 39: 313–315. doi: 10.5152/tpd.2015.4040 [DOI] [PubMed] [Google Scholar]
- 3.Dykstra J. A., Rogers L. L., Mansfield S. A., Wünschmann A.2012. Fatal disseminated blastomycosis in a free-ranging American black bear (Ursus americanus). J. Vet. Diagn. Invest. 24: 1125–1128. doi: 10.1177/1040638712461788 [DOI] [PubMed] [Google Scholar]
- 4.Esteruelas N. F., Malmsten J., Bröjer C., Grandi G., Lindström A., Brown P., Swenson J. E., Evans A. L., Arnemo J. M.2016. Chewing lice Trichodectes pinguis pinguis in Scandinavian brown bears (Ursus arctos). Int. J. Parasitol. Parasites Wildl. 5: 134–138. doi: 10.1016/j.ijppaw.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison L.1915. Mallophaga from apteryx, and their significance; with a note on the genus rallicola. Parasitol. 8: 88–100. doi: 10.1017/S0031182000010428 [DOI] [Google Scholar]
- 6.Harnos A., Lang Z., Petrás D., Bush S. E., Szabó K., Rózsa L.2017. Size matters for lice on birds: Coevolutionary allometry of host and parasite body size. Evolution 71: 421–431. doi: 10.1111/evo.13147 [DOI] [PubMed] [Google Scholar]
- 7.Hazumi T., Maruyama N.1987. Movements and habitat use of Japanese black bears in Nikko. Bears: Their Biology and Management 7: 275–279. [Google Scholar]
- 8.Hazumi T.1994. Status of Japanese black bear. Int. Conf. Bear Res. and Manage.9: 145–148.
- 9.Honda T., Kozakai C.2020. Mechanisms of human-black bear conflicts in Japan: In preparation for climate change. Sci. Total Environ. 739: 140028. doi: 10.1016/j.scitotenv.2020.140028 [DOI] [PubMed] [Google Scholar]
- 10.Ikawa K., Aoki M., Ichikawa M., Itagaki T.2011. The first detection of Babesia species DNA from Japanese black bears (Ursus thibetanus japonicus) in Japan. Parasitol. Int. 60: 220–222. doi: 10.1016/j.parint.2011.02.005 [DOI] [PubMed] [Google Scholar]
- 11.Kadosaki M., Kawahara A., Egusa S., Hayashi H., Wakana S.1990. Ectoparasites of the brown and black bears of Japan (I). J. Japanese Wildl. Res. Soc. for Hares 17: 59–76. [Google Scholar]
- 12.Kubo M., Uni S., Agatsuma T., Nagataki M., Panciera R. J., Tsubota T., Nakamura S., Sakai H., Masegi T., Yanai T.2008. Hepatozoon ursi n. sp. (Apicomplexa: Hepatozoidae) in Japanese black bear (Ursus thibetanus japonicus). Parasitol. Int. 57: 287–294. doi: 10.1016/j.parint.2008.01.002 [DOI] [PubMed] [Google Scholar]
- 13.Kumar S., Stecher G., Tamura K.2016. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33: 1870–1874. doi: 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maestri R., Fiedler M. S., Shenbrot G. I., Surkova E. N., Medvedev S. G., Khokhlova I. S., Krasnov B. R.2020. Harrison’s rule scales up to entire parasite assemblages but is determined by environmental factors. J. Anim. Ecol. 89: 2888–2895. doi: 10.1111/1365-2656.13344 [DOI] [PubMed] [Google Scholar]
- 15.Manville A. M., 2nd1978. Ecto- and endoparasites of the black bear in northern Wisconsin. J. Wildl. Dis. 14: 97–101. doi: 10.7589/0090-3558-14.1.97 [DOI] [PubMed] [Google Scholar]
- 16.Ministry of the Environment. Number of bears permitted to be hunted (preliminary figures) (Kuma no Kyokahokakusu (Sokuhouchi)) (in Japanese). http://www.env.go.jp/nature/choju/effort/effort12/capture-qe.pdf [accessed on November 30, 2021].
- 17.Ministry of the Environment. Recent trends in bears in Japan (Zenkoku no Kumarui no Kinnen no Doukou ni Tsuite) (in Japanese). https://www.env.go.jp/nature/choju/conf/conf_wp/conf04-h30/mat01.pdf [accessed on April 19, 2022].
- 18.Rogers L. L.1975. Parasites of black bears of the Lake Superior region. J. Wildl. Dis. 11: 189–192. doi: 10.7589/0090-3558-11.2.189 [DOI] [PubMed] [Google Scholar]
- 19.Simon C., Frati F., Beckenbach A., Crespi B., Liu H., Flook P.1994. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 87: 651–701. doi: 10.1093/aesa/87.6.651 [DOI] [Google Scholar]
- 20.Tamura K., Nei M.1993. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10: 512–526. [DOI] [PubMed] [Google Scholar]
- 21.Yokohata Y., Fujita O., Kamiya M., Fujita T., Kaneko K., Ohbayashi M.1990. Parasites from the Asiatic black bear (Ursus thibetanus) on Kyushu Island, Japan. J. Wildl. Dis. 26: 137–138. doi: 10.7589/0090-3558-26.1.137 [DOI] [PubMed] [Google Scholar]
- 22.Yoshizawa K., Johnson K. P.2003. Phylogenetic position of Phthiraptera (Insecta: Paraneoptera) and elevated rate of evolution in mitochondrial 12S and 16S rDNA. Mol. Phylogenet. Evol. 29: 102–114. doi: 10.1016/S1055-7903(03)00073-3 [DOI] [PubMed] [Google Scholar]