Abstract
The acquisition of iron by pathogenic bacteria is often a crucial step in establishing infection. To accomplish this, many bacteria, including Staphylococcus aureus, produce low-molecular-weight iron-chelating siderophores. However, the secretion and transport of these molecules in gram-positive organisms are poorly understood. The sequence, organization, and regulation of genes involved in siderophore transport are conserved among gram-negative bacteria. We used this information to identify a putative siderophore transport locus from an S. aureus genomic sequence database. This locus contains three predicted open reading frames with a high degree of homology to genes involved in siderophore uptake in several bacterial species, in particular the cbr locus of the plant pathogen Erwinia chrysanthemi. The first gene in the locus, which we have designated sir for staphylococcal iron regulated, encodes a putative lipoprotein with a molecular mass of 37 kDa. The open reading frame is preceded by a 19-bp region of dyad symmetry with homology for operator sequences controlling iron-regulated expression of genes in other bacteria. Fur titration experiments indicate that this region of dyad symmetry is sufficient for Fur-dependent regulation in Escherichia coli. The expression of this gene was repressed, in a dose-dependent manner, by the addition of iron to the S. aureus culture medium. sir-encoded proteins may be involved in iron acquisition in vivo and therefore may be targets for antimicrobial agents.
Iron is an essential nutrient required by virtually all bacteria (30). Because the concentration of free iron in tissue has been estimated to be as low as 10−12 μM (3), most pathogenic bacteria have developed elaborate mechanisms to obtain iron at concentrations sufficient for growth. One of these mechanisms involves the production of low-molecular-weight chelators, collectively called siderophores. Once complexed with iron, siderophores are transported into cells by specific receptors. Both siderophores and their receptors have been well characterized for gram-negative organisms, but their production and utilization in gram-positive bacteria is less well understood.
The production of siderophores by staphylococcal strains has recently been demonstrated. Staphylococcus hyicus has been shown to produce at least two siderophores, staphyloferrins A and B (8, 17). Both staphyloferrins A and B were also found to be produced by certain strains of Staphylococcus aureus. Recently, Courcol et al. (5) identified a third siderophore produced by S. aureus called aureochelin. Modun et al. (18) described the binding of human transferrin by intact S. aureus cells and identified a 42-kDa protein that is presumably responsible for this binding. In addition, several proteins of unknown function have also been shown to be regulated by the available iron concentration. These include proteins with apparent molecular masses of 120, 88, 57, 35, and 33 kDa (5) and of 36 and 39 kDa (15), all of which are repressed by high iron concentrations.
We report here the molecular cloning and characterization of a locus (called sir, for staphylococcal iron regulated) from S. aureus with homology to the siderophore acquisition locus cbr of Erwinia chrysanthemi. We examined the expression of the product of the first gene in this locus, sirA, under iron-limiting conditions and describe a regulatory region upstream of the gene which is similar to ferric uptake regulator (Fur) boxes of gram-negative bacteria. We also provide evidence that production of this protein correlates with accumulation of siderophore activity in supernatants of S. aureus cultures grown under iron-limited conditions. These results suggest that SirA may be a membrane-associated siderophore-binding protein and may serve as an effective target for antimicrobial agents or vaccines.
(A preliminary account of this work was presented at the 98th General Meeting of the American Society for Microbiology [12].)
MATERIALS AND METHODS
Strains and plasmids.
The strains and plasmids used in this study are listed in Table 1. Escherichia coli clones were grown in Luria-Bertani medium containing 50 μg of carbenicillin per ml or on the same medium solidified with 1.5% agar. S. aureus and Staphylococcus epidermidis strains were grown on Trypticase soy agar or in Trypticase soy broth (Difco, Detroit, Mich.) or in defined medium as described below.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Reference or source | Comment(s) |
|---|---|---|
| S. aureus | ||
| ISP3 | J. Iandolo | Strain used for genomic sequencing |
| 8325-4 | 20 | NCTC 8325 cured of prophages, plasmid free |
| S. epidermidis | ||
| 35983 | ATCCa | Blood isolate from patient with intravascular catheter |
| 35984 | ATCC | Catheter sepsis isolate |
| 49134 | ATCC | Clinical isolate |
| E. coli | ||
| H1717 | 9 | aroB fhuF::λplacMu |
| XL1-Blue | Stratagene | Highly transformable strain |
| M15(pREP4) | Qiagen | Host strain for pQE10 |
| H1717(pFur7,8) | This study | E. coli H1717 carrying pFur7,8 |
| H1717(pFur9,10) | This study | E. coli H1717 carrying pFur9,10 |
| H1717(pFur11,12) | This study | E. coli H1717 carrying pFur11,12 |
| Plasmids | ||
| pUC18 | 31 | E. coli cloning vector |
| pQE10 | Qiagen | E. coli expression vector |
| pFur7,8 | This study | pUC18 containing E. coli Fur1 sequence (26) (complementary oligonucleotides Fur 7 and Fur 8) |
| pFur9,10 | This study | pUC18 containing S. aureus sirA Fur-like sequence (complementary oligonucleotides Fur 9 and Fur 10) |
| pFur11,12 | This study | pUC18 containing scrambled sirA Fur-like sequence (complementary oligonucleotides Fur 11 and Fur 12) |
ATCC, American Type Culture Collection.
Cloning and expression of sirA.
The genome of S. aureus ISP3 was sequenced by the whole-genome random-sequencing method essentially as previously described for the sequencing of other microbial genomes (7). An S. aureus homolog of the cbrABC locus of E. chrysanthemi was identified from the collection of genomic DNA sequences based on sequence homology. The open reading frame encoding SirA beginning at amino acid 22 (serine) was amplified by PCR with the N-terminal primer 5′ ACTGTCGACCAGTGGGAATTCAAATAAACAATCATC 3′ and the C-terminal primer 5′ AGTCTGCAGTTTTGATTGTTTTTCAATATTTAAC 3′. The PCR product was digested with the restriction endonucleases SalI and PstI (Boehringer Mannheim, Indianapolis, Ind.), ligated into the same sites in the expression vector pQE10 (Qiagen, Chatsworth, Calif.), and transformed into the E. coli host strain M15(pREP4). The identity of the cloned DNA fragment was verified by completely sequencing both strands of the DNA. A recombinant polyhistidine-tagged SirA fusion protein (His6SirA) was purified under denaturing conditions by using Ni-nitrilotriacetic acid resin (Qiagen) as described by the manufacturer, and denatured protein was refolded by slowly dialyzing it into phosphate-buffered saline (PBS) (pH 7.5) (33). The purity of the recombinant protein was evaluated by Sypro-Red (Molecular Probes, Eugene, Oreg.) staining of a sodium dodecyl sulfate (SDS)-polyacrylamide gel followed by visualization of the fluorescently stained band on a Storm imaging system and quantitation with ImageQuant software (Molecular Dynamics, Sunnyvale, Calif.) and was determined to be 91.4%. The identity of the recombinant protein was confirmed by N-terminal sequencing of the first 20 amino acids.
Generation of antisera.
Polyclonal antiserum to His6SirA was generated at Covance, Inc. (Denver, Pa.). Briefly, a New Zealand White rabbit was immunized with 250 μg of recombinant protein in Freund’s complete adjuvant by intradermal injection. Three and 6 weeks later, the rabbit was boosted with 125 μg of protein in Freund’s incomplete adjuvant by subcutaneous injection. The animal was sacrificed, and serum was collected, 14 days following the second boost. As a negative control, antiserum against an irrelevant recombinant histidine-tagged protein from Streptococcus agalactiae was generated in the same way.
Growth of S. aureus under iron-limited conditions.
Bacteria were grown in disposable plastic labware in staphylococcal siderophore detection (SSD) medium (15), containing 2 mM KH2PO4, 7.9 mM NaCl, 17.2 mM NH4Cl, 2% (vol/vol) 1.5 M Tris-HCl (pH 8.8) solution, 20 mM glucose, 0.6% (wt/vol) Casamino Acids (Difco), 39 μM tryptophan, 32 μM nicotinic acid, and 6 μM thiamine-HCl. Iron was removed from the medium by treatment with 10 g of Chelex resin (Bio-Rad, Hercules, Calif.) per liter for 1 h at room temperature. The medium was sterilized and the resin was removed by filtration, and MgCl2 was added to the medium to 50 μM. Inoculum cultures for iron limitation experiments were grown overnight in SSD medium supplemented with 2 μM FeCl3 at 37°C with shaking (200 rpm) and then diluted 1:100 into fresh medium containing a range of FeCl3 concentrations. In some experiments, CaCl2 was also added at concentrations of between 1 and 10 μM. Growth was continued at 37°C for the specified times. Cells were collected by centrifugation. Cell pellets were used for production of cell lysates, while culture supernatants were filtered through a 0.2-μm-pore-size filter and siderophore activity was determined by the chrome azurol S (CAS) assay (25).
SDS-polyacrylamide gel electrophoresis and immunoblot analysis.
SDS-polyacrylamide gel electrophoresis was performed with gels purchased from Novex (San Diego, Calif.) or cast by the method of Laemmli (14). Proteins were transferred to nitrocellulose membranes, and unbound membrane sites were blocked with PBS containing 0.1% Triton X-100 (PBS-T) with 5% nonfat dry milk and thimerosol (0.01%). To reduce binding of antibody molecules to protein A in cell lysates of S. aureus, normal human serum was added to the blocking solution to 5% (vol/vol). SirA was detected by incubating the membrane for 1 h at room temperature in His6SirA-specific rabbit antiserum diluted to 1:20,000 in PBS-T. Bound antibody was detected by incubation for 1 h at room temperature with a goat anti-rabbit immunoglobulin G alkaline phosphatase-conjugated secondary antibody diluted 1:10,000 in PBS-T or with a goat anti-rabbit immunoglobulin G horseradish peroxidase-conjugated secondary antibody diluted 1:20,000 in PBS-T (both from Kirkegaard & Perry Laboratories, Gaithersburg, Md.). Alkaline phosphatase activity was visualized fluorescently by using Vistra reagent (Amersham, Arlington Heights, Ill.) and a Storm imaging system. Horseradish peroxidase was detected by exposure to film with ECL reagent (Amersham). Molecular weight markers (Rainbow markers) were purchased from Amersham.
Detection of siderophore activity.
Siderophore activity in culture supernatants was estimated by the liquid CAS assay described by Schwyn and Neilands (25). Five-hundred-microliter portions of culture supernatants, or dilutions of supernatants, were mixed with 500 μl of CAS assay solution. Ten microliters of 0.2 M 5-sulfosalicylic acid was then added, and after 5 min of incubation at room temperature, the absorbance at 630 nm was determined. SSD medium was used as a blank, and deferroxamine mesylate (Sigma, St. Louis, Mo.) was used as a reference standard for siderophore activity.
Fur titration assay.
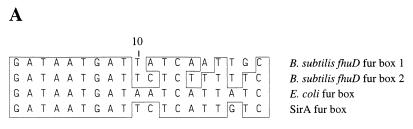
To determine whether the presumptive regulatory sequence upstream of the sirA gene is recognized by the ferric uptake regulator (Fur) protein of E. coli, a Fur titration assay as described by Stojiljkovic et al. (26) was utilized. Oligonucleotide pairs (Fur 7 and Fur 8) were designed to encode the E. coli Fur box designated Fur1 by Stojiljkovic et al. (26) (5′ AATTCGAGATAATGAGAATCATTTTCACG 3′ [Fur 7] and 5′ GATCCGTGAAAATGATTCTCATTATCTCG 3′ [Fur 8]), the sirA Fur box-like sequence (Fur 9 and Fur 10) (see Fig. 2A) (5′ GATCCGATAATGATTCTCATTGTCG 3′ [Fur 9] and 5′ AATTCGACAATCAGAATCATTATCG 3′ [Fur 10]), or a scrambled version of the sirA Fur box-like sequence (Fur 11 and Fur 12) (5′ GATCCTATAATGATTCTGTTACTCG 3′ [Fur 11] and 5′ AATTCGAGTAACAGAATCATTATCG 3′ [Fur 12]). The oligonucleotides were designed to generate restriction endonuclease site 5′ overhangs when annealed to their complementary strands (Fur 7, 10, and 12 contain an EcoRI overhang [single underline], and Fur 8, 9, and 11 contain a BamHI overhang [double underline]). Oligonucleotides (0.25 pmol each) were annealed to their complementary pairs (Fur 7 to Fur 8, Fur 9 to Fur 10, and Fur 11 to Fur 12) by being placed in boiling water for 5 min and then allowed to cool slowly to room temperature. Double-stranded oligonucleotides were then ligated into the vector pUC18, which had previously been digested with BamHI and EcoRI, by using standard cloning techniques (23) and then were transformed into E. coli XL1-Blue (Stratagene, La Jolla, Calif.). Inserts were confirmed by PCR and DNA sequencing, and the plasmids were isolated and transformed by electroporation into E. coli H1717 (26). Transformants were selected on Luria-Bertani agar containing carbenicillin (50 μg/ml) and then streaked onto MacConkey agar plates (Difco) containing carbenicillin and 20 μM Fe(NH4)2(SO4)2. Cultures were incubated overnight at 37°C and examined for brick-red colonies, indicating fermentation of lactose.
FIG. 2.
(A) Analysis of the putative sirA Fur-binding site. An alignment of the presumptive Fur regulon-binding site (Fur box) of the sirA gene against two proposed Fur boxes of the B. subtilis fhuD gene (24) and the E. coli consensus binding site (6) is shown. Nucleotides that are identical to the E. coli sequence are boxed. (B) Nucleotide and deduced amino acid sequences of sirA. The putative Fur box and Shine-Dalgarno (SD) sequences are indicated. The proposed lipoprotein signal sequence is shown, and the cysteine residue that presumably becomes lipid modified is indicated with an arrowhead.
Detergent extraction and phase partitioning.
A mid-log-phase culture of S. aureus 8325-4 was harvested by centrifugation, washed two times in PBS-T, and resuspended in 800 μl of PBS. Cells were lysed by incubation at 37°C with 50 μg of lysostaphin (Applied Microbiology, Inc., Tarrytown, N.Y.) followed by sonication. Triton X-114 phase partitioning was performed essentially as described by Stover et al. (27). Proteins in cellular fractions were precipitated with acetone and suspended in Laemmli sample buffer, and aliquots were subjected to electrophoresis on SDS-polyacrylamide gels followed by Western blotting.
Nucleotide sequence accession number.
The nucleotide sequence described here has been deposited with GenBank and is available under accession number AF079518.
RESULTS AND DISCUSSION
Identification and analysis of the sir locus.
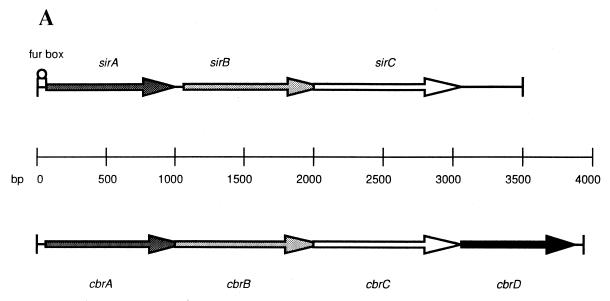
Analysis of the S. aureus ISP3 genome revealed a locus containing three open reading frames with homology for siderophore transport genes in other bacteria. This locus was designated sir (for staphylococcal iron regulated) because of its presumed role in iron uptake. Analysis of the deduced amino acid sequences of the open reading frames revealed that they are most homologous to an iron acquisition locus termed cbr that was previously identified in the plant pathogen E. chrysanthemi (16) (Fig. 1). Iron-mediated control of gene expression in E. chrysanthemi is regulated, at the transcriptional level, by the intracellular iron concentration, which is indirectly dependent on the cbr locus. The cbr locus contains four genes encoding proteins involved in the transport of siderophores. These include cbrA (encoding a periplasmic component), cbrB and cbrC (encoding integral membrane proteins), and cbrD (encoding an ATP-binding protein) (16). This transporter allowed for the accumulation of iron by E. chrysanthemi, via a siderophore distinct from the previously described chrysobactin, when the medium was supplemented with iron as 59FeCl3 or 59Fe dicitrate.
FIG. 1.
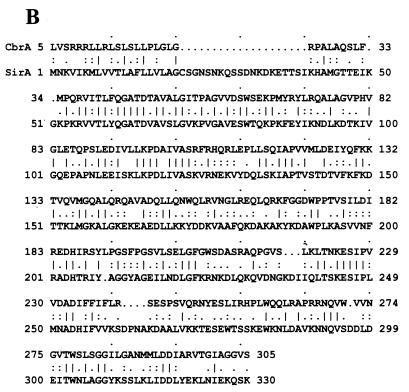
(A) Genetic organization of the sir and cbr loci. The three open reading frames encompassing the sir locus and four open reading frames contained within the cbr locus are indicated. Open reading frames with significant amino acid sequence homology are shown with identical shading patterns. The location of the putative sirA Fur box-like sequence is indicated. (B) Comparison of SirA and CbrA amino acid sequences. The deduced amino acid sequence of SirA was aligned to the CbrA sequence by using the BestFit analysis program of the Wisconsin Sequence Analysis Package (version 8.0). Identical residues are indicated by vertical lines.
The open reading frames located within the sir locus were designated (from 5′ to 3′) sirA, sirB, and sirC. They encode proteins with predicted molecular masses of 36,700 Da (SirA), 35,500 Da (SirB), and 35,700 Da (SirC). SirA was initially identified through its homology to the siderophore-binding protein CbrA, suggesting that SirA may also be involved in the transport of siderophore-iron complexes into staphylococci. Sequence similarity of the SirA protein to CbrA is 61%, with 39% identity (Fig. 1B). The second (sirB) and third (sirC) open reading frames encode putative proteins that also have substantial sequence homology to proteins in the cbr locus (35% identity and 54% similarity to the CbrB protein and 38% identity and 61% similarity to the CbrC protein, respectively). Additionally, the presence of multiple stop codons in other reading frames suggests that the reading frames assigned for sirA, sirB, and sirC are likely to be correct.
The sirA gene is preceded by a region of dyad symmetry resembling a ferric uptake regulator binding site (Fur box) from E. coli and Bacillus subtilis (Fig. 2A). The gene is also preceded by a presumed staphylococcal Shine-Dalgarno sequence (AGGAGGC) (19) ending 10 bp before the start codon (Fig. 2B). The sirB gene begins 16 bp downstream from the termination codon for sirA and contains a putative Shine-Dalgarno sequence (AAGGAGTT) 6 bp upstream of the start codon. Interestingly, the start codon of the sirC gene overlaps the stop codon of sirB, and the Shine-Dalgarno sequence is within the coding region of the sirB gene (GAAAGGA). The amino acid sequence encoded by the sirA open reading frame contains a typical lipoprotein signal peptide with a predicted cleavage site (18 LAGC 21) that conforms to the consensus sequence LA(G,A)C for this class of bacterial proteins as described by von Heijne (32). Both SirA and CbrA contain the three-amino-acid signature sequence described for iron complex-binding proteins (SirA, 105 APNLEEISKLKPDLIV 120) (29).
The gene encoding the mature SirA amino acid sequence (beginning at position 22) was cloned and expressed in E. coli fused to an N-terminal polyhistidine tag. The purity of the recombinant SirA protein, after purification by affinity chromatography with an Ni-nitrilotriacetic acid column, was determined to be 91.4%. The purified protein’s apparent molecular mass (37 kDa) agreed well with that predicted from the amino acid sequence.
Evidence for posttranslational modification of SirA.
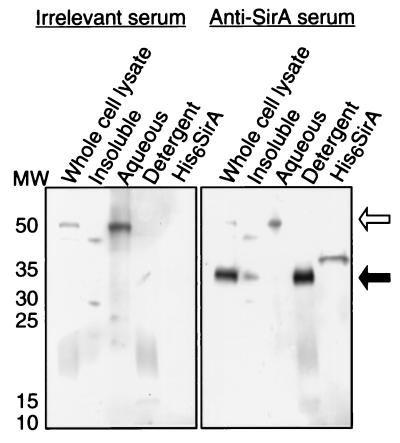
Immunoblot analysis of cell lysates of S. aureus 8325-4 with rabbit polyclonal antiserum generated against recombinant SirA revealed a single band of 37 kDa. Phase partitioning of the Triton X-114-solubilized proteins revealed that SirA was amphipathic, being found predominantly in the detergent phase (Fig. 3). However, Kyte-Doolittle analysis of the SirA sequence predicted that SirA is fairly hydrophilic overall (results not shown). These observations were similar to those made for other bacterial lipoproteins (1, 21) and suggested that the presumptive signal peptidase II cleavage site at cysteine 21 was indeed acylated by S. aureus, thereby conferring an amphipathic character to the native SirA molecule.
FIG. 3.
Fractionation of proteins from whole-cell lysates of S. aureus 8325-4 by phase partitioning with the detergent Triton X-114. Proteins in cellular fractions were analyzed by Western blotting with rabbit polyclonal antisera generated against the recombinant SirA protein or against an irrelevant recombinant protein cloned from S. agalactiae. The filled arrow indicates the position of SirA. The higher-molecular-weight band (open arrow) is presumably due to binding of the antibody molecules to protein A. Lane His6SirA, 100 ng of recombinant SirA; lane MW, molecular weight markers (in thousands).
The molecular mass of the mature SirA protein is similar to the apparent masses of several proteins (35, 36, and 39 kDa) from S. aureus previously described as showing enhanced expression under iron-limited conditions (5, 15) and may represent one of these proteins. Cockayne et al. (4) recently identified two iron-regulated proteins from both S. aureus and S. epidermidis with approximate molecular masses of 32 and 35 kDa. Both of these proteins were extracted by Triton X-114 phase partitioning and were metabolically labeled with tritiated palmitic acid, providing strong evidence that they are lipoproteins and are potentially cytoplasmic membrane associated. Sequencing of the DNA fragment encompassing the gene for the 32 kDa S. epidermidis protein suggested that this gene is part of a locus encoding a presumptive ABC-type transporter, which, in addition to the lipoprotein, also contains a predicted cytoplasmic membrane protein and an ATPase. None of the predicted products of this locus showed any substantial sequence homology to the three proteins encoded by the sir locus, nor were any regulatory control elements identified as was the case for the sirABC locus. Cockayne et al. (4) did not further characterize the 35-kDa iron-regulated lipoprotein from S. aureus, but considering that we found that SirA expression is repressed by elevated iron levels (see below), SirA is a likely candidate for this protein.
In gram-negative bacteria, ABC-type ATPases consist of two transmembrane proteins (each of which spans the membrane approximately six times), one or two ATP-binding proteins that are localized on the cytoplasmic side of the cell membrane, and a periplasmic ligand-binding protein. In gram-positive bacteria, this solute-binding protein is predicted to be a lipoprotein whose lipidated N terminus functions to anchor the protein to the outer leaflet of the cell membrane (22, 28). It seems likely that SirA functions as a membrane-anchored solute-binding protein with specificity for one of the siderophores of S. aureus. SirB and SirC are predicted to be highly hydrophobic proteins by Kyte-Doolittle analysis and may combine to form a transmembrane pore through which the iron-siderophore complex is transported across the membrane. The absence of a gene encoding an ATP-binding protein in the sir locus may simply indicate that this gene was not represented in the genomic library constructed from the S. aureus chromosome.
Growth of S. aureus and S. epidermidis under iron-limited conditions.
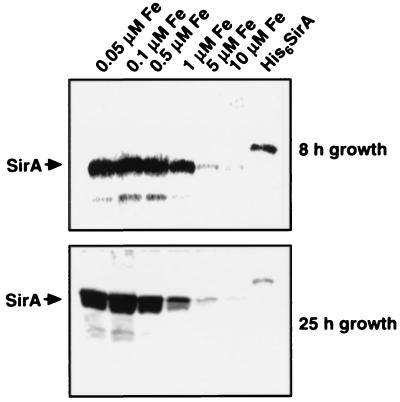
To evaluate the expression of SirA under iron-limited conditions, S. aureus 8325-4, growing in SSD medium under iron-replete conditions (2 μM FeCl3), was subcultured in minimal medium supplemented with FeCl3 to iron concentrations of between 0.05 and 10 μM. Cell lysates were made and normalized for cell numbers. Lysates were examined for the presence of SirA by immunoblotting with rabbit antiserum raised against the recombinant protein. A dramatic increase in production of this protein under low-iron conditions was observed (Fig. 4). Production of SirA was nearly completely repressed in this culture medium at iron concentrations of ≥5 μM. Since Chelex treatment may have also removed other divalent cations, such as Ca2+, from the SSD medium, we examined the effect of Ca2+ supplementation on production of SirA. Supplementation of culture medium with CaCl2, at levels of between 1 and 10 μM, had no effect on the production of SirA (results not shown).
FIG. 4.
Expression of SirA under reduced-iron conditions. S. aureus 8325-4 was grown for 8 or 25 h in deferrated minimal medium supplemented with FeCl3 at concentrations of between 0.05 and 10 μM. SirA was detected in cellular lysates with rabbit polyclonal antiserum raised against His6SirA. Lane His6SirA, 100 ng of recombinant protein.
Protein expression in S. aureus has been shown to be controlled temporally by at least two global regulatory loci, agr and sar (11, 13). S. aureus 8325-4 produced similar amounts of the SirA protein in both the logarithmic and stationary phases of growth, indicating that production of this protein may be independent of the sar and agr loci (results not shown). Although a single passage of S. aureus 8325-4 through iron-deficient medium appeared to derepress SirA expression and production of siderophore activity, this was not sufficient iron limitation to substantially affect cell density (see Fig. 6B). Interestingly, growth of S. aureus under very iron-limited conditions (≤0.1 μM) led to proteolytic degradation of SirA. The basis for this proteolysis is not understood at this time; however, it may be due to the high level of expression seen for this protein at low iron concentrations or may be the consequence of a general stress response under these growth conditions.
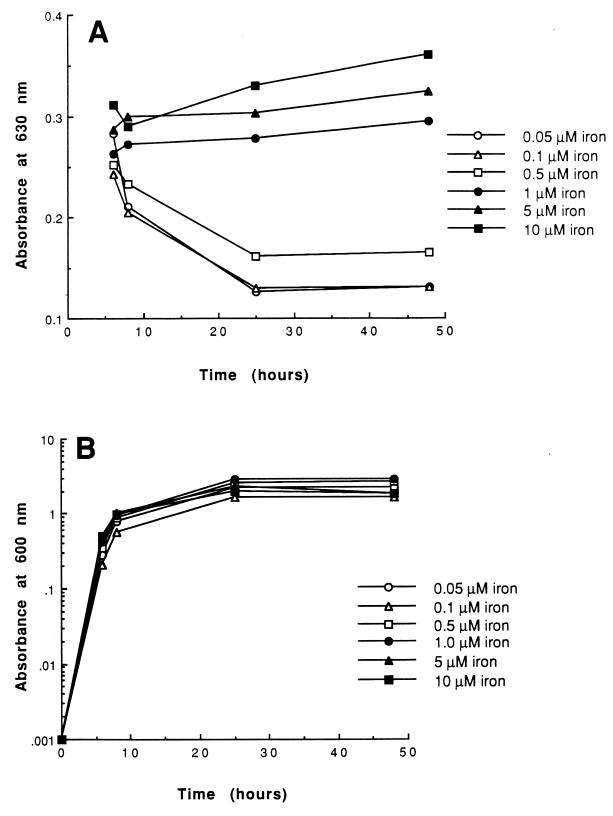
FIG. 6.
(A) Expression of siderophore activity by S. aureus 8325-4. Bacteria were grown in deferrated minimal medium supplemented with FeCl3. At 6, 8, 25, and 48 h of growth, bacteria were removed by centrifugation and siderophore activity present in culture supernatants was detected by the CAS assay (25). (B) Growth was monitored by measuring the absorbance at 600 nm.
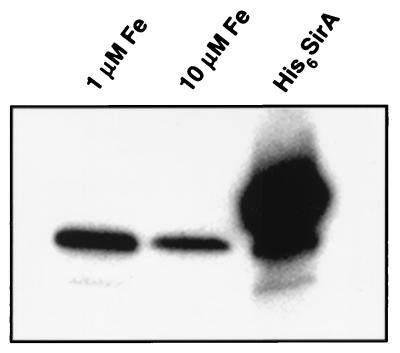
We also examined cell lysates of S. epidermidis by immunoblotting to determine whether a SirA homolog is expressed in this species. An immunoreactive protein with approximately the same molecular mass, 37 kDa, was detected in cell lysates of three clinical isolates of S. epidermidis (ATCC 35983, 35984, and 49134) (results not shown). These included two isolates recovered from patients with catheter-related sepsis (Table 1). We also evaluated the effect of iron concentration on expression of the putative S. epidermidis SirA homolog (Fig. 5). S. epidermidis 35984 required FeCl3 supplements of 1 μM or greater to grow in SSD medium, limiting the range of iron concentrations that could be evaluated. Expression of the putative SirA homolog appeared to be somewhat repressed at the highest FeCl3 concentration tested (10 μM), but the repression of S. aureus 8325-4 SirA expression was greater under these conditions. S. aureus 8325-4 and S. epidermidis 35984 seem to differ in their responses to iron limitation.
FIG. 5.
Expression of a SirA homolog in S. epidermidis. S. epidermidis 35984 was grown in deferrated minimal medium supplemented with either 1 or 10 μM FeCl3. Cells were normalized for optical density and lysed, and expression of the SirA homolog was determined by Western blotting. Lane His6SirA, 100 ng of recombinant SirA.
Correlation of siderophore activity in culture supernatants with SirA expression.
The CAS assay was employed to detect and quantify the accumulation of siderophore activity in culture supernatants of S. aureus. In this assay, a decrease in absorbance indicates the presence of siderophore activity which competes with the CAS dye for binding iron. The production of siderophore activity decreased with increasing concentrations of iron and approached baseline levels at 1 μM iron or greater (Fig. 6A). Decreasing concentrations of iron in culture media had little effect on the growth rate of the bacteria throughout the logarithmic and stationary phases under these conditions (Fig. 6B). The increase in the levels of siderophore activity paralleled an increase in the intensity of bands in immunoblots probed with antiserum specific for SirA (Fig. 4). At iron concentrations of 1 μM or higher, no siderophore activity could be detected by the CAS assay at any time point during growth. Cell lysates of S. aureus cells grown in 1 μM iron still produce a considerable amount of SirA. This may reflect the different levels of control over expression of SirA and siderophore activity or may be due to a relative insensitivity of the CAS assay for staphylococcal siderophore detection. It is also true that the CAS assay indicates the total siderophore activity, which, in this strain of S. aureus, is probably contributed to by more than one type of siderophore (8). The correlation of siderophore production with SirA translation suggests that these two functions may be physiologically related. However, confirmation of the function of SirA awaits identification of the individual siderophore which is presumably transported by this protein.
Evaluation of the Fur-like box in E. coli.
At the outset of these studies, an S. aureus homolog of the E. coli Fur repressor had not been identified but was presumed to exist. We used a Fur activity reporter system in a heterologous E. coli background to evaluate the functionality of the presumptive operator region upstream of the sirA gene (26). A synthetic DNA fragment encompassing the putative Fur-like box upstream of sirA was cloned into pUC18. In addition, the DNA sequence of the putative operator was scrambled and cloned to serve as a negative control. Plasmids containing these synthetic DNA fragments were transformed into the E. coli reporter strain H1717 to determine whether the potential sirA operator sequences contained on a multicopy plasmid could successfully compete for binding of the E. coli Fur protein to the promoter region of the chromosomally encoded fhuF::lacZ fusion. Titration of the Fur protein away from the fhuF::lacZ operator leads to derepression of transcription and expression of the lacZ gene, encoding β-galactosidase activity. When E. coli H1717(pFur9,10), containing a DNA fragment encompassing the putative sirA Fur-binding site, was streaked to a MacConkey agar plate containing 20 μM ferrous ammonium sulfate, the resulting brick-red colonies surrounded by red-pigmented agar indicated that they were capable of fermenting lactose (Fig. 7). These results were similar to those with E. coli H1717(pFur7,8) carrying pUC18 containing the Fur box described by Stojilkovic et al. (Fur1) (26). Neither E. coli H1717(pFur11,12), which contained a scrambled S. aureus Fur box in pUC18, nor E. coli H1717 carrying pUC18 without an insert was able to derepress expression of the lacZ reporter gene, suggesting that the correct sequence of the SirA Fur box was essential for derepression, presumably through binding of the Fur protein in the H1717 host strain.
FIG. 7.
Fur titration analysis of the cloned S. aureus Fur-like sequence in the E. coli reporter strain H1717. E. coli H1717 clones were streaked to MacConkey agar plates supplemented with 20 μM ferrous ammonium sulfate and carbenicillin. Clockwise from top: pFur9,10 (sirA Fur-like sequence), pFur11,12 (scrambled sirA), pFur7,8 (E. coli Fur box), and pUC18 without an insert.
A Fur-like protein has recently been identified in S. epidermidis, although this protein had only a weak affinity for E. coli Fur boxes (10). Taken together, these data suggest that a Fur homolog is expressed in staphylococci and functions in a manner analogous to that of E. coli Fur in iron-dependent gene regulation.
We investigated whether SirA is surface localized in S. aureus, as would be consistent with its proposed function, by labeling surface-exposed proteins with biotin and purifying these proteins by streptavidin chromatography. Immunoblot analysis with SirA-specific antiserum revealed that SirA was among the proteins recovered from lysates of S. aureus cells after labeling of intact cells with biotin (results not shown). Although the localization of SirA needs to be confirmed in a more rigorous manner, biotin labeling of this molecule indicates that it may be surface accessible.
Antisera collected from mice that had been inoculated with S. aureus 8325-4 intraperitoneally revealed that SirA was highly immunogenic during infection (results not shown). This protein was more strongly recognized by antiserum generated through infection with live bacteria than with antiserum generated by immunization with formalin-fixed bacteria, even though the fixed bacteria had been immunized in the presence of Freund’s adjuvant. Although we have not examined the effect of formalin fixation on recognition of SirA by antisera, this evidence suggests that SirA may be abundantly expressed in vivo during the infection process, a scenario that seems plausible considering the protein’s proposed role in iron acquisition.
Our characterization of SirA is consistent with predictions from the sir locus sequence data that this protein is likely to be involved in iron acquisition. Iron acquisition pathways have been targeted for anti-infective agents in the past. In particular, the iron-containing antibiotic albomycin has been shown to be effective against penicillin-resistant pneumococci and staphylococci (2). SirA, or other products of the sir locus, may be a target for development of antibacterial agents because of its potential surface accessibility and apparent expression in vivo. It is not known whether SirA is required for in vivo survival of staphylococci, as more than one siderophore has been identified in some strains of S. aureus (8). However, the fact that this protein also is expressed in strains of S. epidermidis and may be iron regulated in this organism suggests that antimicrobial agents targeted against this protein might have broad specificity for staphylococcal species, including S. aureus.
ACKNOWLEDGMENTS
We thank Ambrose Cheung for providing S. aureus 8325-4, John Iandolo for S. aureus ISP3, Igor Stojilkovic for E. coli H1717, and Scott Koenig, Olaf Schneewind, and Alex Hromockyj for helpful discussions. We also acknowledge Steve Barash for bioinformatics analysis, Cathy Kletke and John Hope for N-terminal sequence analysis, Donni Leach for assistance in preparation of the manuscript, and the National Cancer Institute for allocation of computing time and support staff at the Frederick Biomedical Supercomputing Center of the Frederick Cancer Research and Development Center. The contributions of members of the DNA sequencing facility at Human Genome Sciences are acknowledged.
REFERENCES
- 1.Akins D R, Purcell B K, Mitra M M, Norgard M V, Radolf J D. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993;61:1202–1210. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Budavari S, editor. The merck index. 11th ed. Rahway, N.J: Merck & Co., Inc.; 1989. [Google Scholar]
- 3.Bullen J J, Rogers H J, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 4.Cockayne A, Hill P J, Powell N B L, Bishop K, Sims C, Williams P. Molecular cloning of a 32-kilodalton lipoprotein component of a novel iron-regulated Staphylococcus epidermidis ABC transporter. Infect Immun. 1998;66:3767–3774. doi: 10.1128/iai.66.8.3767-3774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courcol R, Trivier D, Bissinger M-C, Martin G R, Brown M R W. Siderophore production by Staphylococcus aureus and identification of iron-regulated proteins. Infect Immun. 1997;65:1944–1948. doi: 10.1128/iai.65.5.1944-1948.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earhart C F. Uptake and metabolism of iron and molybdenum. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1075–1090. [Google Scholar]
- 7.Fleischmann R D, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:469–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 8.Haag H, Fiedler H-P, Meiwes J, Drechsel H, Jung G, Zähner H. Isolation and biological characterization of staphyloferrin B, a compound with siderophore activity from staphylococci. FEMS Microbiol Lett. 1994;115:125–130. doi: 10.1111/j.1574-6968.1994.tb06626.x. [DOI] [PubMed] [Google Scholar]
- 9.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 10.Heidrich C, Hantke K, Bierbaum G, Sahl H-G. Identification and analysis of a gene encoding a Fur-like protein of Staphylococcus epidermidis. FEMS Microbiol Lett. 1996;140:253–259. doi: 10.1111/j.1574-6968.1996.tb08345.x. [DOI] [PubMed] [Google Scholar]
- 11.Heinrichs J H, Bayer M G, Cheung A L. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J Bacteriol. 1996;178:418–423. doi: 10.1128/jb.178.2.418-423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinrichs J H, Gatlin L G, Hanson M S, Hromockyj A E, Kunsch C. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Identification and characterization of an iron regulated protein from Staphylococcus aureus; p. 60. [Google Scholar]
- 13.Kornblum J, Kreiswirth B, Projan S J, Novick R P. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay J A, Riley T V. Staphylococcal iron requirements, siderophore production, and iron-regulated protein expression. Infect Immun. 1994;62:2309–2314. doi: 10.1128/iai.62.6.2309-2314.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahé B, Masclaux C, Rauscher L, Enard C, Expert D. Differential expression of two siderophore-dependent iron-acquisition pathways in Erwinia chrysanthemi 3937: characterization of a novel ferrisiderophore permease of the ABC transporter family. Mol Microbiol. 1995;18:33–43. doi: 10.1111/j.1365-2958.1995.mmi_18010033.x. [DOI] [PubMed] [Google Scholar]
- 17.Meiwes J, Fiedler H P, Haag H, Zähner H, Konetschny, Rapp A, Jung G. Isolation and characterization of staphyloferrin A, a compound with siderophore activity from Staphylococcus hyicus DSM 20459. FEMS Microbiol Lett. 1990;55:201–205. doi: 10.1111/j.1574-6968.1990.tb13863.x. [DOI] [PubMed] [Google Scholar]
- 18.Modun B, Kendall D, Williams P. Staphylococci express a receptor for human transferrin: identification of a 42-kilodalton cell wall transferrin-binding protein. Infect Immun. 1994;62:3850–3858. doi: 10.1128/iai.62.9.3850-3858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novick R P. Genetic systems in staphylococci. Methods Enzymol. 1991;204:587–636. doi: 10.1016/0076-6879(91)04029-n. [DOI] [PubMed] [Google Scholar]
- 20.Novick R P. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 21.Purcell B K, Swancutt M A, Radolf J D. Lipid modification of the 15 kilodalton major membrane immunogen of Treponema pallidum. Mol Microbiol. 1990;4:1371–1379. doi: 10.1111/j.1365-2958.1990.tb00716.x. [DOI] [PubMed] [Google Scholar]
- 22.Saier M H, Jr, Fagan M J, Hoischen C, Reizer J. Transport mechanisms. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria. Washington, D.C: American Society for Microbiology; 1993. pp. 133–156. [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Schneider R, Hantke K. Iron-hydroxamate uptake systems in Bacillus subtilis: identification of a lipoprotein as part of binding protein-dependent transport system. Mol Microbiol. 1993;8:111–121. doi: 10.1111/j.1365-2958.1993.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–55. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 26.Stojiljkovic I, Bäumler A J, Hantke K. Fur regulon in gram-negative bacteria. Identification and characterization of new iron-regulated Escherichia coli genes by a fur titration assay. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 27.Stover C K, Bansal G P, Hanson M S, Burlein J E, Palaszynski S R, Young J F, Koenig S, Young D B, Sadziene A, Barbour A G. Protective immunity elicited by recombinant Bacille Calmette-Guerin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J Exp Med. 1993;178:197–209. doi: 10.1084/jem.178.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sutcliffe I C, Russell R R B. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tam R, Saier M H., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trivier D, Courcol R J. Iron depletion and virulence in Staphylococcus aureus. FEMS Microbiol Lett. 1996;141:117–127. doi: 10.1111/j.1574-6968.1996.tb08373.x. [DOI] [PubMed] [Google Scholar]
- 31.Vieria J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 32.von Heijne G. The structure of signal peptides from bacterial lipoproteins. Protein Eng. 1989;2:531–534. doi: 10.1093/protein/2.7.531. [DOI] [PubMed] [Google Scholar]
- 33.Wingfield P T, Palmer I, Liang S. Folding and purification of insoluble (inclusion body) proteins from Escherichia coli. In: Coligan J E, Dunn B M, Ploegh H L, Speicher D W, Wingfield P T, editors. Current protocols in protein science. Vol. 1. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 6.5.1–6.5.27. [Google Scholar]