Case Presentation
In July 2020, a previously healthy 6-year-old boy was evaluated in a pulmonary clinic in New York after two episodes of pneumonia in the previous 3 months. For each episode, the patient presented with cough, fever, and hemoptysis, all of which resolved with antibiotic therapy and supportive care. The patient never experienced dyspnea during these episodes of pneumonia. He was asymptomatic at the current visit. The patient had no history of travel, sick contacts, asthma, or bleeding disorders.
Physical Examination
On examination, the patient was afebrile, normotensive, and had a respiratory rate of 16 breaths per minute and an oxygen saturation >99% on room air. Lungs were clear to auscultation bilaterally. Chest wall excursion was equal bilaterally. He had no rash, petechiae, or joint swelling. Results of the rest of the physical examination were within normal limits.
Diagnostic Studies
At the initial presentation of pneumonia (April 2020), laboratory results were significant for a hemoglobin of 9.6 g/dL, elevated WBC 15.9 k/μL, D-dimer 879 ng/mL, and C-reactive protein 1.2 mg/dL. COVID-19 polymerase chain reaction was negative. Point-of-care hemoglobin increased to 11.6 g/dL 1 month later. Chest radiographs demonstrated a right lower lobe opacity at the initial episode and again at the second episode of pneumonia 2 months later.
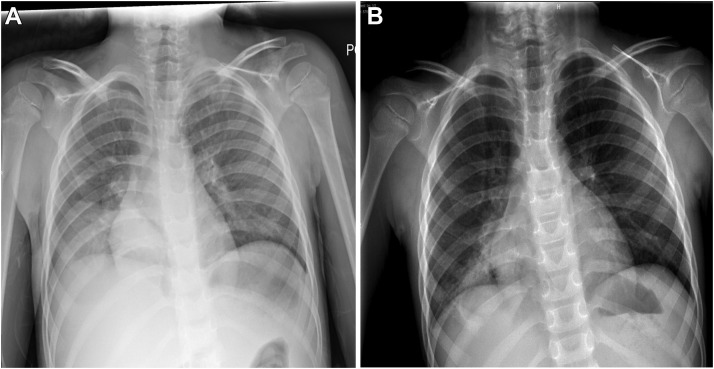
One week after the current clinic visit (July 2020), a repeat hemoglobin was 7.0 g/dL with a mean corpuscular volume of 70.4 fL and red cell distribution width of 21.1%. A repeat chest radiograph performed while the patient asymptomatic again demonstrated a persistent right lower lobe opacity (Fig 1 ).
Figure 1.
Chest radiography demonstrating right lower lobe opacity in (A) Initial chest radiograph and (B) chest radiograph at 2 months.
What Study Should Be Conducted Next?
CT chest with and without IV contrast
What is the diagnosis?
Diagnosis: Endobronchial mucoepidermoid carcinoma (MEC)
Discussion
The differential diagnosis for recurrent pneumonia in children is wide; causes can include immunodeficiency, impaired mucociliary clearance, mechanical airway obstruction, systemic and immune-mediated disease, congenital pulmonary airway malformation/sequestration, and nonpulmonary causes. Involvement of tumors or surgical solutions are rare. Recurrent pneumonia associated with a persistent lesion on imaging warrants cross-sectional imaging to evaluate for anatomical abnormalities.
Endobronchial tumors, as described in this case, are rare in the pediatric population. Such tumors have mostly been described in case reports or in small case series. The largest case series, comprising 14 total patients, included five carcinoid tumors, three mucoepidermoid carcinomas, and one adenoid cystic carcinoma.
MEC accounts for only 0.1% of primary lung tumors in patients across the age spectrum. It is the most common malignant tumor of the salivary gland in adults but also can be found in the thyroid, lacrimal gland, and the lung. This tumor is most often seen in young adults; however, it has been reported in patients ranging from 3 to 72 years of age. In children, bronchial MEC occurs in 10% of malignant lung tumors. Only 145 pediatric cases of MEC are reported in literature.
Definitive diagnosis of MEC is by immunohistochemistry and histology. Although MEC is normally a salivary gland tumor, it can originate from large airways, such as the trachea and primary/secondary bronchi. MEC is a slow-growing, firm, unencapsulated tumor with low potential for malignancy. Histologically MEC contains three cell types: squamous, mucus secreting, and intermediate cells. Immunohistochemistry is positive for CK7, MUc5Ac, p40, and p63 and negative for thyroid transcription factor 1, calponin, human epidermal growth factor receptor 2, and anaplastic lymphoma kinase. These markers distinguish bronchial MEC from other primary lung tumors. Mastermind like transcriptional coactivator 2 (MAML2) is the most frequently identified gene rearrangement associated with MEC and occurs in 77% of the cases, whereas epidermal growth factor receptor mutations also have been reported. Prognosis is determined by histological grade. High-grade MEC demonstrates nuclear pleomorphism, abnormal mitotic activity, and necrosis and is associated with increased metastasis and mortality. Low-grade MEC has a complete absence of these histologic characteristics and has a good clinical outcome.
Diagnosis of MEC and other endobronchial lesions occurs, on average, 11 months after symptom onset. Diagnosis is based on clinical findings of bronchial obstruction, chest radiographs, and chest CT. Large endobronchial lesions cause bronchial obstruction and frequently present as recurrent obstructive pneumonia distal to the lesion. Other clinical manifestations of large airway involvement include hemoptysis, cough, wheezing, bronchitis, chest pain, and clubbed fingers. CT is the most sensitive and specific examination to detect parenchymal lung disease; therefore, it is the test of choice if a mass is suspected. PET-CT also can be useful to determine metastasis, and the use of Gallium-68 may help distinguish the lesion from carcinoid tumor. Serum markers such as chromogranin A and 5-hydroxyindoleacetic acid also may help identify carcinoid tumors. Flexible fiber-optic bronchoscopy is useful for visualization of the mass; however, MEC lesions are friable and may result in bleeding after biopsy or manipulation.
Surgical resection is the treatment of choice for bronchial MEC, with a primary goal of negative surgical margins. In children and adolescents, 5-year survival is 96%. Local recurrence is extremely rare and limited to high-grade disease. In one case series, five deaths out of 120 cases (4%) resulted from metastatic disease secondary to high-grade tumor. Adjuvant chemotherapy or radiation therapy are usually not indicated when complete resection was achieved because of the benign clinical course of MEC.
Clinical Course
CT chest revealed a 3.1 × 2.3 × 2.1-cm mass in the right lower lobe bronchus with postobstructive mucoceles and bronchiectasis (e-Fig 1). A subsequent PET-CT scan with Gallium-68 demonstrated moderate tracer uptake and no sign of metastatic disease. Chromogranin A was normal. Surgical resection was delayed by a third episode of hemoptysis, fever, and dyspnea, which improved with IV antibiotics. Preoperative flexible bronchoscopy demonstrated an endobronchial mass distal to the right middle lobe bronchus. The patient underwent an uncomplicated right middle and lower lobectomy 5 months after initial presentation, with complete resolution of symptoms.
Histopathology revealed a low-grade, mucus-secreting tumor (e-Fig 2) positive for CK7, and negative for thyroid transcription factor 1, CK20, and p63. A positive MAML2 (11q21) rearrangement confirmed the diagnosis of MEC. After an uneventful recovery from surgery, the patient was asymptomatic at 1-year follow-up. All laboratory values have normalized. Repeat CT does not demonstrate recurrence of disease.
Clinical Pearls
-
1.
In the setting of recurrent childhood pneumonia with uncommon clinical findings, including hemoptysis, profound anemia, lack of dyspnea, and a persistent opacity/mass on imaging, rare diagnoses such as an endobronchial mass should be included in the differential diagnosis.
-
2.
Diagnosis is made with cross-sectional imaging or flexible bronchoscopy. Tumor markers and the MAML2 gene rearrangements can distinguish MEC from other primary lung tumors.
-
3.
Surgical resection with negative margins is the definitive treatment for bronchial MEC, with excellent long-term prognosis.
Acknowledgments
Financial/nonfinancial disclosure: None declared.
Other contributions:CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met.
Additional information: The e-Figures are available online under “Supplementary Data.”
Supplementary Data
Suggested Readings
- Al-Qahtani A.R., Di Lorenzo M., Yazbeck S. Endobronchial tumors in children: institutional experience and literature review. J Pediatr Surg. 2003;38(5):733–736. doi: 10.1016/jpsu.2003.50195. [DOI] [PubMed] [Google Scholar]
- Achcar Rde O., Nikiforova M.N., Dacic S., et al. Mammalian mastermind like 2 11q21 gene rearrangement in bronchopulmonary mucoepidermoid carcinoma. Hum Pathol. 2009;40(6):854–860. doi: 10.1016/j.humpath.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Roby B.B., Drehner D., Sidman J.D. Pediatric tracheal and endobronchial tumors: an institutional experience. Arch Otolaryngol Head Neck Surg. 2011;137(9):925–929. doi: 10.1001/archoto.2011.153. [DOI] [PubMed] [Google Scholar]
- Stillwell P.C. In: Pediatric Pulmonology: American Academy of Pediatrics. Schechter M.S., editor. 2011. Recurrent pneumonia; pp. 451–458. [Google Scholar]
- Alsidawi S., Morris J.C., Wikenheiser-Brokamp K.A., et al. Mucoepidermoid carcinoma of the lung: a case report and literature review. Case Rep Oncol Med. 2013;2013 doi: 10.1155/2013/625243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Sun Z., Pan W., et al. Childhood bronchial mucoepidermoid tumors: a case report and literature review. Oncol Lett. 2013;6(5):1409–1412. doi: 10.3892/ol.2013.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyssartier E., Ang P., Bonnemaison E., et al. Characteristics of endobronchial primitive tumors in children. Pediatr Pulmonol. 2014;49(6):E121–E125. doi: 10.1002/ppul.22987. [DOI] [PubMed] [Google Scholar]
- Huo Z., Wu H., Li J., et al. Primary pulmonary mucoepidermoid carcinoma: histopathological and moleculargenetic studies of 26 cases. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0143169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo S., Rojas Y., Slater B.J., et al. Childhood and adolescent tracheobronchial mucoepidermoid carcinoma (MEC): a case-series and review of the literature. Pediatr Surg Int. 2016;32(4):417–424. doi: 10.1007/s00383-015-3849-y. [DOI] [PubMed] [Google Scholar]
- Jichlinski A., Kilaikode S., Koumbourlis A.C. Case 1: recurrent pneumonia in a 15-year-old girl. Pediatr Rev. 2018;39(9):464. doi: 10.1542/pir.2017-0205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.