A 45-year-old man who was admitted to the ICU for treatment of critical COVID-19 infection developed spontaneous subcutaneous emphysema 2 days after being intubated for acute hypoxemic respiratory failure. In the chest radiograph the patient presented with subcutaneous emphysema and pneumomediastinum (Fig 1 ).
Figure 1.

Chest radiograph with evidence of pneumomediastinum and subcutaneous emphysema.
The patient evolved with severe ARDS requiring two prone positioning sessions of nearly 18 hours. Positive end-expiratory pressure (PEEP) was titrated using EXPRESS trial up-titration, complying with current recommendations of high PEEP in moderate-to-severe ARDS (PEEP = 18 cm H2O, plateau pressure = 28 cm H2O). After the second repositioning to a supine position, he sustained a Pao 2/Fio 2 ratio of 150 with lung protective ventilation and high PEEP, stage 2 acute kidney injury, and no other organ dysfunctions. Approximately 40 min after being unproned for the second time, he developed sudden hypotension. A point-of-care lung ultrasound showed no pneumothorax, and a point-of-care echocardiogram for undifferentiated shock was obtained, although the apical and parasternal views could not be obtained because of artifacts (diffuse A lines) (Video 1, subcostal long axis window; Video 2, inferior vena cava long axis window; Video 3, subcostal short axis window) (Figs 2, 3 ).
Figure 2.
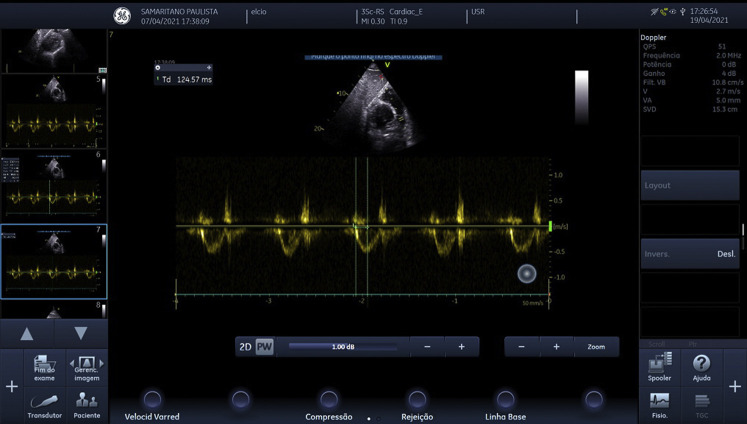
Right ventricular outflow tract pulsatile Doppler image with calculated velocity-time integral.
Figure 3.
Right ventricular outflow tract pulsatile Doppler image with calculated acceleration time.
Question: Given the clinical presentation and the findings of point-of-care ultrasound, what is the likely cause of hemodynamic instability?
Answer: Obstructive shock caused by tension pneumomediastinum
Video 1 (subcostal long axis window) demonstrates normal systolic function for both left and right ventricles, without signs of pericardial effusion. Video 2 (inferior vena cava long axis window) shows a distended inferior vena cava with no respiratory variability. Video 3 (subcostal short axis window) shows signs of extrinsic compression in the right ventricle caused by the pneumomediastinum and probable pneumopericardium and image artifact on the right because of air in the mediastinum (A lines). Figure 2 shows the pulmonary artery velocity time integral; it was calculated as 11 cm/sec, with a cardiac rate of 77 beats/min. Using a pulmonary artery diameter of 2.4 cm obtained from a chest CT scan, we calculated a cardiac output of approximately 3.8 L/min. Figure 3 shows a right ventricular outflow tract acceleration time of 124 msec.
Hemodynamic stability was achieved with norepinephrine infusion and PEEP reduction. The patient evolved with a protracted ICU course with prolonged mechanical ventilation and eventually died, 3 weeks after ICU admission, unrelated to this hemodynamic event.
Discussion
Spontaneous pneumomediastinum is a relatively common complication in patients wtih COVID-19, presenting in approximately 13% of cases.1 The higher incidence of spontaneous pneumomediastinum in patients with COVID-19 when compared with non-COVID ARDS may be attributable to marked lung frailty rather than only barotrauma itself.1 The most common pathophysiological mechanism leading to this event is known as the Macklin effect. Pneumomediastinum starts with a rupture along the alveolar tree, followed by release of the alveolar air that centripetally dissects through the pulmonary interstitium along the bronchovascular sheaths toward the pulmonary hila, into the mediastinum.2
Point-of-care ultrasound is a powerful tool for the intensivist in the evaluation of shock. Provided an adequate window is obtained, it can provide information on preload, afterload, systolic and diastolic function of the heart, and cardiac output, and sometimes it can even visualize obstructive causes of shock such as pericardial effusion and pulmonary embolism. In the setting of pneumomediastinum, the parasternal window is usually unavailable, but the subcostal window can provide similar and valuable information.3 In this case, the presence of a distended inferior vena cava with no respiratory variability associated with low cardiac output and adequate systolic function for both the left and right ventricles suggested obstructive shock was the likely cause of hemodynamic instability. To assess for possible causes of obstructive shock, we evaluated the right ventricle outflow tract with a pulsatile Doppler, which revealed a dome shape with long acceleration time (124 ms), suggesting absence of pulmonary hypertension.4 There was no sign of pericardial effusion or of right ventricle afterload increase. However, there was a visible extrinsic compression of the right ventricle (Video 3) and a known pneumomediastinum, allowing us to diagnose a tension pneumomediastinum. Point-of-care ultrasound in this case excluded both tension pneumothorax and massive pulmonary embolism as causes of obstructive shock, but advanced echocardiography (right ventricle and pulmonary hypertension evaluation) knowledge was necessary to be confident in the next steps in treatment decisions. This highlights the need for critical care physicians to pursue advanced critical care echocardiography competence in daily practice.
The management of spontaneous pneumomediastinum is usually symptomatic, with a possible benefit of oxygen therapy in the reabsorption of the free air.5 We also opted to lower PEEP in an effort to ease venous return and lower intrathoracic pressure to reduce possible overdistension and allow reabsorption of the pneumomediastinum. See Narration Video for a detailed explanation of Videos 1-3.
Reverberations
-
1.
Obstructive shock is a potential cause of hemodynamic instability in ARDS, and the most common causes are pulmonary embolism, acute cor pulmonale secondary to ARDS, and tension pneumothorax.
-
2.
Pneumothorax and pneumomediastinum are relatively common complications in patients with COVID-19 and should be considered as potential causes of sudden hemodynamic instability.
-
3.
In the presence of pneumomediastinum, when the parasternal windows are unavailable because of air in the mediastinum, the subcostal window can provide similar and valuable information.
-
4.
The diagnosis of obstructive shock caused by tension pneumomediastinum can be done after observing normal biventricular function, a plethoric inferior vena cava, and signs of compression of the right ventricle without signs of pulmonary hypertension.
Acknowledgments
Financial/nonfinancial disclosure: The authors have reported to CHEST the following: B. A. M. P. Besen reports receiving fees for lectures from CRISTALIA and Hill-Rom unrelated to the contents of this manuscript. None declared (A. C. G. Hirano, L. M. G. Melro)
Other contributions:CHEST worked with the authors to ensure that the Journal policies on patient consent to report information were met.
Additionalinformation:Videos for this case are available under "Supplementary Data."
Supplementary Data
Subcostal long axis view demonstrating normal systolic function for both left and right ventricles, without signs of pericardial effusion.
Video 2. Inferior vena cava long axis view showing a distended inferior vena cava with no variability.
Video 3. Subcostal short axis view, with signs of extrinsic compression of the right ventricle caused by the pneumomediastinum and image artifact on the right caused by air in the mediastinum.
References
- 1.Lemmers D.H.L., Abu Hilal M., Bnà C. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6(4):00385–2020. doi: 10.1183/23120541.00385-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murayama S., Gibo S. Spontaneous pneumomediastinum and Macklin effect: overview and appearance on computed tomography. World J Radiol. 2014;6(11):850–854. doi: 10.4329/wjr.v6.i11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zachariah S., Gharahbaghian L., Perera P., Joshi N. Spontaneous pneumomediastinum on bedside ultrasound: case report and review of the literature. West J Emerg Med. 2015;16(2):321. doi: 10.5811/westjem.2015.1.24514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parasuraman S., Walker S., Loudon B.L., et al. Assessment of pulmonary artery pressure by echocardiography: a comprehensive review. Int J Cardiol Heart Vasc. 2016;12:45–51. doi: 10.1016/j.ijcha.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sahni S., Verma S., Grullon J., Esquire A., Patel P., Talwar A. Spontaneous pneumomediastinum: time for consensus. N Am J Med Sci. 2013;5(8):460–464. doi: 10.4103/1947-2714.117296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subcostal long axis view demonstrating normal systolic function for both left and right ventricles, without signs of pericardial effusion.
Video 2. Inferior vena cava long axis view showing a distended inferior vena cava with no variability.
Video 3. Subcostal short axis view, with signs of extrinsic compression of the right ventricle caused by the pneumomediastinum and image artifact on the right caused by air in the mediastinum.