Abstract
3-Hydroxylaminophenol mutase from Ralstonia eutropha JMP134 is involved in the degradative pathway of 3-nitrophenol, in which it catalyzes the conversion of 3-hydroxylaminophenol to aminohydroquinone. To show that the reaction was really catalyzed by a single enzyme without the release of intermediates, the corresponding protein was purified to apparent homogeneity from an extract of cells grown on 3-nitrophenol as the nitrogen source and succinate as the carbon and energy source. 3-Hydroxylaminophenol mutase appears to be a relatively hydrophobic but soluble and colorless protein consisting of a single 62-kDa polypeptide. The pI was determined to be at pH 4.5. In a database search, the NH2-terminal amino acid sequence of the undigested protein and of two internal sequences of 3-hydroxylaminophenol mutase were found to be most similar to those of glutamine synthetases from different species. Hydroxylaminobenzene, 4-hydroxylaminotoluene, and 2-chloro-5-hydroxylaminophenol, but not 4-hydroxylaminobenzoate, can also serve as substrates for the enzyme. The enzyme requires no oxygen or added cofactors for its reaction, which suggests an enzymatic mechanism analogous to the acid-catalyzed Bamberger rearrangement.
The recognition that synthetic nitroaromatic compounds are environmental hazards has led to a considerable amount of research on their biodegradation (for reviews, see references 18, 21, 32, 47, and 48). As a result, a variety of novel enzymatic mechanisms for the degradation or transformation of nitroarenes have been discovered. Oxidative elimination of the nitro group as nitrite seems to be a key reaction in the catabolism of many mononitroaromatic and some dinitroaromatic compounds (49). In general, the electron-withdrawing character of the nitro group favors biological reduction, giving rise to ring hydrogenation (28, 29, 54, 55) or transformation of the nitro group to either nitroso, hydroxylamino, or amino derivatives (47, 48). Each of these products can be subjected to further transformation or mineralization (47).
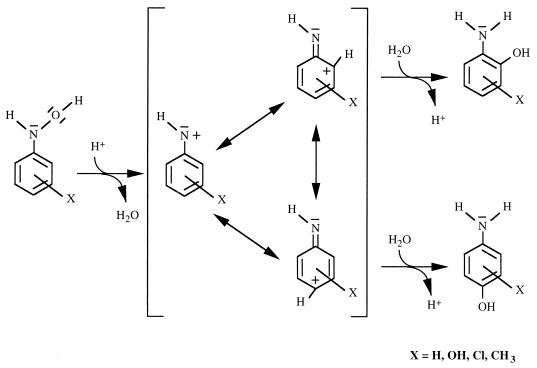
Recent evidence suggests that the hydroxylaminoaromatic compounds are key intermediates in a variety of metabolic pathways of mononitroaromatic compounds. For example, an enzymatic rearrangement of aromatic hydroxylamines and hydroxamic acids to their corresponding ortho-aminophenol derivatives was observed in rabbit liver (6) and rat liver homogenates (51). In the latter report, a hepatic isomerase-catalyzed mechanism that corresponded to the acid-catalyzed chemical reaction formerly described by Bamberger was proposed (3). Figure 1 shows the mechanism of the Bamberger rearrangement of phenylhydroxylamines (45, 46), which was carried out in aqueous sulfuric acid. The ability to catalyze a Bamberger-type rearrangement was also discussed for some thiamine-dependent and/or metal cation-containing enzymes (15, 25, 33), and even bovine serum albumin was suspected to catalyze such a reaction (26). Furthermore, Corbett and Corbett showed that a yeast cometabolized 4-chloronitrobenzene via 4-chlorohydroxylaminobenzene to 2-hydroxy-4-chloroaniline and 4-aminophenol (13). In all these studies, the described rearrangement reactions were rather nonspecific, since the aminophenolic compounds were not the only products formed from aromatic hydroxylamines or hydroxamic acids.
FIG. 1.
Proposed mechanism of the Bamberger rearrangement of hydroxylaminoaromatic compounds based on the mechanism analyzed in aqueous sulfuric acid (45, 46).
Partial reduction of nitrobenzene to hydroxylaminobenzene and subsequent rearrangement to 2-aminophenol leads to complete mineralization of nitrobenzene by the bacterium Pseudomonas pseudoalcaligenes JS45 (37). The key enzyme, hydroxylaminobenzene mutase, specifically catalyzes the isomerization of hydroxylaminobenzene to 2-aminophenol in a reaction analogous to the acid-catalyzed Bamberger rearrangement (3, 45, 46). The mutase was not purified from P. pseudoalcaligenes JS45, and nothing is known about the characteristics of the enzyme or the mechanism of the reaction. Thus, it is not known whether the conversion of the hydroxylamine to the corresponding aminophenol is catalyzed by a single enzyme or requires multiple steps. Ralstonia eutropha JMP134 was shown to degrade 3-nitrophenol (3NP) by reduction of the nitro group, yielding 3-hydroxylaminophenol (3HAP), which was further converted to aminohydroquinone (43). The latter reaction proceeded in cell extracts of induced R. eutropha JMP134 in the absence of oxygen and without any cofactors; therefore, a rearrangement analogous to that observed for hydroxylaminobenzene in P. pseudoalcaligenes JS45 (37) was postulated. Although R. eutropha JMP134 was not able to mineralize nitrobenzene, enzymes in extracts of 3NP-grown cells catalyzed the rearrangement of hydroxylaminobenzene. However, whereas the mutase enzyme(s) from P. pseudoalcaligenes JS45 produced ortho-aminophenol almost exclusively, the enzyme(s) from R. eutropha JMP134 produced both the ortho and para isomers (43).
Several authors proposed that the mechanism of the enzymatic reaction corresponds to that of the Bamberger reaction (Fig. 1) but provided no experimental evidence (14, 49, 51). To date, none of the enzymes whose physiological role is the rearrangement of an aromatic hydroxylamine to an aminophenol has been isolated or characterized. To gain insight into the reaction mechanism and to determine whether the enzymatic isomerization of 3-hydroxylaminophenol to aminohydroquinone in R. eutropha JMP134 was catalyzed by a single enzyme, we have purified and characterized the 3HAP mutase.
MATERIALS AND METHODS
Culture conditions.
R. eutropha JMP134 (39) was grown in a 30-liter bioreactor (Bioengineering) containing 20 liters of nitrogen-free mineral salt medium (10). Two 1-liter cultures pregrown on 3NP (0.5 mM) as the nitrogen source and succinate (10 mM) as the carbon and energy source served as the inoculum. During fed-batch fermentation, addition of 3NP was controlled by use of a syringe pump, so that the concentration of the nitroaromatic compound never exceeded 0.5 mM. The culture was stirred at 150 rpm and aerated with 150 liters of air/h. After 37.5 mmol of 3NP was consumed (absorbance at 546 nm = 1.6) the cells were harvested by centrifugation and washed twice in 50 mM phosphate buffer (pH 7.4). The wet cell paste (19.5 g) was stored frozen at −20°C overnight.
Enzyme purification.
The protein was purified by ultracentrifugation, anion-exchange chromatography, and hydrophobic interaction chromatography. All steps were performed in 50 mM sodium-potassium phosphate buffer (pH 7.5) at 4°C unless stated otherwise. The thawed cells were resuspended in 55 ml of buffer and lysed by two passes through a French pressure cell at 20,000 lb · in−2. The resulting lysate was centrifuged at 100,000 × g for 1 h, and the pellet was discarded. The supernatant was applied to a DEAE CL-6B (weak anion-exchange resin) column (HK 16/30; bed volume, 55 ml; diameter, 16 mm [Pharmacia, Uppsala, Sweden]) equilibrated with buffer. After the column was washed with 55 ml of buffer, proteins were eluted with 275 ml of a linear NaCl gradient (0 to 0.5 M) in buffer at a flow rate of 2.5 ml/min. Mutase activity eluted at 0.21 M NaCl. Fractions with mutase activity were pooled (30 ml), and 4 M ammonium sulfate (pH 7.5) was added to a final concentration of 1 M. The solution was centrifuged at 30,000 × g for 10 min, and the pellet was discarded. The supernatant (45 ml) was applied to a butyl agarose column (bed volume, 7.5 ml; diameter, 10 mm [Landgraf]) preequilibrated with buffer containing 1 M ammonium sulfate. The butyl group of the resin (Sigma, Deisenhofen, Germany) was attached via a neutral ether linkage and had a spacer of 3 carbon atoms. The column was washed with 7.5 ml of buffer containing 1 M ammonium sulfate to elute unbound protein. Bound protein was eluted with 20 mM phosphate buffer (pH 7.5) at a flow rate of 0.3 ml/min as follows. A linear gradient with 10 ml of ammonium sulfate (1 to 0.5 M) was followed by a wash step with 7.5 ml of 0.5 M ammonium sulfate. Then a gradient with 37.5 ml of ammonium sulfate (0.5 to 0 M) eluted the protein containing 3HAP mutase activity at 0.2 M ammonium sulfate. Fractions (3 ml) containing mutase were tested for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as described by Laemmli (27) with a 10% ready-to-use polyacrylamide gel (Bio-Rad, Munich, Germany). Proteins were made visible by silver staining (Silver Stain Plus kit; Bio-Rad). For extended storage, aliquots of purified mutase were kept frozen at −70°C. The protein contents of lysates and enzyme fractions were determined by the method of Bradford (9).
Molecular weight determination.
The mass of subunits was determined by SDS-PAGE (see above). Protein molecular weight standards for SDS-PAGE were purchased from Pharmacia. Proteins were stained as described above. The number of subunits forming the native 3HAP mutase was determined by ultrafiltration with a Centriprep-100 filter unit (Amicon, Witten, Germany), which excludes proteins with a molecular mass of ≥100 kDa from passing through the membrane. The filter unit loaded with a sample of 3HAP mutase was centrifuged at 500 × g and 4°C for 45 min.
Determination of 3HAP mutase activity.
The activity of the 3HAP mutase was estimated spectrophotometrically by measuring the increase of the absorption at 300 nm concomitant with the accumulation of aminohydroquinone. 3HAP does not absorb at this wavelength. The standard assay mixture contained 0.4 mM 3HAP in 100 mM phosphate buffer (pH 7). Since both the product and substrate of the enzymatic reaction were autoxidizable, the phosphate buffer was made anaerobic by sparging with argon. Spontaneous 3HAP decomposition led to slow formation of products absorbing at 300 nm, but aminohydroquinone was not found. The rate of spontaneous decomposition was subtracted from the rate of the enzyme-catalyzed reaction. Some assay mixtures included 1 mM 3HAP and 2 mM dithiothreitol (DTT) to stabilize 3HAP. The stoichiometry of the reaction was estimated by relating the decrease of 3HAP to the increase in the absorption at 300 nm due to accumulation of aminohydroquinone. Various concentrations of 3HAP were used in the standard mutase assay (see above), and after the reaction was complete (<2 min), samples of the reaction mixtures were analyzed by high-pressure liquid chromatography (HPLC). The increase in the absorption at 300 nm was proportional to the concentrations of aminohydroquinone formed and 3HAP consumed. Aminohydroquinone was the only product of the reaction detectable by HPLC. Thus, the increase of 0.48 absorption unit at 300 nm corresponded to the formation of 0.1 mM aminohydroquinone. One unit of enzyme activity was defined as the production of 1 μmol of aminohydroquinone per min.
Inactivation-activation experiments.
For testing the activity of 3HAP mutase with potential effectors, portions of 3HAP mutase were preincubated with different reagents at 1 mM (unless stated otherwise) for at least 1 h. Some reagents were added directly to the assay buffer (final concentration, 1 mM). Standard assays with 2 mM DTT and 16.5 μg of 3HAP mutase (3.8 U/mg) were performed as described above. Instead of purified enzyme, some of the measurements were performed with cell extract (0.12 mg of protein; 0.52 U/mg). The activity without the addition of effectors was set to 100%.
Kinetics and pI determination.
The optimum pH for enzyme activity was determined by using the standard assay with 100 mM phosphate buffer in the range from pH 5.5 to 8 and 200 mM succinate buffer in the range from pH 4 to 6.
The Vmax and Km were measured spectrophotometrically as described above with 0.05, 0.1, 0.25, 0.5, and 1 mM 3HAP and 2 mM DTT in 100 mM phosphate buffer (pH 7). For this purpose, 20 mM 3HAP was freshly prepared as described below and diluted appropriately in aqueous HCl (1:100 [vol/vol]), which stabilized 3HAP. The dilutions were stored on ice, and no spontaneous Bamberger rearrangement was observed under these conditions. The apparent Vmax and Km were estimated by linear regression in a Lineweaver-Burk plot (5).
The pI of 3HAP mutase was determined by chromatofocusing. A partially purified sample of mutase was applied to a Mono P HR 5/20 column (Pharmacia) after it was equilibrated with 25 mM bis-Tris HCl buffer (pH 6.3). Then the eluent was changed to Polybuffer 74 (1:10 with water [pH 3.5]; Pharmacia), and protein was eluted by the pH gradient (pH 6.1 to 3.8, measured at room temperature) in 36 ml at a flow rate of 1 ml/min.
Turnover experiments.
Turnover experiments of nitro and hydroxylamino aromatics in the absence of oxygen were conducted as described previously (43). Conversion of 1 mM 3HAP by 0.6 U (11.4 μg) of 3HAP mutase was performed in 100 mM phosphate buffer (pH 7). After an appropriate incubation period, samples were treated with concentrated HCl (approximately 1:30 [vol/vol]) to stop the enzymatic reaction and to stabilize the substrate and product. Samples were stored on ice until analyzed. 3HAP was stable under these conditions. Hydroxylaminobenzene (1 mM) conversion was carried out in the same way, except that more 3HAP mutase (3.8 U [157 μg]) was added. The concentrations of substrate and products were measured as described previously (43).
Anaerobic conversion of 4-nitrotoluene was carried out with an extract from 3NP-grown cells (2.8 mg of protein) in 20 ml of 50 mM phosphate buffer (pH 7) containing 2 mM NADPH and 0.5 mM nitroaromatic compound. The substrate and products were detected by HPLC (43) with 50% methanol and 50% water, each containing hexane sulfonate (Pic B6; Waters) as the solvent. The same conditions were used to convert 4-nitrobenzoate, except that 2.5 mg of cell extract protein was added to the medium. Conversion was monitored by using an HPLC gradient method as described previously (43), but 0.34% (vol/vol) phosphoric acid instead of hexane sulfonate was added to the solvents.
Determination of amino acid sequences.
A partial tryptic digestion of the purified enzyme was carried out by the method of Stone and Williams (52). The peptides were separated on a Smart system (Pharmacia) with a reversed-phase Hypersil 3μ ODS column (50 by 2.1 mm). The liquid phase consisted of solvent A (H2O containing 0.1% trifluoroacetic acid), and solvent B (70% acetonitrile, 30% H2O, 0.1% trifluoroacetic acid). The peptides were eluted by a gradient which started from 100% solvent A and changed linearly over 10 ml to 25% solvent A–75% solvent B and then changed over 2 ml to 100% solvent B at a flow rate of 0.2 ml/min. For NH2-terminal amino acid sequencing of undigested protein and of isolated peptides, the preparations were directly spotted onto a BioPrene membrane (ABI, Foster City, Calif.) and sequenced with a model 473A protein-sequencing system (ABI). The sequences were compared with those in the nonredundant GenBank CDS translations-PDB-SwissProt-SPupdate-PIR database (as of 15 August 1998) by using the BlastP program (2).
Chemical Bamberger reaction of 3HAP.
Rearrangement of phenylhydroxylamines to p-aminophenols was observed in aqueous sulfuric acid solutions (46). To find which products are formed from 3HAP by the chemical reaction, a solution of 3HAP (18 ml of approximately 36 mM 3HAP) was synthesized under argon (43) and 1 ml of sulfuric acid (96%) was added to the aqueous solution. The reaction mixture was placed in a serum bottle closed with a gas-tight rubber septum. Then the solution was evacuated and flushed with argon before an overpressure of 0.5 × 105 Pa was set with argon. The bottle was incubated overnight in a shaking water bath at 30°C. The next day, samples were analyzed by HPLC (43) with 5% methanol and 95% water each containing hexane sulfonate as the solvent.
Chemicals.
3HAP was synthesized as described previously (43), except that the 3NP concentration in the reaction mixture was 20 or 50 mM. Frozen stock solutions, which were acidified by HCl (1:100 [vol/vol]), could be stored for approximately 4 weeks at −70°C under an argon atmosphere, and thawed solutions were not used for longer than 2 h. Hydroxylaminobenzene was provided by Shirley Nishino (Tyndall Air Force Base, Fla.), and 4-aminocatechol was provided by Andreas Stolz (Universität Stuttgart, Stuttgart, Germany).
RESULTS
Purification and molecular mass of 3HAP mutase.
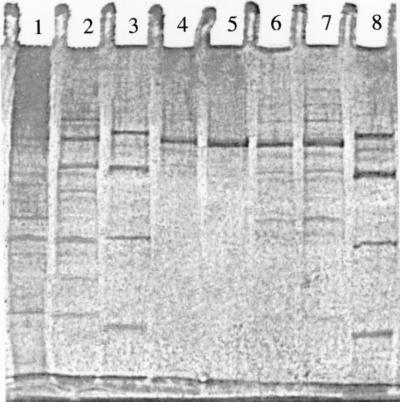
Table 1 summarizes how the 3HAP mutase was purified to homogeneity. The specific activity increased only 12-fold, either because of inactivation during the course of purification or because the enzyme may already make up a substantial part of the protein in the cell extract. On the assumption that no inactivation occurred, the mutase could have made up more than 8% of the cell protein. As shown in Fig. 2, lane 5, the SDS-PAGE gel exhibited one single band demonstrating that the enzyme consists of a single type of subunit with a molecular mass of 62 kDa. Attempts to determine the molecular mass of the native enzyme by gel filtration chromatography failed, although different resins and buffer systems were tested. The results were not reproducible, and the activity eluted mostly in several overlapping protein bands. Gel electrophoresis under nondenaturing conditions revealed inconsistent results concerning the size of the native enzyme. However, ultrafiltration of the purified enzyme showed that it passed through a membrane that had an exclusion limit of 100 kDa. This indicates, together with the result from SDS-PAGE, that the native 3HAP mutase consists of a single 62-kDa polypeptide. Gel electrophoresis under nondenaturing conditions gave inconsistent results concerning the size of the native enzyme.
TABLE 1.
Purification of the 3HAP mutase
| Purification step | Total amt of protein (mg) | Total activity (U)a | Sp act (U · mg−1) | Yield (%) |
|---|---|---|---|---|
| Cell extract | 1,102 | 452 | 0.41 | 100 |
| Lysate after ultracentrifugation | 941 | 420 | 0.44 | 92 |
| DEAE chromatography | 162 | 259 | 1.6 | 57 |
| Butyl agarose chromatography | 11.4 | 54 | 4.7 | 12 |
One unit is defined as the production of 1 μmol of aminohydroquinone per min.
FIG. 2.
SDS-PAGE analysis during purification of the 3HAP mutase from R. eutropha JMP134. Lanes 3 and 8 contained the following molecular mass standards (from top to bottom): bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), and soybean trypsin inhibitor (20 kDa). All other lanes contained 0.5 μg of protein from the respective preparations of the mutase. Lane 1, cell extract after ultracentrifugation; lane 2, pooled fractions from DEAE anion-exchange chromatography; lanes 4, 6, and 7, pooled fractions from butyl agarose hydrophobic interaction chromatography, each containing 3HAP mutase with minor contaminations; lane 5, pooled fraction from butyl agarose hydrophobic interaction chromatography containing purified 3HAP mutase.
Amino acid sequences.
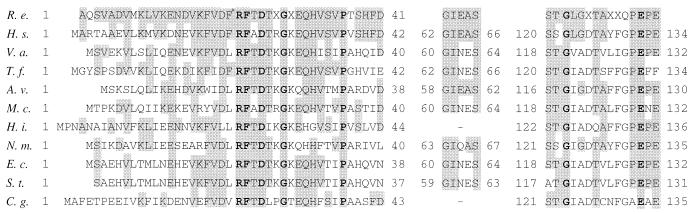
Amino acid sequences of the NH2 terminal of 3HAP mutase and of three internal peptides obtained by tryptic digestion of the enzyme were determined. The NH2 terminal and one of the internal peptides were overlapping at positions 30 to 34. The obtained sequences of 3HAP mutase are highly similar to sequences from different bacterial glutamine synthetases (glutamate-ammonia ligase; EC 6.3.1.2). Figure 3 shows an alignment of sequences from 3HAP mutase and those from 10 different glutamine synthetases.
FIG. 3.
Comparison of amino acid sequences from 3HAP mutase and from different bacterial glutamine synthetases. Amino acid residues from glutamine synthetases that are identical to those of 3HAP mutase (R. e., R. eutropha 134) at the particular sequence positions are shaded. Amino acid residues conserved across all 10 sequences are shown in boldface type. A dash means that no corresponding amino acid sequence was found. X means an unidentified amino acid. (H. s., Herbaspirillum seropedicae [42], V. a., Vibrio alginolyticus [31]; T. f., Thiobacillus ferrooxidans [40]; A. v., Azotobacter vinelandii [53]; M. c., Methylococcus capsulatus [11]; H. i., Haemophilus influenzae (16); N. m., Neisseria meningitidis [56]; E. c., Escherichia coli [12]; S. t., Salmonella typhimurium [1]; C. g., Corynebacterium glutamicum [23]).
Characteristics of the enzyme.
The purified 3HAP mutase stored on ice retained 87% of its initial activity after 14 days and 63% after 38 days. After 30 days of storage at −70°C, the enzyme lost 7% of its activity. Since the enzyme was remarkably stable, no additional attempts at optimizing the storage conditions were made. Heating of cell extract to 60°C for 1 min abolished 72% of the original mutase activity, and no activity remained after 8 min.
The purified protein had no specific color and no relevant absorption at wavelengths above the 280-nm maximum. Therefore, the presence of flavins or other visible-light-absorbing prosthetic groups attached to the enzyme could be excluded. The isoelectric point of the protein was 4.5 as shown by chromatofocusing. The pH optimum of the purified enzyme was determined to be 6.5. The temperature optimum could not be determined, since the substrate was too unstable at temperatures higher than 30°C. However, the highest transformation rate was measured at 30°C.
Kinetics and inhibition by decomposition products of 3HAP.
3HAP mutase had a Vmax of approximately 4.8 μmol min−1 mg−1 and an apparent Km of approximately 0.1 mM. Experiments to determine the exact kinetic data of the enzyme failed because 3HAP stock solutions rapidly developed colored decomposition products. Depending on the decomposition state, Lineweaver-Burk plots showed parallel curves, indicating that there was not a competitive effect. Under optimized conditions, however, the measured rates were reproducible. Inhibition could be partly reversed by the addition of 2 mM DTT and/or 10 mM hydroxylamine to the assay buffer. This indicated that spontaneous autoxidation products of 3HAP, e.g., 3-nitrosophenol, could have been formed and interfered with 3HAP mutase. Hydroxylamine reacts rapidly with nitrosoaromatic compounds to form benzenediazonium salts (17), whereas DTT would reduce the nitroso group. Additionally, azoxy compounds could have been formed from 3HAP and 3-nitrosophenol (24). However, it could not be clarified which autoxidation products present in 3HAP stock solutions interacted with the enzyme.
Influence of possible activators or inhibitors on the enzyme.
To obtain information about the mechanism of the enzymatic reaction, the influence of reagents having a possible effect on the 3HAP mutase was investigated. 1,10-Phenanthroline inhibited the activity slightly, but other metal cation chelators such as EDTA or tiron had no effect on the activity. The results indicated that metal cations play no role in the reaction mechanism, although the presence of a tightly associated metal ion cannot be ruled out. AgNO3 destroyed mutase activity when 1 mM AgNO3 was preincubated with the enzyme, but preincubation with low concentrations (e.g., 0.01 mM) had no effect. H2O2 (0.3 μM) destroyed mutase activity, which indicated the presence of relevant sulfhydryl groups in the protein. Inhibition was observed with l-cysteine and was dependent on the preincubation time: 3HAP mutase activity was reduced by 39% after 90 min and by 83% after 240 min of exposure. In contrast, the reducing agents DTT (10 mM) and NaBH4 (26 μM) had no effect on activity. Furthermore, a preincubation of 3HAP mutase with the electron transport inhibitors KOCN and NaN3 did not affect the enzyme activity when present at ≤0.1 mM. No inhibition or activation of 3HAP mutase activity was observed by preincubation of the enzyme with Co2+, Cu2+, Fe2+, Fe3+, Mg2+, Mn2+, Ni2+, Zn2+, NaNO2, or hydroxylamine. Addition of hydroxylaminobenzene as alternative substrate of 3HAP mutase to the assay buffer inhibited the enzyme activity slightly (25%). Due to the relationship of the protein with glutamine synthetases, glutamate and glutamine were tested as inhibitors. Neither preincubation of the compounds with the enzyme nor their addition to the assay buffer affected 3HAP mutase activity.
3HAP conversion by the purified enzyme and its substrate specificity.
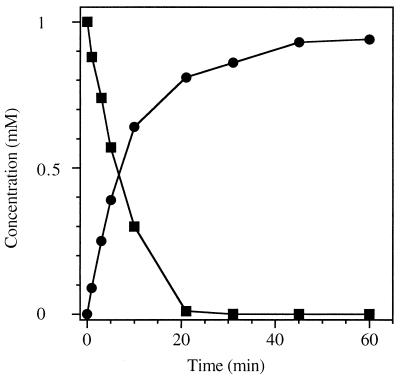
Preliminary studies (43) indicated that cell extracts from induced cells of R. eutropha JMP134 formed aminohydroquinone from 3HAP during 3NP degradation. It was not clear whether a single enzyme was responsible for the complex conversion of 3HAP to aminohydroquinone. A reduction of 3HAP to 3-aminophenol and subsequent hydroxylation of 3-aminophenol by a monooxygenase could be an alternative mechanism for the formation of aminohydroquinone. Therefore, the enzyme was incubated with 3HAP under anaerobic conditions (Fig. 4). The fast decrease in the concentration of 3HAP was concomitant with the production of aminohydroquinone. 3HAP mutase required neither a cofactor nor oxygen for the enzymatic reaction. This indicated that the enzyme catalyzes a Bamberger-type rearrangement. Three isomeric dihydroxyanilines (aminohydroquinone, 4-aminocatechol, and 3-aminocatechol) can theoretically be formed from 3HAP based on the mechanism of a Bamberger rearrangement (Fig. 1). The chemical reaction of 3HAP in dilute sulfuric acid formed 4-aminocatechol, which could be clearly identified by comparison of the UV spectrum and the chromatographic patterns with those of an authentic standard. The yield of 4-aminocatechol was low (approximately 10%), and the reaction mixture adopted a deep black coloration. Because no products other than 4-aminocatechol were detected in relevant amounts by HPLC analysis, formation of aminohydroquinone and 3-aminocatechol from 3HAP by the nonenzymatic reaction seems unlikely. The conversion of 3HAP by 3HAP mutase yielded exclusively aminohydroquinone instead of aminocatechol, which clearly supported the enzymatic nature of the reaction.
FIG. 4.
Conversion of 3HAP (■) to aminohydroquinone (●) by 3HAP mutase from R. eutropha JMP134. The enzyme (0.6 U) and 3HAP (1 mM) were incubated in 100 mM phosphate buffer (pH 7) under an argon atmosphere at 30°C. The conversion was analyzed by HPLC.
Extracts from induced cells of R. eutropha JMP134 converted hydroxylaminobenzene to a mixture of 2- and 4-aminophenol (43). The purified enzyme gave similar results (data not shown). The reaction rate with hydroxylaminobenzene was 44-fold lower than that with 3HAP. Additionally, extracts of R. eutropha JMP134 containing 3NP nitroreductase and 3HAP mutase in the presence of excess NADPH converted 4-nitrotoluene anaerobically to two metabolites. One was identified as 6-amino-m-cresol by comparing its chromatographic patterns and its UV spectrum with those of an authentic standard. The second metabolite could be reduced to 4-aminotoluene by treatment with zinc in HCl solution. The results indicated that the reaction involved partial reduction of 4-nitrotoluene to 4-hydroxylaminotoluene, which then underwent rearrangement to 6-amino-m-cresol by the cell extract containing 3HAP mutase.
In contrast, the transformation of 4-nitrobenzoate under the same conditions did not lead to production of 3-hydroxy-4-aminobenzoate, although 4-nitrobenzoate was completely converted. The only metabolite which accumulated could be reduced to 4-aminobenzoate by treatment with zinc in HCl solution, which suggested that the metabolite was 4-hydroxylaminobenzoate (not available as authentic compound). The accumulation of 4-nitrosobenzoate could be excluded, since it would have been spontaneously reduced to 4-hydroxylaminobenzoate by NADPH (4, 30).
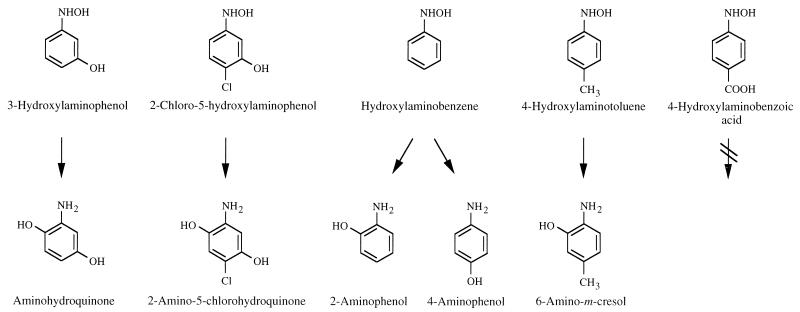
In Fig. 5, the above-described reactions catalyzed by 3HAP mutase are summarized. The conversion of 2-chloro-5-hydroxylaminophenol to 2-amino-5-chlorohydroquinone has been reported previously (44). Here, the reaction rate was 1.6-fold higher than that with 3HAP.
FIG. 5.
Conversion of hydroxylaminoaromatic compounds by 3HAP mutase from R. eutropha JMP134.
DISCUSSION
Purification and characterization of 3HAP mutase revealed clearly that the conversion of 3HAP to aminohydroquinone is catalyzed by a single 62-kDa enzyme. Since oxygen or cofactors were not required, it was confirmed that the enzymatic transformation corresponds to the acid-catalyzed Bamberger rearrangement (Fig. 1). The enzymatic reaction is regiospecific: the enzyme directs the formation of aminohydroquinone exclusively, whereas the chemical reaction forms the isomeric 4-aminocatechol from 3HAP.
The hydroxylaminobenzene mutase from P. pseudoalcaligenes JS45 grown on nitrobenzene directs the conversion of hydroxylaminobenzene almost exclusively to 2-aminophenol (37). In contrast, 3HAP mutase from R. eutropha JMP134 transformed hydroxylaminobenzene to a mixture of 2-aminophenol and 4-aminophenol.
The 3HAP mutase of JMP134 is active with substrates with different substituents in positions 4 and 5 (Fig. 5). For example, it catalyzed the conversion of 2-chloro-5-hydroxylaminophenol to 2-amino-5-chlorohydroquinone (44) and that of 4-hydroxylaminotoluene to 6-amino-m-cresol. The rearrangement of 4-hydroxylaminotoluene to 6-amino-m-cresol is also a key reaction in a new pathway for 4-nitrotoluene degradation by Mycobacterium sp. strain HL 4-NT-1. The pathway involves initial reduction of 4-nitrotoluene to 4-hydroxylaminotoluene (50). In contrast, 4-nitrotoluene degradation by several Pseudomonas spp. (20, 41) is initiated by a stepwise oxidation of the methyl group, yielding 4-nitrobenzoate, which is reduced to 4-hydroxylaminobenzoate. Finally, protocatechuate (3,4-dihydroxybenzoate) is formed from 4-hydroxylaminobenzoate with concomitant release of ammonia by a lyase reaction. The same reaction has been described by Groenewegen and de Bont (19) for 4-nitrobenzoate degradation by Comamonas acidovorans NBA-10. The corresponding enzyme was partially purified and had a narrow substrate specificity (34). Although the mechanism of the reaction was unknown, a mechanism similar to that of a Bamberger rearrangement was discussed (34). 4-Hydroxylaminobenzoate lyase had a molecular mass of 45 kDa, and reducing agents were required to restore activity. The different chemoselectivity, together with the failure of 3HAP mutase to attack 4-hydroxylaminobenzoate, clearly demonstrates that the 4-hydroxylaminobenzoate lyase from C. acidovorans NBA-10 and the 3HAP mutase from R. eutropha JMP134 are different enzymes. In contrast to our results with R. eutropha JMP134, Pseudomonas putida B2 also degrades 3NP via 3HAP (35), but a subsequent lyase reaction converts 3HAP to 1,2,4-trihydroxybenzene and ammonia. In contrast, 3NP-grown cells of R. eutropha JMP134 release ammonia only when oxygen is present (43).
Inhibition studies performed with 3HAP mutase did not elucidate the enzymatic reaction mechanism. Inhibition of the enzyme was observed by finding that 3HAP stock solutions contained impurities resulting from spontaneous and rapid decomposition of the compound in the presence of oxygen, but the inhibitory compound(s) could be not identified. Inactivation of 3HAP mutase by high concentrations of H2O2 indicated the presence of structurally important sulfhydryl groups in the enzyme. Inhibition by cysteine, which was dependent on the preincubation time, cannot be explained, since other reducing agents did not affect the enzyme. All other compounds tested had no significant effect on the mutase activity.
A striking similarity of the amino acid sequence of the purified enzyme to those of glutamine synthetases exists. Glutamine synthetase catalyzes an ATP-dependent amidation of glutamic acid to glutamine plus ammonia (36). A mechanistic analogy between the mutase and the amidase reaction is difficult to identify, particularly since neither glutamate nor glutamine inhibited the 3HAP mutase, so that a potential phylogenetic relationship among these enzymes would require a complete analysis of the amino acid sequence of the 3HAP mutase.
Arylhydroxylamines are highly reactive and hence cytotoxic, mutagenic, and carcinogenic. Interestingly, the formation of nitrenium/carbenium cations from hydroxylaminoarenes plays an important role in these effects, because the reactive cations are also susceptible to nucleophilic attack by nucleic acid bases and other nucleophiles (7, 8, 38). Enzymes that convert arylhydroxylamines to harmless products are useful detoxification tools and are essential to survival for a biological system. In the case of bacterial metabolism of nitroarenes, such enzymes allow the conversion of a highly reactive intermediate to compounds that can serve as growth substrates. Several recent reports indicate that enzymes catalyzing Bamberger-type rearrangements can play key roles in the bacterial metabolism of nitroarenes via the highly reactive hydroxylamino derivatives (22, 37, 43, 44, 50). 3HAP mutase from R. eutropha JMP134 is the first of these enzymes to be purified and characterized. The reported characteristics of the enzyme may be useful for comparison with enzymes from other organisms which catalyze analogous reactions.
ACKNOWLEDGMENTS
We gratefully acknowledge U. Göttert, U. Lendenmann, and M. Schlömann for valuable discussions. We thank H. Weber for determining the amino acid sequences. We thank C. M. Vogel for her interest and help in facilitating the research project.
This work was sponsored by the Air Force Office of Scientific Research, Air Force Systems Command USAF, under grant AFOSR-91-0237.
REFERENCES
- 1.Almassy R J, Janson C A, Hamlin R, Xuong N H, Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. Nature. 1986;323:304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamberger E. Über das Phenylhydroxylamin. Chem Ber. 1984;27:1548–1557. [Google Scholar]
- 4.Becker A R, Sternson L A. Nonenzymatic reduction of nitrosobenzene to phenylhydroxylamine by NAD(P)H. Bioorg Chem. 1980;9:305–312. [Google Scholar]
- 5.Bisswanger H. Theorie und Methoden der Enzymkinetik. Weinheim, Germany: Verlag Chemie; 1979. [Google Scholar]
- 6.Booth J, Boyland E. The biochemistry of aromatic amines. 10. Enzymic N-hydroxylation of arylamines and conversion of arylhydroxylamines into o-aminophenols. Biochem J. 1964;91:362–369. doi: 10.1042/bj0910362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosold F, Boche G. The ultimate carcinogen, O-acetyl-N-2-(fluorenyl)-hydroxylamine (“N-acetoxy-2-aminofluorene”), and its reaction in vitro to form 2-[N-(deoxyguanosin-8-yl)amino]fluorene. Angew Chem Int Ed Engl. 1990;29:63–64. [Google Scholar]
- 8.Boteju L W, Hanna P E. Bioactivation of N-hydroxylaminofluorenes by N,O-acyltransferase: substituent effects on covalent binding to DNA. Carcinogenesis. 1993;14:1651–1675. doi: 10.1093/carcin/14.8.1651. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantification of micrograms quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Bruhn C, Lenke H, Knackmuss H-J. Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl Environ Microbiol. 1987;53:208–210. doi: 10.1128/aem.53.1.208-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cardy D L, Murrell J C. Cloning, sequencing and expression of the glutamine synthetase structural gene (glnA) from the obligate methanotroph Methylococcus capsulatus (Bath) J Gen Microbiol. 1990;136:343–352. doi: 10.1099/00221287-136-2-343. [DOI] [PubMed] [Google Scholar]
- 12.Colombo G, Villafranca J J. Amino acid sequence of Escherichia coli glutamine synthetase deduced from the DNA nucleotide sequence. J Biol Chem. 1986;261:10587–10591. [PubMed] [Google Scholar]
- 13.Corbett M D, Corbett B R. Metabolism of 4-chloronitrobenzene by the yeast Rhodosporidium sp. Appl Environ Microbiol. 1981;41:942–949. doi: 10.1128/aem.41.4.942-949.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbett M D, Corbett B R. Bioorganic chemistry of the arylhydroxylamine and nitrosoarene functional groups. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 151–182. [Google Scholar]
- 15.Corbett M D, Corbett B R, Doerge D R. Hydroxamic acid production and active-site induced Bamberger rearrangement from the action of α-ketoglutarate dehydrogenase on 4-chloronitrosobenzene. J Chem Soc Perkin Trans I. 1982;1982:345–350. [Google Scholar]
- 16.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J-F, Dougherty B A, Merrick J M, McKenney K, Sutton G, Fitzhugh W, Fields C A, Gocayne J D, Scott J D, Shirley R, Liu L-I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Fritchman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 17.Furniss B S, Hannaford A J, Smith P W G, Tatchell A R. Vogel’s textbook of practical organic chemistry. 5th ed. New York, N.Y: John Wiley & Sons, Inc.; 1989. [Google Scholar]
- 18.Gorontzky T, Drzyzga O, Kahl M W, Bruns-Nagel D, Breitung J, von Loew E, Blotevogel K-H. Microbial degradation of explosives and related compounds. Crit Rev Microbiol. 1994;20:265–284. doi: 10.3109/10408419409113559. [DOI] [PubMed] [Google Scholar]
- 19.Groenewegen P E J, de Bont J A M. Degradation of 4-nitrobenzoate via 4-hydroxylaminobenzoate and 3,4-dihydroxybenzoate in Comamonas acidovorans NBA-10. Arch Microbiol. 1992;158:381–386. doi: 10.1099/00221287-138-8-1599. [DOI] [PubMed] [Google Scholar]
- 20.Haigler B E, Spain J C. Biodegradation of 4-nitrotoluene by Pseudomonas sp. strain 4NT. Appl Environ Microbiol. 1993;59:2239–2243. doi: 10.1128/aem.59.7.2239-2243.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higson F K. Microbial degradation of nitroaromatic compounds. Adv Appl Microbiol. 1992;37:1–19. doi: 10.1016/s0065-2164(08)70250-8. [DOI] [PubMed] [Google Scholar]
- 22.Hughes, J. Personal communication.
- 23.Jakoby M, Tesch M, Sahm H, Kraemer R, Burkovski A. Isolation of the Corynebacterium glutamicum glnA gene encoding glutamine synthetase I. FEMS Microbiol Lett. 1997;154:81–88. doi: 10.1111/j.1574-6968.1997.tb12627.x. [DOI] [PubMed] [Google Scholar]
- 24.Knight G T, Saville B. Hydrogen transfer from N-arylhydroxylamines to nitrosoarenes: an accompaniment to azoxyarene formation. J Chem Soc Perkin Trans II. 1973;1973:1550–1553. [Google Scholar]
- 25.Koerber S C, Schack P, Au A M-J, Dunn M F. Investigations of a novel liver alcohol dehydrogenase catalyzed redox-elimination reaction involving arylnitroso substrate analogues. Biochemistry. 1980;19:731–738. doi: 10.1021/bi00545a019. [DOI] [PubMed] [Google Scholar]
- 26.Kolanczyk R C, Gutmann H R, Rutks I R. Effect of bovine serum albumin on the extent of ortho rearrangement of N-(sulfooxy)-2-fluorenylacetamide and of enzymatically activated N-hydroxy-2-fluorenylacetamide and on the binding of reactive esters to nucleic acids. Chem Res Toxicol. 1992;5:274–279. doi: 10.1021/tx00026a020. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Lenke H, Knackmuss H-J. Initial hydrogenation during catabolism of picric acid by Rhodococcus erythropolis HL 24-2. Appl Environ Microbiol. 1992;58:2933–2937. doi: 10.1128/aem.58.9.2933-2937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenke H, Knackmuss H-J. Initial hydrogenation and extensive reduction of substituted 2,4-dinitrophenols. Appl Environ Microbiol. 1996;62:784–790. doi: 10.1128/aem.62.3.784-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leskovac V, Svircevic J, Trivic S, Popovic M, Radulovic M. Reduction of aryl-nitroso compounds by pyridine and flavin coenzymes. Int J Biochem. 1989;21:825–834. doi: 10.1016/0020-711x(89)90279-6. [DOI] [PubMed] [Google Scholar]
- 31.Maharaj R, Rumbak E, Jones W A, Robb S M, Robb F T, Woods D R. Nucleotide sequence of the Vibrio alginolyticus glnA region. Arch Microbiol. 1989;152:542–549. doi: 10.1007/BF00425484. [DOI] [PubMed] [Google Scholar]
- 32.Marvin-Sikkema F D, de Bont J A M. Degradation of nitroaromatic compounds by microorganisms. Appl Microbiol Biotechnol. 1994;42:499–507. doi: 10.1007/BF00173912. [DOI] [PubMed] [Google Scholar]
- 33.Maskos Z, Winston G W. Alcohol dehydrogenase-dependent reduction of 2-nitrosofluorene and rearrangement of N-hydroxy-2-aminofluorene. Biochemistry. 1993;32:12768–12773. doi: 10.1021/bi00210a028. [DOI] [PubMed] [Google Scholar]
- 34.Meulenberg R, de Bont J A M. Microbial production of catechols from nitroaromatic compounds. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 37–52. [Google Scholar]
- 35.Meulenberg R, Pepi M, de Bont J A M. Degradation of 3-nitrophenol by Pseudomonas putida B2 occurs via 1,2,4-benzenetriol. Biodegradation. 1996;7:303–311. doi: 10.1007/BF00115744. [DOI] [PubMed] [Google Scholar]
- 36.Müller O. Grundlagen der Biochemie. 1. Biochemische Reaktionen. Stuttgart, Germany: Thieme; 1977. [Google Scholar]
- 37.Nishino S F, Spain J C. Degradation of nitrobenzene by a Pseudomonas pseudoalcaligenes. Appl Environ Microbiol. 1993;59:2520–2525. doi: 10.1128/aem.59.8.2520-2525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak M, Kahley M J, Eiger E, Helmick J S, Peters H E. Reactivity and selectivity of nitrenium ions derived from ester derivatives of carcinogenic N-(4-biphenyl)hydroxylamine and the corresponding hydroxamic acid. J Am Chem Soc. 1993;115:9453–9460. [Google Scholar]
- 39.Pemberton J M, Corney B, Don R H. Evolution and spread of pesticide degrading ability among soil micro-organisms. In: Timmis K N, Pühler A, editors. Plasmids of medical, environmental and commercial importance. Amsterdam, The Netherlands: Elsevier/North Holland Biomedical Press; 1979. pp. 287–299. [Google Scholar]
- 40.Rawlings D E, Jones W A, O’Neill E G, Woods D R. Nucleotide sequence of the glutamine synthetase gene and its controlling region from the acidophilic autotroph Thiobacillus ferrooxidans. Gene. 1987;53:211–217. doi: 10.1016/0378-1119(87)90009-6. [DOI] [PubMed] [Google Scholar]
- 41.Rhys-Williams W, Taylor S C, Williams P A. A novel pathway for the catabolism of 4-nitrotoluene by Pseudomonas. J Gen Microbiol. 1993;139:1967–1972. doi: 10.1099/00221287-139-9-1967. [DOI] [PubMed] [Google Scholar]
- 42.Rigo, L. U., M. B. R. Steffens, E. M. Souza, and F. O. Pedrosa. Sequence and structural organization of the glnAntrBC operon from Herbaspirillum seropedicae strain Z78. Submitted for publication.
- 43.Schenzle A, Lenke H, Fischer P, Williams P A, Knackmuss H-J. Catabolism of 3-nitrophenol by Ralstonia eutropha JMP134. Appl Environ Microbiol. 1997;63:1421–1427. doi: 10.1128/aem.63.4.1421-1427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schenzle A, Lenke H, Knackmuss H-J. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Catabolism of 2-chloro-5-nitrophenol by Ralstonia eutropha JMP 134, abstr. Q-117; p. 475. [Google Scholar]
- 45.Shine H J. The rearrangement of phenylhydroxylamines. In: Eaborn C, Chapmann N B, editors. Reaction mechanisms in organic chemistry. Amsterdam, The Netherlands: Elsevier Biomedical Publishing Co.; 1967. pp. 182–190. [Google Scholar]
- 46.Sone T, Tokuda Y, Sakai T, Shinkai S, Manabe O. Kinetics and mechanisms of the Bamberger rearrangement. 3. Rearrangement of phenylhydroxylamines to p-aminophenols in aqueous sulphuric acid solutions. J Chem Soc Perkin Trans II. 1981;1981:298–302. [Google Scholar]
- 47.Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. [DOI] [PubMed] [Google Scholar]
- 48.Spain J C. Biodegradation of nitroaromatic compounds. Annu Rev Microbiol. 1995;49:523–555. doi: 10.1146/annurev.mi.49.100195.002515. [DOI] [PubMed] [Google Scholar]
- 49.Spain J C. Bacterial degradation of nitroaromatic compounds under aerobic conditions. In: Spain J C, editor. Biodegradation of nitroaromatic compounds. New York, N.Y: Plenum Press; 1995. pp. 19–35. [Google Scholar]
- 50.Spiess T, Desiere F, Fischer P, Spain J C, Knackmuss H-J, Lenke H. A novel degradative pathway of 4-nitrotoluene by a Mycobacterium sp. strain. Appl Environ Microbiol. 1998;64:446–452. doi: 10.1128/aem.64.2.446-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sternson L A, Gammans R E. A mechanistic study of aromatic hydroxylamine rearrangement in the rat. Bioorg Chem. 1975;4:58–63. [Google Scholar]
- 52.Stone K L, Williams K R. 2. Enzymatic digestion of proteins. In: Matsudaira P, editor. A practical guide to protein and peptide purification for microsequencing. 2nd ed. New York, N.Y: Academic Press, Inc.; 1993. pp. 54–68. [Google Scholar]
- 53.Toukdarian A, Saunders G, Selman-Sosa G, Santero E, Woodley P, Kennedy C. Molecular analysis of the Azotobacter vinelandii glnA gene encoding glutamine synthetase. J Bacteriol. 1990;172:6529–6539. doi: 10.1128/jb.172.11.6529-6539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vorbeck C, Lenke H, Fischer P, Knackmuss H-J. Identification of a hydride-Meisenheimer complex as a metabolite of 2,4,6-trinitrotoluene by a Mycobacterium strain. J Bacteriol. 1994;176:932–934. doi: 10.1128/jb.176.3.932-934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vorbeck C, Lenke H, Fischer P, Spain J C, Knackmuss H-J. Initial reductive reactions in aerobic microbial metabolism of 2,4,6-trinitrotoluene. Appl Environ Microbiol. 1998;64:246–252. doi: 10.1128/aem.64.1.246-252.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J, Bowler L D, Spratt B G. Interspecies recombination, and phylogenetic distortions, within the glutamine synthetase and shikimate dehydrogenase genes of Neisseria meningitidis and commensal Neisseria species. Mol Microbiol. 1997;23:799–812. doi: 10.1046/j.1365-2958.1997.2681633.x. [DOI] [PubMed] [Google Scholar]