Abstract
Individual studies have suggested that upper airway dysbiosis may be associated with asthma or its severity. We aimed to systematically review studies that evaluated upper airway bacterial microbiota in relation to asthma, compared to nonasthmatic controls. Searches used MEDLINE, Embase, and Web of Science Core Collection. Eligible studies included association between asthma and upper airway dysbiosis; assessment of composition and diversity of upper airway microbiota using 16S rRNA or metagenomic sequencing; upper airway samples from nose, nasopharynx, oropharynx or hypopharynx. Study quality was assessed and rated using the Newcastle-Ottawa scale. A total of 249 publications were identified; 17 in the final analysis (13 childhood asthma and 4 adult asthma). Microbiome richness was measured in 6 studies, species diversity in 12, and bacterial composition in 17. The quality of evidence was good and fair. The alpha-diversity was found to be higher in younger children with wheezing and asthma, while it was lower when asthmatic children had rhinitis or mite sensitization. In children, Proteobacteria and Firmicutes were higher in asthmatics compared to controls (7 studies), and Moraxella, Streptococcus, and Haemophilus were predominant in the bacterial community. In pooled analysis, nasal Streptococcus colonization was associated with the presence of wheezing at age 5 (p = 0.04). In adult patients with asthma, the abundance of Proteobacteria was elevated in the upper respiratory tract (3 studies). Nasal colonization of Corynebacterium was lower in asthmatics (2 studies). This study demonstrates the potential relationships between asthma and specific bacterial colonization in the upper airway in adult and children with asthma.
Keywords: Asthma, Dysbiosis, Microbiota, Upper airway, Wheezing
INTRODUCTION
Research in recent decades has shown the role of human microbiome in health and disease pathogenesis. The respiratory tract is colonized by distinct microbial species directly after birth [1], and functional or compositional perturbations of the microbiome have consequences to chronic respiratory conditions, including asthma [2]. Although the mechanism of the association between asthma and microbiome has not been fully explored, relationships between airway microbial dysbiosis and disease progression, exacerbations and response to treatment have been observed [3,4,5].
Bacterial burden in the upper airway is greater than in the lower respiratory tract, and the local airway inflammatory milieu influences the lower airway health through postnasal drip or aspiration, and leads translocation of pathogens downwards [6,7]. Individual studies have reported the major microbiome communities in asthmatics’ upper and lower airways that showed similarities in major colonizers including enriched Proteobacteria and Firmicutes, and reduced Actinobacteria and Bacteroidetes [4,8,9]. Depending on the bacterial species, the immunomodulation patterns differ; Proteobacteria is associated with T helper (Th)17-related gene expression and IL-17-driven inflammation may invoke noneosinophilic/nontype 2 asthma that is less responsive to corticosteroids [10], whereas certain Actinobacteria (i.e., Tropheryma whipplei) is abundant in poorly controlled eosinophilic asthma [11]. In children with asthma, nasal microbiota dominated by Corynebacterium and Dolosigranulum may reduce loss of asthma control, compared to clusters dominated with Moraxella, Staphylococcus, and Streptococcus [3]. While the relative abundances of nasal Proteobacteria is higher in young adult asthma, the genus Moraxella is less prevalent in elderly asthma [8]. Although these findings highlight the close interactions between asthma and airway microbial communities, the precise relationship between upper airway microbiome and asthma is still not conclusive.
This systematic review aimed to summarize studies that have evaluated the association between upper airway microbiota and asthma in children and adults. Childhood asthma was subdivided into 2 groups (birth to less than 3 years and 3 to 18 years) as studies have shown that airway microbial composition before age 3 is highly variable, and then appears to be more stable and to persist into adulthood [12,13].
A review of the literature with the following objectives was conducted:
(1) To systematically identify and review the current evidence for associations between asthma and upper respiratory tract (URT) microbiome through assessing the changes in microbial diversity, richness and composition in asthmatics comparing to healthy controls.
(2) To identify URT microbiome characteristics that are commonly associated with asthma.
(3) To provide contemporary understanding of the URT microbiota and its potential impact on increased risk of having asthma.
Search strategy
We performed a systematic literature review following the PRISMA (Preferred Reporting Items from Systematic Reviews and Meta-Analyses) guidelines [14]. A review protocol was registered to PROSPERO, a database of systematic review protocols (registration number: CRD42021247965).
An electronic search of 3 databases, MEDLINE, Embase, and Web of Science Core Collection, was performed on 4 June 2021. Searches in Google Scholar and cited reference searches were also done. The search was without date and language limitations. Details on the search strategy are provided in Supplementary Table 1.
Eligibility criteria
Articles meeting the following criteria were included: studies of any changes in the upper airway microbiome associated with wheezing or asthma; assessment of composition and diversity of the upper airway microbiome using advanced molecular techniques including next-generation sequencing platforms including pyrosequencing, HiSeq, MiSeq, whole metagenome sequencing; studies with asthma and control groups and adequate statistical analyses. Studies used upper airway samples, including anterior nares, nasal cavity, sinuses, nasopharynx, oropharynx, or hypopharyngeal swabs/aspirates [15]. When results were derived from the same study population, we considered the sample collection period for microbiome analysis, and included if the collection period differed. The use of the same population in different studies was determined by verifying the name and affiliation of authors and source of study participants.
Articles were excluded if microbiome composition was measured in sputum or lower airway samples; participants with respiratory diseases other than asthma; if the study tested the effect of medicine or environmental exposures on asthma microbiome; absence of healthy control group; and if sample size were less than 5 participants. Conference papers, letters, editorials, case reports, animal research, or review articles were not considered.
Titles, abstracts and full-text of articles were screened independently by 2 reviewers (PL and HSP) for eligibility. Discrepancies were resolved through discussion among the reviewers. Identified studies underwent for data extraction and qualitative synthesis.
Data extraction
The following information was collected from each included study: author, country, publication year, number of participants, asthma definition, asthma/wheezing rate, comorbidity, sampling period and site, sequence variation, detection instrument, confounding factors, and study outcomes.
Risk of bias assessment
The quality of observational studies was assessed using a modified Newcastle-Ottawa scale [16]. This method is used to assess the quality and biases of nonrandomized studies in systematic review and meta-analysis by evaluating 9 items grouped in domains: selection of participants (max 4 scores), comparability of groups (max 2), and ascertainment of the outcome (max 3). A study with a lower score indicates a higher risk of bias. The assessment was performed by 2 independent authors and the disagreements were resolved via discussion.
STUDY OUTCOME
Study selection
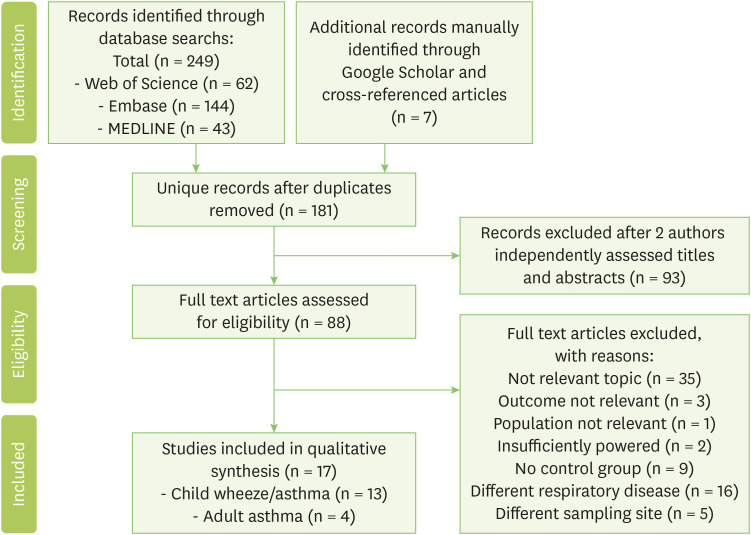
A literature search identified 249 articles, and 7 additional studies were identified from Google Scholar and cited reference search. Seventeen studies met the eligibility criteria and remained for qualitative synthesis (Fig. 1).
Fig. 1. PRISMA (Preferred Reporting Items from Systematic Reviews and Meta-Analyses) figure demonstrating literature excluded and examined in systematic review.
Studies characteristics
The characteristics and results of the observational studies are summarized in Table 1 for childhood asthma and Table 2 for adult asthma. All included studies had a cross-sectional observational design and were published between 2012 and 2021.
Table 1. Summary of studies investigating the association between upper airway microbiota and childhood asthma.
| Study | Country | Participant (n) | Asthma definition | Wheezing & asthma rate | Age at sample collection | Sample | Bacterial sequence, region | Method | Confounding factors | Changes in diversity and richness |
|---|---|---|---|---|---|---|---|---|---|---|
| Powell et al., 2019 [17] | UK | 98 | Physician diagnosed wheeze | 26.5% wheeze at 24 mo | 6 wk, 6, 9, 12, 18, and 24 mo | Oropharyngeal swab | 16S rRNA V3-V5 | Roche 454 pyrosequencing | Ethnicity, family history of atopy, presence of fever, use of antibiotics in the 4 wk prior to visit | Increased α-diversity (p < 0.001) |
| Ta et al., 2018 [19] | Singapore | 122 | Symptom-based wheeze | 27.8% rhinitis with wheezing at first 18 mo | 3 wks, 3, 6, 9, 12, 15, and 18 mo | Nasal swab | 16S rRNA V3-V6 | Illumina HiSeq | Gender, family history of respiratory disease, presence of siblings, mode of delivery, use of intrapartum antibiotic prophylaxis, postnatal antibiotics and breastfeeding pattern | Decreased α-diversity (p = 0.025) in rhinitis with wheeze |
| Cardenas et al., 2012 [25] | Ecuador | 48 | Physician diagnosed | 50% early-onset wheezing | Once at age 10.2 mo (mean) | Oropharyngeal swab | 16S rRNA V3-V5 | Roche 454 pyrosequencing | NA | No changes in diversity and richness |
| Teo et al., 2018 [26] | Australia | 244 | Questionnaire based | 10.6% early-sensitized children with wheezing at 5 yr | 2, 6, and 12 mo | Nasopharyngeal sample | 16S rRNA V4 | Illumina MiSeq | Gender and lower respiratory infection | NA |
| Teo et al., 2015 [28] | Australia | 234 | Symptom-based | 28% wheezing at 5 yr | 7, 8 and 9 wks, and 2, 6, and 12 mo | Nasopharyngeal sample | 16S rRNA V4 | Illumina MiSeq | Gender, maternal and paternal history of atopic disease | NA |
| Thorsen et al., 2019 [18] | Denmark | 644 | Symptoms-based | 22.7% asthma in the first 6 yr | 1 wk, 1, and 3 mo | Hypopharyngeal aspirate | 16S rRNA V4 | Illumina MiSeq | NA | At age 1 month: increased Shannon index p = 0.0046, Richness p = 0.0017 and Bray-Curtis p = 0.016 |
| Toivonen et al., 2020 [22] | Finland | 704 | Physician diagnosed and medication | 8% at age 7 yr | 2, 13 and 24 mo | Nasal swab | 16S rRNA V4 | Illumina MiSeq | Gender, siblings, parental asthma and child’s eczema by age 13 mo | No changes in α and β-diversity measures. |
| Tang et al., 2021 [27] | USA | 285 | Physician diagnosed and medication | 6–63% asthma at age 6, 8, 11, 13, and 18 yr | 2, 4, 6, 9, 12, 18, and 24 mo | Nasopharyngeal sample | 16S rRNA V4 | Illumina MiSeq | Age, gender, and season | NA |
| Chiu et al., 2017 [20] | Taiwan | 87 | Questionnaire based | 36.7% asthma | Once at ages 3–5 yr | Throat swab | 16S rRNA V3-V4 | Illumina MiSeq | Age, gender, maternal atopy, passive smoking, older siblings, and household income OR FDR-adjusted | Lower Chao1 (p = 0.014) and Shannon (p = 0.023) indeces in mite sensitized asthma |
| Kim et al., 2017 [21] | Korea | 92 | Physician diagnosed | 33.6% asthma | Once at ages 7.1–8 (mean) | Nasopharyngeal swab | 16S rRNA V1-V3, whole metagenome | Roche 454 pyrosequencing, Illumina HiSeq | NA | No change in α-diversity. Increased β-diversity (p < 0.001) |
| Birzele et al., 2017 [31] | Austria | 86 | Physician diagnosed, symptom and questionnaire based | 22.9% asthma | Once at ages 6–12 yr | Nasal swab | 16S rRNA V3-V5 | Roche 454 pyrosequencing | Farming | Decreased richness OR=0.63, p = 0.087 and Shannon index OR=0.66, p = 0.129 |
| Depner et al., 2017 [29] | Germany | 68 | Physician diagnosed, symptom and questionnaire based | 57.3% asthma | Once at ages 7–12 yr | Nasal swab | 16S rRNA V3-V5 | Roche 454 pyrosequencing | Farming | Lowered richness p = 0.052 |
| Castro-Nallar et al., 2015 [30] | USA | 14 | Physician diagnosed | 57.1% asthma | Once at 11–15 years (mean) | Nasal brush | Whole metagenome | HiSeq metagenome sequencing | NA | High richness and low evenness |
NA, not applicable; V, variable regions.
Table 2. Summary of studies investigating the association between upper airway microbiota and adult asthma.
| Study | Country | Participant (n) | Asthma definition | Asthma rate (%) | Age (yr) | Comorbidity | Sample | Bacterial sequence, region | Method | Changes in diversity and richness | Taxonomical changes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Durack et al., 2018 [32] | USA | 45 | Lung function test | 48.8 | 27–45 | Rhinitis 55% | Nasal brushing | 16S rRNA V4 | Illumina MiSeq | No changes in α-diversity | Decreased Corynebacterium (p = 0.07) |
| Fazlollahi et al., 2018 [23] | USA | 72 | Physician diagnosed and self-report | 70.8 | 35.8 ± 16 | Rhinitis 70.5% | Nasal swab | 16S rRNA V3-V4 | Illumina MiSeq | Increased α-diversity (not significant) | Increased Bacteroidetes (r = 0.33, p = 5.1×10-3) and Proteobacteria (r = 0.29, p = 1.4×10-2). |
| Prevotella buccalis (p = 1.0×10-2), Gardnerella vaginalis (p = 2.8×10-3), Alkanindiges hongkongensis (p = 2.6×10-3) | |||||||||||
| Dialister invisus (p = 9.1×10-3) | |||||||||||
| Lee et al., 2019 [8] | Korea | 80 | Physician diagnosis, symptom and lung test | 75 | 18–45, > 65 | NA | Nasopharyngeal swab | 16S rRNA V1-V3, whole metagenome | Roche 454 pyrosequencing, Illumina HiSeq | No changes in Shannon diversity | Increased Proteobacteria (p < 0.05), decreased Corynebacteriales (p < 0.01) |
| Moraxella (p < 0.05) | |||||||||||
| Park et al., 2014 [24] | Korea | 47 | Symptoms-based | 38.2 | 23–79 | NA | Oropharyngeal sample | 16S rRNA V1-V3 | Roche 454 pyrosequencing, | Shannon diversity decreased (2.4 ± 1) vs. control (3.5 ± 0.7) | Increased Pseudomonas spp. and Lactobacillus spp. (p < 0.0001), decreased Streptococcus spp., Neisseria spp., Veillonella spp., and Prevotella spp. (p < 0.0001) |
In childhood asthma, 7 studies collected samples at more than 3 time points in first 24 months, and assessed asthma outcome once between ages 10 months to 7 years, and 1 study followed up and assessed asthma outcome at ages 6, 8, 11, 13, and 18 (Table 1). Upper airway samples were collected from nose/nasopharynx in 9 studies, oropharynx in 2 studies, and throat or hypopharynx in 2 studies. Frequently accounted confounding variables were gender (46%), family history of respiratory disease/atopy (38%), presence of siblings (23%), age (15%), antibiotic use (15%), whereas ethnicity, presence of fever, mode of delivery, breastfeeding pattern, lower respiratory infection, child’s eczema, season, passive smoking, and household income were accounted for once. A total of 11 studies used hypervariable regions of 16S rRNA gene sequencing for the evaluation of taxonomic composition, one study used whole metagenome RNA sequencing and one used both methods.
In adult asthma, rhinitis was reported in 55% and 70.5% of subjects as a comorbidity (Table 2). Samples were collected at one-time point from the nose/nasopharynx in 3 studies and from the oropharynx in one study. Taxonomic composition was identified using 16S rRNA gene sequencing in all studies and one study performed both 16S rRNA and whole metagenome sequencing.
Quality of the included studies
The quality of the 17 studies included was rated according to the modified Newcastle-Ottawa scale (Supplementary Table 2). The mean score was 7.2 (range, 5–9 points).
Microbiome diversity and richness
The changes in microbiome diversity and richness in asthmatic children are summarized in Table 1 and Table 3. The main diversity outcome measure was alpha-diversity (61%). Alpha-diversity was reported to be higher in younger children (1–24 months old) with wheezing and asthma [17,18], which was inconsistent with other reports when asthmatic children had rhinitis or mite sensitization [19,20]. Increased bacterial richness (high diversity) was observed to be first elevated at one month of age in subjects who developed asthma by 6 years, though this was not observed at 3 months of age [18]. In contrast, the richness estimated by the Chao 1 score was observed to be reduced in a separate study of mite sensitized asthmatics at ages 3–5 years [20]. The changes in alpha-diversity and richness did not differ significantly between groups in 6 studies.
Table 3. Summary of differences in the composition of the upper airway microbiota associated with childhood asthma.
| Sample collection | Relative abundance | Sample | Actinobacteria | Bacteroidetes | Firmicutes | Proteobacteria |
|---|---|---|---|---|---|---|
| ≤ 24 months | Increased | Nasal/nasopharyngeal swab | Aerococcaceae [19] (p < 0.01) Streptococcus [27] (OR = 1.7 [1.3–2.2], p = 5.70E-05) Streptococcus [28] (OR = 3.8 [1.3–12], p = 0.017) | Haemophilus [22] (FDR = 0.03) Oxalobacteraceae [19] (p < 0.01) Moraxella, Streptococcus and Haemophilus [26] (OR = 2.5 [1.3–4.6, p < 0.0054]) | ||
| Oropharyngeal swab | Actinomyces [25] (OR = 1.10, p = 1.89×10-2) | Flavobacteriaceae [25] (OR = 12.07, p = 4.02×10-31) | Staphylococcus [25] (OR = 124.11, p = 1.87×10-241) Veillonella [18] (HR = 1.45 [1.21–1.73], p < 0.0001) | Neisseriaceae [25] (OR = 1.19, p = 5.84×10-5) | ||
| Atopobium [25] (OR = 2.27, p = 8.99×10-20) | Prevotella [25] (OR = 1.38, p = 3.24×10-13) | Haemophilus [25] (OR = 2.12, p = 5.46×10-23) | ||||
| Corynebacterium [25] (OR = 24.99, p = 1.37×10-129) | Prevotella [18] (HR = 1.32 [1.13–1.55], p = 0.0005) | Neisseria [17] (p = 0.003) | ||||
| Decreased | Nasal/nasopharyngeal swab | Corynebacteriaceae [19] (p < 0.01) | Staphylococcaceae [19] (p < 0.05) Dolosigranulum [27] [OR = 0.42 (0.29–0.61) p = 8.50E-06] | Moraxella [22] (OR = 2.74 [1.20–6.27]) | ||
| Oropharyngeal swab | Bacteroidales [25] (OR = 0.55, p = 9.57×10-8) | Gemella [25] (OR = 0.40, p = 4.29×10-21) | Pasteurellaceae [25] (OR = 0.20, p = 1.13×10-20) | |||
| Porphyromonas [25] (OR = 0.20, p = 2.81×10-32) | Lachnospiraceae [25] (OR = 0.39, p = 7.79×10-14) | Moraxella [25] (OR = 0.79, p = 4.54×10-06) | ||||
| Prevotella [17] (p = 0.018) | Veilonella [25] (OR = 0.59, p = 8.06×10-86) | |||||
| Leptotrichia [25] (OR = 0.42, p = 9.37×10-14) Granulicatella [17] (p = 0.012) | ||||||
| > 24 months | Increased | Nasal/nasopharyngeal sample | Staphylococcus [21] (p < 0.05) | Moraxella catarrhalis [30] (14-fold) | ||
| E. coli [30] (p < 0.05) | ||||||
| Psychrobacter [30] (p < 0.05) | ||||||
| Moraxella [29] (OR = 3.78, p = 9.76×10-5) | ||||||
| Throat swab | Selenomonas [20] (p = 0.020) | |||||
| Decreased | Nasal swab | Prevotella [31] (OR = 0.44 [0.21–0.93], p = 0.0345) | ||||
| Throat swab | Butyrivibrio [20] (FDR p = 0.030) | |||||
| Parvimonas [20] (p = 0.020) |
FDR, false discovery rate; HR, hazard ratio; OR, odds ratio.
Beta-diversity, variation of communities between samples, was reported in 3 studies using different metric measures. The intergroup microbiota composition according to UniFrac distances were greater in asthma and remission groups than that in control group at ages 7–8 [21]. In an individual study, the Bray-Curtis dissimilarity index was higher during the first month of age in asthmatic children [18]; however, this was not confirmed in another study when assessed at ages 2, 13, and 24 months [22].
In adult asthma, 2 studies reported inconsistent changes in alpha-diversity, but none of them reached statistical significance [23,24].
Taxonomic composition
The major phyla changes reported in studies of asthma were Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria as summarized in Table 3.
Upper airway microbiota at first 2 years in asthmatic children
A total of 8 studies investigated the taxonomic changes in the upper airway microbiota during the first 2 years of life. Proteobacteria was the most abundant phylum in the community and its association with asthma was examined in 5 studies. The prevalent abundance of families Oxalobacteraceae, Neisseriaceae, and decreased abundance of Pasteurellaceae were associated with wheezing before age 2 [19,25]. At the genus level, Haemophilus [22,25,26], Moraxella [26], and Neisseria [17] were enriched in children with wheezing. In contrast, a profile of persistent sparsity of Moraxella from age 2-13 months increased the risk of developing asthma at age 7 in comparison to a persistent Moraxella dominance profile as a reference group [22].
Six studies identified significant changes of Firmicutes phylum in association with wheezing or asthma. Children who had wheezing and asthma had a greater abundance of Aerococcaceae [19], Staphylococcus [25], Streptococcus [27,28], Veillonella [18], and decreased abundance of Lachnospiraceae [25], Staphylococcaceae [19], Gemella, Veilonella, Leptotrichia [25], Granulicatella [17], and Dolosigranulum [27]. In a pooled analysis, nasopharyngeal colonization of Streptococcus at first 7 weeks was associated with wheezing at age 5 (p = 0.04) (Supplementary Fig. 1).
The association between the phylum Bacteroidetes and asthma was reported in 2 studies. Prevalent Flavobacteriaceae family, and less frequent order Bacteroidales and genera Porphyromonas were associated with wheezing in infants [25]. Two studies reported an increased abundance of Prevotella at ages 1 and 10.2 months [18,25], and one study reported a decreased abundance of this genus at age 18 months [17].
Two studies identified significant changes in the phylum Actinobacteria. The abundance of oropharyngeal Actinomyces, Atopobium, and Corynebacterium were reported to increase in children with wheezing [25], whereas nasal Corynebacteriaceae was decreased in the first 18 months of life [19].
Upper airway microbiota at ages 3–18 years in asthmatic children
The association between asthma and microbial community structure at ages 3–18 was assessed in 5 studies. The abundance of phylum Proteobacteria including genera Moraxella [29], Psychrobacter [30], and species E. coli [30], and M. catarrhalis [30] were dominant in school-age children with asthma.
The relative abundance of Firmicutes phylum was identified in 2 studies. At genera level, a higher abundance of Selenomonas and Staphylococcus [20,21], and lower abundance of Butyrivibrio and Parvimonas were reported in asthma group [20].
Phylum Bacteroidetes (Prevotella genus) was decreased in children with asthma [31].
Upper airway microbiota in adult asthma
Four studies investigated the association between upper airway microbiota and asthma in adults (Table 2). The abundance of Proteobacteria including genus Pseudomonas and species Alkanindiges hongkongensis was reported to be enriched [8,23,24], but the genera Neisseria and Moraxella were decreased in 2 independent studies [8,24]. Among phylum Firmicutes, the genus Lactobacillus and species Dialister invisus were frequent in the asthmatics airway [23,24], whereas the genera Streprococcus and Veillonella were lower when compared to healthy controls [24]. The phylum Bacteroidetes and its species Prevotella buccalis were reported to be significantly elevated in the nasal microbiome community [23]. However, the genera Prevotella was significantly lower when assessed in the oropharynx in an individual study [24]. Among Actinobacteria phylum, the abundance of species Gardnerella vaginalis was associated with asthma exacerbation [23], and the abundance of order Corynebacteriales and genera Corynebacterium were lower in nasal bacterial community of asthmatics [8,32].
DISCUSSION AND FUTURE DIRECTIONS
Key findings
In this systematic review, we synthesized the evidence of the association between asthma and bacterial microbiome changes in URT. The diversity and richness of the microbiota were less consistent in childhood asthma. In the first 2 years, the alpha-diversity was higher in childhood asthma [17,18]; however, asthmatics with early-onset rhinitis and mite sensitization showed an inverse association [19,20]. URT microbial composition was highly variable at this age and Proteobacteria (Moraxella, Haemophilus, Neisseria) and Firmicutes (Staphylococcus, Streptococcus) were the most prevalent phyla in the URT in children with asthma. The most abundant URT microbiota in adult asthmatics was Proteobacteria. Less consistent changes were observed in phyla Bacteroidetes and Actinobacteria in both age groups. In a pooled analysis of 2 studies, nasopharyngeal colonization of Streptococcus at first 7 weeks was associated with the risk of having wheeze at age 5, and this requires further validation in a larger cohort of patients. A reduction of presumed commensal bacteria, Corynebacterium, was reported in the nasal cavity in adult patients [8,32].
Comparison to existing literatures
We observed inconsistent observations with respect to alpha-diversity which may differ depending on asthma inflammatory phenotypes. Previous findings have shown variation in microbial diversity and richness in the lower airway among eosinophilic asthma and healthy controls [33], and neutrophilic and non-neutrophilic asthma [34]. This finding needs to be validated in studies with larger sample sizes and in pooled analysis with consistent asthma definition or asthma endotypes.
Enriched pathogenic microbiota were also found in the URT in childhood asthma. In particular, nasopharyngeal Streptococcus colonization at early ages increased risk of having asthma at age 5. When authors evaluated this change with respect to persistent asthma, only a Staphylococcus-dominant microbiome in the first 6 months increased the risk of recurrent wheezing by age 3 and asthma that persisted throughout childhood [27]. Other illness-associated taxa including Streptococcus- and Moraxella-dominant groups did not show any associations with persistent asthma phenotype in children. In addition, asymptomatic colonization of Streptococcus, Haemophilus and Moraxella in the URT increased risk of chronic wheeze at age 5 in early-sensitized children [26]. Infants who were atopic by age 2 and developed chronic wheeze at age 5 also had early Streptococcus colonization [28]. Thus, early Streptococcus colonization in the URT may predict wheeze or asthma risk in preschool children with atopic condition. These findings were not replicated when wheeze was defined in the first 18 months [19], and at 7 years [22], and when microbiota was assessed in oropharyngeal samples using different sequencing region and platform (V4 region of the 16S rRNA and Illumina MiSeq [27,28] vs. V3–V5 region and Roche 454 pyrosequencing [17]). These pathogens (Moraxella, Streptococcus, and Haemophilus) localized in the lower airway have been associated with neutrophilic airway inflammation in young children with persistent wheezing and in adults with severe asthma [35,36].
In adult upper airway, nasal colonization of microbiota varied according to asthma activity [23,37]. However, the observations in adult asthma were not replicated in other studies except enriched Proteobacteria phylum. Some genera, including Prevotella were inconsistent among studies, and this could be due to different localization and disease severity [38]. While anaerobic bacteria Prevotella is identified in the healthy oropharynx and lungs, children with asthma presented a greater abundance of Prevotella in the oropharynx and hypopharynx, and adult asthmatics presented higher in the nasal cavity [18,23,25]. Inverse association were also observed in nasal and oropharyngeal samples obtained from children with asthma [17,31]. In asthma, mucus hypersecretion is common and is associated with rhinosinusitis, polyps and exacerbation. Excessive mucus secretion may provide anaerobic niches in the airways leading to increased bacterial colonization in these patients [39,40]. Asthma patients with rhinitis had an increased relative abundance of Prevotella spp. in their nasal microbiota [23].
There was also evidence that reduction of nasal Corynebacterium was associated with asthma in adult patients [8,32]. In children, oropharyngeal Corynebacterium was higher in asthma group [19], whereas nasal Corynebacteriaceae was lower in disease group [25]. This genus has previously been recognized as having beneficial effect in children with asthma exacerbation along with genus Dolosigranulum [3].
Confounding factors
While feeding type, siblings, antibiotic use, respiratory viral infection, animal exposure, day care attendance, season and antibiotic have shown to influence the airway microbiome in children [28,41,42], disease process, smoking, and season potentially affected the composition of airway microbiome in adults [8,23,43,44]. In current review, the confounding factors considered among studies were diverse, and were accounted for only in studies of childhood asthma. Among them, gender, family history of respiratory disease or atopy, presence of siblings, age, and antibiotic use were the most commonly accounted factors in analyses. The presence of heterogeneity and inadequate accounting for potential confounding factors in studies may dilute the statistical estimates of effect sizes of the microbiome [45], and may have affected the results of the studies. To tackle these challenges and increase the complexity of the model, application of a robust variable selection method would be a better approach in future studies.
Methodologies
To define a complete taxonomic composition of the microbiome inhabiting the upper airways, a vast majority of studies used 16S rRNA gene sequencing targeting different hypervariable subregions of this gene. This method limits the taxonomic classification of bacteria up to a genus-level composition, whereas metagenomic sequencing identifies genomes of microbiota providing species and strain-level identification and offers more advantages, especially for designing microbiome-based therapeutic interventions. Recent evaluation of 16S rRNA gene sequencing analysis revealed unmatched taxonomic accuracy in some subregions of 16S gene [46]. For example, V4 and V3–V5, which were the most commonly targeted regions in current systematic review, showed lower performance to recreate the number of sequences, and at classifying sequences belonging to the phylum Actinobacteria. Similarly, when 16S rRNA amplicon sequencing data generated using 3 different platforms (Illumina MiSeq, Ion Torrent PGM, Roche 454) and 7 bioinformatics pipelines were compared, the average relative abundance of specific taxa varied depending on platform, library preparation method, and bioinformatics analysis [47]. Therefore, application of standardized protocols on study designs, consistent sample processing, full-length 16S sequencing, and appropriate computational analysis will be essential to enable accurate resolution for classification of individual organisms and their potential effects on disease development.
CONCLUSION
Microbiota colonizing the URT potentially contribute to the development of asthma. The microbial community in the first 2 years of life is more diverse, and may increase, or act as a biomarker for, subsequent risk of asthma development in childhood. Nasopharyngeal colonization of Streptococcus in the first 7 weeks may predict wheezing in preschool children. The most abundant phylum in the URT of asthmatics were Proteobacteria in all age groups. The relative abundance of phyla Firmicutes, Bacteroidetes, and Actinobacteria were inconsistent among studies and remains to be evaluated in further studies. Cohesive validation and standardization of protocols for microbiome studies are essential to reduce the inconsistency between studies, and provide more accurate information on the association of microbiome dysbiosis with asthma development and progression.
Supplementary Tables 1, 2 and Fig. 1 can be found via DOI.
ACKNOWLEDGMENTS
This work was supported by Brain Pool Program through the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (grant number 2019H1D3A2A02102333).
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Purevsuren Losol, Sae-Hoon Kim, Yoon-Seok Chang.
- Formal analysis: Purevsuren Losol, Sae-Hoon Kim, Hee-Sun Park, Woo-Jung Song, Yoon-Seok Chang.
- Investigation: Purevsuren Losol, Hee-Sun Park, Yu-Kyoung Hwang.
- Methodology: Purevsuren Losol, Hee-Sun Park, Yu-Kyoung Hwang, Woo-Jung Song.
- Project administration: Purevsuren Losol, Yoon-Seok Chang.
- Writing - original draft: Purevsuren Losol, Yu-Kyoung Hwang.
- Writing - review & editing: Purevsuren Losol, Hee-Sun Park, Woo-Jung Song, Yu-Kyoung Hwang, Sae-Hoon Kim, John W Holloway, Yoon-Seok Chang.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1, 2 and Fig. 1 can be found via 10.5415/apallergy.2022.12.e32.
Database search strategies for MEDLINE, Embase, and Web of Science
Quality assessment
Forest plots of odds ratio for the wheezing prevalence and nasopharyngeal Streptococcus colonization at age 5. SE, standard error; CI, confidence interval.
References
- 1.Dogra S, Sakwinska O, Soh SE, Ngom-Bru C, Brück WM, Berger B, Brüssow H, Karnani N, Lee YS, Yap F, Chong YS, Godfrey KM, Holbrook JD. Rate of establishing the gut microbiota in infancy has consequences for future health. Gut Microbes. 2015;6:321–325. doi: 10.1080/19490976.2015.1078051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peroni DG, Nuzzi G, Trambusti I, Di Cicco ME, Comberiati P. Microbiome Composition and Its Impact on the Development of Allergic Diseases. Front Immunol. 2020;11:700. doi: 10.3389/fimmu.2020.00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Jackson D, Bacharier LB, Mauger D, Boushey H, Castro M, Durack J, Huang Y, Lemanske RF, Jr, Storch GA, Weinstock GM, Wylie K, Covar R, Fitzpatrick AM, Phipatanakul W, Robison RG, Beigelman A. The upper-airway microbiota and loss of asthma control among asthmatic children. Nat Commun. 2019;10:5714. doi: 10.1038/s41467-019-13698-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Q, Cox M, Liang Z, Brinkmann F, Cardenas PA, Duff R, Bhavsar P, Cookson W, Moffatt M, Chung KF. Airway microbiota in severe asthma and relationship to asthma severity and phenotypes. PLoS One. 2016;11:e0152724. doi: 10.1371/journal.pone.0152724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barcik W, Boutin RCT, Sokolowska M, Finlay BB. The role of lung and gut microbiota in the pathology of asthma. Immunity. 2020;52:241–255. doi: 10.1016/j.immuni.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P, Armstrong-James DPH, Adcock IM, Chotirmall SH, Chung KF, Hansbro PM. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med. 2019;7:907–920. doi: 10.1016/S2213-2600(18)30510-1. [DOI] [PubMed] [Google Scholar]
- 7.Corren J. The impact of allergic rhinitis on bronchial asthma. J Allergy Clin Immunol. 1998;101(2 Pt 2):S352–S356. doi: 10.1016/s0091-6749(98)70218-0. [DOI] [PubMed] [Google Scholar]
- 8.Lee JJ, Kim SH, Lee MJ, Kim BK, Song WJ, Park HW, Cho SH, Hong SJ, Chang YS, Kim BS. Different upper airway microbiome and their functional genes associated with asthma in young adults and elderly individuals. Allergy. 2019;74:709–719. doi: 10.1111/all.13608. [DOI] [PubMed] [Google Scholar]
- 9.Hufnagl K, Pali-Schöll I, Roth-Walter F, Jensen-Jarolim E. Dysbiosis of the gut and lung microbiome has a role in asthma. Semin Immunopathol. 2020;42:75–93. doi: 10.1007/s00281-019-00775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR, Boushey H. The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol. 2015;136:874–884. doi: 10.1016/j.jaci.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson JL, Daly J, Baines KJ, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, Hugenholtz P, Willner D, Gibson PG. Airway dysbiosis: Haemophilus influenzae and Tropheryma in poorly controlled asthma. Eur Respir J. 2016;47:792–800. doi: 10.1183/13993003.00405-2015. [DOI] [PubMed] [Google Scholar]
- 12.Renz H, Skevaki C. Early life microbial exposures and allergy risks: opportunities for prevention. Nat Rev Immunol. 2021;21:177–191. doi: 10.1038/s41577-020-00420-y. [DOI] [PubMed] [Google Scholar]
- 13.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG The PRISMA Group, editor. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alamri A. Diversity of microbial signatures in asthmatic airways. Int J Gen Med. 2021;14:1367–1378. doi: 10.2147/IJGM.S304339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norris JM, Simpson BS, Ball R, Freeman A, Kirkham A, Parry MA, Moore CM, Whitaker HC, Emberton M. A modified Newcastle-Ottawa Scale for assessment of study quality in genetic urological research. Eur Urol. 2021;79:325–326. doi: 10.1016/j.eururo.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Powell EA, Fontanella S, Boakes E, Belgrave D, Shaw AG, Cornwell E, Fernandez-Crespo R, Fink CG, Custovic A, Kroll JS. Temporal association of the development of oropharyngeal microbiota with early life wheeze in a population-based birth cohort. EBioMedicine. 2019;46:486–498. doi: 10.1016/j.ebiom.2019.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorsen J, Rasmussen MA, Waage J, Mortensen M, Brejnrod A, Bønnelykke K, Chawes BL, Brix S, Sørensen SJ, Stokholm J, Bisgaard H. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat Commun. 2019;10:5001. doi: 10.1038/s41467-019-12989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ta LDH, Yap GC, Tay CJX, Lim ASM, Huang CH, Chu CW, De Sessions PF, Shek LP, Goh A, Van Bever HPS, Teoh OH, Soh JY, Thomas B, Ramamurthy MB, Goh DYT, Lay C, Soh SE, Chan YH, Saw SM, Kwek K, Chong YS, Godfrey KM, Hibberd ML, Lee BW. Establishment of the nasal microbiota in the first 18 months of life: correlation with early-onset rhinitis and wheezing. J Allergy Clin Immunol. 2018;142:86–95. doi: 10.1016/j.jaci.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu CY, Chan YL, Tsai YS, Chen SA, Wang CJ, Chen KF, Chung IF. Airway microbial diversity is inversely associated with mite-sensitized rhinitis and asthma in early childhood. Sci Rep. 2017;7:1820. doi: 10.1038/s41598-017-02067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BS, Lee E, Lee MJ, Kang MJ, Yoon J, Cho HJ, Park J, Won S, Lee SY, Hong SJ. Different functional genes of upper airway microbiome associated with natural course of childhood asthma. Allergy. 2018;73:644–652. doi: 10.1111/all.13331. [DOI] [PubMed] [Google Scholar]
- 22.Toivonen L, Karppinen S, Schuez-Havupalo L, Waris M, He Q, Hoffman KL, Petrosino JF, Dumas O, Camargo CA, Jr, Hasegawa K, Peltola V. Longitudinal changes in early nasal microbiota and the risk of childhood asthma. Pediatrics. 2020;146:e20200421. doi: 10.1542/peds.2020-0421. [DOI] [PubMed] [Google Scholar]
- 23.Fazlollahi M, Lee TD, Andrade J, Oguntuyo K, Chun Y, Grishina G, Grishin A, Bunyavanich S. The nasal microbiome in asthma. J Allergy Clin Immunol. 2018;142:834–43.e2. doi: 10.1016/j.jaci.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H, Shin JW, Park SG, Kim W. Microbial communities in the upper respiratory tract of patients with asthma and chronic obstructive pulmonary disease. PLoS One. 2014;9:e109710. doi: 10.1371/journal.pone.0109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cardenas PA, Cooper PJ, Cox MJ, Chico M, Arias C, Moffatt MF, Cookson WO. Upper airways microbiota in antibiotic-naïve wheezing and healthy infants from the tropics of rural Ecuador. PLoS One. 2012;7:e46803. doi: 10.1371/journal.pone.0046803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teo SM, Tang HHF, Mok D, Judd LM, Watts SC, Pham K, Holt BJ, Kusel M, Serralha M, Troy N, Bochkov YA, Grindle K, Lemanske RF, Jr, Johnston SL, Gern JE, Sly PD, Holt PG, Holt KE, Inouye M. Airway microbiota dynamics uncover a critical window for interplay of pathogenic bacteria and allergy in childhood respiratory disease. Cell Host Microbe. 2018;24:341–52.e5. doi: 10.1016/j.chom.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang HHF, Lang A, Teo SM, Judd LM, Gangnon R, Evans MD, Lee KE, Vrtis R, Holt PG, Lemanske RF, Jr, Jackson DJ, Holt KE, Inouye M, Gern JE. Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J Allergy Clin Immunol. 2021;147:1683–1691. doi: 10.1016/j.jaci.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E, Bochkov YA, Grindle K, Johnston SL, Gern JE, Peter DS, Holt PG, Holt KE, Inouye M. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Depner M, Ege MJ, Cox MJ, Dwyer S, Walker AW, Birzele LT, Genuneit J, Horak E, Braun-Fahrländer C, Danielewicz H, Maier RM, Moffatt MF, Cookson WO, Heederik D, von Mutius E, Legatzki A. Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol. 2017;139:826–34.e13. doi: 10.1016/j.jaci.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Nallar E, Shen Y, Freishtat RJ, Pérez-Losada M, Manimaran S, Liu G, Johnson WE, Crandall KA. Integrating microbial and host transcriptomics to characterize asthma-associated microbial communities. BMC Med Genomics. 2015;8:50. doi: 10.1186/s12920-015-0121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birzele LT, Depner M, Ege MJ, Engel M, Kublik S, Bernau C, Loss GJ, Genuneit J, Horak E, Schloter M, Braun-Fahrländer C, Danielewicz H, Heederik D, von Mutius E, Legatzki A. Environmental and mucosal microbiota and their role in childhood asthma. Allergy. 2017;72:109–119. doi: 10.1111/all.13002. [DOI] [PubMed] [Google Scholar]
- 32.Durack J, Huang YJ, Nariya S, Christian LS, Ansel KM, Beigelman A, Castro M, Dyer AM, Israel E, Kraft M, Martin RJ, Mauger DT, Rosenberg SR, King TS, White SR, Denlinger LC, Holguin F, Lazarus SC, Lugogo N, Peters SP, Smith LJ, Wechsler ME, Lynch SV, Boushey HA National Heart, Lung and Blood Institute’s “AsthmaNet”. Bacterial biogeography of adult airways in atopic asthma. Microbiome. 2018;6:104. doi: 10.1186/s40168-018-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sverrild A, Kiilerich P, Brejnrod A, Pedersen R, Porsbjerg C, Bergqvist A, Erjefält JS, Kristiansen K, Backer V. Eosinophilic airway inflammation in asthmatic patients is associated with an altered airway microbiome. J Allergy Clin Immunol. 2017;140:407–17.e11. doi: 10.1016/j.jaci.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Li H, Ma Q, Zhang Q, Wang C. Neutrophilic asthma is associated with increased airway bacterial burden and disordered community composition. BioMed Res Int. 2018;2018:9230234. doi: 10.1155/2018/9230234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green BJ, Wiriyachaiporn S, Grainge C, Rogers GB, Kehagia V, Lau L, Carroll MP, Bruce KD, Howarth PH. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One. 2014;9:e100645. doi: 10.1371/journal.pone.0100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Schutter I, Dreesman A, Soetens O, De Waele M, Crokaert F, Verhaegen J, Piérard D, Malfroot A. In young children, persistent wheezing is associated with bronchial bacterial infection: a retrospective analysis. BMC Pediatr. 2012;12:83. doi: 10.1186/1471-2431-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Losol P, Choi JP, Kim SH, Chang YS. The role of upper airway microbiome in the development of adult asthma. Immune Netw. 2021;21:e19. doi: 10.4110/in.2021.21.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Man WH, de Steenhuijsen Piters WA, Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10:299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai HY, Rogers DF. Mucus hypersecretion in asthma: intracellular signalling pathways as targets for pharmacotherapy. Curr Opin Allergy Clin Immunol. 2010;10:67–76. doi: 10.1097/ACI.0b013e328334643a. [DOI] [PubMed] [Google Scholar]
- 41.Biesbroek G, Tsivtsivadze E, Sanders EA, Montijn R, Veenhoven RH, Keijser BJ, Bogaert D. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med. 2014;190:1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 42.Moore HC, Jacoby P, Taylor A, Harnett G, Bowman J, Riley TV, Reuter K, Smith DW, Lehmann D Kalgoorlie Otitis Media Research Project Team. The interaction between respiratory viruses and pathogenic bacteria in the upper respiratory tract of asymptomatic Aboriginal and non-Aboriginal children. Pediatr Infect Dis J. 2010;29:540–545. doi: 10.1097/INF.0b013e3181d067cb. [DOI] [PubMed] [Google Scholar]
- 43.Habibi N, Mustafa AS, Khan MW. Composition of nasal bacterial community and its seasonal variation in health care workers stationed in a clinical research laboratory. PLoS One. 2021;16:e0260314. doi: 10.1371/journal.pone.0260314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charlson ES, Chen J, Custers-Allen R, Bittinger K, Li H, Sinha R, Hwang J, Bushman FD, Collman RG. Disordered microbial communities in the upper respiratory tract of cigarette smokers. PLoS One. 2010;5:e15216. doi: 10.1371/journal.pone.0015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shankar J. Insights into study design and statistical analyses in translational microbiome studies. Ann Transl Med. 2017;5:249. doi: 10.21037/atm.2017.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson JS, Spakowicz DJ, Hong BY, Petersen LM, Demkowicz P, Chen L, Leopold SR, Hanson BM, Agresta HO, Gerstein M, Sodergren E, Weinstock GM. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat Commun. 2019;10:5029. doi: 10.1038/s41467-019-13036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allali I, Arnold JW, Roach J, Cadenas MB, Butz N, Hassan HM, Koci M, Ballou A, Mendoza M, Ali R, Azcarate-Peril MA. A comparison of sequencing platforms and bioinformatics pipelines for compositional analysis of the gut microbiome. BMC Microbiol. 2017;17:194. doi: 10.1186/s12866-017-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Database search strategies for MEDLINE, Embase, and Web of Science
Quality assessment
Forest plots of odds ratio for the wheezing prevalence and nasopharyngeal Streptococcus colonization at age 5. SE, standard error; CI, confidence interval.