Abstract
Surgical site infections remain a significant cause of morbidity following colon cancer surgery. Although diabetes has been recognised as a risk factor, patients with asymptomatic diabetes are likely underdiagnosed. The aim of the present study was to determine the relationship between preoperative glycated haemoglobin (HbA1C), clinicopathological characteristics and the influence on surgical site infection in a cohort of patients undergoing potentially curative colon cancer surgery. Patients who underwent elective, potentially curative colon cancer surgery between January 2011 and December 2014 were assessed for HbA1C levels (mmol/mol) measured within 3 months preoperatively. Clinicopathological data were recorded in a maintained database. A multivariate binary logistic regression model was used to assess the relationship between HbA1C, clinicopathological characteristics and surgical site infections. A total of 362 patients had HbA1C levels preoperatively recorded. HbA1C was significantly associated with body mass index (BMI), diabetes, smoking status, visceral fat area and skeletal muscle index. As determined by multivariate analysis, preoperative HbA1C levels remained independently associated with an increased risk of surgical site infections (OR 1.69, 95% CI 1.05-2.7; P=0.031) together with BMI (OR 1.91, 95% CI 1.36-2.67; P<0.001). Notably, in the present study, tumour-based factors, such as tumour location and TNM status, were not associated with infective complications. By contrast, host factors, such as BMI and pre-operative HbA1C were associated with surgical site infections suggesting that these factors were of more importance in determining short-term outcomes. In conclusion, objective measurements of BMI and HbA1C effectively stratified the risk of developing surgical site infection from 8 to 59%; therefore, HbA1C levels should be determined to allow for preoperative optimisation.
Keywords: pre-operative glycated haemoglobin, surgical site infection, surgery, colon, cancer
Introduction
Surgical resection remains the primary management for Stage I–III colon cancer (1) but not for Stage IV colon cancer (2). Cancer Research UK reports that approximately 90% of patients with Stage I–III colon cancer undergo curative surgery compared to approximately 75% of those in a Stage I–III rectal cancer (3). Nevertheless, post-operative complications particularly surgical site infections remain a significant sequela following both colon and rectal cancer resection. Post-operative complications not only intensifies the burden on the healthcare system but are increasingly recognised to influence long term oncological outcomes (4).
The presence of diabetes has also been recognised to affect the long-term outcome in patients in colon cancer (5–7) but the mechanism remains unclear. The global prevalence of diabetes has increased at a rapid rate. Over the next two decades, the International Diabetes Federation predicts a 51% increase in the diabetes prevalence worldwide from 463 million in 2019 (8). Nevertheless, there are no national screening programmes for diabetes and tests are usually performed for diagnostic purposes in symptomatic patients. A study extrapolated that patients are likely to have the onset of diabetes at least 4-7 years prior to diagnosis, long after dysglycaemia have taken effect (9). The World Health Organisation endorsed several methods for the diagnosis of diabetes including a random venous plasma glucose concentration (≥11.1 mmol/l), a fasting plasma glucose concentration (≥7.0 mmol/l), a two-hour plasma glucose concentration (≥11.1 mmol/l) after an oral glucose tolerance test (10) or haemoglobin A1C (≥48 mmol/mol or 6.5% mmol/l) (11).
Glycated haemoglobin represents the average plasma glucose over a three-month period and tests can be performed at any time (12) and therefore, eliminates the effect of day-to-day variability and the need for preceding dietary restrictions while creating the opportunity for identification of asymptomatic diabetics in the preoperative setting. The National Institute of Health and Care Excellence (NICE) currently only recommends glycated haemoglobin testing in known diabetes preoperatively. Approximately 30% of the adult population in the United Kingdom are obese (BMI >30) and 17% of patients who are obese are diagnosed with diabetes (13). However, the International Federation of Diabetes estimates that almost 1 in 2 adults live with undiagnosed diabetes in 2021 (14). This raises the question of the proportion of undiagnosed diabetics especially in those of the higher BMI range.
As diabetes is not readily diagnosed in asymptomatic patients, the aim of the present study is to examine the relationship between glycated haemoglobin, clinicopathological factors and surgical site infections in a cohort of patients undergoing elective potentially curative colon cancer resection.
Patients and methods
Study population
A dataset is maintained by the West of Scotland Colorectal Cancer Managed Clinical Network (MCN) for all patients diagnosed with colorectal cancer in the West of Scotland. The West of Scotland Colorectal Cancer Managed Clinical Network (MCN) is a collaborative made up of four NHS health boards (NHS Greater Glasgow and Clyde, NHS Lanarkshire, NHS Forth Valley and NHS Ayrshire and Arran) and serves approximately 50% of the Scottish population (15). The Academic Unit of Surgery based at the Glasgow Royal Infirmary is part of the School of Medicine, Dentistry and Nursing within the University of Glasgow. The interface between the Academic Unit of Surgery and NHS partners allows for collaborative surgical research and teaching. These patients are usually followed up for a period of 3-5 years and receive treatment in line with national guidelines. The analysis for the clinicopathological data from the MCN dataset and complication data were performed retrospectively.
Inclusion and exclusion criteria
Patients diagnosed with colon cancer between January 2011 and December 2014 within the West of Scotland were identified from the MCN database. Patients who underwent curative surgery for diagnosis of TNM Stage I–III colon cancer were included. Only patients who had preoperative glycated haemoglobin (HbA1C) levels measured in mmol/mol within 3 months prior to date of surgery were included. Patients with emergency presentation, Stage IV disease(distant metastasis) (16), rectal cancer, macroscopically involved margins (R2 resections), those who did not have surgical resection or those who underwent palliative procedures were excluded. Patients with a histological diagnosis of rectal cancer have been reported to demonstrate different clinical, pathological, and genetic abnormalities compared to those with histological diagnosis of colon cancer (17). More recently, the management of rectal cancer has diverged from colon cancer with the availability of neo-adjuvant treatment (18). Similarly, for patients with macroscopically involved margins, the management would no longer be for curative intent due to residual disease and therefore excluded from the study.
Data collection
Patients were assessed for any postoperative complication, infective complication, and surgical site infections. Infective complications included respiratory tract infections, urinary tract infections or any surgical site infections. Surgical site infections were defined as the presence of superficial skin infection, deep organ or space infections including anastomotic leaks and abdominal collections. Abdominal collections are intra-abdominal collection of pus or infected material classified either by radiological or intraoperative findings. The data was collected utilising all available electronic patient medical record including referral letters, preassessment records, anaesthetic records, inpatient medical notes, discharge letters, clinic letters, radiology reports, blood test results and microbiology test results.
Comorbidities were classified using the American Society of Anesthesiologists (ASA) Physical Status Classification System (19). Socioeconomic deprivation was stratified using the Scottish Index of Multiple Deprivation (SIMD). BMI was calculated from height and weight measured within 6 months prior to surgery and the BMI was categorised according to thresholds as follows; normal range <25, overweight 25-29.9, obese 30-34.9 and morbidly obese ≥35. In the present study, the majority of the BMI results were calculated from height and weight measurements taken at the pre-operative admission episode for surgery (n=217, 85%). The mean of time height and weight measured before surgery was 27.6 days with a standard deviation of 36.6 days and a range of 165 days. However, in order to minimise missing data, height and weight recorded in the initial referral letter from general practitioner with the maximal extension up to 6 months prior to surgery were included. Tumours were staged using the TNM classification system (16). The preoperative systemic inflammatory response was categorised by Systemic Inflammatory Grade (SIG) as previously described (20).
Surgical resections were performed either via an open or laparoscopic procedure. Open procedures included procedures that involved incisions made via a midline or transverse laparotomy, with resection of the cancer specimen (right hemicolectomy, transverse colectomy, left hemicolectomy, sigmoid colectomy, or subtotal colectomy), extraction of the specimen and colonic anastomosis via a single incision. Laparoscopic procedures were performed using multiple small incision of approximately 1 to 1.5 cm based on surgeon's preference, with resection of the cancer specimen completed laparoscopically (right hemicolectomy, transverse colectomy, left hemicolectomy, sigmoid colectomy, or subtotal colectomy) and colonic anastomosis intracorporeally. The specimens were extracted via a transverse incision usually between 4-8 cm in length. However, if laparoscopic procedures required conversion to an open procedure during any stage of the operation aside from specimen extraction, the procedures were classified as open/converted to open procedure. No robotic procedures were performed in all four NHS health boards during the study period.
Body composition measurements were derived from the preoperative computerised tomography (CT) image slice at the level of the third lumbar vertebra included total fat area (TFA), visceral fat area (VFA), and skeletal muscle area (SMA). Each CT image was individually analysed using ImageJ-a free to download, Java-based program developed by NIH (NIH ImageJ version 1.47; http://rsbweb.nih.gov/ij/) shown to provide reliable measurements (21). Attenuation thresholds were from −190 to +30 Hounsfield units (HU) for fat and −29 to +150 HU for muscle. The TFA was quantified by depicting the outer contours of the abdominal wall, compared with the inner contour of the psoas and abdominal wall muscles for VFA. Similarly, SMA was measured by manually delineating muscle areas including the quadratus lumborum, psoas, rectus abdominus, and erector spinae muscles, and the internal transverse and external oblique muscle groups. Skeletal muscle density (SMD) was calculated (in Hounsfield units) as the mean of the measured muscle area used to calculate SMI. Subcutaneous fat area (SFA) was calculated by subtraction of the VFA from TFA. SFA and SMA measurements were then normalised by division of the patient's height in meters squared to generate a subcutaneous fat index (SFI: centimetres squared/meters squared) and skeletal muscle index (SMI: centimetres squared/meters squared). These indices were then compared with established thresholds for body composition status (Table I).
Table I.
Computed tomography-derived body composition measures and thresholds.
| A, Obesity | |
|---|---|
|
| |
| Body composition measurement | Value |
| Subcutaneous fat index | |
| Higha | |
| Male | >50 cm2m2 |
| Female | >42 cm2m2 |
| Visceral fat area | |
| High bc | |
| Male | >160 cm2 |
| Female | >80 cm2 |
|
| |
| B, Sarcopenia | |
|
| |
| Body composition measurement | Value |
|
| |
| SMI | |
| Lowb | |
| Male | BMI ≤25 kg2m2 and |
| SMI <43 cm2m2 or | |
| BMI >25 kg2m2 and | |
| SMI <53 cm2m2 | |
| Female | BMI ≤25 kg2m2 and |
| SMI <41 cm2m2 or | |
| BMI >25 kg2m2 and | |
| SMI <41 cm2m2 | |
| SMD | |
| Lowb | BMI <25 kg/m2 and |
| SMD <41 HU or | |
| BMI ≥25 kg/m2 and | |
| SMD <33 HU | |
Ethics
The Caldicott Guardian plays an organisational role to ensure confidentiality and the highest standard of practice for the handling of patient identifiable information are adhered (22). All NHS organisations are required to have a Caldicott Guardian regulating the use of and transfer of person identifiable information between NHS organisations and between NHS organisations and non-NHS bodies. Ethical approval (ref. no. 1617-0079) was granted for this project from the Public Benefit and Privacy Panel (NHS Scotland) for Health and Social Care (PBPP) and Caldicott Guardian Approval.
Statistical analysis
The relationship between the clinicopathological characteristics and preoperative HbA1C were compared using the Chi-squared test and Fisher's exact test for significance where appropriate. P<0.05 were considered statistically significant. Surgical site complications were analysed using univariate and multivariate binary logistic regression model. Those variables associate to a degree of P<0.10 on univariate analysis were entered into a backward conditional multivariate model where variables with a P<0.05 were considered statistically significant. Correlation between body mass index and preoperative HbA1C was performed via bivariate analysis using Pearson's correlation coefficient. The relationship between HbA1c, BMI and surgical site infection was assessed using Fisher's exact test. Statistical analysis was performed using IBM SPSS Statistics for Windows Version 28 (IBM Corporation, Armonk, New York, USA).
Results
Study population
A total of 2,261 patients underwent elective curative resection for TNM Stage I–III colon cancer in the West of Scotland from January 2011 to December 2014. Following exclusion of patients with missing data, 362 patients had HbA1C levels available within 3 months preoperatively (Fig. 1). Most of the patients who had HbA1C levels (n=315) available had a pre-existing diagnosis of diabetes, representing 87% of the eligible study population.
Figure 1.
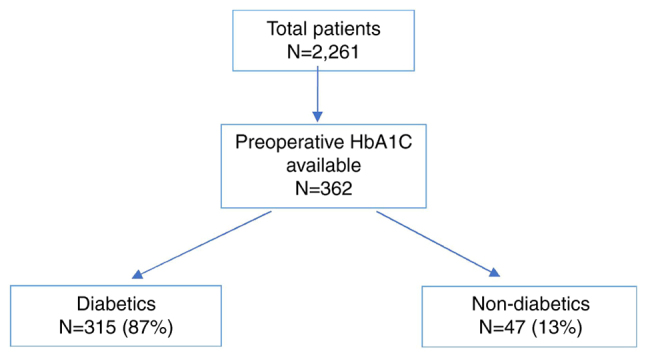
Preoperative HbA1c recorded for patients undergoing elective curative colon cancer surgery. The medical records for patients who underwent elective curative resection for TNM Stage I–III colon cancer in the West of Scotland from January 2011-2014 were examined to determine the presence of preoperative HbA1C levels. The proportion of diabetic and non-diabetic patients with preoperative HbA1C levels available are shown in the flow diagram. HbA1C, glycated haemoglobin.
Clinicopathological characteristics
The relationship between the clinicopathological characteristics with preoperative HbA1C are shown in Table II. Approximately 57% of the patients had a HbA1C >48 mmol/mol. Preoperative HbA1C was significantly associated with body mass index (P<0.05), diabetes status (P<0.001), type of diabetic control (P<0.001), smoking status (P<0.001), visceral fat area (P<0.01), skeletal muscle index (P<0.05) and surgical site infections (P<0.05). However, when surgical site infection was subdivided into wound infection, abdominal collection and anastomotic leak, only wound infections (P<0.05) were significantly associated with preoperative HbA1C.
Table II.
Clinical characteristics and preoperative HbA1C for patients undergoing elective potentially curative resection for colon cancer.
| Preoperative HbA1C, mmol/mol | |||||
|---|---|---|---|---|---|
|
|
|||||
| Characteristics | Total (%) | <42 | 42-48 | >48 | P-valuea |
| Age, years | 362 | 69 (19) | 86 (24) | 207 (57) | 0.051 |
| <65 | 80 (22) | 16 (23) | 12 (14) | 52 (25) | |
| 65-74 | 154 (43) | 22 (32) | 40 (47) | 92 (45) | |
| ≥75 | 128 (35) | 31 (45) | 34 (39) | 63 (30) | |
| Sex | 362 | 69 (19) | 86 (24) | 207 (57) | 0.179 |
| Male | 209 (58) | 33 (48) | 52 (61) | 124 (60) | |
| Female | 153 (42) | 36 (52) | 34 (39) | 83 (40) | |
| ASA | 345 | 66 (19) | 85 (25) | 194 (56) | 0.294b |
| 1 | 2 (3) | 2 (2) | 2 (2) | 12 (6) | |
| 2 | 32 (49) | 32 (38) | 32 (38) | 95 (49) | |
| 3 | 29 (44) | 48 (57) | 48 (57) | 79 (41) | |
| 4 | 3 (4) | 3 (3) | 3 (3) | 8 (4) | |
| SIMD | 362 | 69 (19) | 86 (24) | 207 (57) | 0.87 |
| Most deprived | 113 (31) | 22 (32) | 28 (32) | 63 (30) | |
| 2 | 82 (23) | 18 (27) | 16 (19) | 48 (23) | |
| 3 | 63 (17) | 8 (11) | 18 (21) | 37 (18) | |
| 4 | 47 (13) | 8 (11) | 12 (14) | 27 (13) | |
| Least deprived | 57 (18) | 13 (19) | 12 (14) | 32 (16) | |
| BMI | 255 | 41 (16) | 57 (22) | 157 (62) | 0.032 |
| <25 | 41 (16) | 13 (32) | 4 (7) | 24 (15) | |
| 25-29.9 | 79 (31) | 7 (17) | 19 (33) | 53 (34) | |
| 30-34.9 | 78 (31) | 14 (34) | 20 (35) | 44 (28) | |
| ≥35 | 57 (22) | 7 (17) | 14 (25) | 36 (23) | |
| Diabetes | 362 | 69 (19) | 86 (24) | 207 (57) | <0.001 |
| No | 47 (13) | 19 (28) | 11 (13) | 17 (8) | |
| Yes | 315 (87) | 50 (72) | 75 (87) | 190 (92) | |
| Type of diabetic control | 306 | 47 (15) | 72 (24) | 187 (61) | <0.001b |
| Diet | 89 (29) | 28 (60) | 25 (35) | 36 (19) | |
| Oral antihyperglycaemic medication (Metformin/Sulphonylurea) | 181 (59) | 17 (36) | 45 (63) | 119 (64) | |
| Insulin | 21 (7) | 0 (0) | 2 (3) | 19 (10) | |
| Insulin + oral antihyperglycaemic medication | 15 (5) | 2 (4) | 0 (0) | 13 (7) | |
| Metformin | 306 | 47 (15) | 72 (24) | 187 (61) | 0.101 |
| No | 139 (45) | 28 (60) | 32 (44) | 79 (42) | |
| Yes | 167 (55) | 19 (40) | 40 (56) | 108 (28) | |
| Sulphonylurea | 306 | 47 (15) | 72 (24) | 187 (61) | 0.055 |
| No | 218 (71) | 40 (85) | 52 (72) | 126 (67) | |
| Yes | 88 (29) | 7 (15) | 20 (28) | 61 (33) | |
| Smoking status | 346 | 63 (18) | 83 (24) | 200 (58) | 0.001 |
| Non-smoker | 155 (45) | 27 (43) | 36 (43) | 92 (46) | |
| Ex-smoker | 159 (46) | 22 (35) | 44 (53) | 93 (47) | |
| Smoker | 32 (9) | 14 (22) | 3 (4) | 15 (7) | |
| Tumour location | 359 | 68 (19) | 86 (24) | 205 (57) | 0.173 |
| Right | 207 (58) | 45 (66) | 44 (51) | 118 (58) | |
| Left | 152 (42) | 23 (34) | 42 (49) | 87 (42) | |
| TNM | 362 | 69 (19) | 86 (24) | 207 (57) | 0.06 |
| I | 81 (22) | 17 (25) | 16 (19) | 48 (23) | |
| II | 139 (38) | 35 (50) | 33 (38) | 71 (34) | |
| III | 142 (39) | 17 (25) | 37 (43) | 88 (43) | |
| Preop SIG | 226 | 46 (20) | 62 (27) | 118 (52) | 0.988b |
| 0 | 89 (39) | 17 (37) | 26 (42) | 46 (39) | |
| 1 | 59 (26) | 12 (26) | 16 (26) | 31 (26) | |
| 2 | 44 (20) | 8 (17) | 13 (21) | 23 (20) | |
| 3 | 21 (9) | 6 (13) | 4 (7) | 11 (9) | |
| 4 | 13 (6) | 3 (7) | 3 (5) | 7 (6) | |
| Procedure | 360 | 69 (19) | 86 (24) | 206 (57) | 0.066 |
| Laparoscopic | 236 (66) | 37 (54) | 59 (69) | 140 (68) | |
| Open or converted | 124 (34) | 32 (46) | 26 (31) | 66 (32) | |
| SFI | 316 | 63 (20) | 76 (24) | 177 (56) | 0.637 |
| Normal | 38 (12) | 9 (14) | 7 (9) | 22 (12) | |
| High | 278 (88) | 54 (86) | 69 (91) | 155 (88) | |
| VFA | 346 | 68 (20) | 82 (24) | 196 (56) | 0.002 |
| Normal | 30 (9) | 13 (19) | 3 (4) | 14 (7) | |
| High | 316 (91) | 55 (81) | 79 (96) | 182 (93) | |
| SMI | 244 | 40 (16) | 56 (23) | 148 (61) | 0.044 |
| Normal | 124 (51) | 19 (48) | 21 (37) | 84 (57) | |
| Low | 120 (49) | 21 (52) | 35 (64) | 64 (43) | |
| SMD | 244 | 40 (16) | 56 (23) | 148 (61) | 0.397 |
| Normal | 62 (25) | 9 (23) | 11 (20) | 42 (28) | |
| Low | 182 (75) | 31 (78) | 45 (80) | 106 (72) | |
| All complications | 289 | 56 (19) | 58 (24) | 165 (57) | 0.14 |
| No | 134 (46) | 27 (48) | 38 (56) | 69 (42) | |
| Yes | 155 (54) | 29 (52) | 30 (44) | 96 (58) | |
| Infective complication | 289 | 56 (19) | 68 (24) | 165 (57) | 0.084 |
| No | 174 (60) | 40 (71) | 43 (63) | 91 (55) | |
| Yes | 115 (40) | 16 (29) | 25 (37) | 74 (45) | |
| Surgical site infections | 289 | 56 (19) | 68 (24) | 165 (57) | 0.049 |
| No | 205 (71) | 47 (84) | 48 (71) | 110 (67) | |
| Yes | 84 (29) | 9 (16) | 20 (29) | 55 (33) | |
| Wound infection | 289 | 56 (19) | 68 (24) | 165 (57) | 0.041b |
| No | 231 (80) | 51 (91) | 55 (81) | 125 (76) | |
| Yes | 58 (20) | 5 (9) | 13 (19) | 40 (24) | |
| Abdominal collection | 289 | 56 (19) | 68 (24) | 165 (57) | 0.449b |
| No | 275 (95) | 52 (93) | 64 (94) | 159 (96) | |
| Yes | 14 (5) | 4 (7) | 4 (6) | 6 (4) | |
| Anastomotic leak | 289 | 56 (19) | 68 (24) | 165 (57) | 0.103b |
| No | 268 (93) | 55 (98) | 60 (88) | 153 (93) | |
| Yes | 21 (7) | 1 (2) | 8 (12) | 12 (7) | |
| 30-day mortality | 362 | 69 (19) | 86 (24) | 207 (57) | 0.447b |
| No | 67 (97) | 81 (94) | 201 (97) | 349 (96) | |
| Yes | 2 (3) | 5 (6) | 6 (3) | 13 (4) | |
ASA, American Society of Anesthesiologists; BMI, body mass index; SIMD, Scottish Index of Multiple Deprivation; TNM, tumour, nodal and metastasis; SIG, systemic inflammatory grade; SFI, subcutaneous fat index; VFA, visceral fat area; SMI, skeletal muscle index; SMD, skeletal muscle density.
P-value calculated using Chi-squared analysis except forb
P-value calculated using Fisher's exact test.
Surgical site infection
Binary logistic regression of factors associated with surgical site infections are shown in Table III. On univariate analysis, surgical site infection was associated with age (P<0.10), BMI (P<0.001), HbA1C (P<0.05) and SMI (P<0.10). On multivariate analysis, surgical site infection was independently associated with BMI (OR 1.9, 95% CI 1.36-2.37; P<0.001) and preoperatively HbA1C (OR 1.69, 95% CI 1.05-2.7; P=0.031).
Table III.
Factors associated with surgical site infections in patients undergoing elective potentially curative resection for colon cancer.
| Surgical site infection | ||||
|---|---|---|---|---|
|
|
||||
| Clinical characteristics | Univariate OR (95% CI) | P-value | Multivariate OR (95% CI) | P-value |
| Age, years (<65/65-74/>74) | 0.74 (0.53-1.03) | 0.072 | 1.04 (0.67-1.60) | 0.848 |
| BMI (<25/25-29.9/30-34.9/≥35) | 1.80 (1.31-2.49) | <0.001 | 1.91 (1.36-2.67) | <0.001 |
| Diabetes (no/yes) | 1.32 (0.57-3.06) | 0.518 | ||
| Preoperative HbA1C (<42/42-48/>48) | 1.51 (1.07-2.15) | 0.020 | 1.69 (1.05-2.70) | 0.031 |
| Type of diabetic control (diet/oral antihyperglycaemic medication/insulin/insulin + oral antihyperglycaemic medication) | 1.04 (0.73-1.48) | 0.842 | ||
| Metformin (no/yes) | 1.23 (0.72-2.11) | 0.446 | ||
| Sulphonylurea (no/yes) | 1.22 (0.98-2.21) | 0.503 | ||
| Smoking status (non-smoker/Ex-smoker/Smoker) | 0.76 (0.50-1.15) | 0.195 | ||
| Tumour location (right/left) | 1.31 (0.78-2.20) | 0.305 | ||
| TNM (I/II/III) | 0.87 (0.63-1.20) | 0.383 | ||
| Procedure (laparoscopic/open or converted) | 0.89 (0.52-1.53) | 0.684 | ||
| VFA (normal/high) | 1.29 (0.46-3.66) | 0.627 | ||
| SMI (normal/high) | 0.60 (0.33-1.10) | 0.099 | 0.94 (0.48-1.85) | 0.881 |
BMI, body mass index; CI, confidence interval; HbA1C, glycated haemoglobin; OR, odds ratio; TNM, tumour, nodal and metastasis; VFA, visceral fat area; SMI, skeletal muscle index.
Rate of surgical site infection based on HbA1C and BMI
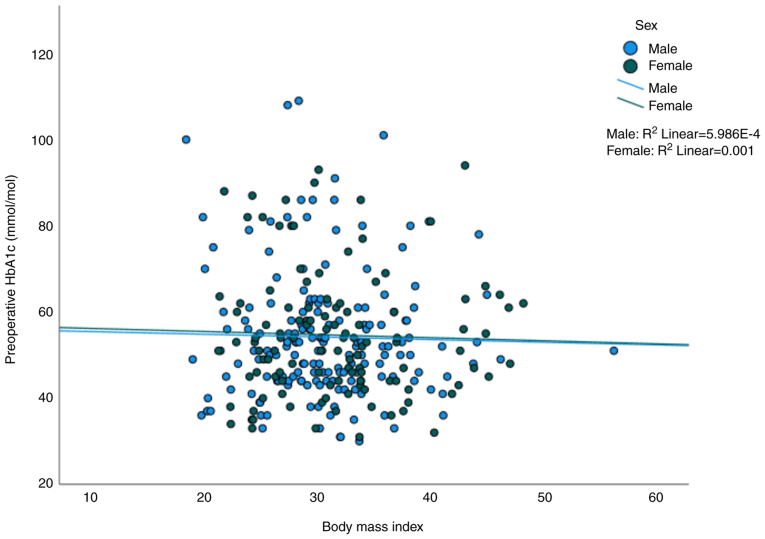
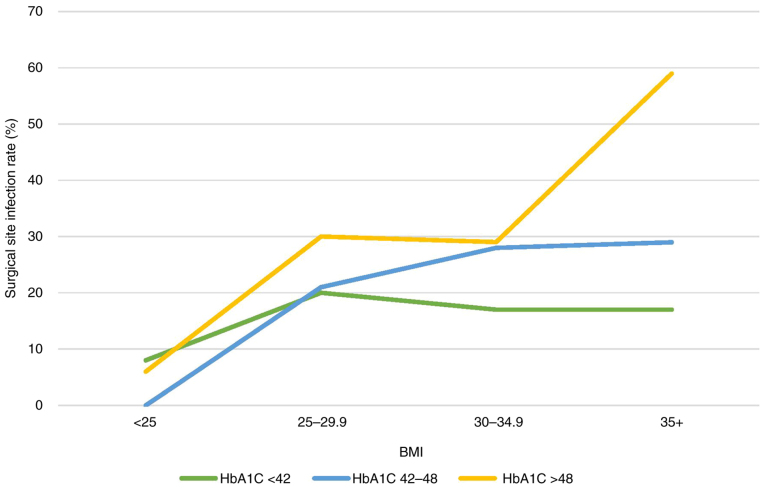
A scatter plot of BMI vs. preoperative HbA1C levels is shown in Fig. 2. The Pearson's correlation analysis showed no statistically significant linear correlation between BMI and HbA1C for both sexes. The relationship between preoperative HbA1c and BMI on surgical site infection rate is shown in Table IV represented in a line graph in Fig. 3. The presence of both, high preoperative HbA1c and high BMI was associated with an increased the rate of surgical site infection compared with a lower BMI and HbA1c (59% vs. 8%; P<0.001).
Figure 2.
Scatter plot of body mass index vs. preoperative HbA1C level (mmol/mol). The blue dots represent males and green dots represent females with corresponding best fit line for both sexes. Pearson's correlation analysis performed using SPSS Statistics software showed r=−0.24 (P=0.751) for males and r=−0.32 (P=0.736) for females. HbA1C, glycated haemoglobin.
Table IV.
Relationship between preoperative HbA1C, BMI and SSI rate.
| BMI | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| HbA1C | <25 All/SSI (%) | 25-29.9 All/SSI (%) | 30-34.9 All/SSI (%) | ≥35 All/SSI (%) | Total All/SSI (%) | P-valuea |
| <42 | 13/1 (8) | 5/1 (20) | 12/2 (17) | 6/1 (17) | 36/5 (14) | 0.844 |
| 42-48 | 3/0 (−) | 14/3 (21) | 18/5 (28) | 14/4 (29) | 49/12 (25) | 0.891 |
| >48 | 18/1 (6) | 46/14 (30) | 41/13 (29) | 32/19 (59) | 137/47 (34) | <0.001 |
| Total | 34/2 (6) | 65/18 (28) | 71/20 (28) | 52/24 (46) | 222/64 (29) | <0.001c |
| P-valueb | 1 | 0.898 | 0.666 | 0.059 | 0.042d | |
BMI, body mass index; HbA1C, glycated haemoglobin; SSI, surgical site infection in category.
Fisher's exact test was used to analyse P-value for SSI in individual HbA1C category against all BMI categories aside from total;
Fisher's exact test was used to analyse P-value for SSI in individual BMI category against all HbA1C categories aside from total;
Fisher's exact test was used to analyse the P-value between SSI and body mass index for all patients with HbA1c available;
Fisher's exact test was used to analyse the P-value between SSI and HbA1c for all patients with BMI measurements available.
Figure 3.
Relationship between pre-operative HbA1C (mmol/mol), BMI and surgical site infection. The green line shows the rate of surgical site infection for patients with preoperative HbA1C <42 with corresponding BMI. The blue line shows the rate of surgical site infection for patients with preoperative HbA1C 42-28 with corresponding BMI. The yellow line shows the rate of surgical site infection for patients with preoperative HbA1C >48 with corresponding BMI. BMI, body mass index; HbA1C, glycated haemoglobin.
Discussion
The present study shows the relationship between preoperative HbA1C and clinicopathological characteristics in patients who underwent potentially curative colon cancer surgery. As the preoperative level of HbA1C and BMI became greater, a greater proportion of patients developed surgical site infection. Therefore, HbA1C would appear to have clinical utility as an objective pre-operative measure to evaluate the risk of a post-operative surgical site infection.
In the present study it was of interest that there was discrepancy between BMI, HbA1c and a diagnosis of diabetes. The basis of this discrepancy is not clear however currently the diagnosis of diabetes mellitus tends to present after a patient becomes symptomatic and so it is likely that the onset of diabetes began earlier, in some cases years before. The present study identified that 5% of patients examined had HbA1C levels above the threshold for diabetes diagnosis and who were not known to be diabetic prior to surgery. Given that this was in patients who were not screened using HbA1c this is likely to be an underestimation of the discrepancy. Indeed, in the Diabetes Control and Complication Trial, 25% of patients were reported to have a high abnormal HbA1C level (>6% or >42 mmol/l) in a screened undiagnosed population undergoing major colorectal surgery (23). Despite this, HbA1c levels are currently rarely used as a pre-operative assessment tool due to the lack of evidence on the prognostic effect in post-operative outcomes in undiagnosed diabetics (24). Nevertheless, the results of the present study support the measurement of HbA1c in patients undergoing surgery for colorectal cancer, particularly in those patients defined as obese patients by BMI.
A diagnosis of diabetes has been long recognised to be associated with poor post-surgery outcomes (25). However, the mechanism by which diabetes influences post-operative complications is not clear. One potential mechanism relates to hyperglycaemia (26) where its effect on the innate immune response leads to impairment of neutrophil and monocyte dysfunction such as adherence, chemotaxis and phagocytosis (27). This in turn lead to increasing inflammatory markers and oxidative stress, lowering the host immune defences against infection. Furthermore, there is good evidence that correction of hyperglycaemia reduces postoperative complications in general surgery patients (28). Taken together there is considerable potential for HbA1c as a prognostic factor particularly in those patients who are undiagnosed diabetics undergoing colon cancer resection.
The incidence of surgical site infections varies dependent on specialty. Nevertheless, diabetes has been consistently reported as an independent risk factor with an overall pooled odd ratio of 1.53 (95% CI 1.11-2.12) across multiple specialties in a previous meta-analysis (29). In oncological resections, there are similar trends towards higher rates of surgical site infection in patients with pre-existing diabetes. In abdominal gynaecological oncology, surgical site infection rates range from 16-35% but elevated rates of 45% have been reported in patients with a diagnosis of diabetes (30–32). While in breast cancer surgery, the incidence was reported to ranges from 3-15% (33) to 20% in diabetic patients (34). Whereas the incidence in the diabetic population following elective colorectal cancer resection is less well studied, reported rates of surgical site infection range between 3-23% in colon cancer and 14-27% in rectal cancer surgery (35,36). Despite the wide range in reported rates of surgical site infection, the expected rates are usually between 10-13% (37). Of note, in the present study the reported rate of surgical site infection was slightly higher compared to average. This could be explained by the patient selection for the study based on patients with available preoperative glycated haemoglobin that included a relatively higher prevalence of diabetic patients, majority of open or converted procedures (38,39) as well as patients with malignancy (40) that have been shown to increase incidences of surgical site infection in previous work.
The main limitation of the present study was the retrospective nature and relatively small sample size which raises the risk of selection bias. However, the clinicopathological data for the patient cohort was derived from a prospective MCN dataset and the complication data collection and analysis were performed retrospectively. In the present cohort there was a relatively high prevalence of diabetes (87%). This was in part due to selection of patients who had pre-operative glycated haemoglobin measurement. The Scottish Diabetes Survey reports a diabetes prevalence of 5.7% in the Scottish population (41) while the prevalence of diabetics in the overall surgical population is approximately 15% (42). Based on NICE guidelines, HbA1C testing is only recommended in patients with known diabetes however several patients had HbA1C levels measured preoperatively. Another potential limitation of the present study was the potential confounding effect of immunosuppression agents that have been implicated in impaired wound healing. However, a report by the World Health Organisation would suggest that only low quality evidence to support discontinuation of immunosuppressant agents in the pre-operative period would be of benefit in reducing surgical site infection rate (43). Nevertheless, the present study yielded new information on the relationship between BMI, body composition, HbA1C (all objective measures) and surgical site infections and future prospective studies examining these relationships are warranted.
The present results are consistent with a previous study that reported that the level of preoperative HbA1c rather than the diagnosis of diabetes was independently associated with clinical outcome following surgery (44). HbA1c has the potential to predict complications with glycaemic control in those with elevated levels pre-operatively. However, to date, there are no studies examining the clinical effectiveness of optimising glycated haemoglobin preoperatively in patients undergoing colon cancer surgery. This would be of considerable interest in future studies. In conclusion, objective measurements of BMI and glycated haemoglobin effectively stratified the risk of developing surgical site infection from 8 to 59%. Of interest, approximately 8% of our study population who were not known to have a diabetes had HbA1C levels above 42 mmol/l. Therefore, glycated haemoglobin levels should be determined in all patients regardless of diabetes status to allow for preoperative optimisation.
Acknowledgements
The authors would like to thank Mr. David Mansouri (Department of Surgery, Glasgow Royal Infirmary, NHS Greater Glasgow and Clyde) who carried out the initial application for ethical approval that has been amended by Mr. Allan Golder (Academic Unit of Surgery, University of Glasgow, Glasgow Royal Infirmary) for this study.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
CMC and DCM designed the research. CMC and AMG conducted the research and confirm the authenticity of all the raw data. CSDR, PGH and DCM conceptualised the research. CSDR and PGH provided access to database and facilities to conduct research, revision of the draft and final review of the manuscript. CMC analysed data. CMC and DCM wrote the paper, and DCM had primary responsibility for final content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Ethics approval was granted for this project from the Public Benefit and Privacy Panel (NHS Scotland) for Health and Social Care (PBPP) and Caldicott Guardian Approval.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chakrabarti S, Peterson CY, Sriram D, Mahipal A. Early stage colon cancer: Current treatment standards, evolving paradigms, and future directions. World J Gastrointest Oncol. 2020;12:808–832. doi: 10.4251/wjgo.v12.i8.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Research UK. Journal; 2020. Chemotherapy, radiotherapy and surgical tumour resection in England. [Google Scholar]
- 4.McSorley ST, Horgan PG, McMillan DC. The impact of the type and severity of postoperative complications on long-term outcomes following surgery for colorectal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;97:168–177. doi: 10.1016/j.critrevonc.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Mills KT, Bellows CF, Hoffman AE, Kelly TN, Gagliardi G. Diabetes mellitus and colorectal cancer prognosis: A meta-analysis. Dis Colon Rectum. 2013;56:1304–1319. doi: 10.1097/DCR.0b013e3182a479f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker DJ, Iyengar AD, Punekar SR, Kaakour D, Griffin M, Nicholson J, Gold HT. Diabetes mellitus and colorectal carcinoma outcomes: A meta-analysis. Int J Colorectal Dis. 2020;35:1989–1999. doi: 10.1007/s00384-020-03666-z. [DOI] [PubMed] [Google Scholar]
- 7.Zhu B, Wu X, Wu B, Pei D, Zhang L, Wei L. The relationship between diabetes and colorectal cancer prognosis: A meta-analysis based on the cohort studies. PLoS One. 2017;12:e0176068. doi: 10.1371/journal.pone.0176068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IDF Diabetes Atlas, corp-author. 9th edition. Brussels: 2019. International Diabetes Federation. [Google Scholar]
- 9.Duan D, Kengne AP, Echouffo-Tcheugui JB. Screening for diabetes and prediabetes. Endocrinol Metab Clin North Am. 2021;50:369–385. doi: 10.1016/j.ecl.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization, International Diabetes Federation, corp-author. World Health Organization; 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: Report of a WHO/IDF consultation. [Google Scholar]
- 11.World Health Organisation, corp-author. World Health Organization; 2011. Use of glycated haemoglobin(HbA1c) in the diagnosis of diabetes mellitus: Abbreviated Report of a WHO Consultation. [PubMed] [Google Scholar]
- 12.McPherson RA, Pincus MR. Henry's clinical diagnosis and management by laboratory methods. In: Klein MJ, Zhang Y, editors. 22nd edition. Elsevier; 2022. pp. 232–233. [Google Scholar]
- 13.Public Health England, corp-author. Adult Obesity and Type 2 Diabetes. 2014 [Google Scholar]
- 14.Federation ID, corp-author. IDF Diabetes Atlas 2021. 2021 [Google Scholar]
- 15.West of Scotland Cancer Network (WoSCAN), corp-author West of Scotland Cancer Network. 2017 [Google Scholar]
- 16.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. 7th edition. Springer; New York, NY: 2010. AJCC Cancer Staging Manual. [Google Scholar]
- 17.van der Sijp MP, Bastiaannet E, Mesker WE, van der Geest LG, Breugom AJ, Steup WH, Marinelli AW, Tseng LN, Tollenaar RA, van de Velde CJ, Dekker JW. Differences between colon and rectal cancer in complications, short-term survival and recurrences. Int J Colorectal Dis. 2016;31:1683–1691. doi: 10.1007/s00384-016-2633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feeney G, Sehgal R, Sheehan M, Hogan A, Regan M, Joyce M, Kerin M. Neoadjuvant radiotherapy for rectal cancer management. World J Gastroenterol. 2019;25:4850–4869. doi: 10.3748/wjg.v25.i33.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American Society of Anesthesiologists, corp-author. ASA Physical Status Classification system. 2014 [Google Scholar]
- 20.Golder AM, McMillan DC, Park JH, Mansouri D, Horgan PG, Roxburgh CS. The prognostic value of combined measures of the systemic inflammatory response in patients with colon cancer: An analysis of 1700 patients. Br J Cancer. 2021;124:1828–1835. doi: 10.1038/s41416-021-01308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irving BA, Weltman JY, Brock DW, Davis CK, Gaesser GA, Weltman A. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring) 2007;15:370–376. doi: 10.1038/oby.2007.573. [DOI] [PubMed] [Google Scholar]
- 22.NHSScotland Caldicott Guardians, corp-author. Principles Into Practice. 2010 [Google Scholar]
- 23.Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg. 2009;96:1358–1364. doi: 10.1002/bjs.6724. [DOI] [PubMed] [Google Scholar]
- 24.National Institute for Health, Care Excellance, corp-author. Routine Preoperative Test for Elective Surgery. 2016 [PubMed] [Google Scholar]
- 25.Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NE, Dhatariya K, Greenhaff PL, Hiesmayr M, Hjort Jakobsen D, Klek S, et al. Perioperative nutrition: Recommendations from the ESPEN expert group. Clin Nutr. 2020;39:3211–3227. doi: 10.1016/j.clnu.2020.03.038. [DOI] [PubMed] [Google Scholar]
- 26.Kotagal M, Symons RG, Hirsch IB, Umpierrez GE, Dellinger EP, Farrokhi ET, Flum DR, SCOAP-CERTAIN Collaborative Perioperative hyperglycemia and risk of adverse events among patients with and without diabetes. Ann Surg. 2015;261:97–103. doi: 10.1097/SLA.0000000000000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turina M, Miller FN, Tucker C, Polk HC. Effects of hyperglycemia, hyperinsulinemia, and hyperosmolarity on neutrophil apoptosis. Surg Infect (Larchmt) 2006;7:111–121. doi: 10.1089/sur.2006.7.111. [DOI] [PubMed] [Google Scholar]
- 28.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34:256–261. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin ET, Kaye KS, Knott C, Nguyen H, Santarossa M, Evans R, Bertran E, Jaber L. Diabetes and risk of surgical site infection: A systematic review and meta-analysis. Infection Infect Control Hosp Epidemiol. 2016;37:88–99. doi: 10.1017/ice.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nugent EK, Hoff JT, Gao F, Massad LS, Case A, Zighelboim I, Mutch DG, Thaker PH. Wound complications after gynecologic cancer surgery. Gynecol Oncol. 2011;121:347–352. doi: 10.1016/j.ygyno.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 31.Al-Niaimi AN, Ahmed M, Burish N, Chackmakchy SA, Seo S, Rose S, Hartenbach E, Kushner DM, Safdar N, Rice L, Connor J. Intensive postoperative glucose control reduces the surgical site infection rates in gynecologic oncology patients. Gynecol Oncol. 2015;136:71–76. doi: 10.1016/j.ygyno.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell RL, Angelopoulos G, Beirne JP, Biliatis I, Bolton H, Bradbury M, Craig E, Gajjar K, Mackintosh ML, MacNab W, et al. Impact of surgical site infection (SSI) following gynaecological cancer surgery in the UK: A trainee-led multicentre audit and service evaluation. BMJ Open. 2019;9:e024853. doi: 10.1136/bmjopen-2018-024853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallagher M, Jones DJ, Bell-Syer SV. Prophylactic antibiotics to prevent surgical site infection after breast cancer surgery. Cochrane Database Syst Rev. 2019;9:CD005360. doi: 10.1002/14651858.CD005360.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olsen MA, Lefta M, Dietz JR, Brandt KE, Aft R, Matthews R, Mayfield J, Fraser VJ. Risk factors for surgical site infection after major breast operation. J Am Coll Surg. 2008;207:326–335. doi: 10.1016/j.jamcollsurg.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serra-Aracil X, García-Domingo MI, Parés D, Espin-Basany E, Biondo S, Guirao X, Orrego C, Sitges-Serra A. Surgical site infection in elective operations for colorectal cancer after the application of preventive measures. Arch Surg. 2011;146:606–612. doi: 10.1001/archsurg.2011.90. [DOI] [PubMed] [Google Scholar]
- 36.Eto K, Urashima M, Kosuge M, Ohkuma M, Noaki R, Neki K, Ito D, Takeda Y, Sugano H, Yanaga K. Standardization of surgical procedures to reduce risk of anastomotic leakage, reoperation, and surgical site infection in colorectal cancer surgery: A retrospective cohort study of 1189 patients. Int J Colorectal Dis. 2018;33:755–762. doi: 10.1007/s00384-018-3037-3. [DOI] [PubMed] [Google Scholar]
- 37.Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev. 2014 May 9; doi: 10.1002/14651858.CD001181.pub4. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kulkarni N, Arulampalam T. Laparoscopic surgery reduces the incidence of surgical site infections compared to the open approach for colorectal procedures: A meta-analysis. Tech Coloproctol. 2020;24:1017–1024. doi: 10.1007/s10151-020-02293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z, Qu H, Kanani G, Guo Z, Ren Y, Chen X. Update on risk factors of surgical site infection in colorectal cancer: A systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:2147–2156. doi: 10.1007/s00384-020-03706-8. [DOI] [PubMed] [Google Scholar]
- 40.Kamboj M, Childers T, Sugalski J, Antonelli D, Bingener-Casey J, Cannon J, Cluff K, Davis KA, Dellinger EP, Dowdy SC, et al. Risk of surgical site infection (SSI) following colorectal resection is higher in patients with disseminated cancer: An NCCN member cohort study. Infect Control Hosp Epidemiol. 2018;39:555–562. doi: 10.1017/ice.2018.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Group SDD, corp-author. Scottish Diabetes Survey 2020. 2022 [Google Scholar]
- 42.Centre for Perioperative Care, corp-author. Guideline for perioperative care for people with diabetes mellitus undergoing elective and emergency surgery. 2021 [Google Scholar]
- 43.World Health Organization, corp-author. World Health Organization; Geneva: 2018. Global guidelines for the prevention of surgical site infection. [PubMed] [Google Scholar]
- 44.O'Sullivan CJ, Hynes N, Mahendran B, Andrews EJ, Avalos G, Tawfik S, Lowery A, Sultan S. Haemoglobin A1c (HbA1C) in non-diabetic and diabetic vascular patients. Is HbA1C an independent risk factor and predictor of adverse outcome? Eur J Vasc Endovasc Surg. 2006;32:188–197. doi: 10.1016/j.ejvs.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 45.Doyle SL, Bennett AM, Donohoe CL, Mongan AM, Howard JM, Lithander FE, Pidgeon GP, Reynolds JV, Lysaght J. Establishing computed tomography-defined visceral fat area thresholds for use in obesity-related cancer research. Nutr Res. 2013;33:171–179. doi: 10.1016/j.nutres.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 47.Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.