Abstract
Glioblastoma (GBM) is the most common and fatal malignant tumor type of the central nervous system. GBM affects public health and it is important to identify biomarkers to improve diagnosis, reduce drug resistance and improve prognosis (e.g., personalized targeted therapies). Hedgehog (HH) signaling has an important role in embryonic development, tissue regeneration and stem cell renewal. A large amount of evidence indicates that both normative and non-normative HH signals have an important role in GBM. The present study reviewed the role of the HH signaling pathway in the occurrence and progression of GBM. Furthermore, the effectiveness of drugs that target different components of the HH pathway was also examined. The HH pathway has an important role in reversing drug resistance after GBM conventional treatment. The present review highlighted the relevance of HH signaling in GBM and outlined that this pathway has a key role in the occurrence, development and treatment of GBM.
Keywords: Hedgehog, glioblastoma, Sonic, patched-1, smoothened, drug resistance, therapeutics
1. Introduction
Primary central nervous system (PCNS) tumors account for 12% of all neoplasms (1). Glioblastoma (GBM) is the most common type of primary malignant CNS tumor, representing ~48% of all primary malignant CNS tumors and 57% of all gliomas (2). In spite of the progress made in the treatment of GBM in recent years (including surgery, radiotherapy, chemotherapy and targeted therapy), the overall prognosis is still not ideal and the long-term survival rate is low. Certain studies indicated that teenagers and young adults account for 27% of all PCNS tumor cases and the average age was 29 years (1,3). If patients are diagnosed with cancer in those busy years of their life, this may take a serious toll on both their body and mind, and in turn on their spouses and offspring (4). Upon diagnosis, the prognosis of GBM is poor, with months to a year left to live, so that this may also have detrimental effects on the patients' dependents and family (4).
In recent years, important advances have been made in the exploration of the molecular pathogenesis of tumorigenesis and progression, but this has not been applied to significantly improve patient prognosis. It is thus essential to identify biomarkers for diagnosis, as well as means of reducing drug resistance and delivering treatments, including personalized targeted therapies in the study of GBM. The role of Hedgehog (HH) signaling in the pathophysiology of GBM is underscored by a growing number of publications (5–7). The HH pathway is increasingly being revealed to have an important role in the growth, progression, prognosis and treatment of GBM (8–10).
The present review will discuss the contribution of HH signaling in the development and treatment of GBM. Chemotherapy, targeted therapy and radiotherapy in the HH pathway will also be discussed and the issue of improving partial drug resistance through this pathway will be addressed.
2. Overview of GBM
GBM originates from the glial stem or progenitor cells and is characterized by molecular heterogeneity, with a mean survival of only 15 months after diagnosis (11). Commonly mutated genes and core pathways in sporadic GBM were identified based on molecular mapping and three major GBM subpopulations were identified in combination with other dimensions (gene expression, DNA methylation). The DNA methylated α group amplified cyclin-dependent kinase (CDK)4 and platelet-derived growth factor in the three ways (classical gene expression; classical like; receptor tyrosine kinase II). High-frequency amplification of EGFR and homozygous deletion of CDK inhibitor 2A/B occurred in the DNA methylation group. Mesenchymal/mesenchymal subtypes are abundant in tumors with loss of neurofibromatosis type 1 and increased tumor macrophage infiltration (12,13). The above three types are the most common types of GBM and all involve mutations in telomerase reverse transcriptase promoters (14,15). In addition, characteristic epigenetic patterns are associated with certain putative driving mutations that are important in GBM, according to recent studies (16,17). Examples include mutated isocitrate dehydrogenase (IDH)1 and IDH2, H3.3 histone A or H3 clustered histone 2 mutations, particularly H3K27M in diffuse midline glioma and H3G34R/H3G34V mutations in young patients with GBM (16,17). However, their clinical implication for these GBM subtypes has not been proven. These studies indicate that different subtypes of GBM are caused by different oncogenes, which paves the way for the exploration of highly specific personalized targets.
GBM is characterized by continuous vascularization, tissue invasion and metastasis, metabolic recombination or alteration, immune regulation and promotion of the tumor microenvironment. All of the above characteristics lead to high resistance of GBM to radiotherapy and chemotherapy, which brings a non-negligible challenge to the treatment of the disease (18).
GBM has different subtypes, but the current international treatment methods mainly include chemotherapy [such as temozolomide (TMZ)], radiotherapy (RT) and surgical treatment. Monotherapy may be well tolerated in elderly patients (>65 years) with poor functional status. It has been reported that low-grade RT (40 Gy/15 doses of 2.67 Gy over 3 weeks) was higher than the standard 60 Gy for 6 weeks (19,20). Relapsing patients may be treated with surgery (as palliative care only) or other options include TMZ reactivation, nutrition and bevacizumab. However, there is no specific clinical evidence of prolonged survival (21,22). Several valuable clinical trials are under development for the treatment of GBM, including targeted molecular (precise) therapies (targeting gene mutations and associated signaling pathways, DNA damage repair, tumor metabolism), checkpoint inhibitors/immunomodulation agents and viral therapies. Despite the GBM treatment options available, metastatic disease remains a great concern. Therefore, it is of marked importance to find novel therapeutic targets and new drugs targeting the HH signaling pathway to regulate the occurrence, development, treatment and chemotherapy resistance of GBM (23,24).
3. The HH signaling pathway
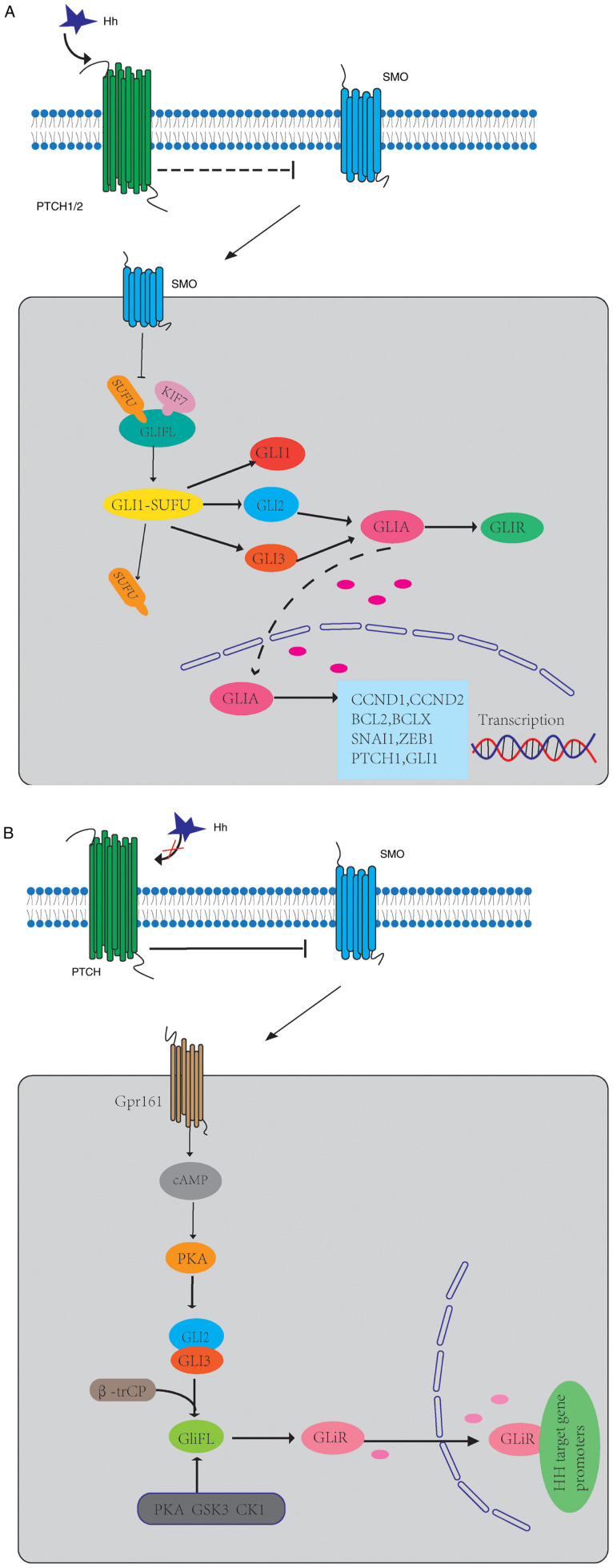
HH is a morphogenetic gene, which is highly conserved from drosophila to humans. The HH signaling pathway has an important role in embryonic development, cell proliferation, differentiation and maintenance of tissue polarity (25). Inactivation of this pathway during development may lead to congenital defects, while over-activation in adults is related to tumorigenesis (26,27). The HH protein family includes Sonic HH (SHH), Indian HH (IHH) and desert HH (DHH) (28). In mammals, the mechanism of HH signaling is complex and occurs in primary cilia (PC) (29). In PC, HH protein binds to 12 transmembranes (TM) receptors [Patch1 (PTCH1) and PTCH2] to activate pathways, so that 7-TM protein smoothened (SMO) is inhibited (30). The HH signal is transmitted downstream of SMO through the complex composed of kinesin 7 (KIF7), suppressor of fused homolog (SUFU) and full-length glioma-associated oncogenes (GliFL), which promotes the dissociation of SUFU from GLI protein and then releases transcription factors (GLI1, GLI2 and GLI3) (31,32). GLI2 and GLI3 constitute GliFL, which act as both GLI activators (GLIA) and GLI inhibitors (GLIR) (33,34). After activation of SMO, GLI2/3 P1-6 clusters were dephosphorylated and separated from SUFU (35), which facilitates the transfer of GLIA into the nucleus and the initiation of the transcription of target genes, and their pathway genes (PTCH1, GLI1) (36,37). GLI1 is the main HH target gene and its expression further promotes the activation of the HH signaling pathway at the transcriptional level (38). KIF7, in turn, coordinates HH signaling at the top of the PC and avoids GLI3 from cracking into an inhibited form in response to HH (39). This GLI transcription factor signal transduction pathway is the canonical HH signaling pathway (Fig. 1A).
Figure 1.
Schematics of the mechanisms of HH signaling in GBM. (A) The HH protein activates a signaling cascade by binding to the 12-TM receptors PTCH1 and PTCH2 and leading to derepression of the seven-TM protein SMO. The HH signaling may now proceed downstream of SMO via a cytoplasmic protein complex consisting of Kif7, SUFU and GLIFL. When the signal reaches SUFU, the GLI1-SUFU complex dissociates, allowing it to release transcription factors (GLI1, GLI2 and GLI3). GLI2 and GLI3 are constitutively expressed and serve as transcriptional activators, GLIA, in their full-length form and as transcriptional repressors, GLIR, after partial proteasomal processing. Activation of SMO leads to the dephosphorylation of GLI2/3 P1-6 clusters and their dissociation from SUFU, which facilitates the transfer of GLIA into the nucleus and the initiation of transcription of target genes. (B) In the absence of the HH ligand, Ptch represses the activity of SMO by inhibiting its translocation into the PC. Gpr161 localizes to the PC to maintain high CAMP levels and PKA activity, which phosphorylates P1-6 clusters located on GLI2/3. Subsequently, GliFL is phosphorylated by PKA, GSK3 and CK1 and recognized by β-trCP. This results in the proteolytic cleavage of GliFL into the form of a C-terminal truncated repressor known as a GLiR. GLiR is translocated to the nucleus, where it binds to HH target gene promoters and inhibits their expression. HH, hedgehog; SMO, smoothened; PTCH, patched; TM, transmembrane; Kif7, kinesin family member protein 7; SUFU, suppressor of fused; GPR161, G-protein coupled receptor 61; CAMP, cyclic adenosine monophosphate; PKA, protein kinase A; GliFL, full-length glioma-associated oncogene; GSK3, glycogen synthase kinase-3; CK1, casein kinase 1; GLIR, GLI repressor; GLIA, GLI activator; PC, primary cilia.
When HH ligand is absent, PTCH inhibits the activity of SMO by inhibiting the translocation of SMO in PC (40). GLIFL is phosphorylated by protein kinase A (PKA), glycogen synthase kinase 3 (GSK3) and casein kinase 1 (CK1), and then recognized by β-trCP and cleaved into GLIR (41,42). This results in the proteolytic cleavage of GLIFL into the form of a C-terminal truncated repressor known as a GLIR (33). SUFU is a negative regulator that binds to GLI proteins and prevents them from migrating to the nucleus (43). GLIR enters the nucleus, binds to HH target gene promoters and inhibits their expression (Fig. 1B).
HH signaling, canonical and non-canonical signaling, exist in parallel, and the mechanisms are complex. Non-canonical HH signaling is the most common HH-dependent reaction process, independent of GLI transcription factors or PC (44). Non-canonical HH signaling pathways may be divided into type I (independent of SMO) and type II (dependent on SMO) (45).
The canonical HH pathway is related to tumorigenesis and detransformation development. In adults, this signal abnormality has a key role in promoting the proliferation and differentiation of numerous tumor types. Its carcinogenic mechanisms mainly include abnormal cell differentiation, neovascularization, epithelial-mesenchymal transition (EMT) and enhanced invasiveness (46–48). Initially, HH signaling was mainly studied in brain cancer, skeletal muscle and skin cancer (49–51). However, in recent years, studies have indicated that this pathway is abnormal in numerous tumor types, including stomach, pancreas, lung and breast tumors (52–54). As HH signaling is activated in various types of cancer and contributes to cancer proliferation, progression and invasiveness, the HH signaling pathway is anticipated to provide new targets for cancer therapy.
4. Molecular mechanisms of the HH signaling pathway in GBM
HH signaling pathway and GBM microenvironment
The tumor microenvironment/stroma is closely related to tumorigenesis, metastasis and invasion (55,56). The tumor microenvironment/stroma is mainly composed of endothelial cells, adipocytes, immune cells and cancer-associated fibroblasts (CAFs) (57). CAFs are able to secrete soluble factors to stimulate cancer cells, thereby triggering tumor metastasis and chemotherapy resistance (58–60). Recombinant human Sonic HH N-terminal peptide (rhSHH) enhances HH signaling, accompanied by increased mRNA and protein levels of matrix metalloproteinase-2 (MMP2) and MMP9. Furthermore, the protein expression of GLI1 was positively associated with the protein expression of MMP-2 and −9, which promoted the adhesion and invasion of GBM cells (60). It has been reported that gap junctions have a role in tumor growth and progression. Torrisi et al (61) modulated SHH signaling and connexin 43 (CX43)-based intercellular communication in an in vitro model. Modulation of SMO with the use of a known agonist (i.e., taxamine) and a known antagonist (i.e., cyclodopamine) affected CX43 expression levels and thus affected related functions. In addition, SMO activation also promoted cell proliferation and migration. Of note, inhibition of the CX43 channel prevented the SMO-induced effects (61).
Therefore, further exploration of the mechanisms of the HH signaling pathway in the tumor microenvironment may lead to better targeting of this pathway to fight cancer.
Regulatory mechanism and role of SHH in GBM cells
In the development of GBM disease, PC serve as cell antennae to transmit and regulate a variety of signaling pathways and SHH is one of the most important pathways. SHH levels are significantly increased in GBM cells compared with normal brain tissue and SHH overexpression induced neuroectodermal angiogenesis during mouse embryonic development (62–64). A study has indicated that Fms-related tyrosine kinase 1 (FLT1) is significantly increased in GBM cells and overexpression of FLT1 increased the expression of SHH in cells (64). Knockdown of SHH reduced the migration and invasion mediated by FLT1 overexpression, while overexpression of SHH restored the migration and invasive ability of FLT1 knockdown (64). FLT1 is a tyrosine kinase receptor that binds VEGF-A with several times the affinity of other kinases inserted into domain receptors and has been reported to promote tumor growth and metastasis (65). VEGF-A is one of the key factors promoting tumor angiogenesis and activation of the VEGF-A pathway requires the binding of VEGF-A to its receptor FLT1 to generate downstream signals to stimulate the proliferation and development of tumor cells and provide tumor blood vessels for the growth and metastasis of GBM (64,66). In addition, brain tumorigenic-initiating cells produce DHH ligands to realize the paracrine DHH/PTCH2 signaling cascade, transmit high permeability and angiogenesis, and also promote GBM growth (6). Chen et al (67) reported that C-terminal binding protein 2 (CtBP2) expression was increased and zinc finger and BTB domain containing 18 (ZBTB18) expression was decreased in GBM tissues, and the two were negatively correlated. CtBP2 short hairpin (sh)RNA interacts with ZBTB18 to block cells in G0/G1 phase, inhibit the SHH-Gli1 pathway and reduce the tumor volume (67). However, whether this effect is exerted by increasing SHH gene expression has remained to be elucidated. Therefore, targeted FLT1 or CtBP2 therapy may be a promising direction to develop anti-metastasis agents.
GBM develops through a complex interlocking signaling pathway. RhSHH enhances the HH signaling pathway, which increases the production of MMP-2 and −9 through the PI3K/AKT pathway, thereby regulating migration and invasion of basal membrane cells and promoting GBM cell adhesion, invasion and migration (60). By contrast, triggering the vasoactive intestinal peptide receptor system is triggered to reduce GBM cell migration and invasion through PKA-dependent PI3K/AKT and SHH/GLI1 pathway blocking (68). Similarly, Henao-Restrepo et al (69) reported that PI3K/AKT/mammalian target of rapamycin complex 1 (mTORC1) and SHH/GLI signaling pathway proteins were expressed differently in human gliomas with different tumor types and grades, suggesting that the activation of these signaling networks is related to the occurrence and development of high-grade gliomas.
Multiple studies have indicated that the SHH signaling pathway promotes the plasticity of cancer cells by regulating the adhesion between cells and the extracellular matrix, thus increasing the motility and aggressiveness of cells, leading to poor prognosis of patients (60,65,67,68). Statistical analysis of the The Cancer Genome Atlas (TCGA) dataset (TCGA-Glioblastoma June 2016) suggested that SHH upregulation was associated with decreased overall survival (64).
Hedgehog-interacting protein (HHIP), which is located on chromosome 4q31.21-31.3, is defined as an antagonist of SHH, IHH and DHH. Chang et al (9) were the first to demonstrate that the expression of HHIP determined by immunohistochemistry is an independent prognostic marker of favorable outcomes in patients with GBM.
Expression and role of GLI1 in GBM
Although GLI1 was originally identified as the amplified gene in malignant human gliomas (70), GLI1 amplification is infrequent in most cancers such as GBM (71). However, since GLI1 is a vital downstream target of the HH pathway, the mRNA level of GLI1 is a reliable indicator of HH pathway activity, this suggests that control of GLI1 protein conversion is critical for GLI-dependent transcription and regulation of the HH signaling pathway (53). And GLI1 protein levels are upregulated in a variety of cancers, and high levels of GLI1 are often associated with tumor progression (72,73). Low GLI1 mRNA levels were similarly negatively correlated with survival in patients with GBM. GLI1 mRNA expression in GBM was significantly lower than in patients with high-HH-medulloblastoma (MB) but significantly higher than in patients with low-HH-MB, and GLI1 mRNA expression is a single continuous distribution, rather than being discrete high/low clusters (5,74). GLI1 promotes the nuclear import of GLI1 into GBM multiforme cells through its transcription factor Forkhead box M1 (FOXM1), thereby increasing the expression of its target genes (75).
Zhou et al (73) reported that in GBM cells, ubiquitin specific peptidase 48 (USP48) gene knockout inhibited cell proliferation and downstream GLI1 target gene expression, thereby inhibiting glioblastoma by USP48 removing ubiquitin-binding compounds on GLI1 and thereby inhibiting GLI1-dependent proteasome degradation. In addition, to a certain extent, GLI1 determines the effect of USP48 on cell proliferation and tumorigenesis and the HH pathway also induces USP48 expression via GLI1 trans-activation, thus forming a mutual feedback loop (73). Similarly, Chang et al (8) indicated that Engraile 1 (EN1) was highly expressed in GBM cells and tissues and positively regulated GLI1 levels. In addition, EN1 also affected HH signal transduction by regulating PC length and the PC transport-related protein TUB-like protein 3, a PC transport-related protein, to control the proliferation, colony formation, migration and tumorigenesis of GBM cells in vivo. Truncated GLI1 (TGLI1) acted as a functionally enhanced GLI1 with an enhanced ability to promote angiogenic heparanase expression. In vivo and in vitro, TGLI1 is more likely to promote GBM angiogenesis and growth than GLI1. Therefore, TGLI1 is a novel mediator promoting GBM angiogenesis through the HH signaling pathway and heparinase is a novel transcriptional target of TGLI1, providing new clues for molecular pathways of tumor angiogenesis and invasive growth (76).
It was observed that both the activation of metabolic glutamate receptor subtype 4 and naringin are able to inhibit the expression of GLI-1 in cells and affect HH signaling pathway transduction, thus inhibiting cell proliferation and promoting cell apoptosis to inhibit the growth of GBM cells (77,78). These may be potential drug targets for controlling GBM cell growth by blocking HH signaling.
Expression and role of GLI2 in GBM
Molecular crosstalk is present between mTORC1/2 and HH pathway activity (71,79,80). In GBM, higher mTORC2 activity enhances the expression of several HH pathway molecules (GLI1, GLI2 and PTCH1). A further study by Maiti et al (80) indicated that mTORC2 inhibits GLI2 ubiquitination by inactivating GSK3β, thereby promoting GLI2 stability and nuclear translocation, then modulating the role of HH pathway activity in GBM angiogenesis, metastasis, cell proliferation and cancer stem cell (CSC) regeneration. In addition to influencing mTORC1/2 and HH pathway interactions, GLI2 also affects HH and Wnt pathways and has an important role in GBM stem cell (GSC) maintenance. GLI2 knockdown using lentiviral-mediated shRNA downregulated HH-related and Wnt signaling pathway-related genes, including leucine-rich repeat-containing G-protein coupled receptor 5, inhibited tumor cell proliferation and invasive capacity, and induced apoptosis (81). Takezaki et al (7) indicated that overexpression of GLI2DC, a C-terminal truncated form of GLI2, antagonized GLI transcription factor function, inhibited glioma-initiating cell proliferation in culture and neoplasms occurring in organisms; glioma-initiating cell proliferation was prevented by clipping glial downstream factor cell division cycle 2 (CDC2). These results suggested that the HH/GLI/CDC2 signaling cascade has an important role in glioma-initiating cell proliferation and malignancy. Since GLI2 affects downstream multiple carcinogenic and cancer-inhibiting pathways and is a key player in the network of neoplasmic microenvironments, the possibility of blocking multiple pathways by targeting GLI2 may be a promising strategy.
Expression and role of PTCH in GBM. PTCH is the receptor of HH protein
In vertebrates, two PTCH homologs have been isolated: PTCH1 and PTCH2 (82). PTCH1 is mainly expressed in SHH protein-producing mesenchymal cells, while PTCH2 is expressed in skin and testicular epithelial cells (83). A large clinical cohort study using the TCGA-GBM database detected GLI1 expression in relation to PTCH1. The strong correlation between GLI1 and PTCH1 expression was indicated to be a potential marker of HH-pathway activity (84), since PTCH1 is a true target of GLI1 transcription factors (85) and its expression is expected to increase with the activity of GLI1 (5). Marjanovic Vicentic et al (86) reported increased expression of HH ligand-receptor PTCH and HH effectors GLI1 and GLI2 in U87 and U251 cells overexpressing SOX3. BBF2H7 is an endoplasmic reticulum stranded transmembrane basic leucine zipper transcription factor that binds to HH ligand and PTCH1 to promote the formation of ligand-receptor complexes, thereby activating HH signal transduction (87). Iwamoto et al (88) further indicated that the c-terminal end of secreted lumen BBF2H7 participates in HH ligand-dependent GBM proliferation by binding to HH ligands and PTCH1 to activate HH signaling. Therefore, SOX3 and BBF2H7C terminals may become novel targets for anticancer drug development.
HH signaling pathway and the role of GSC in GBM
The HH, mTOR, Notch and Wnt/β-catenin signaling pathways are important signaling pathways that regulate GSC stemness and self-renewal (27,89,90), However, the self-renewal and abnormal differentiation of GSCs and their ability to promote the formation of drug resistance to RT and chemotherapy are the main reasons for the recurrence and invasion of GBM after conventional treatment (91,92). The mechanisms of how GSCs during invasion through the HH pathway, particularly in the face of complex and changing brain tissue anatomy, are presented in Fig. 2.
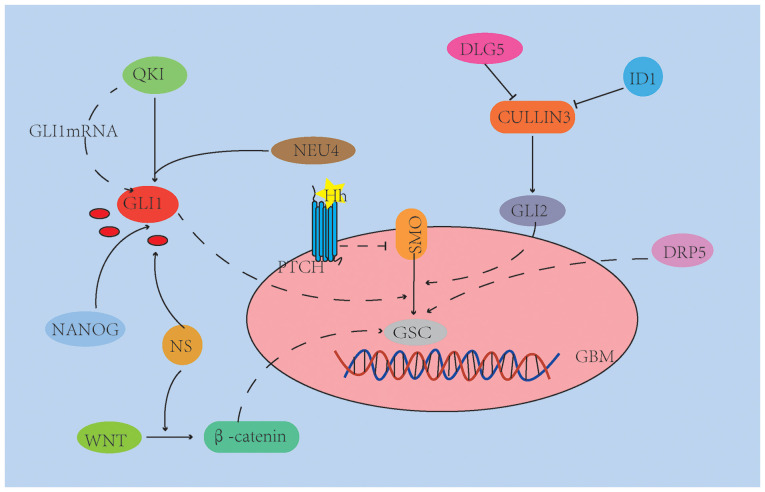
Figure 2.
Related homolog genes (e.g. QKI), transcription factors (e.g. NANOG) and sialidase (e.g. NEU4) are able to activate the HH signaling pathway to maintain the self-renewal ability of GSCs by increasing SHH/GLI1 expression. ID1 and DLG5 inhibit cullin-3 ubiquitin ligase, activate HH signaling and promote GSC proliferation and tumorigenicity. DRP5 is specifically upregulated in the proneural subtype of GSC. NS activates the Wnt and HH signaling pathways by regulating the β/AKT axis of β-catenin and Gli1, respectively. Vascular endothelial cells in the tumor microenvironment may provide SHH to further activate HH signaling pathways, thereby promoting GSC properties. QKI, Quaking homolog; DRP5, dihydro pyrimidine-associated protein 5; SHH, Sonic Hedgehog; GBM, glioblastoma; SMO, smoothened; Gli, glioma-associated oncogene; GSC, glioblastoma stem cells; ID1, differentiation inhibitor 1; NS, microenvironmental nutritional stress; DLG5, discs large homolog 5.
It has been reported that related homolog genes [e.g. Quaking homolog I)] (93), as well as transcription factors (e.g. Nanog homeobox) (94) and sialidase (e.g. neuroaminidase 4 (95) are able to activate the HH signaling pathway to maintain the self-renewal ability of GSCs by increasing SHH/GLI1 expression. This may promote the development of stem-like traits of GSCs and the formation and migration of GBM cell spheres (93–95). In addition, scaffold protein discs large homolog 5 (DLG5) and differentiation inhibitor 1 (ID1) regulate HH pathways by inhibiting downstream target ubiquitination (e.g. GLI1/2) and reducing GLI1/2 degradation (27,96). Cullin-3 interacts with GLI1/2 and dishevelled segment polarity protein 2 and induces their degradation through ubiquitination (27). ID1 and DLG5 inhibit cullin-3 ubiquitin ligase, activate HH signaling and promote GSC proliferation and tumorigenicity (27,96). Park et al (97) demonstrated that dihydro pyrimidine-associated protein 5 (DRP5) is particularly upregulated in the proneural (PN) subtype of GSC and has a key role in maintaining GSC characteristics, including tumor globule formation, stem cell marker expression and xenograft tumor growth, and DRP5 is considered to be a functional biomarker of GBM derived from PN-GSCs. The emergence and maintenance of CSCs are usually controlled by the tumor microenvironment. The tumor microenvironment always provides metabolic challenges to cancer cells and CSCs, mainly due to tissue hypoxia. Mondal et al (98) revealed that nutritional deprivation-induced enhanced the expression of specific biomarkers for GSCs, with higher invasiveness and angiogenic characteristics. These cells induced by microenvironmental nutritional stress (NS) have a high xenoefflux capacity and are therefore resistant to numerous anticancer drugs. The mechanism is that NS activates the Wnt and HH signaling pathways by regulating the β/AKT axis of β-catenin and GLI1, respectively. Vascular endothelial cells in the tumor microenvironment may provide SHH to further activate HH signaling pathways, thereby promoting GSC properties (99).
In summary, the neurobiology and basement membrane invasiveness of neural stem cells involves multiple molecular pathways that are interrelated. Therefore, targeting cross-signaling pathways (e.g., Wnt/HH signaling) and specific markers may be a better therapeutic approach for GSCs.
5. Targeting the HH signaling pathway in GBM
The increased understanding of the key role of HH signaling in cancer has led to the development of pathway-specific inhibitors and the reuse of existing drugs that regulate HH/GLI (Table I). Drugs currently used in the clinic target SMO; among them, Vismodegib and Sonidegib have been approved by the US Food and Drug Administration for the treatment of basal cell carcinoma (BCC) (100,101) and medulloblastoma (101). However, mutations leading to drug resistance may occur, and thus, compounds that inhibit HH signaling downstream of SMO are urgently required and further research on the effects of HH/GLI pathway modulators in combination with anticancer drugs should be performed in order to provide evidence to pave the road for the future use of the combination of HH/GLI inhibitors and anticancer drugs.
Table I.
List of hedgehog pathway inhibitors used in GBM.
| Inhibitor name | Drug combination | Target | Mode of action | Reverse resistance | (Refs.) |
|---|---|---|---|---|---|
| Dynarrestin | (−) | PC | Inhibition of the flow of SMO in PC | (−) | (112) |
| O6-benzylamine | Honokiol(+) | SHH | Antagonist of MGMT | (+) | (117) |
| LDE225 | (−) | SHH | Downregulated PTCH1 and GLI1 | (−) | (120) |
| PEI-SNAs | (−) | GLI1 | Binding to clearance receptors on GBM cells | (+) | (123) |
| GANT-61 | TMZ | SHH | Increases production of ROS | (+) | (124) |
| GANT-61 | (−) | SHH | Increases the expression of LC3 II and cleaved caspase 3 and 9 | (−) | (125) |
| Curcumin | MicroRNA-326 | SHH/GLI1 | Antagonist of SHH/GLI1 | (−) | (126) |
| XH30 | (−) | GLI1 | Decreases GLI1 activity | (+) | (127) |
| Phosphorylated peptides | (−) | GLI2 | Decreases GLI2 activity | (−) | (128) |
| Tubasatin A | (−) | SHH/GLI1 | Downregulation of GLI1 and PTCH1/2 receptors | (−) | (129,130) |
| CGP-2 | (−) | GLI1 | Antagonist of SMO | (−) | (132) |
| Capsulated | TMZ | GLI1 | Inhibition of GLI1 expression | (+) | (136) |
| propylamine PF403 | (−) | SMO/GLI1 | Antagonist of SMO/GLI1 | (+) | (137) |
PC, primary cilia; TMZ, temozolomide; SMO, smoothened; MGMT, methylguanine methyltransferase; PEI-SNAs, polyethylene imine-coated spherical nucleic acid nanoparticles; ROS, reactive oxygen species; CGP-2, cyclodopamine glucuronoside precursor drugs; PF403, 13A (S)-3-hydroxyl-6,7-dimethoxyphenanthro[9,10-b]-indolizidine; Gli, glioma-associated oncogene; GBM, glioblastoma; SHH, Sonic Hedgehog; PTCH, patched; LC, light chain.
Targeted therapy for GBM microenvironment
The mechanisms of GBM cell migration and invasion are complex and involve a series of mechanisms, including adhesion of GBM cells to the extracellular matrix (ECM) and ECM remodeling and degradation (102). As with other malignant tumor types, the growth, metastasis and invasion of GBM also depend on tumor angiogenesis. Although gliomas are characterized by hypervascularization, there are unavoidable disadvantages to anti-angiogenesis, such as reactive resistance mediated by the tumor microenvironment, and invasion and metastasis of tumor cells activated by hypoxia responses (103,104). During invasion and metastasis, GBM cells lose the polarized phenotype of epithelial cells and acquired mesenchymal characteristics, which is referred to as EMT (105). EMT is an active, drug-resistant, low-proliferative transient state that is frequently a feature of cancer as a whole but is seen in GBM in particular (106–108). Tubasatin A, a histone deacetylase 6 (HDAC6) inhibitor, reduced the expression of mesenchymal markers in GBM cells and contributed to the reversal of EMT (109). Feng et al (110) developed a pegylated poly (lactic acid) based nano-drug delivery system (nanoparticles) and modified CK peptides on its surface via GYG connectors to promote multitargeted delivery of Paclitaxel vasculogenic mimicry channels, tumor neovascularization and glioma cells. Similarly, Kast et al (111) proposed the EIS regimen (combination of itraconazole, metformin, naproxen, pirfenidone, quetiapine and rifampicin) that was able to safely and effectively block EMT of GBM. GBM progression may be inhibited by targeting tumor angiogenesis and EMT. Although these animal models are not perfect, they may be used to explore the effectiveness of new treatments for GBM prior to clinical phase I/II studies.
The HH signaling pathway is closely related to PC function, and thus, inhibiting PC function may help inhibit GBM proliferation, malignant development and treatment resistance (112). A previous study reported that the development of resistance to acquired kinase inhibitors is associated with upregulation of PC, uncontrolled PC length and abnormal activation of SHH signaling. Knockdown of KIF7 was observed to control the length and integrity of the PC and re-sensitize GBM cells (113). In addition, Dynarrestin was able to reversely inhibit intraflagellar transport of SMO flux in PC and inhibit HH pathway-dependent neuronal precursors and tumor cell proliferation (23). Therefore, Dynarrestin is a promising compound for the pharmacochemical development of anticancer drugs.
Inhibition of the HH/GLI pathway
HH signaling has been reported to be abnormally activated in >30% of solid tumor types, including GBM (62,114). Abnormal activation of the SHH pathway is associated with GBM resistance to temozolomide (TMZ) and the reason is the high expression of methylguanine methyltransferase (MGMT), which reverses the effects of TMZ on DNA (115,116) and confirms cell protection from TMZ-induced death by silencing three genes: MutS homolog 2 (a DNA repair protein involved in MMR), PTCH2 and chloride channel accessory 2 (a type 1 transmembrane protein that inhibits the Wnt pathway) (24). Resistance to TMZ was only slightly reversed by MGMT inhibitor O6-benzylamine, but a marked further enhancement was achieved by addition of Honokiol (117). Furthermore, the invasion of GBM was reported to be associated with the presence of CSCs and the SHH pathway has an important role in the maintenance and proliferation of CSCs (118,119). After inhibiting SHH, LDE225 slowed down the growth of GBM and downregulated PTCH1 and GLI1 in vivo (120). CSCs preferentially activate the DNA damage checkpoint response and exhibit enhanced DNA repair ability; thus, SHH signaling via GLI1 in CSC has a role in GBM resistance to TMZ (121).
Glabrescione B is the first small molecule to bind to GLI1 zinc fingers, impelling GLI1 activity by interfering with its interaction with DNA. Thus, it inhibits the ability of HH-dependent tumor stem cells to self-renew and cladogenesis. The determination of the structural requirements for GLI1/DNA interactions highlights their relevance to drug interference with GLI signaling (10). Melamed et al (122) developed polyethylene imine-coated spherical nucleic acid nanoparticles (PEI-SNAs) targeting GLI1. GLI1 PEI-SNAs bind scavenger receptors on GBM cells and undergo endocytosis in a pit/lipid raft/dynein-dependent manner, promoting the silencing of HH pathway genes and downstream target genes. These genes promote an aggressive, drug-resistant GBM phenotype. GLI1 PEI-SNAs not only significantly increased the sensitivity of nerve spheres to chemotherapy, but also further impaired the formation of dry nerve spheres (123). Arsenic trioxide also significantly reduced the cladogenesis of tumor neuroglobules by inhibiting the HH pathway, inhibiting the proliferation of GBM neuroglobules and promoting apoptosis (124). The combination of the SHH inhibitor GANT-61 with TMZ increased the cytotoxicic effect of TMZ and the combination of GANT-61 with TMZ increased the production of reactive oxygen species in GBM cells, suggesting that inhibition of the SHH pathway may sensitize GBM cells to the effects of TMZ by increasing oxidative stress (114,124). GANT-61 induced apoptosis and autophagy in GBM cells by increasing the expression of light chain 3II and lysed Caspase-3 and −9 (125). Furthermore, GLI inhibition combined with TMZ increased the apoptosis rate of glioma stem cells by 6.8-fold, thereby reducing the size and number of nerve spheres grown from glioma stem cells (115). Yin et al (126) reported that the combination of tumor suppressor gene miR-326 and curcumin significantly inhibited the SHH/GLI1 pathway of glioma cells, independent of the P53 status, significantly increased apoptosis and reduced the proliferation and migration of glioma cells. Similarly, Ji et al (127) reported that a novel PI3K inhibitor, XH30, inhibited tumor growth that was resistant to TMZ. In terms of the mechanism, the role of XH30 may be to reverse the activation of GLI1 induced by SHH by atypical HH signaling and to reduce GLI1 activation by insulin-like growth factor 1 (127). Thus, XH30 may be a novel treatment option for TMZ-resistant GBM.
Traditional treatments for GBM include systemic chemotherapy, RT and surgery. Han et al (128) synthesized three phosphorylated peptides derived from GLI2 and combined them with the cell-penetrating peptide TAT-[47–57]AYGRKKRRQRRR. The three mixed phosphorylated polypeptides derived from GLI2 significantly increased the level of GLI2 phosphorylation and decreased the transcriptional activation of GLI2, and the radiation sensitization of GBM cells was significantly higher than that in the control group (128). HDAC6 was upregulated in GSCs and inhibited HDAC6 down-regulated glioma-associated oncogene GLI1 and PTCH1/2 receptors, as well as SHH signaling components, expression and activity, thereby inhibiting GSC proliferation, inducing differentiation and increasing the apoptosis rate through the SHH/GLI1 signaling pathway (109,129). Inhibition of HDAC6 by Tubasatin A enhanced the radiosensitivity of GBM tumor cells. The mechanism may be that HDAC6 inhibits checkpoint kinase (CHK)1 degradation induced by down-regulation of X-linked inhibitor of apoptosis, which reduced the DNA damage repair ability of GSCs, leading to increased radiosensitivity (109,130).
In summary, target genes associated with the SHH/GLI pathway provide promising new drug targets for inhibiting GBM proliferation, as well as overcoming drug resistance and radiation resistance of GSCs.
SMO inhibitors
The steroidal alkaloid cyclopamine, an antagonist of the HH coreceptor SMO, acts as an inhibitor of the HH pathway (131). To limit the toxicity of cyclodopamine to HH-dependent non-tumor cells, cyclodopamine precursor drugs [e.g., cyclodopamine glucuronoside precursor drugs (CGP-2) and 1b] are commonly used (132,133). It was indicated that CGP-2 inhibits the HH pathway more effectively than conventional TMZ adjuvants (131). In the presence of β-glucuronidase, the activated prodrug 1b was toxic and downregulated the HH target gene GLI1 in C6 cells and C6-CSCs (132). In U251 cells, tyramine not only inhibited the HH/GLI1 signal transduction pathway, leading to decreased MGMT expression, but also induced cell apoptosis by activating caspase-3 cleavage, thus leading to increased sensitivity of GBM to TMZ (133). However, the combination of acepromazine and TMZ enhanced the dryness and drug resistance of GBM cells by inducing the expression of SOX-2 and OCT-4 and may lead to tumor recurrence in patients (134). Therefore, the best therapeutic strategy is to first inhibit the SHH pathway and then administer TMZ (134,135). Liu et al (136) found that the combination of capsulated propylamine and TMZ had synergistic cytotoxic effects and was more likely to inhibit the ability to induce apoptosis and eliminate neuroglobin formation by inhibiting GLI1 expression. Therefore, MCyp may be used as a tumor stem cell inhibitor to prevent tumor recurrence. Future efforts should be made to investigate the possibility of using HH pathway inhibitors prior to conventional chemotherapy in patients with GBM. Future efforts should focus on the efficacy of HH pathway inhibitors prior to systemic chemotherapy in patients with GBM.
Chen et al (137) indicated that PF403 inhibits cell surface Smoothened (Smo) receptor aggregation at the molecular level by directly binding or enhancing the interaction between Smo and the suppressor PTCH1. In addition, PF403 significantly inhibited the transcription of GLI1 and its accumulation in the nucleus by promoting the interaction between SUFU-GLI1 and PKA-GLI1, blocking the HH signaling pathway of T98G MGMT-expressing cells, and downregulated the expression of MGMT. Inhibition of the HH pathway by PF403 counteracted TMZ resistance and the precursor Cat3 of PF403 enhanced the anti-tumor activity of TMZ in vivo (137,138). In summary, Cat3 is a promising therapeutic agent for HH-driven GBM.
6. HH pathway and immunotherapy
The key to antitumor immunity is that antigen-presenting cells (APCs) engulf tumor cells. TMZ may induce an endoplasmic reticulum stress response, and the combination of CD47 blocker and TMZ may produce significant prophagocytosis (139,140). Increased tumor cell phagocytosis, enhanced antigenic cross-presentation in APC and activation of cyclic GMP-AMP interferon gene synthase stimulation leads to more efficient T-cell effects. This connection between innate and adaptive responses inhibits GBM growth while also activating immune checkpoints. Sequential administration of an anti-programmed cell death protein 1 (anti-PD1) antibody overcomes this potential adaptive resistance (140). However, the mechanism by which anti-PD1 antibodies reverse GBM resistance through HH signaling remains to be elucidated. It has been reported that GANT-61 is able to reduce the expression of PD-L1 and the proliferation of tumor cells in vivo and in vitro by using organic compound drugs for human gastric cancer. Of note, anti-PD-L1 antibodies induced apoptosis of tumor cells in organs of GLI2-expressing mice. Studies suggested that GLI2 expressed in gastric cancer cells is an internal regulator of PD-L1 and promotes tumor growth by inhibiting the anti-tumor response (141,142). In summary, the HH pathway may become a new immunotherapy target for GBM after further study.
7. Discussion
The biological treatment of GBM has been studied for numerous years, but the treatment of deadly cancers still poses a great challenge. GBMs are highly invasive and susceptible to drug resistance, resulting in a high mortality rate, and GBM accounts for 2.9% of cancer-related deaths (143).
A key treatment issue for GBM is the high degree of heterogeneity within the tumor. This heterogeneity further complicates the differences among patients with GBM. In addition to heterogeneity, GBM also has GSCs that contribute to tumor proliferation, maintenance and drug resistance (144,145), and GSCs may respond differently to TMZ or ionizing radiation (146). All of this makes routine treatment difficult. Further research is required on the impact of GBM heterogeneity on modern therapies, including molecular immunotherapy and personalized therapy. The lymphocytes present in GBM have an increased proportion of CD4+T cells and FOXP3+ regulatory T cells may induce signaling pathways that inhibit immune responses (147,148), e.g., the expression of IDO enzyme and STAT3 signals (149,150). However, GBM tumor-infiltrating effector lymphocytes were observed to be rare (151,152). This may also be the reason why a clinical trial of immune checkpoint blocking using the anti-PD1 antibody nivolumab (NCT02017717) used in patients with newly diagnosed or relapsed unmethylated GBM (153), have not been successful. The currently used immunotherapy for GBM may be broadly divided into vaccine therapy, immune checkpoint blocking, oncolytic virus therapy and chimeric antigen receptor T-cell therapy (154–156). In addition to immunotherapy, EGFR using tyrosine kinase inhibitors (TKI), VEGF TKI and targeted therapies for the PI3K/mTOR pathway have also been explored in GBM. However, a phase 3 trial of deatuxizumab mandolin in combination with standard therapy for the treatment of newly diagnosed EGFR-amplified GBM was terminated early for being ineffective (157), and mTOR inhibitors such as everolimus (NCCTGN057K) and Taxiolimus (EORTC26082) also proved to lack efficacy in phase 2 trials (158,159). A phase 2 trial of regorafenib (REGOMA) in a relapsed setting indicated a therapeutic OS benefit compared to lomustine, but the drug had minimal activity; thus, VEGF monotherapy may have a limited effect in a non-selected population (160).
It is necessary to study new targets for the treatment of GBM. HH signaling has emerged as an attractive target for cancer therapy and several HH inhibitors have been designed. To date, SMO inhibitors were proven to have satisfactory efficacy in BCC and medulloblastoma (100,101), but clinical trials for other cancer types, such as colorectal, pancreatic or lung cancers, have yielded poor results (161–163). In preclinical studies, compared with HH and SMO inhibitors, GLI inhibitors had better anticancer efficacy (164,165). In addition, GLI inhibitors effectively inhibited the growth of numerous GLI-dependent cancers by targeting the GLI-regulated SMO-independent pathway (166). As for inhibitors of GLI1 and GLI2 transcription factors, the anticancer drug arsenic trioxide is currently the only drug undergoing clinical trials in solid tumors and hematological malignancies (167). Although the use of HH inhibitors in GBM has not been extensively investigated, numerous studies suggested that HH inhibitors in combination with conventional therapies may markedly increase efficacy and reduce the incidence of drug resistance (124–126,133,137). Of course, this also requires a large number of clinical trials to further verify whether HH inhibitors are beneficial to the therapeutic efficacy of GBM.
Epigenetic regulators interact with drivers of GBM stem cell-like cell proliferation. These drivers include Notch, HH and WNT pathways. Previous studies suggested that these signaling pathways may perform cross-talk with SHH signaling pathways (27,71,80-81,89,90), which means that these signaling pathways may be activated simultaneously in different tumor types. WNT/β-catenin interacts with the SHH pathway through GLI1 and GLI2 by regulating the expression of secreted crimp-related proteins. SHH signaling was inhibited by GSK3β, a component of the WNT signaling pathway. In certain tumor types, upregulation of the WNT signaling pathway occurs sequentially when the SHH pathway is inhibited (168). In addition, the synergistic effect of the inhibition of the SHH and PI3K/AKT/mTOR signaling pathways may inhibit the proliferation of glioblastoma-initiating cells (GICs), tumor growth and the formation of neural spheres and clones, and induce cell apoptosis (169). Combined drug action targeting two pathways or inhibition at the intersection of two pathways may be a good breakthrough point for targeted therapy.
8. Conclusion
Current conventional therapies for GBM are ineffective due to drug resistance issues and resistance may be overcome through a combination of HH inhibitors or multilevel HH signaling cascades, such as combinations of multiple targeted HH drugs and multi-target HH inhibitors. In addition to pioneering new approaches based on existing scientific theories, the effectiveness of evaluating these therapies in clinical trials requires to be further improved. This includes increasing the number of patients with GBM in phase I trials of HH pathway inhibitors, thereby providing more complete clinical trial data for the development of more effective targeted therapeutic strategies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- GBM
glioblastoma
- CNS
central nervous system
- HH
Hedgehog
- PCNS
primary central nervous system
- GLI
glioma-associated oncogenes
- CDK4
cyclin-dependent kinase 4
- SHH
Sonic HH
- PC
primary cilia
- SUFU
suppressor of fused homolog
- KIF7
kinesin 7
- SMO
smoothened
- PKA
protein kinase A
- GSK3
glycogen synthase kinase 3
- CK1
casein kinase 1
- EMT
epithelial-mesenchymal transition
- CAFs
cancer-associated fibroblasts
- MMP2
matrix metalloproteinase-2
- FLT1
Fms-related tyrosine kinase 1
- HHIP
HH-interacting protein
- mTORC1
mammalian target of rapamycin complex 1
- TMZ
temozolomide
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HW performed the literature search and selection. XX was responsible for the conception, analysis and design of the study. HW and XX were major contributors in writing of the manuscript. DW and JP participated in the coordination of the study and reviewed the manuscript. BT and YG were responsible for the revision of the manuscript. QL and ZG were responsible for the literature search and design of the study. All authors read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, Stearns DS, Wolff JE, Liu M, Wolinsky Y, et al. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2016;18((Suppl 1)):i1–i50. doi: 10.1093/neuonc/nov297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20((Supp l4)):iv1–iv86. doi: 10.1093/neuonc/noy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vadgaonkar R, Epari S, Chinnaswamy G, Krishnatry R, Tonse R, Gupta T, Jalali R. Distinct demographic profile and molecular markers of primary CNS tumor in 1873 adolescent and young adult patient population. Childs Nerv Syst. 2018;34:1489–1495. doi: 10.1007/s00381-018-3785-y. [DOI] [PubMed] [Google Scholar]
- 4.Husson O, Zebrack B, Block R, Embry L, Aguilar C, Hayes-Lattin B, Cole S. Personality traits and health-related quality of life among adolescent and young adult cancer patients: The role of psychological distress. J Adolesc Young Adult Oncol. 2017;6:358–362. doi: 10.1089/jayao.2016.0083. [DOI] [PubMed] [Google Scholar]
- 5.Chandra V, Das T, Gulati P, Biswas NK, Rote S, Chatterjee U, Ghosh SN, Deb S, Saha SK, Chowdhury AK, et al. Hedgehog signaling pathway is active in GBM with GLI1 mRNA expression showing a single continuous distribution rather than discrete high/low clusters. PLoS One. 2015;10:e0116390. doi: 10.1371/journal.pone.0116390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzi S, Treps L, Leclair HM, Ngo HM, Harford-Wright E, Gavard J. Desert Hedgehog/Patch2 axis contributes to vascular permeability and angiogenesis in glioblastoma. Front Pharmacol. 2015;6:281. doi: 10.3389/fphar.2015.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu J, Kondo T. Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Sci. 2011;102:1306–1312. doi: 10.1111/j.1349-7006.2011.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang J, Guo C, Li J, Liang Z, Wang Y, Yu A, Liu R, Guo Y, Chen J, Huang S. EN1 regulates cell growth and proliferation in human glioma cells via Hedgehog signaling. Int J Mol Sci. 2022;23:1123. doi: 10.3390/ijms23031123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang L, Zhang P, Zhao D, Liu H, Wang Q, Li C, Du W, Liu X, Zhang H, Zhang Z, Jiang C. The Hedgehog antagonist HHIP as a favorable prognosticator in glioblastoma. Tumour Biol. 2016;37:3979–3986. doi: 10.1007/s13277-015-3442-y. [DOI] [PubMed] [Google Scholar]
- 10.Infante P, Mori M, Alfonsi R, Ghirga F, Aiello F, Toscano S, Ingallina C, Siler M, Cucchi D, Po A, et al. Gli1/DNA interaction is a druggable target for Hedgehog-dependent tumors. EMBO J. 2015;34:200–217. doi: 10.15252/embj.201489213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network, corp-author. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Q, Hu B, Hu X, Kim H, Squatrito M, Scarpace L, deCarvalho AC, Lyu S, Li P, Li Y, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2017;32:42–56.e6. doi: 10.1016/j.ccell.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sturm D, Bender S, Jones DT, Lichter P, Grill J, Becher O, Hawkins C, Majewski J, Jones C, Costello JF, et al. Paediatric and adult glioblastoma: Multiform (epi)genomic culprits emerge. Nat Rev Cancer. 2014;14:92–107. doi: 10.1038/nrc3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, Koelsche C, Sahm F, Chavez L, Reuss DE, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555:469–474. doi: 10.1038/nature26000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torrisi F, Alberghina C, D'Aprile S, Pavone AM, Longhitano L, Giallongo S, Tibullo D, Di Rosa M, Zappalà A, Cammarata FP, et al. The hallmarks of glioblastoma: Heterogeneity, intercellular crosstalk and molecular signature of invasiveness and progression. Biomedicines. 2022;10:806. doi: 10.3390/biomedicines10040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, Barnholtz-Sloan JS. Neuro Oncol 21 (Suppl 5):v1-v100; 2019. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, Fisher B, Fulton D, Gulavita S, Hao C, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: A prospective randomized clinical trial. J Clin Oncol. 2004;22:1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 21.Suchorska B, Weller M, Tabatabai G, Senft C, Hau P, Sabel MC, Herrlinger U, Ketter R, Schlegel U, Marosi C, et al. Complete resection of contrast-enhancing tumor volume is associated with improved survival in recurrent glioblastoma-results from the DIRECTOR trial. Neuro Oncol. 2016;18:549–556. doi: 10.1093/neuonc/nov326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F, Dbalý V, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–2202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Höing S, Yeh TY, Baumann M, Martinez NE, Habenberger P, Kremer L, Drexler HCA, Küchler P, Reinhardt P, Choidas A, et al. Dynarrestin, a novel inhibitor of cytoplasmic dynein. Cell Chem Biol. 2018;25:357–369.e6. doi: 10.1016/j.chembiol.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocha CRR, Reily Rocha A, Molina Silva M, Rodrigues Gomes L, Teatin Latancia M, Andrade Tomaz M, de Souza I, Karolynne Seregni Monteiro L, Menck CFM. Revealing temozolomide resistance mechanisms via genome-wide CRISPR libraries. Cells. 2020;9:2573. doi: 10.3390/cells9122573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebrahimi A, Larijani L, Moradi A, Ebrahimi MR. Hedgehog signalling pathway: Carcinogenesis and targeted therapy. Iran J Cancer Prev. 2013;6:36–43. [PMC free article] [PubMed] [Google Scholar]
- 26.Jin X, Jeon HM, Jin X, Kim EJ, Yin J, Jeon HY, Sohn YW, Oh SY, Kim JK, Kim SH, et al. The ID1-CULLIN3 axis regulates intracellular SHH and WNT signaling in glioblastoma stem cells. Cell Rep. 2016;16:1629–1641. doi: 10.1016/j.celrep.2016.06.092. [DOI] [PubMed] [Google Scholar]
- 27.Huynh DL, Koh H, Chandimali N, Zhang JJ, Kim N, Kang TY, Ghosh M, Gera M, Park YH, Kwon T, Jeong DK. BRM270 inhibits the proliferation of CD44 positive pancreatic ductal adenocarcinoma cells via downregulation of sonic Hedgehog signaling. Evid Based Complement Alternat Med. 2019;2019:8620469. doi: 10.1155/2019/8620469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marigo V, Tabin CJ. Regulation of patched by sonic Hedgehog in the developing neural tube. Proc Natl Acad Sci USA. 1996;93:9346–9351. doi: 10.1073/pnas.93.18.9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mastronardi FG, Dimitroulakos J, Kamel-Reid S, Manoukian AS. Co-localization of patched and activated sonic Hedgehog to lysosomes in neurons. Neuroreport. 2000;11:581–585. doi: 10.1097/00001756-200002280-00030. [DOI] [PubMed] [Google Scholar]
- 31.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–1033. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 32.Alexandre C, Jacinto A, Ingham PW. Transcriptional activation of Hedgehog target genes in Drosophila is mediated directly by the cubitus interruptus protein, a member of the GLI family of zinc finger DNA-binding proteins. Genes Dev. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- 33.Pan Y, Bai CB, Joyner AL, Wang B. Sonic Hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/S0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 35.Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R. Gli protein activity is controlled by multisite phosphorylation in vertebrate Hedgehog signaling. Cell Rep. 2014;6:168–181. doi: 10.1016/j.celrep.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonnissen A, Isebaert S, Haustermans K. Targeting the Hedgehog signaling pathway in cancer: Beyond smoothened. Oncotarget. 2015;6:13899–13913. doi: 10.18632/oncotarget.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skoda AM, Simovic D, Karin V, Kardum V, Vranic S, Serman L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn J Basic Med Sci. 2018;18:8–20. doi: 10.17305/bjbms.2018.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sabol M, Trnski D, Musani V, Ozretić P, Levanat S. Role of GLI transcription factors in pathogenesis and their potential as new therapeutic targets. Int J Mol Sci. 2018;19:2562. doi: 10.3390/ijms19092562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endoh-Yamagami S, Evangelista M, Wilson D, Wen X, Theunissen JW, Phamluong K, Davis M, Scales SJ, Solloway MJ, de Sauvage FJ, Peterson AS. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Curr Biol. 2009;19:1320–1326. doi: 10.1016/j.cub.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 40.Denef N, Neubüser D, Perez L, Cohen SM. Hedgehog induces opposite changes in turnover and subcellular localization of patched and smoothened. Cell. 2000;102:521–531. doi: 10.1016/S0092-8674(00)00056-8. [DOI] [PubMed] [Google Scholar]
- 41.Tschaikner P, Enzler F, Torres-Quesada O, Aanstad P, Stefan E. Hedgehog and Gpr161: Regulating cAMP signaling in the primary cilium. Cells. 2020;9:118. doi: 10.3390/cells9010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price MA, Kalderon D. Proteolysis of the Hedgehog signaling effector cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell. 2002;108:823–835. doi: 10.1016/S0092-8674(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 43.Méthot N, Basler K. Suppressor of fused opposes Hedgehog signal transduction by impeding nuclear accumulation of the activator form of cubitus interruptus. Development. 2000;127:4001–4010. doi: 10.1242/dev.127.18.4001. [DOI] [PubMed] [Google Scholar]
- 44.Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical Hedgehog signaling. Vitam Horm. 2012;88:55–72. doi: 10.1016/B978-0-12-394622-5.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Awasthi A, Woolley AG, Lecomte FJ, Hung N, Baguley BC, Wilbanks SM, Jeffs AR, Tyndall JD. Variable expression of GLIPR1 correlates with invasive potential in melanoma cells. Front Oncol. 2013;3:225. doi: 10.3389/fonc.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K, Pan L, Che X, Cui D, Li C. Sonic Hedgehog/GLI1 signaling pathway inhibition restricts cell migration and invasion in human gliomas. Neurol Res. 2010;32:975–980. doi: 10.1179/016164110X12681290831360. [DOI] [PubMed] [Google Scholar]
- 48.Lo HW, Zhu H, Cao X, Aldrich A, Ali-Osman F. A novel splice variant of GLI1 that promotes glioblastoma cell migration and invasion. Cancer Res. 2009;69:6790–6798. doi: 10.1158/0008-5472.CAN-09-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reifenberger J, Wolter M, Weber RG, Megahed M, Ruzicka T, Lichter P, Reifenberger G. Missense mutations in SMOH in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58:1798–1803. [PubMed] [Google Scholar]
- 50.Lee Y, Miller HL, Jensen P, Hernan R, Connelly M, Wetmore C, Zindy F, Roussel MF, Curran T, Gilbertson RJ, McKinnon PJ. A molecular fingerprint for medulloblastoma. Cancer Res. 2003;63:5428–5437. [PubMed] [Google Scholar]
- 51.Lim CB, Prêle CM, Cheah HM, Cheng YY, Klebe S, Reid G, Watkins DN, Baltic S, Thompson PJ, Mutsaers SE. Mutational analysis of Hedgehog signaling pathway genes in human malignant mesothelioma. PLoS One. 2013;8:e66685. doi: 10.1371/journal.pone.0066685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 53.Noman AS, Uddin M, Rahman MZ, Nayeem MJ, Alam SS, Khatun Z, Wahiduzzaman M, Sultana A, Rahman ML, Ali MY, et al. Overexpression of sonic Hedgehog in the triple negative breast cancer: Clinicopathological characteristics of high burden breast cancer patients from Bangladesh. Sci Rep. 2016;6:18830. doi: 10.1038/srep18830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2013;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 55.Riobo-Del Galdo NA, Lara Montero Á, Wertheimer EV. Role of Hedgehog signaling in breast cancer: Pathogenesis and therapeutics. Cells. 2019;8:375. doi: 10.3390/cells8040375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 57.Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez-Outschoorn U, Sotgia F, Lisanti MP. Tumor microenvironment and metabolic synergy in breast cancers: Critical importance of mitochondrial fuels and function. Semin Oncol. 2014;41:195–216. doi: 10.1053/j.seminoncol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Martinez-Outschoorn UE, Lin Z, Ko YH, Goldberg AF, Flomenberg N, Wang C, Pavlides S, Pestell RG, Howell A, Sotgia F, Lisanti MP. Understanding the metabolic basis of drug resistance: Therapeutic induction of the Warburg effect kills cancer cells. Cell Cycle. 2011;10:2521–2528. doi: 10.4161/cc.10.15.16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang L, Zhao D, Liu HB, Wang QS, Zhang P, Li CL, Du WZ, Wang HJ, Liu X, Zhang ZR, Jiang CL. Activation of sonic Hedgehog signaling enhances cell migration and invasion by induction of matrix metalloproteinase-2 and −9 via the phosphoinositide-3 kinase/AKT signaling pathway in glioblastoma. Mol Med Rep. 2015;12:6702–6710. doi: 10.3892/mmr.2015.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torrisi F, Alberghina C, Lo Furno D, Zappalà A, Valable S, Li Volti G, Tibullo D, Vicario N, Parenti R. Connexin 43 and Sonic Hedgehog pathway interplay in glioblastoma cell proliferation and migration. Biology (Basel) 2021;10:767. doi: 10.3390/biology10080767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cherepanov SA, Cherepanova KI, Grinenko NF, Antonova OM, Chekhonin VP. Effect of Hedgehog signaling pathway activation on proliferation of high-grade gliomas. Bull Exp Biol Med. 2016;161:674–678. doi: 10.1007/s10517-016-3483-2. [DOI] [PubMed] [Google Scholar]
- 63.Rowitch DH, S-Jacques B, Lee SM, Flax JD, Snyder EY, McMahon AP. Sonic Hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. J Neurosci. 1999;19:8954–8965. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jiang K, Wang YP, Wang XD, Hui XB, Ding LS, Liu J, Liu D. Fms related tyrosine kinase 1 (Flt1) functions as an oncogene and regulates glioblastoma cell metastasis by regulating sonic Hedgehog signaling. Am J Cancer Res. 2017;7:1164–1176. [PMC free article] [PubMed] [Google Scholar]
- 65.Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J, Jr, Fischer W, Lukas J, et al. Autocrine VEGF-VEGFR2-neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–520. doi: 10.1084/jem.20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci USA. 2016;113:4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Wang L, Qin J, Wei DS. CtBP2 interacts with ZBTB18 to promote malignancy of glioblastoma. Life Sci. 2020;262:118477. doi: 10.1016/j.lfs.2020.118477. [DOI] [PubMed] [Google Scholar]
- 68.Bensalma S, Turpault S, Balandre AC, De Boisvilliers M, Gaillard A, Chadéneau C, Muller JM. PKA at a cross-road of signaling pathways involved in the regulation of glioblastoma migration and invasion by the neuropeptides VIP and PACAP. Cancers (Basel) 2019;11:123. doi: 10.3390/cancers11010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Henao-Restrepo J, Caro-Urrego YA, Barrera-Arenas LM, Arango-Viana JC, Bermudez-Munoz M. Expression of activator proteins of SHH/GLI and PI3K/Akt/mTORC1 signaling pathways in human gliomas is associated with high grade tumors. Exp Mol Pathol. 2021;122:104673. doi: 10.1016/j.yexmp.2021.104673. [DOI] [PubMed] [Google Scholar]
- 70.Kinzler KW, Bigner SH, Bigner DD, Trent JM, Law ML, O'Brien SJ, Wong J, Vogelstein B. Identification of an amplified, highly expressed gene in a human glioma. Science. 1987;236:70–73. doi: 10.1126/science.3563490. [DOI] [PubMed] [Google Scholar]
- 71.Bigner SH, Wong AJ, Mark J, Muhlbaier LH, Kinzler KW, Vogelstein B, Bigner DD. Relationship between gene amplification and chromosomal deviations in malignant human gliomas. Cancer Genet Cytogenet. 1987;29:165–170. doi: 10.1016/0165-4608(87)90045-8. [DOI] [PubMed] [Google Scholar]
- 72.ten Haaf A, Bektas N, von Serenyi S, Losen I, Arweiler EC, Hartmann A, Knuchel R, Dahl E. Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer. 2009;9:298. doi: 10.1186/1471-2407-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou A, Lin K, Zhang S, Ma L, Xue J, Morris SA, Aldape KD, Huang S. Gli1-induced deubiquitinase USP48 aids glioblastoma tumorigenesis by stabilizing Gli1. EMBO Rep. 2017;18:1318–1330. doi: 10.15252/embr.201643124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y, Do IG, Hong M, Suh YL. Negative prognostic effect of low nuclear GLI1 expression in glioblastomas. J Neurooncol. 2017;133:69–76. doi: 10.1007/s11060-017-2426-8. [DOI] [PubMed] [Google Scholar]
- 75.Xue J, Zhou A, Tan C, Wu Y, Lee HT, Li W, Xie K, Huang S. Forkhead box M1 is essential for nuclear localization of glioma-associated oncogene homolog 1 in glioblastoma multiforme cells by promoting importin-7 expression. J Biol Chem. 2015;290:18662–18670. doi: 10.1074/jbc.M115.662882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu H, Carpenter RL, Han W, Lo HW. The GLI1 splice variant TGLI1 promotes glioblastoma angiogenesis and growth. Cancer Lett. 2014;343:51–61. doi: 10.1016/j.canlet.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang Z, Zheng X, Luan Y, Liu Y, Li X, Liu C, Lu H, Chen X, Liu Y. Activity of metabotropic glutamate receptor 4 suppresses proliferation and promotes apoptosis with inhibition of Gli-1 in human glioblastoma cells. Front Neurosci. 2018;12:320. doi: 10.3389/fnins.2018.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sargazi ML, Juybari KB, Tarzi ME, Amirkhosravi A, Nematollahi MH, Mirzamohammdi S, Mehrbani M, Mehrabani M, Mehrabani M. Naringenin attenuates cell viability and migration of C6 glioblastoma cell line: A possible role of Hedgehog signaling pathway. Mol Biol Rep. 2021;48:6413–6421. doi: 10.1007/s11033-021-06641-1. [DOI] [PubMed] [Google Scholar]
- 79.Chantaravisoot N, Wongkongkathep P, Loo AJ, Mischel SP, Tamanoi F. Significance of filamin A in mTORC2 function in glioblastoma. Mol Cancer. 2015;14:e127. doi: 10.1186/s12943-015-0396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maiti S, Mondal S, Satyavarapu EM, Mandal C. mTORC2 regulates Hedgehog pathway activity by promoting stability to Gli2 protein and its nuclear translocation. Cell Death Dis. 2017;8:e2926. doi: 10.1038/cddis.2017.296. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Tanigawa S, Fujita M, Moyama C, Ando S, Ii H, Kojima Y, Fujishita T, Aoki M, Takeuchi H, Yamanaka T, et al. Inhibition of Gli2 suppresses tumorigenicity in glioblastoma stem cells derived from a de novo murine brain cancer model. Cancer Gene Ther. 2021;28:1339–1352. doi: 10.1038/s41417-020-00282-5. [DOI] [PubMed] [Google Scholar]
- 82.Zaphiropoulos PG, Undén AB, Rahnama F, Hollingsworth RE, Toftgård R. PTCH2, a novel human patched gene, undergoing alternative splicing and up-regulated in basal cell carcinomas. Cancer Res. 1999;59:787–792. [PubMed] [Google Scholar]
- 83.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 84.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 86.Marjanovic Vicentic J, Drakulic D, Garcia I, Vukovic V, Aldaz P, Puskas N, Nikolic I, Tasic G, Raicevic S, Garros-Regulez L, et al. SOX3 can promote the malignant behavior of glioblastoma cells. Cell Oncol (Dordr) 2019;42:41–54. doi: 10.1007/s13402-018-0405-5. [DOI] [PubMed] [Google Scholar]
- 87.Saito A, Kanemoto S, Zhang Y, Asada R, Hino K, Imaizumi K. Chondrocyte proliferation regulated by secreted luminal domain of ER stress transducer BBF2H7/CREB3L2. Mol Cell. 2014;53:127–139. doi: 10.1016/j.molcel.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 88.Iwamoto H, Matsuhisa K, Saito A, Kanemoto S, Asada R, Hino K, Takai T, Cui M, Cui X, Kaneko M, et al. Promotion of cancer cell proliferation by cleaved and secreted luminal domains of ER stress transducer BBF2H7. PLoS One. 2015;10:e0125982. doi: 10.1371/journal.pone.0125982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guen VJ, Chavarria TE, Kröger C, Ye X, Weinberg RA, Lees JA. EMT programs promote basal mammary stem cell and tumor-initiating cell stemness by inducing primary ciliogenesis and Hedgehog signaling. Proc Natl Acad Sci USA. 2017;114:E10532–E10539. doi: 10.1073/pnas.1711534114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brandner S. Nanog, Gli, and p53: A new network of stemness in development and cancer. EMBO J. 2010;29:2475–2476. doi: 10.1038/emboj.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rich JN, Eyler CE. Cancer stem cells in brain tumor biology. Cold Spring Harb Symp Quant Biol. 2008;73:411–420. doi: 10.1101/sqb.2008.73.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Groot JF, Fuller G, Kumar AJ, Piao Y, Eterovic K, Ji Y, Conrad CA. Tumor invasion after treatment of glioblastoma with bevacizumab: Radiographic and pathologic correlation in humans and mice. Neuro Oncol. 2010;12:233–242. doi: 10.1093/neuonc/nop027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han B, Wang R, Chen Y, Meng X, Wu P, Li Z, Duan C, Li Q, Li Y, Zhao S, et al. QKI deficiency maintains glioma stem cell stemness by activating the SHH/GLI1 signaling pathway. Cell Oncol (Dordr) 2019;42:801–813. doi: 10.1007/s13402-019-00463-x. [DOI] [PubMed] [Google Scholar]
- 94.Yuan Y, Zhang M, Yan G, Ma Q, Yan Z, Wang L, Yang K, Guo D. Nanog promotes stem-like traits of glioblastoma cells. Front Biosci (Landmark Ed) 2021;26:552–565. doi: 10.2741/4907. [DOI] [PubMed] [Google Scholar]
- 95.Silvestri I, Testa F, Zappasodi R, Cairo CW, Zhang Y, Lupo B, Galli R, Di Nicola M, Venerando B, Tringali C. Sialidase NEU4 is involved in glioblastoma stem cell survival. Cell Death Dis. 2014;5:e1381. doi: 10.1038/cddis.2014.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kundu S, Nandhu MS, Longo SL, Longo JA, Rai S, Chin LS, Richardson TE, Viapiano MS. The scaffolding protein DLG5 promotes glioblastoma growth by controlling sonic Hedgehog signaling in tumor stem cells. Neuro Oncol: noac001. 2022 doi: 10.1093/neuonc/noac001. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park MG, Seo S, Ham SW, Choi SH, Kim H. Dihydropyrimidinase-related protein 5 controls glioblastoma stem cell characteristics as a biomarker of proneural-subtype glioblastoma stem cells. Oncol Lett. 2020;20:1153–1162. doi: 10.3892/ol.2020.11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mondal S, Bhattacharya K, Mandal C. Nutritional stress reprograms dedifferention in glioblastoma multiforme driven by PTEN/Wnt/Hedgehog axis: A stochastic model of cancer stem cells. Cell Death Discov. 2018;4:110. doi: 10.1038/s41420-018-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan GN, Yang L, Lv YF, Shi Y, Shen LL, Yao XH, Guo QN, Zhang P, Cui YH, Zhang X, et al. Endothelial cells promote stem-like phenotype of glioma cells through activating the Hedgehog pathway. J Pathol. 2014;234:11–22. doi: 10.1002/path.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Robinson GW, Orr BA, Wu G, Gururangan S, Lin T, Qaddoumi I, Packer RJ, Goldman S, Prados MD, Desjardins A, et al. Vismodegib exerts targeted efficacy against recurrent sonic Hedgehog-subgroup medulloblastoma: Results from phase II pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J Clin Oncol. 2015;33:2646–2654. doi: 10.1200/JCO.2014.60.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wong R, Turlova E, Feng ZP, Rutka JT, Sun HS. Activation of TRPM7 by naltriben enhances migration and invasion of glioblastoma cells. Oncotarget. 2017;8:11239–11248. doi: 10.18632/oncotarget.14496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, Feng J, Stewart AE, Hu-Lowe DD, Christensen JG. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–10100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 104.Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, et al. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li L, Li W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol Ther. 2015;150:33–46. doi: 10.1016/j.pharmthera.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–654. doi: 10.1007/s10555-016-9648-7. [DOI] [PubMed] [Google Scholar]
- 107.Tang H, Massi D, Hemmings BA, Mandalà M, Hu Z, Wicki A, Xue G. AKT-ions with a TWIST between EMT and MET. Oncotarget. 2016;7:62767–62777. doi: 10.18632/oncotarget.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yeung KT, Yang J. Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol. 2017;11:28–39. doi: 10.1002/1878-0261.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Urdiciain A, Erausquin E, Meléndez B, Rey JA, Idoate MA, Castresana JS. Tubastatin A, an inhibitor of HDAC6, enhances temozolomide-induced apoptosis and reverses the malignant phenotype of glioblastoma cells. Int J Oncol. 2019;54:1797–1808. doi: 10.3892/ijo.2019.4739. [DOI] [PubMed] [Google Scholar]
- 110.Feng X, Yao J, Gao X, Jing Y, Kang T, Jiang D, Jiang T, Feng J, Zhu Q, Jiang X, Chen J. Multi-targeting peptide-functionalized nanoparticles recognized vasculogenic mimicry, tumor neovasculature, and glioma cells for enhanced anti-glioma therapy. ACS Appl Mater Interfaces. 2015;7:27885–27899. doi: 10.1021/acsami.5b09934. [DOI] [PubMed] [Google Scholar]
- 111.Kast RE, Skuli N, Karpel-Massler G, Frosina G, Ryken T, Halatsch ME. Blocking epithelial-to-mesenchymal transition in glioblastoma with a sextet of repurposed drugs: The EIS regimen. Oncotarget. 2017;8:60727–60749. doi: 10.18632/oncotarget.18337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li M, Zhang J, Zhou H, Xiang R. Primary cilia-related pathways moderate the development and therapy resistance of glioblastoma. Front Oncol. 2021;11:718995. doi: 10.3389/fonc.2021.718995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jenks AD, Vyse S, Wong JP, Kostaras E, Keller D, Burgoyne T, Shoemark A, Tsalikis A, de la Roche M, Michaelis M, et al. Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep. 2018;23:3042–3055. doi: 10.1016/j.celrep.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Carpenter RL, Lo HW. Hedgehog pathway and GLI1 isoforms in human cancer. Discov Med. 2012;13:105–113. [PMC free article] [PubMed] [Google Scholar]
- 115.Honorato JR, Hauser-Davis RA, Saggioro EM, Correia FV, Sales-Junior SF, Soares LOS, Lima LDR, Moura-Neto V, Lopes GPF, Spohr TCLS. Role of sonic Hedgehog signaling in cell cycle, oxidative stress, and autophagy of temozolomide resistant glioblastoma. J Cell Physiol. 2020;235:3798–3814. doi: 10.1002/jcp.29274. [DOI] [PubMed] [Google Scholar]
- 116.Melamed JR, Morgan JT, Ioele SA, Gleghorn JP, Sims-Mourtada J, Day ES. Investigating the role of Hedgehog/GLI1 signaling in glioblastoma cell response to temozolomide. Oncotarget. 2018;9:27000–27015. doi: 10.18632/oncotarget.25467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai IC, Shih PH, Yao CJ, Yeh CT, Wang-Peng J, Lui TN, Chuang SE, Hu TS, Lai TY, Lai GM. Elimination of cancer stem-like cells and potentiation of temozolomide sensitivity by honokiol in glioblastoma multiforme cells. PLoS One. 2015;10:e0114830. doi: 10.1371/journal.pone.0114830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sarkaria JN, Kitange GJ, James CD, Plummer R, Calvert H, Weller M, Wick W. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14:2900–2908. doi: 10.1158/1078-0432.CCR-07-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Munoz JL, Rodriguez-Cruz V, Walker ND, Greco SJ, Rameshwar P. Temozolomide resistance and tumor recurrence: Halting the Hedgehog. Cancer Cell Microenviron. 2015;2:e747. doi: 10.14800/ccm.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hung HC, Liu CC, Chuang JY, Su CL, Gean PW. Inhibition of sonic Hedgehog signaling suppresses glioma stem-like cells likely through inducing autophagic cell death. Front Oncol. 2020;10:1233. doi: 10.3389/fonc.2020.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen J, Fu X, Wan Y, Wang Z, Jiang D, Shi L. miR-125b inhibitor enhance the chemosensitivity of glioblastoma stem cells to temozolomide by targeting Bak1. Tumour Biol. 2014;35:6293–6302. doi: 10.1007/s13277-014-1821-4. [DOI] [PubMed] [Google Scholar]
- 122.Melamed JR, Ioele SA, Hannum AJ, Ullman VM, Day ES. Polyethylenimine-spherical nucleic acid nanoparticles against Gli1 reduce the chemoresistance and stemness of glioblastoma cells. Mol Pharm. 2018;15:5135–5145. doi: 10.1021/acs.molpharmaceut.8b00707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ding D, Lim KS, Eberhart CG. Arsenic trioxide inhibits Hedgehog, notch and stem cell properties in glioblastoma neurospheres. Acta Neuropathol Commun. 2014;2:31. doi: 10.1186/2051-5960-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, Huang S, Tian R, Chen J, Gao H, Xie C, Shan Y, Zhang Z, Gu S, Xu M. The protective autophagy activated by GANT-61 in MYCN amplified neuroblastoma cells is mediated by PERK. Oncotarget. 2018;9:14413–14427. doi: 10.18632/oncotarget.24214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carballo GB, Ribeiro JH, Lopes GPF, Ferrer VP, Dezonne RS, Pereira CM, Spohr TCLSE. GANT-61 induces autophagy and apoptosis in glioblastoma cells despite their heterogeneity. Cell Mol Neurobiol. 2021;41:1227–1244. doi: 10.1007/s10571-020-00891-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin S, Du W, Wang F, Han B, Cui Y, Yang D, Chen H, Liu D, Liu X, Zhai X, Jiang C. MicroRNA-326 sensitizes human glioblastoma cells to curcumin via the SHH/GLI1 signaling pathway. Cancer Biol Ther. 2018;19:260–270. doi: 10.1080/15384047.2016.1250981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ji M, Zhang Z, Lin S, Wang C, Jin J, Xue N, Xu H, Chen X. The PI3K inhibitor XH30 enhances response to temozolomide in drug-resistant glioblastoma via the noncanonical Hedgehog signaling pathway. Front Pharmacol. 2021;12:749242. doi: 10.3389/fphar.2021.749242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Han L, Tang L, Jiang Z, Jiang Y. Enhanced radiosensitization of human glioblastoma multiforme cells with phosphorylated peptides derived from Gli2. Neuropeptides. 2018;70:87–92. doi: 10.1016/j.npep.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 129.Urdiciain A, Erausquin E, Zelaya MV, Zazpe I, Lanciego JL, Meléndez B, Rey JA, Idoate MA, Riobo-Del Galdo NA, Castresana JS. Silencing of histone deacetylase 6 decreases cellular malignancy and contributes to primary cilium restoration, epithelial-to-mesenchymal transition reversion, and autophagy inhibition in glioblastoma cell lines. Biology (Basel) 2021;10:467. doi: 10.3390/biology10060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang W, Liu Y, Gao R, Yu H, Sun T. HDAC6 inhibition induces glioma stem cells differentiation and enhances cellular radiation sensitivity through the SHH/Gli1 signaling pathway. Cancer Lett. 2018;415:164–176. doi: 10.1016/j.canlet.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 131.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. Hedgehog-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Balbous A, Renoux B, Cortes U, Milin S, Guilloteau K, Legigan T, Rivet P, Boissonnade O, Martin S, Tripiana C, et al. Selective release of a cyclopamine glucuronide prodrug toward stem-like cancer cell inhibition in glioblastoma. Mol Cancer Ther. 2014;13:2159–2169. doi: 10.1158/1535-7163.MCT-13-1038. [DOI] [PubMed] [Google Scholar]
- 133.Bensalma S, Chadeneau C, Legigan T, Renoux B, Gaillard A, de Boisvilliers M, Pinet-Charvet C, Papot S, Muller JM. Evaluation of cytotoxic properties of a cyclopamine glucuronide prodrug in rat glioblastoma cells and tumors. J Mol Neurosci. 2015;55:51–61. doi: 10.1007/s12031-014-0395-3. [DOI] [PubMed] [Google Scholar]
- 134.Wang K, Chen D, Qian Z, Cui D, Gao L, Lou M. Hedgehog/Gli1 signaling pathway regulates MGMT expression and chemoresistance to temozolomide in human glioblastoma. Cancer Cell Int. 2017;17:117. doi: 10.1186/s12935-017-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carballo GB, Matias D, Ribeiro JH, Pessoa LS, Arrais-Neto AM, Spohr TCLSE. Cyclopamine sensitizes glioblastoma cells to temozolomide treatment through sonic Hedgehog pathway. Life Sci. 2020;257:118027. doi: 10.1016/j.lfs.2020.118027. [DOI] [PubMed] [Google Scholar]
- 136.Liu YJ, Ma YC, Zhang WJ, Yang ZZ, Liang DS, Wu ZF, Qi XR. Combination therapy with micellarized cyclopamine and temozolomide attenuate glioblastoma growth through Gli1 down-regulation. Oncotarget. 2017;8:42495–42509. doi: 10.18632/oncotarget.17205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen J, Lv H, Hu J, Ji M, Xue N, Li C, Ma S, Zhou Q, Lin B, Li Y, et al. CAT3, a novel agent for medulloblastoma and glioblastoma treatment, inhibits tumor growth by disrupting the Hedgehog signaling pathway. Cancer Lett. 2016;381:391–403. doi: 10.1016/j.canlet.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 138.Ji M, Wang L, Chen J, Xue N, Wang C, Lai F, Wang R, Yu S, Jin J, Chen X. CAT3, a prodrug of 13a(S)-3-hydroxyl-6,7-dimethoxyphenanthro[9,10-b]-indolizidine, circumvents temozolomide-resistant glioblastoma via the Hedgehog signaling pathway, independently of O6-methylguanine DNA methyltransferase expression. Onco Targets Ther. 2018;11:3671–3684. doi: 10.2147/OTT.S163535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Horrigan SK, Reproducibility Project Cancer Biology: Replication study: The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Elife. 2017;6:e18173. doi: 10.7554/eLife.18173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.von Roemeling CA, Wang Y, Qie Y, Yuan H, Zhao H, Liu X, Yang Z, Yang M, Deng W, Bruno KA, et al. Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat Commun. 2020;11:1508. doi: 10.1038/s41467-020-15129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chakrabarti J, Holokai L, Syu L, Steele NG, Chang J, Wang J, Ahmed S, Dlugosz A, Zavros Y. Oncotarget. Vol. 9. 37439-37457; 2018. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grund-Gröschke S, Stockmaier G, Aberger F. Hedgehog/GLI signaling in tumor immunity-new therapeutic opportunities and clinical implications. Cell Commun Signal. 2019;17:172. doi: 10.1186/s12964-019-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.SurveillanceEpidemiology, End Results Program, corp-author. Cancer stat facts: Brain and other nervous system cancer. https://seer.cancer.gov/statfacts/html/brain.html. [ July 12; 2019 ];2019 [Google Scholar]
- 144.Braun S, Oppermann H, Mueller A, Renner C, Hovhannisyan A, Baran-Schmidt R, Gebhardt R, Hipkiss A, Thiery J, Meixensberger J, Gaunitz F. Hedgehog signaling in glioblastoma multiforme. Cancer Biol Ther. 2012;13:487–495. doi: 10.4161/cbt.19591. [DOI] [PubMed] [Google Scholar]
- 145.Filbin MG, Dabral SK, Pazyra-Murphy MF, Ramkissoon S, Kung AL, Pak E, Chung J, Theisen MA, Sun Y, Franchetti Y, et al. Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: New therapeutic opportunities. Nat Med. 2013;19:1518–1523. doi: 10.1038/nm.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lan X, Jörg DJ, Cavalli FMG, Richards LM, Nguyen LV, Vanner RJ, Guilhamon P, Lee L, Kushida MM, Pellacani D, et al. Fate mapping of human glioblastoma reveals an invariant stem cell hierarchy. Nature. 2017;549:227–232. doi: 10.1038/nature23666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Sun W, Qiao W, Hiraoka N, Fuller GN. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14:5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 149.Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim CK, Tobias A, Cheng Y, Kim JW, Qiao J, Zhang L, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heimberger AB, Sun W, Hussain SF, Dey M, Crutcher L, Aldape K, Gilbert M, Hassenbusch SJ, Sawaya R, Schmittling B, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: Case study. Neuro Oncol. 2008;10:98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: Lessons from glioblastoma. Nat Immunol. 2019;20:1100–1109. doi: 10.1038/s41590-019-0433-y. [DOI] [PubMed] [Google Scholar]
- 152.Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020;20:12–25. doi: 10.1038/s41568-019-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, Baehring J, Ahluwalia MS, Roth P, Bähr O, et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1003–1010. doi: 10.1001/jamaoncol.2020.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chiocca EA, Nassiri F, Wang J, Peruzzi P, Zadeh G. Viral and other therapies for recurrent glioblastoma: Is a 24-month durable response unusual? Neuro Oncol. 2019;21:14–25. doi: 10.1093/neuonc/noy170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Iorgulescu JB, Reardon DA, Chiocca EA, Wu CJ. Immunotherapy for glioblastoma: Going viral. Nat Med. 2018;24:1094–1096. doi: 10.1038/s41591-018-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lassman A, Pugh S, Wang T, Aldape K, Gan H, Preusser M, Vogelbaum M, Sulman E, Won M, Zhang P, et al. ACTR-21. A randomized, double-blind, placebo-controlled phase 3 trial of depatuxizumab mafodotin (ABT-414) in epidermal growth factor receptor (EGFR) amplified (AMP) newly diagnosed glioblastoma (nGBM) Neuro Oncol. 2019;21((Suppl 6)):vi17. doi: 10.1093/neuonc/noz175.064. [DOI] [Google Scholar]
- 158.Ma DJ, Galanis E, Anderson SK, Schiff D, Kaufmann TJ, Peller PJ, Giannini C, Brown PD, Uhm JH, McGraw S, et al. A phase II trial of everolimus, temozolomide, and radiotherapy in patients with newly diagnosed glioblastoma: NCCTG N057K. Neuro Oncol. 2015;17:1261–1269. doi: 10.1093/neuonc/nou328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wick W, Gorlia T, Bady P, Platten M, van den Bent MJ, Taphoorn MJ, Steuve J, Brandes AA, Hamou MF, Wick A, et al. Phase II study of radiotherapy and temsirolimus versus radiochemotherapy with temozolomide in patients with newly diagnosed glioblastoma without MGMT promoter hypermethylation (EORTC 26082) Clin Cancer Res. 2016;22:4797–4806. doi: 10.1158/1078-0432.CCR-15-3153. [DOI] [PubMed] [Google Scholar]
- 160.Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, Lolli I, Pace A, Daniele B, Pasqualetti F, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019;20:110–119. doi: 10.1016/S1470-2045(18)30675-2. [DOI] [PubMed] [Google Scholar]
- 161.Kim EJ, Sahai V, Abel EV, Griffith KA, Greenson JK, Takebe N, Khan GN, Blau JL, Craig R, Balis UG, et al. Pilot clinical trial of Hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin Cancer Res. 2014;20:5937–5945. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berlin J, Bendell JC, Hart LL, Firdaus I, Gore I, Hermann RC, Mulcahy MF, Zalupski MM, Mackey HM, Yauch RL, et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin Cancer Res. 2013;19:258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 163.Belani CP, Dahlberg SE, Rudin CM, Fleisher M, Chen HX, Takebe N, Velasco MR, Jr, Tester WJ, Sturtz K, Hann CL, et al. Vismodegib or cixutumumab in combination with standard chemotherapy for patients with extensive-stage small cell lung cancer: A trial of the ECOG-ACRIN cancer research group (E1508) Cancer. 2016;122:2371–2378. doi: 10.1002/cncr.30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zubčić V, Rinčić N, Kurtović M, Trnski D, Musani V, Ozretić P, Levanat S, Leović D, Sabol M. GANT61 and lithium chloride inhibit the growth of head and neck cancer cell lines through the regulation of GLI3 processing by GSK3β. Int J Mol Sci. 2020;21:6410. doi: 10.3390/ijms21176410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Miyazaki Y, Matsubara S, Ding Q, Tsukasa K, Yoshimitsu M, Kosai K, Takao S. Efficient elimination of pancreatic cancer stem cells by Hedgehog/GLI inhibitor GANT61 in combination with mTOR inhibition. Mol Cancer. 2016;15:49. doi: 10.1186/s12943-016-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pietrobono S, Gagliardi S, Stecca B. Non-canonical Hedgehog signaling pathway in cancer: Activation of GLI transcription factors beyond smoothened. Front Genet. 2019;10:556. doi: 10.3389/fgene.2019.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim J, Lee JJ, Kim J, Gardner D, Beachy PA. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the Gli2 transcriptional effector. Proc Natl Acad Sci USA. 2010;107:13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yanai K, Nakamura M, Akiyoshi T, Nagai S, Wada J, Koga K, Noshiro H, Nagai E, Tsuneyoshi M, Tanaka M, Katano M. Crosstalk of Hedgehog and Wnt pathways in gastric cancer. Cancer Lett. 2008;263:145–156. doi: 10.1016/j.canlet.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 169.Nanta R, Shrivastava A, Sharma J, Shankar S, Srivastava RK. Inhibition of sonic Hedgehog and PI3K/Akt/mTOR pathways cooperate in suppressing survival, self-renewal and tumorigenic potential of glioblastoma-initiating cells. Mol Cell Biochem. 2019;454:11–23. doi: 10.1007/s11010-018-3448-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.