Abstract
Background
There have been over 200 million cases and 4.4 million deaths from COVID-19 worldwide. Despite the lack of robust evidence one potential treatment for COVID-19 associated severe hypoxaemia is inhaled pulmonary vasodilator (IPVD) therapy, using either nitric oxide (iNO) or prostaglandins. We describe the implementation of, and outcomes from, a protocol using IPVDs in a cohort of patients with severe COVID-19 associated respiratory failure receiving maximal conventional support.
Methods
Prospectively collected data from adult patients with SARS-CoV-2 admitted to the intensive care unit (ICU) at a large teaching hospital were analysed for the period 14th March 2020 - 11th February 2021. An IPVD was considered if the PaO2/FiO2 (PF) ratio was less than 13.3kPa despite maximal conventional therapy. Nitric oxide was commenced at 20ppm and titrated to response. If oxygenation improved Iloprost nebulisers were commenced and iNO weaned. The primary outcome was percentage changes in PF ratio and Alveolar-arterial (A-a) gradient.
Results
Fifty-nine patients received IPVD therapy during the study period. The median PF ratio before IPVD therapy was commenced was 11.33kPa (9.93-12.91). Patients receiving an IPVD had a lower PF ratio (14.37 vs. 16.37kPa, p = 0.002) and higher APACHE-II score (17 vs. 13, p = 0.028) at ICU admission. At 72 hours after initiating an IPVD the median improvement in PF ratio was 33.9% (-4.3-84.1). At 72 hours changes in PF ratio (70.8 vs. −4.1%, p < 0.001) and reduction in A-a gradient (44.7 vs. 14.8%, p < 0.001) differed significantly between survivors (n = 33) and non-survivors (n = 26).
Conclusions
The response to IPVDs in patients with COVID-19 associated acute hypoxic respiratory failure differed significantly between survivors and non-survivors. Both iNO and prostaglandins may offer therapeutic options for patients with severe refractory hypoxaemia due to COVID-19. The use of inhaled prostaglandins, and iNO where feasible, should be studied in adequately powered prospective randomised trials.
Keywords: COVID-19, Intensive care, ARDS, Hypoxic respiratory failure, Pulmonary vasodilators, Iloprost, Nitric oxide
Introduction
The novel coronavirus SARS-CoV-2 was first detected in Wuhan, China, in December 2019. The consequent illness referred to as COVID-19 has emerged as a global pandemic accounting for over 200 million confirmed cases and 4.4 million deaths worldwide.1 The first COVID-19 case in the United Kingdom (UK) was diagnosed in January 2020, and since then more than 6.4 million people have tested positive, with over half a million requiring hospitalisation. Although most people experience either no symptoms or a mild illness, with an overall mortality of around 2-3%, the mortality for patients requiring mechanical ventilation can be high as 50%.2,3 Despite improvements in treatment strategies with systemic corticosteroids and immune modulation with anti-IL-6 therapies, there was little difference in the observed 28-day mortality between the first and second waves of the pandemic in the UK.3 This large data set from all intensive care unit admissions in the UK implies that there is an urgent need to identify other effective therapeutic interventions to reduce mortality and disease progression among this critically ill COVID-19 phenotype.
Due to the evolving understanding of the pathophysiology of COVID-19 and the rapidly changing evidence surrounding therapeutics, it is unsurprising that there is significant variation in the reported treatment of COVID-19 patients with severe acute hypoxic respiratory failure (AHRF).4 One potential treatment strategy for patients with severe hypoxaemia is inhaled pulmonary vasodilator (IPVD) therapy either in the form of nitric oxide (iNO) or prostaglandin analogues. During the UK pandemic waves, the use of pulmonary vasodilators’ as treatment for acute hypoxic respiratory failure was reported by 35.3% of ICUs.4 Despite the lack of robust clinical evidence pulmonary vasodilators are often considered as recue therapies in extreme refractory hypoxaemia.5 Given the severity of hypoxaemia in patients with COVID-19, and the theoretical benefits of IPVD therapy, we rapidly developed and implemented a protocol at our institution for the use of IPVD therapy on a compassionate basis for patients with severe hypoxaemia who were receiving maximal conventional support. In this study we describe the cohort of patients who received IPVDs and assess the differences in response to our protocolised use of IPVD therapy between survivors and non-survivors in patients admitted to the general intensive care unit with COVID-19 AHRF.
Methods
Design
This is a retrospective analysis of prospectively collected data in patients with polymerase chain reaction (PCR) confirmed SARS-CoV-2 infection admitted to the Intensive Care Unit at a large teaching hospital. The hospital is a tertiary centre for paediatric cardiac surgery and adult pulmonary hypertension and therefore had immediate access to nitric oxide delivery systems and nebulised prostaglandins. Data analysed are for the time period 14th March 2020 - 11th February 2021. Standard care for all patients with confirmed SARS-Cov-2 on the ICU consisted of a trial of non-invasive ventilation followed by mechanical ventilation in the event of deterioration. As the evidence base progressed, corticosteroids (dexamethasone), and subsequently IL-6 inhibitors (tocilizumab/sarilumab), were used in accordance with local guidelines. Once mechanically ventilated, all patients underwent a minimum of three proning cycles unless contra-indicated (16 hours prone, 8 hours supine), received neuromuscular blockade and ventilation titrated to target PaO2 8kPa with permissive hypercapnia. Where this could not be safely achieved appropriate patients were referred to a specialised tertiary centre for consideration of extracorporeal membrane oxygenation (ECMO). During the peak of the pandemic, there were rigorous inclusion criteria for ECMO acceptance due to the availability of ECMO beds. We developed departmental guidelines for the use of inhaled IPVD therapy on a compassionate basis for patients with severe refractory hypoxaemia (PaO2 <13.3kPa) who were on otherwise maximal therapy. We used a combination of volume targeted (Adaptive pressure ventilation controlled mandatory ventilation synchronised (APVcmv)) and airway pressure release ventilation (APRV) as primary invasive ventilation strategies. The target tidal volumes and plateau pressures were limited to 6–8ml/kg predicted body weight and ≤ 30cm H2O respectively. The required PEEP was titrated according to the quasi-static pressure volume curve via a P-V Tool® function (HAMILTON-C6). During APRV, peak inspiratory pressure was limited to ≤ 30 cm H2O.
The objectives of this study were to assess changes in oxygenation following the initiation of IPVD therapy and to evaluate any differences in the response between ICU survivors and non-survivors. Our secondary objective was to assess the differences in characteristics between survivors and non-survivors. The study was completed in accordance with the STROBE guidelines.6 This study was part of a larger observational cohort study (REACT-COVID Observational Database) and has the approval of the local research and development and National Health Research Authority (IRAS: 285145) with a REC reference 20/HRA/2986. Due to the nature of the study consent was waived.
Participants
Patients aged 18 years or older with PCR confirmed SARS-CoV-2 infection admitted to the ICU who received an IPVD during their admission were eligible for inclusion in this analysis. Exclusion criteria included the use of an IPVD for reasons other than SARS-CoV-2 infection and receipt of less than 24 hours of an IPVD. The latter criterion was to allow for titration of therapy as well as mitigate the potential confounding effect of prone positioning.
Interventions
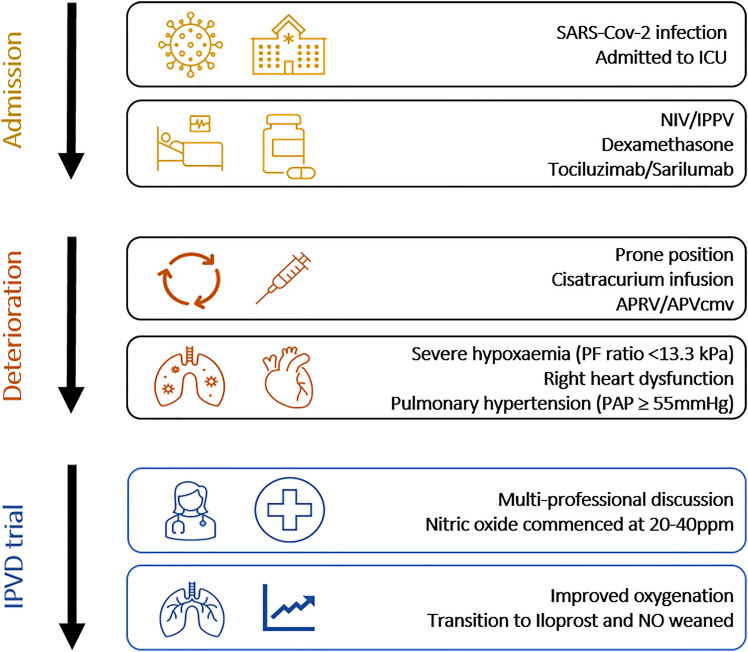
The decision to start an IPVD followed a standard operating procedure (SOP) (Supplemental Material 1). In brief, an IPVD was considered if a patient had a PaO2/FiO2 (PF) ratio <13.3kPa refractory to rescue therapies (prone position, PEEP titration, APRV ventilation) with evidence of right heart strain and/or pulmonary hypertension on trans-thoracic echocardiogram where possible. The iNO was delivered by NOxBOXi® at a starting dose of 20ppm and titrated to their response up to 40ppm. Where there was an improvement in oxygenation Iloprost nebulisers were commenced and NO weaned (Figure 1). Iloprost was commenced at 10mcg every four hours and delivered via the Aerogen® Solo nebuliser delivery system. The dose was increased in 10mcg increments up to a maximum of 30mcg every four hours. If there was no further improvement in oxygenation at 48 hours the Iloprost nebulisers were stopped. We only had 4 iNO delivery systems (n = 4), which were shared between the paediatric (PICU) and the general intensive care units (GICU). In the absence of a NOxBOXi® patients were commenced on Iloprost nebulisers and iNO was started if and when it became available. Where patients did not meet the inclusion criteria for the intervention (eg, patients with NIV as ceiling of care) an IPVD could be commenced following a consensus review from the treating clinicians (eg, nebulised Iloprost during NIV).
Figure 1.
Compassionate use of inhaled pulmonary vasodilators (IPVD) pathway overview.
Outcomes
The following data were prospectively collected from the ICU Clinical Information System (Metavision®, iMDsoft): Hospital and ICU admission and discharge dates, ICU and hospital survival, age, gender, ethnicity, BMI, illness severity scores, admission ventilation indices, admission bloods, therapies received (corticosteroids, prone position ventilation, renal replacement therapy (RRT) and ECMO.) The primary outcome was percentage changes in PF ratio and Alveolar-arterial (A-a) gradient at 2, 6, 12, 24, 48 and 72 hours following initiation of IPVD therapy (A-a gradient calculated as ). Secondary outcomes were differences in patient characteristics, therapies received and changes in PF ratio and A-a gradient between survivors and non-survivors who received an IPVD. We also assessed the number of patients who had an improvement in PF ratio of 10%, 20% and 50% to explore whether different percentage increments were associated with improved survival. Outcomes are up to date as of 6th July 2021.
Statistical Methods
Data were analysed using IBM SPSS Statistics 26.0 for Microsoft Windows. Data were tested for normality using the Shapiro-Wilks test. Data are presented as mean and standard deviation, median and inter-quartile range or number and percentages, depending on their distribution. Continuous data were compared using an independent t-test, non-parametric variables with the Mann–Whitney U and Kruskal-Wallis tests, and categorical variables with the χ2 or Fisher's exact test. A p-value of < 0.05 was used to denote statistical significance.
Results
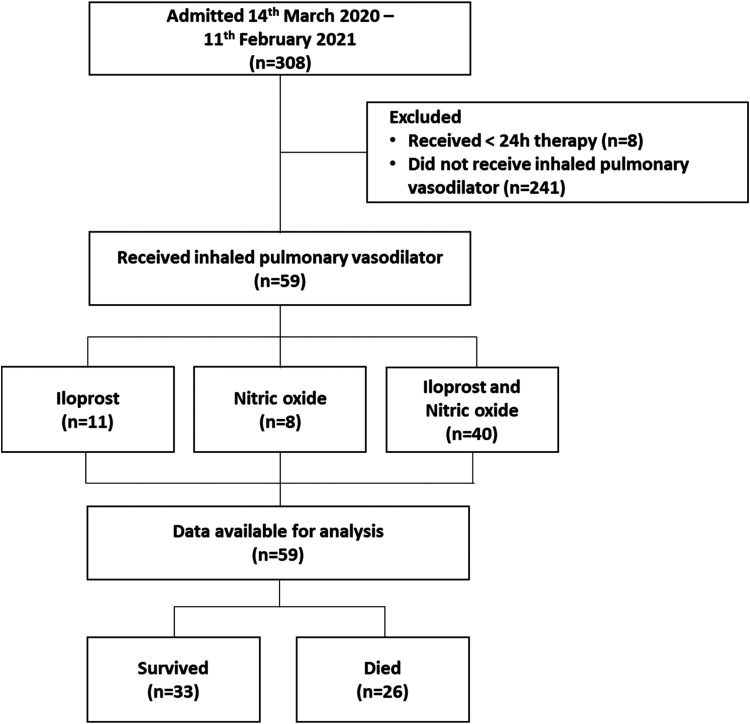
Between 14th March 2020 and 11th February 2021, 308 patients with confirmed SARS-CoV-2 infection were admitted to the ICU. Two hundred forty-one patients did not receive an IPVD and eight received less than 24 hours of therapy. Of the 59 patients who received an IPVD nine received iNO, 11 received Iloprost and 39 received combination of both iNO and Iloprost. Data was available for analysis on all 59 patients (Figure 2).
Figure 2.
STROBE flow chart.
Patient Characteristics
The characteristics of the IPVD group compared to patients who did not receive an IPVD during their admission are detailed in Table 1. Patients receiving an IPVD had a lower PF ratio (14.37 vs. 16.37 kPa, p = 0.002) and higher APACHE-II score (17 vs. 13, p = 0.028) at admission, and were more likely to receive renal replacement therapy (25.4% vs. 13.7%, p = 0.026). There were no other differences in demographic and clinical variables such as age, gender, BMI, ethnicity or baseline co-morbidities between patients who received an IPVD and those that did not. Thirty-three (55.9%) patients in the IPVD group survived to ICU discharge compared to 81.9% of the non-IPVD group (p <0.001).
Table 1.
IPVD Therapy Versus non-IPVD Therapy COVID-19 ICU Patients.
| IPVD (n = 59) | No IPVD (n = 249) | p-value | |
|---|---|---|---|
| Age (years) | 60 (54-66) | 60 (47-71) | 0.753 |
| Gender (n (%)) | |||
| Male | 37 (62.7%) | 164 (65.9%) | 0.648 |
| Female | 22 (37.3%) | 85 (34.1%) | |
| BMI (kg/m2) | 31.1 (27.1-35.9) | 30.5 (26-34.8) | 0.274 |
| Ethnicity (n (%)) | |||
| White | 41 (69.5%) | 190 (76.3%) | 0.609 |
| Asian | 12 (20.3%) | 38 (15.3%) | |
| Black | 4 (6.8%) | 11 (4.4%) | |
| Mixed | 2 (3.4%) | 6 (2.4%) | |
| Unknown | - | 4 (1.6%) | |
| Comorbidities (n (%)) | |||
| T1DM | 0 (0%) | 7 (2.8%) | 0.354 |
| T2DM | 17 (28.8%) | 64 (25.7%) | 0.626 |
| COPD | 3 (5.1%) | 15 (6.0%) | 1.000 |
| Hypertension | 28 (47.5%) | 101 (40.6%) | 0.334 |
| Ischaemic heart disease | 3 (5.1%) | 34 (13.7%) | 0.069 |
| CKD | 5 (8.5%) | 17 (6.8%) | 0.585 |
| Illness severity scores | |||
| APACHE-II | 17 (12-23) | 13 (10-20) | 0.028* |
| Admission oxygenation indices | |||
| FiO2 | 0.7 (0.55-0.8) | 0.6 (0.5-0.7) | 0.001* |
| PaO2 (kPa) | 9.41 (8.55-11.10) | 9.96 (8.50-9.96) | 0.144 |
| PF ratio (kPa) | 14.37 (11.64-18.5) | 16.37 (13.70-21.44) | 0.002* |
| Admission bloods | |||
| Lactate (mmol/L) | 1.2 (1.0-1.6) | 1.0 (0.8-1.4) | 0.013* |
| WCC (109/L) | 8.2 (6.0-11.0) | 8.5 (6.0-12.0) | 0.379 |
| Neutrophils (109/L) | 7.2 (5.2-9.43) | 7.6 (5.1-10.4) | 0.540 |
| Lymphocytes (109/L) | 0.6 (0.5-0.8) | 0.8 (0.5-1.1) | 0.004* |
| Ferritin (μg/L) | 774 (534-1237) | 683 (376-1281) | 0.547 |
| Troponin (ng/L) | 15 (8-43) | 13 (7-44) | 0.698 |
| LDH (U/L) | 1022 (829-1400) | 874 (687-1211) | 0.005* |
| D-Dimer (μg/L) | 583 (283-1141) | 553 (283-1176) | 0.888 |
| Creatinine Kinase (U/L) | 148 (54-381) | 131 (68-342) | 0.997 |
| Treatments (n (%)) | |||
| Transfer for ECMO | 1 (1.7%) | 4 (1.6%) | 1.000 |
| Renal replacement therapy | 15 (25.4%) | 34 (13.7%) | 0.026* |
| Survival (n (%)) | |||
| ICU survival | 33 (55.9%) | 204 (81.9%) | <0.001* |
| Hospital survival | 31 (52.5%) | 199 (79.9%) | <0.001* |
Response to Inhaled Pulmonary Vasodilator Therapy
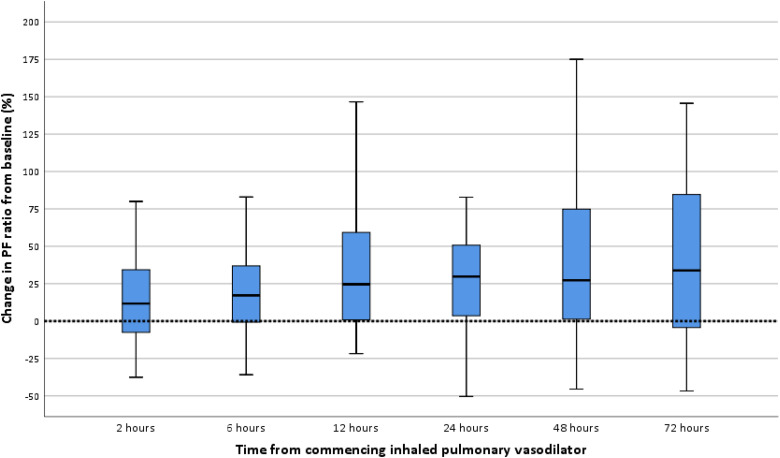
The median PF ratio at commencing of IPVD therapy was 11.33kPa (9.93-12.91). The median time from ICU admission to receipt of an IPVD was 6 days. At 48 hours from commencing an IPVD three patients had died (3/59) [5.1%]) (one receiving iNO and two receiving combined therapy) and one patient had been transferred to the regional ECMO centre. The overall response to IPVD therapy at different time points and the variations between therapies received are detailed in Table 2. The majority of patients showed an improvement in oxygenation when assessed 72 hours following the start of IPVD therapy (median increase in PF ratio 33.9% (-4.3-84.1)). This increase occurred more quickly in patients treated with iNO compared to those treated with Iloprost, with maximal improvement at 12 and 72 hours, respectively. (Table 2) The greatest percentage change was seen in patients receiving iNO only (65.1% at 12 hours), however this data was from a small number of patients. The percentage changes for all patients during therapy are shown in Figure 3.
Table 2.
Percentage Change in PF Ratio from Initiation of IPVD Therapy.
| Change in PF ratio from initiation of IPVD (%) | ||||
|---|---|---|---|---|
| All IPVD (n = 59) | Iloprost (n = 11) | Nitric oxide (n = 9) | Nitric oxide and Iloprost (n = 39) | |
| Time point | ||||
| + 2 hours | 7.1 (-9.5-33.6) | -10.7 (-18.6-25.4) | 1.0 (-5.8-23.0) | 14.0 (-5.5-38.0) |
| + 6 hours | 16.4 (-1.9-36.9) | -1.3 (-21.2-14.0) | 27.2 (13.5-67.5) | 17.2 (-1.9-38.5) |
| + 12 hours | 21.3 (-1.6-65.1) | 17.4 (-1.6-44.3) | 65.1 (51.0-117.2) | 15.4 (-5.5-56.8) |
| + 24 hours | 28.4 (0.6-51.3) | 29.8 (-10.5-44.7) | 51.3 (38.3-77.7) | 25.1 (0.6-47.4) |
| + 48 hoursa | 24.9 (0.5-74.8) | 6.2 (-13.3-107.7) | 40.7 (22.2-96.3) | 24.9 (-2.0-70.5) |
| + 72 hoursa | 33.9 (-4.3-84.1) | 57.1 (19.8-84.8) | 62.0 (31.6-95.5) | 21.0 (-11.3-84.2) |
Data for 4 patients unavailable (3 deceased and 1 transferred to regional ECMO centre).
Figure 3.
Percentage change in PF ratio from initiation of IPVD (all patients).
Characteristics of Survivors versus non-Survivors in Patients Receiving an IPVD
Of the 59 patients who received an IPVD 33 (55.9%) survived to ICU discharge. The differences in characteristics between survivors and non-survivors are detailed in table 3. There were no significant differences in survivors compared to non-survivors with regards to age, gender, BMI, ethnicity, baseline comorbidities, APACHE-II score or admission oxygenation indices. With the exception of a higher creatinine kinase in the survivors there were no differences in admission bloods. There were no differences in receipt of corticosteroids, ECMO or prone position ventilation between the groups. Survivors received renal replacement therapy less frequently than non-survivors (15.2% vs. 38.5%, p = 0.041).
Table 3.
Characteristics of Survivors Versus non-Survivors in Patients Receiving IPVD Therapy.
| Survivors (n = 33) | Non-survivors (n = 26) | p-value | |
|---|---|---|---|
| Age (Years) | 57 (51-64) | 61 (57-67) | 0.093 |
| Gender (n (%)) | |||
| Male | 20 (60.6%) | 17 (65.4%) | 0.706 |
| Female | 13 (39.4%) | 9 (34.6%) | |
| BMI (kg/m2) | 29.7 (27.0-37.3) | 31.7 (27.0-36.1) | 0.832 |
| Ethnicity (n (%)) | |||
| White | 24 (72.7%) | 17 (65.4%) | 0.434 |
| Asian | 5 (15.2%) | 7 (26.9%) | |
| Black | 2 (6.1%) | 2 (7.7%) | |
| Mixed | 2 (6.1%) | - | |
| Comorbidities (n (%)) | |||
| T2DM | 8 (24.2%) | 9 (34.6%) | 0.382 |
| COPD | 2 (6.1%) | 1 (3.8%) | 0.590 |
| Hypertension | 13 (39.4%) | 15 (57.7%) | 0.162 |
| Ischaemic heart disease | 3 (9.1%) | - | 0.168 |
| Heart failure | 1 (3.0%) | - | 0.559 |
| ACEI/ARB | 8 (24.2%) | 7 (26.9%) | 0.814 |
| Immunosuppressed | 2 (6.1%) | 6 (23.1%) | 0.065 |
| CKD | 2 (6.1%) | 3 (11.5%) | 0.386 |
| Illness severity scores | |||
| APACHE-II | 14 (11.5-23.5) | 18 (12.8-22.3) | 0.435 |
| Oxygenation indices at admission | |||
| FiO2 | 0.7 (0.6-0.83) | 0.65 (0.5-0.76) | 0.160 |
| PaO2 (kPa) | 9.31 (8.33-11.25) | 9.69 (8.87-9.96) | 0.541 |
| PF ratio (kPa) | 13.75 (10.49-17.36) | 15.52 (12.41-18.57) | 0.133 |
| Bloods at admission | |||
| Lactate (mmol/L) | 1.2 (0.95-1.55) | 1.40 (0.98-1.83) | 0.287 |
| WCC (109/L) | 8.2 (6.9-10.5) | 8.1 (5.3-11.3) | 0.778 |
| Neutrophils (109/L) | 7.1 (5.9-9.0) | 7.4 (4.5-10.1) | 0.963 |
| Lymphocytes (109/L) | 0.7 (0.4-0.9) | 0.6 (0.5-0.8) | 0.628 |
| CRP (mg/L) | 147 (78-250) | 129 (68-165) | 0.167 |
| Ferritin (μg/L) | 772 (443-1163) | 790 (582-1383) | 0.535 |
| Troponin (ng/L) | 15.5 (7.0-47.8) | 13.0 (9.0-44.0) | 0.766 |
| LDH (U/L) | 975 (810-1391) | 1081 (865-1411) | 0.718 |
| D-Dimer (μg/L) | 583 (269-1039) | 582 (328-1453) | 0.698 |
| Creatinine Kinase (U/L) | 172 (102-582) | 55 (31-286) | 0.008* |
| ICU Treatments (n (%)) | |||
| Dexamethasone | 26 (78.8%) | 19 (73.1%) | 0.609 |
| Methyl prednisolone | 20 (60.6%) | 13 (50.0%) | 0.415 |
| Any steroid | 29 (87.9%) | 24 (92.3%) | 0.457 |
| Prone position ventilation | 30 (90.9%) | 25 (96.2%) | 0.402 |
| Transfer for ECMO | 1 (3.0%) | - | 0.569 |
| Renal replacement therapy | 5 (15.2%) | 10 (38.5%) | 0.041* |
Response to IPVD Therapy in Survivors versus non-Survivors
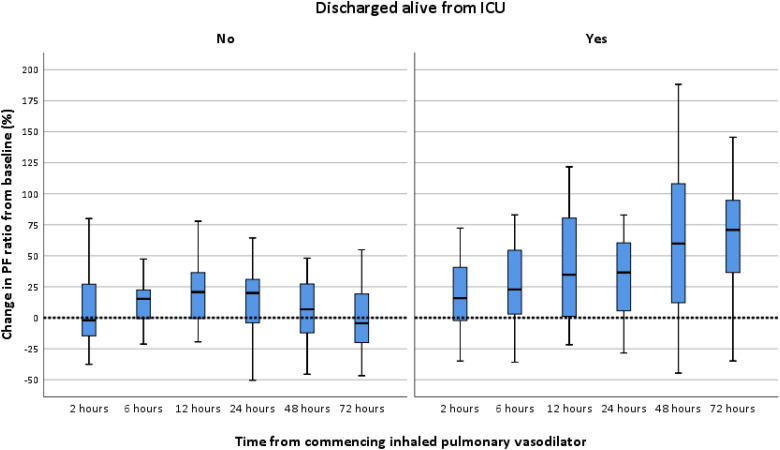
Both groups had severe acute hypoxic respiratory failure with a median PF ratio of 11.33 kPa before commencing IPVD therapy. There were no differences in PF ratio or A-a gradient at initiation of IPVD between both groups. The median time from ICU admission to receipt of IPVD therapy was 6 days and did not differ between survivors and non-survivors. Moreover, there were no differences between the groups in terms of IPVD received (Iloprost, iNO or both) (Table 4). The PF ratio increased significantly after 2 hours of IPVD initiation and the response was more evident after 24 hours. Sustained improvement in PF ratio from baseline was noted in survivors, with a median 70.8% percent increase at 72 hours. In contrast, the non-survivors had a median reduction in PF ratio of 4.1% from baseline at 72 hours (Table 4 and Figure 4). The reduction in A-a gradient was greater in survivors compared to non-survivors at 48 and 72 hours (Table 4). The improvements noted in the A-a gradient was less in non-survivors than survivors.
Table 4.
Response to IPVD Therapy in Survivors Versus non-Survivors.
| Survivors (n = 33) | Non-survivors (n = 26) | p-value | |
|---|---|---|---|
| Days from admission to IPVD | 6 (2-9) | 6 (3-9) | 0.742 |
| PF ratio at commencing therapy (kPa) | 11.39 (9.90-12.92) | 11.28 (9.74-12.71) | 1.000 |
| Therapy received (n (%)) | |||
| Iloprost | 8 (24.2%) | 3 (11.5%) | 0.405 |
| NO | 4 (12.1%) | 5 (19.2%) | |
| NO and Iloprost | 21 (63.6%) | 18 (69.2%) | |
| NIV or MV at initiation of IPVD (n (%)) | |||
| NIV | 5 (15.2%) | 3 (11.5%) | 1.000 |
| MV | 28 (84.8%) | 23 (88.5%) | |
| Percentage change PaO2/FiO2 (%) | |||
| + 2 hours | 14.2 (-5.4-40.6) | -2.3 (-17.3-20.5) | 0.022* |
| + 6 hours | 20.0 (3.0-54.4) | 9.6 (-2.9-25.2) | 0.106 |
| + 12 hours | 31.6 (-0.4-80.4) | 18.1 (-7.2-46.7) | 0.174 |
| + 24 hours | 34.0 (3.3-60.4) | 17.6 (-7.1-44.7) | 0.045* |
| + 48 hours | 59.5 (9.7-108.1) | 6.5 (12.4-27.4) | <0.001* |
| + 72 hours | 70.8 (35.2-95.5) | -4.1 (-19.9-21.3) | <0.001* |
| A-a gradient at commencing therapy (kPa) | 54.3 (48.6-67.0) | 58.9 (50.0-70.3) | 0.376 |
| Percentage reduction A-a gradient (kPa) (%) | |||
| + 2 hours | 4.9 (-7.8-15.5) | 1.8 (-7.8-7.0) | 0.418 |
| + 6 hours | 19.7 (1.8-35.2) | 7.3 (-2.9-23.6) | 0.109 |
| + 12 hours | 26.4 (3.3-40.9) | 21.1 (2.4-32.7) | 0.246 |
| + 24 hours | 23.8 (2.5-48.7) | 21.3 (4.4-29.3) | 0.200 |
| + 48 hours | 38.4 (16.1-62.6) | 11.7 (-5.3-25.8) | <0.001* |
| + 72 hours | 44.7 (28.4-58.0) | 14.8 (-7.6-31.1) | <0.001* |
Figure 4.
Changes in PF ratio during IPVD therapy in non-survivors versus survivors.
Survival in Patients Increasing PF Ratio by 10%, 20% and 50%
A 10% increment in PF ratio was seen in 27 (45.8%) and 38 (69.1%) patients at 2 hours and 72 hours after commencing IPVD therapy, respectively. Similar proportional changes were noted for a 20% response. However, 50% increment in PF ratio was proportionately lower at 10.2% and 43.6% at 2 hours and 72 hours, respectively. An increment in PF ratio of 10% (p = 0.006), 20% (p = 0.001) and 50% (p < 0.001) at 48 hours from commencing IPVD therapy was associated with increased ICU survival. (Table 5).
Table 5.
10%, 20% and 50% Increments in PF Ratio and Survival.
| Change in PF ratio | All patient (n = 59) | Survivors (n = 33) | Non-survivors (n = 26) | p-value |
|---|---|---|---|---|
| 10% increase | ||||
| + 2 hours | 27 (45.8%) | 19 (57.6%) | 8 (30.8%) | 0.040* |
| + 6 hours | 35 (53.9%) | 23 (69.7%) | 12 (46.2%) | 0.068 |
| + 12 hours | 38 (64.4%) | 22 (66.7%) | 16 (61.5%) | 0.683 |
| + 24 hours | 39 (66.1%) | 23 (69.7%) | 16 (61.5%) | 0.511 |
| + 48 hoursa | 34 (61.8%) a | 25 (75.6%) | 9 (39.1%) | 0.006* |
| + 72 hoursa | 38 (69.1%)a | 28 (84.8%) | 10 (45.5%) | <0.001* |
| 20% increase | ||||
| + 2 hours | 21 (35.6%) | 15 (45.5%) | 6 (23.1%) | 0.075 |
| + 6 hours | 26 (44.1%) | 17 (51.5%) | 9 (34.6%) | 0.194 |
| + 12 hours | 32 (54.2%) | 19 (57.6%) | 13 (50.0%) | 0.562 |
| + 24 hours | 36 (61.0%) | 23 (69.7%) | 13 (50.0%) | 0.068 |
| + 48 hoursa | 31 (56.4%) a | 23 (69.7%) | 8 (34.8%) | 0.010* |
| + 72 hoursa | 33 (60.0%)a | 27 (81.8%) | 6 (27.3%) | <0.001* |
| 50% increase | ||||
| + 2 hours | 6 (10.2%) | 5 (15.2%) | 1 (3.8%) | 0.215 |
| + 6 hours | 11 (18.6%) | 9 (27.3%) | 2 (7.7%) | 0.091 |
| + 12 hours | 19 (32.2%) | 13 (39.4%) | 6 (23.1%) | 0.183 |
| + 24 hours | 16 (27.1%) | 11 (33.3%) | 5 (19.2%) | 0.226 |
| + 48 hoursa | 18 (32.7%)a | 17 (51.5%) | 1 (4.3%) | <0.001* |
| + 72 hoursa | 24 (43.6%)a | 21 (63.6%) | 3 (13.6%) | <0.001* |
Data for 4 patients unavailable (3 deceased and 1 transferred to regional ECMO centre).
Discussion
In this retrospective observational study, we analysed the compassionate use of inhaled pulmonary vasodilators (IPVD); nebulised nitric oxide (iNO) in combination with Iloprost in patients with severe hypoxic respiratory failure due to SARS-CoV-2 infection. In our ICU cohort 19% of patients received IPVD for more than 24 hours and were included in the analysis. Patients who received an IPVD had a higher fractional inspired oxygen (0.7 vs. 0.6) and lower PF ratio (14.37 vs. 16.37kPa) at admission, compared to the patients not receiving an IPVD. The group receiving an IPVD were sicker (APACHE-II score at presentation 17 vs. 13) and were more likely to have received renal replacement therapy. At the point of initiation of IPVD therapy, the median PF ratio was 11.33kPa which demonstrates that this cohort had severe lung injury. To our knowledge, this is the first study to report the combined use of iNO and inhaled Iloprost in COVID-19 ICU patients with severe AHRF.
The median time from illness onset and ICU admission to IPVD therapy was 13 days and 6 days, respectively. There was a significant improvement in the PF ratio and A-a gradient following initiation of IPVD therapy. The PF ratio increased by 10% in 69% and by 50% in 44% of patients at 72 hours after the initiation of IPVD therapy. The PF response to IPVD therapy differed significantly between survivors and non-survivors. Although there was some improvement in the PF ratio and A-a gradient among the non-survivors, the effect size was much lower and not sustained. At 72 hours the non-survivors had a median decrease of 4.1% in PF ratio from baseline compared to the survivors, who had a median increase of 70.8% (p < 0.001). It is plausible that these differences in response between survivors and non-survivors were due to other factors that we were unable to quantify in this pragmatic observational study. The significant increase in PF ratio would allow a reduction in FiO2 to meet standard PaO2/SpO2 targets in patients with severe ARHF and thus might limit hyperoxic lung injury caused by exposure to high concentrations of oxygen and limit ventilator and clinician induced additional lung injury while recovery occurs.
The literature describing the use of IPVD therapy in COVID-19 related AHRF is sparse and limited to few observational studies. As far as we are aware, there are no studies reporting the combined use of iNO and inhaled prostaglandins to date. We adopted a pragmatic approach for the use of IPVDs in severe refractory hypoxaemia due to several reasons. First, there were a large number of patients with severe hypoxic respiratory failure with relatively few available iNO delivery systems. Second, nebulised Iloprost is relatively safe and easy to deliver during a demanding pandemic setting. Consequently, we used a trial of iNO as the initial intervention followed by Iloprost nebulisation with subsequent weaning of iNO.
Both iNO and nebulised Iloprost will cause selective vasodilatation of pulmonary arterioles supplying ventilated lung units thus improving the matching of ventilation and perfusion (V/Q) and arterial PaO2. Both agents have other potentially beneficial effects in the context of the inflammation seen in COVID lung disease. Epoprostenol (prostacyclin) and the synthetic prostacyclin analogue Iloprost are prostaglandins (PGI2) which reduce platelet aggregation and cause smooth muscle relaxation.7,8 They are normally indicated for use in scleroderma and idiopathic pulmonary arterial hypertension. The evidence supporting their use in AHRF and ARDS is limited. Iloprost has been shown in small studies to improve oxygenation in patients with refractory hypoxaemia9,10 without effecting haemodynamic stability11 and is more potent in the pulmonary circulation compared to iNO.12–14 In animal models it has been shown to reduce platelet aggregation and thrombus formation in the pulmonary circulation.15,16,8 Given that COVID-19 is characterised by coagulopathy, endothelial disruption, microangiopathy and microthrombi formation17 it is plausible that the improvements in oxygenation seen could have been due to the effects of Iloprost on endotheliopathy and microangiopathy rather than direct pulmonary vasodilation. In keeping with our findings, others have also reported improvements in oxygenation when prostaglandins were given either by inhalation or intravenously for COVID-19.18,19
Nitric oxide is a selective pulmonary vasodilator which activates guanylyl cyclase resulting in increased cyclic GMP and smooth muscle relaxation. This causes reduced pulmonary vascular resistance, reduced pulmonary arterial pressure, and improved V/Q matching. It is indicated in pulmonary hypertension in neonates and adults post cardiac surgery, but has a controversial role in ARDS with some studies showing improvement in oxygenation but not mortality.20 The proposed mechanisms for the potential benefits of iNO in COVID-19 are not only as a pulmonary vasodilator, but also as a direct anti-viral agent through cytotoxic intermediaries.21,22 In health NO is produced from arginine by endothelial, neuronal and inducible NO synthases; all three of which are expressed in the airways. The balance between NO and reactive oxygen species (ROS) are crucial for normal pulmonary vascular reactivity.22,23 Data is emerging to suggest that SARS-Cov-2 may, by inducing endothelial cell apoptosis, reducing local concentrations of Angiotensin II and affecting mitochondrial electron transport, reduce endothelial NO production and increase the production of ROS which in turn will reduce NO bioavailability.24 The use of iNO during SARS-Cov-2 infection may redress this imbalance. The evidence supporting the efficacy of iNO therapy in COVID-19 is limited and primarily focuses on improvements in oxygenation. Tavazzi et al reported no improvement (defined as a 20% increase in PF ratio) in 16 patients at 30 minutes after starting therapy, though there was a trend towards improvement in those with RV dysfunction.25 Abou-arab et al reported a response (20% increase in PF ratio) in 22 out of 34 patients at 30 minutes following initiation of iNO.26 While Longobardo et al found the response to iNO (10% increase in PF ratio) in 24 patients with COVID-19 to be worse than a historical cohort of patients with ARDS (40% vs. 77% response).27 Our studies differ from these in both the earlier introduction of iNO (6 days vs. 12 days in the Longobardo study) and the combined use of Iloprost. As anticipated, the mortality for this group is very high at 44% compared with the non IPVD group (18.1%). Our mortality for this severe hypoxic respiratory failure group is comparable to other published series.21
Despite the retrospective nature of this study its strengths are the accuracy of the data collected (extracted from a computerised clinical information system) and the protocolised implementation of IPVD therapy throughout the pandemic (Supplemental Material 1). Limitations of the study are its small sample size, resulting in a lack of power to detect potentially clinically significant differences in patient characteristics between survivors and non-survivors. Furthermore, due to its’ retrospective nature we cannot exclude residual confounding as an explanation for the association between improvements in PF ratio in survivors compared to non-survivors. Moreover, we did not evaluate additional ventilator variables as PEEP, tidal volumes, compliance and dead space. However, we had a predefined ventilation strategy for all patients guided by the ARDSnet protocol, with adjustments to the PEEP delivered in accordance with recruitability assessed by the pressure/volume curve (P-V Tool®), or APRV with inspiratory pressure limitation of ≤ 30 cmH2O. Nevertheless, it appears that even with a predefined cohort of COVID-19 related severe hypoxaemia, there are responders and non-responders to pulmonary vasodilators and sustained responders are more likely to survive their acute illness. Moreover, IPVD therapy may limit alveolar oxygen toxicity in severe hypoxaemia by enabling a reduction in inspired oxygen concentration. However, the exact reason why only some patients have a positive response and others don't is unclear. Possible explanations include maximal vasodilatation of the pulmonary vasculature in ventilated lung segments or a lack of pulmonary vascular reactivity to inhaled vasodilators. These processes require more detailed cardiovascular assessments and will need further exploration by large randomised controlled studies.
Conclusion
In conclusion, we have found the response to the compassionate use of inhaled pulmonary vasodilators for patients with acute hypoxic respiratory failure due to COVID-19 differs significantly between survivors and non-survivors. Our study differs from previous work in being the first to report the combined use of inhaled iNO alongside an inhaled prostaglandin. Both may offer a therapeutic option for severe hypoxaemia due to COVID-19, with inhaled prostaglandins particularly attractive as they do not require specialist delivery systems; therefore, not limited to cardiac surgical centres and easier to blind in randomised trials. The use of inhaled prostaglandins, and iNO where feasible, should be studied both in isolation and combination in adequately powered prospective randomised trials.
Supplemental Material
Supplemental material, sj-docx-1-jic-10.1177_08850666221086521 for Compassionate use of Pulmonary Vasodilators in Acute Severe Hypoxic Respiratory Failure due to COVID-19 by Lewis Matthews, Laurence Baker, Matteo Ferrari, Weronika Sanchez, John Pappachan, Mike PW Grocott and Ahilanandan Dushianthan in Journal of Intensive Care Medicine
Acknowledgments
We thank the REACT COVID group, the GICU consultants, and all the nursing staff and allied healthcare professionals caring for patients with COVID-19 for their support.
Footnotes
MPWG is in part funded by the NIHR Senior Investigator Scheme. MPWG is in part funded by the Southampton NIHR Biomedical Research Centre.
Ethics Approval and Consent to Participate: This study was part of a larger observational cohort study (REACT-COVID Observational Database) and has the approval of the local research and development and National Health Research Authority (IRAS: 285145) with a REC reference 20/HRA/2986. Due to the nature of the study consent was waived.
Consent for Publication: Not applicable.
Availability of Data and Materials: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors Contributions: Conception and design: LM, AD
Data acquisition: LM, LB, MF, WS, AD
Data analysis and interpretation: LM, AD, JP, MPWG
Drafting the manuscript: LM, LB, MF, WS
Critical revisions of the manuscript: AD, JP, MPWG
Agreement to accountability: All authors
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lewis Matthews https://orcid.org/0000-0001-5967-8615
Supplemental Material: Supplemental material for this article is available online.
References
- 1.WHO. Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed August 19, 2021.
- 2.Cao Y, Hiyoshi A, Montgomery S. COVID-19 case-fatality rate and demographic and socioeconomic influencers: worldwide spatial regression analysis based on country-level data. BMJ Open. 2020;10(11). doi: 10.1136/bmjopen-2020-043560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ICNARC (Intensive Care National Audit and Research Centre). ICNARC Covid-19 Report 2021-08-13. ICNARC Covid-19 Report 2021-08-13. https://www.icnarc.org/DataServices/Attachments/Download/6281b38a-45fc-eb11-9134-00505601089b. Published 2021. Accessed August 20, 2021.
- 4.Dushianthan A, Cumpstey AF, Ferrari M, et al. Intensive care physicians’ perceptions of the diagnosis & management of patients with acute hypoxic respiratory failure associated with COVID-19: a UK based survey. J Intensive Care Soc. March 2021. doi: 10.1177/17511437211002352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno Franco P, Enders F, Wilson G, Gajic O, Pannu SR. A comparative effectiveness study of rescue strategies in 1,000 subjects with severe hypoxemic respiratory failure. Respir Care. 2016;61(2):127-133. doi: 10.4187/respcare.04162 [DOI] [PubMed] [Google Scholar]
- 6.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4(10):1623-1627. doi: 10.1371/journal.pmed.0040296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsou P, Palisoc P, Flavahan N, Khanna D. Dissecting the cellular mechanism of prostacyclin analog iloprost in reversing vascular dysfunction in Scleroderma. Arthritis Rheumatol (Hoboken. NJ. 2021;73(3):520-529. doi: 10.1002/ART.41536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gąsecka A, Banaszkiewicz M, Nieuwland R, et al. Prostacyclin analogues inhibit platelet reactivity, extracellular vesicle release and thrombus formation in patients with pulmonary arterial hypertension. J Clin Med. 2021;10(5):1024. doi: 10.3390/JCM10051024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Afshari A, Bastholm Bille A, Allingstrup M. Aerosolized prostacyclins for acute respiratory distress syndrome (ARDS). Cochrane Database Syst Rev. 2017;2017(7). doi: 10.1002/14651858.CD007733.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Wet CJ, Affleck DG, Jacobsohn E, et al. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxaemia after cardiothoracic surgery. J Thorac Cardiovasc Surg. 2004;127(4):1058-1067. doi: 10.1016/j.jtcvs.2003.11.035 [DOI] [PubMed] [Google Scholar]
- 11.Sawheny E, Ellis AL, Kinasewitz GT. Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest. 2013;144(1):55-62. doi: 10.1378/chest.12-2296 [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Olschewski H, Ghofrani HA, et al. A comparison of the acute hemodynamic effects of inhaled nitric oxide and aerosolized iloprost in primary pulmonary hypertension. J Am Coll Cardiol. 2000;35(1):176-182. doi: 10.1016/S0735-1097(99)00494-5 [DOI] [PubMed] [Google Scholar]
- 13.Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W. Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996;153(3):991-996. doi: 10.1164/ajrccm.153.3.8630585 [DOI] [PubMed] [Google Scholar]
- 14.Walmrath D, Schneider T, Pilch J, Grimminger F, Seeger W. Aerosolised prostacyclin in adult respiratory distress syndrome. Lancet. 1993;342(8877):961-962. doi: 10.1016/0140-6736(93)92004-D [DOI] [PubMed] [Google Scholar]
- 15.Weiss HJ, Turitto VT. Prostaglandin I2 (prostacyclin) inhibits platelet adhesion and thrombus formation on subendothelium. Blood. 1979;53(2):244-350. http://ashpublications.org/blood/article-pdf/53/2/244/581766/244.pdf. Accessed July 20, 2021. [PubMed] [Google Scholar]
- 16.Higgs EA, Higgs GA, Moncada S, Vane JR. Prostacyclin(PGI2) inibits the formation of platelet thrombi in arterioles and venules of the hamster cheek pouch. Br J Pharmacol. 1978;63(3):535-539. doi: 10.1111/j.1476-5381.1978.tb07809.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383(2):120-128. doi: 10.1056/nejmoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moezinia CJ, Ji-Xu A, Azari A, Horlick S, Denton C, Stratton R. Iloprost for COVID-19-related vasculopathy. Lancet Rheumatol. 2020;2(10):e582-e583. doi: 10.1016/S2665-9913(20)30232-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsareva NA, Avdeev SN, Kosanovic D, Schermuly RT, Trushenko N V, Nekludova G V. Inhaled iloprost improves gas exchange in patients with COVID-19 and acute respiratory distress syndrome. Crit Care. 2021;25(1):1-3. doi: 10.1186/s13054-021-03690-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adhikari NKJ, Dellinger RP, Lundin S, et al. Inhaled nitric oxide does not reduce mortality in patients with acute respiratory distress syndrome regardless of severity: systematic review and meta-analysis. Crit Care Med. 2014;42(2):404-412. doi: 10.1097/CCM.0b013e3182a27909 [DOI] [PubMed] [Google Scholar]
- 21.Garfield B, McFadyen C, Briar C, et al. Potential for personalised application of inhaled nitric oxide in COVID-19 pneumonia. Br J Anaesth. 2021;126(2):e72-e75. doi: 10.1016/j.bja.2020.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epstein FH, Moncada S, Higgs A. The L-Arginine-Nitric Oxide Pathway. N Engl J Med. 1993;329(27):2002-2012. doi: 10.1056/nejm199312303292706 [DOI] [PubMed] [Google Scholar]
- 23.Ignarro L. Nitric Oxide: Biology and Pathobiology; 2000. https://books.google.com/books?hl=en&lr=&id=h5FugARr4bgC&oi=fnd&pg=PP1&ots=dZBRM0r9Jj&sig=QgQMSkvgu9H0T2Mb8r9AqssI1Rk. Accessed July 21, 2021.
- 24.Fang W, Jiang J, Su L, et al. The role of NO in COVID-19 and potential therapeutic strategies. Free Radic Biol Med. 2021;163:153-162. doi: 10.1016/j.freeradbiomed.2020.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tavazzi G, Marco P, Mongodi S, Dammassa V, Romito G, Mojoli F. Inhaled nitric oxide in patients admitted to intensive care unit with COVID-19 pneumonia. Crit Care. 2020;24(1):1-2. doi: 10.1186/s13054-020-03222-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abou-Arab O, Huette P, Debouvries F, Dupont H, Jounieaux V, Mahjoub Y. Inhaled nitric oxide for critically ill COVID-19 patients: a prospective study. Crit Care. 2020;24(1):1-3. doi: 10.1186/s13054-020-03371-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longobardo A, Montanari C, Shulman R, Benhalim S, Singer M, Arulkumaran N. Inhaled nitric oxide minimally improves oxygenation in COVID-19 related acute respiratory distress syndrome. Br J Anaesth. 2021;126(1):e44-e46. doi: 10.1016/j.bja.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jic-10.1177_08850666221086521 for Compassionate use of Pulmonary Vasodilators in Acute Severe Hypoxic Respiratory Failure due to COVID-19 by Lewis Matthews, Laurence Baker, Matteo Ferrari, Weronika Sanchez, John Pappachan, Mike PW Grocott and Ahilanandan Dushianthan in Journal of Intensive Care Medicine