Abstract
Osteochondritis dissecans (OCD) is a pathologic condition, most commonly affecting the knee joint in adolescents and young adults, although pathology can also be found at the elbow and ankle. Lesions to the medial femoral condyle are classically associated with varus alignment, while lesions to the lateral femoral condyle are seen in patients with valgus malalignment. Common risk factors for failed fixation of OCD lesions include unstable lesions to the lateral femoral condyle, screw breakage, older age, and closed physes. The purpose of this technical note is to describe the preoperative planning and step-by-step surgical approach for treatment of failed fixation of an OCD lesion of the posterior aspect of the lateral femoral condyle in young, active patients using an osteochondral allograft, a lateral opening wedge distal femoral osteotomy to correct malalignment, and a tibial tubercle osteotomy to facilitate access to the lesion.
Technique Video
Left knee lateral opening wedge distal femoral osteotomy, lateral femoral condyle osteochondral allograft transplantation, and tibial tubercle osteotomy and augmentation with bone marrow aspirate concentrate for failed osteochondral defect fixation in osteochondritis dissecans.
Osteochondritis dissecans (OCD) is a pathologic condition, most commonly affecting adolescents and young adults, in which a portion of the subchondral bone detaches from its surrounding superficial structures and results in subsequent cartilage injury.1,2
Surgical intervention is often reserved for juvenile lesions that fail conservative management, unstable lesions, detached lesions in which physeal closure will occur within 6 to 12 months, and osteochondral fragment nonunion.1,3, 4, 5 Surgical options for juvenile OCD lesions include transarticular drilling for stable lesions and fixation for unstable lesions.6 On the other hand, adult OCD lesions are often treated surgically with fixation of the fragment in combination with drilling the subchondral bone to stimulate healing potential, the application of cancellous bone grafting, or a variety of osteochondral transfer procedures depending on the degree of cartilage damage.1
Commonly, OCD lesions on the lateral femoral condyle (LFC) of the knee can be associated with valgus deformities.1,7 In these clinical scenarios, surgical correction of coronal malalignment and concomitant management of OCD lesions may be considered to reduce the risk of malunion and improve subchondral healing potential.1
As such, the purpose of this technical note is to describe a step-by-step surgical approach for the treatment of failed fixation of an OCD lesion of the posterior aspect of the lateral femoral condyle in young, active patients, using an osteochondral allograft (OCA), a lateral opening wedge distal femoral osteotomy (DFO) to correct malalignment, and a tibial tubercle osteotomy (TTO) to facilitate access to the lesion (Video 1).
Technique
Preoperative Evaluation and Surgical Decision-Making
Anteroposterior (AP), lateral, sunrise, and 45° flexed weightbearing radiographs are obtained to evaluate the joint spaces as well as the size and position of the osteochondral fragment on the lateral femoral condyle. A full-length standing radiograph is also obtained to assess coronal alignment at the knee. An AP radiograph of the knee reveals the location and size of the OCD lesion occupying the lateral femoral condyle; the lateral radiograph may be less revealing (Fig. 1a,b). Using full-length standing radiographs, the mechanical axis, the lateral distal femoral angle (LDFA), and the medial proximal tibial angle (MPTA) are measured to determine whether the deformity is based in the femur or in the tibia (Fig. 1c). An LDFA >90° indicates a varus deformity, and <85° indicates a valgus deformity originating from the femur. An MPTA <85° indicates a varus deformity based in the proximal tibia, and an angle >90° indicates a valgus deformity. Additionally, a computed tomography (CT) scan with 3D reconstruction allows for visualizing the extent of the osteochondral defect in all planes to estimate the size of the required osteochondral allograft (Fig. 1d,e). Magnetic resonance imaging (MRI) is often needed to fully assess the extent of any articular damage.
Figure 1.
Preoperative imaging and planning for left knee osteochondral allograft and distal femoral osteotomy. Standing anteroposterior (A) and lateral (B) radiographs assessing the location of the deformity to the posterolateral femoral condyle. (C) The mechanical lateral distal femoral angle (mLDFA) of 82.1° and mechanical medial proximal tibial angle (mMPTA) of 88.9° with a mechanical valgus of 3.6° is seen from the standing weightbearing radiographs. Sagittal (D) and axial (E) cuts of a computed tomography scan with 3D reconstruction allow for visualizing the extent of the osteochondral defect, which helps with planning for the required size of the osteochondral allograft.
Nonoperative measures including anti-inflammatory medications, activity modification, physical therapy, and use of a lateral unloader brace should be attempted initially to determine whether the patient has improvement in symptoms. Surgical intervention should be considered in patients with continued pain and functional decline refractory to nonoperative treatment. In the setting of previously attempted fixation with subsequent nonunion and progressive erosion and expansion of the chondral defect, the use of an osteochondral allograft to restore the integrity of the femoral condyle, and correction of the femoral-based valgus deformity of the femur is warranted. To facilitate access to the posterior location of the defect, a tibial tubercle osteotomy may be used.
Patient Positioning and Anesthesia
The patient is placed supine on an operating table. After the induction of general anesthesia and antibiotic prophylaxis, a tourniquet is placed high on the operative extremity, and the knee is prepped and draped in a sterile fashion. The ipsilateral anterior iliac crest is also draped to harvest bone marrow aspirate concentrate (BMAC) that will be used to augment osteochondral allograft fixation later in the procedure.
Surgical landmarks including the patella, patellar tendon, tibial tubercle, lateral tibial plateau, and lateral femoral condyle are outlined. High anterolateral and anteromedial parapatellar portals are marked for diagnostic arthroscopy, and the planned surgical incision is drawn as an 8-cm curvilinear incision centered over the lateral aspect of the femur and extending distally to the tibial tubercle (Fig. 2a).
Figure 2.
Surgical landmarks and tibial tubercle osteotomy of the left knee. (A) The planned osteotomy incision is drawn as an 8-cm curvilinear incision centered over the lateral aspect of the femur and extending distally to the tibial tubercle. (B) A layered dissection is carried down to the iliotibial band, and a Cobb elevator is used to bluntly dissect soft tissue from the surface. (C) Kirschner wires (0.045 inch) are drilled from medial to lateral through the tibial tubercle to mark out the planned tibial tubercle osteotomy site. (D) An oscillating saw is used to create the tibial osteotomy, and a straight osteotome is used to release the tibial bone block.
Surgical Approach
Diagnostic Arthroscopy
Diagnostic arthroscopy is performed to evaluate any intra-articular pathology before performing the arthrotomy. Further inspection of the lateral compartment is completed to assess the posterior lateral femoral condyle and associated defect. Before proceeding further, 60 cc of iliac crest aspirate is obtained from the anterior iliac crest and added to a centrifuge to obtain BMAC (Arthrex Angel cPRP & Bone Marrow Processing System; Arthrex, Naples, FL) to be used later in the procedure.
Tibial Tubercle Osteotomy
A tibial tubercle osteotomy is then performed using the previously marked laterally based incision. Layered dissection is carried down to the iliotibial band, and a Cobb elevator is used to bluntly dissect soft tissue from the surface (Fig. 2b). A posteriorly based incision is made in line with the fibers of the iliotibial band, and the underlying vastus lateralis is mobilized and retracted anteriorly to expose the lateral femoral condyle proximally and tibial periosteum distally. Kirschner wires (K-wires; 0.045 inch) are then drilled from medial to lateral and posterior to the tibial tubercle to mark out the planned osteotomy site (Fig. 2c). Future sites of tubercle fixation to the tibial metaphysis are drilled from anterior to posterior using a 4.5-mm drill on the anterior tibial cortex and a 3.2-mm drill bicortically to the posterior tibial cortex to allow for later compression of the osteotomy at the conclusion of the procedure. An oscillating saw is then used to start the tibial osteotomy, and a straight osteotome is used to release the tibial bone block (Fig. 2d). The bone block is retracted proximally, and electrocautery is used to release adherent soft tissue along the edges of the patellar tendon to allow for improved visualization of the entire lateral femoral condyle and access the posteriorly based defect.
Lateral Femoral Condyle Osteochondral Allograft
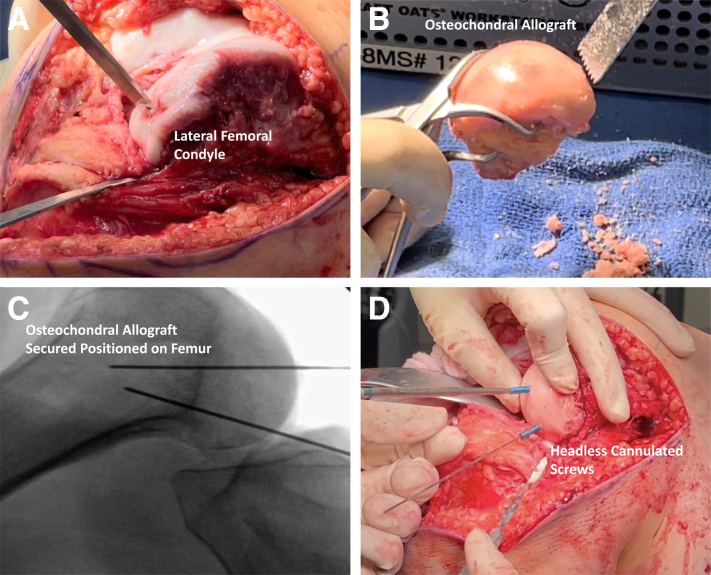
The operative knee is placed in deep flexion, and the posterior aspect of the lateral femoral condyle is visualized and inspected. A straight osteotome is used to remove areas of damaged and unstable cartilage (Fig. 3a). An oblique bony cut is made to reveal healthy bone underneath the damaged cartilaginous area. Care is taken to maintain the integrity of the popliteus that was detached during removal of this portion of the lateral femoral condyle.
Figure 3.
Preparation of the left knee osteochondral allograft of the lateral femoral condyle. (A) A straight osteotome is used to remove areas of damaged and unstable cartilage to the lateral femoral condyle. (B) A lateral femoral condyle osteochondral allograft (JRF Ortho) is measured and prepared. The size of graft is repeatedly compared to the patient’s native condyle and modified with an oscillating bone saw to ensure a congruent fit with the patient’s anatomy. (C) The osteochondral graft is provisionally fixed with Kirschner wires, and the position of the graft is confirmed with intraoperative radiographs. (D) The graft is fixed with two 3.5-mm cannulated headless screws (Fully Threaded Headless Compression Screws; Arthrex).
On the back table, a lateral femoral condyle osteochondral allograft (JRF Ortho, Englewood, CO) is measured and prepared. The size of the graft is repeatedly compared to the patient’s native condyle and modified with an oscillating bone saw to ensure a congruent fit with the patient’s anatomy (Fig. 3b). Once size and position of the graft are confirmed, the graft is soaked in the previously harvested BMAC and provisionally fixed with K-wires (Fig. 3c). Position of the graft is confirmed with intraoperative radiographs and fixed with two 3.5-mm cannulated headless screws (Fully Threaded Headless Compression Screws; Arthrex) (Fig. 3d).
To further restore the patient’s native anatomy, the previously detached popliteus tendon is repaired using a 1.9-mm all-suture anchor double-loaded with 1.3-mm suture tape (FiberTak Soft Anchor; Arthrex) at the native popliteus insertion on the femur. Sutures are passed in a mattress configuration through the remnant popliteus tendon and tied.
Distal Femoral Osteotomy
Attention is then directed to the distal femoral osteotomy to offload the lateral compartment and address the valgus deformity. Two guide pins are placed, aiming toward the adductor tubercle, and confirmed under fluoroscopy. An osteotomy guide is inserted over the guide pins to create a straight line for the osteotomy. An oscillating saw is applied to score the lateral cortex, followed by straight osteotomes to advance the osteotomy site anteriorly, posteriorly, and finally across the center of the femur (Fig. 4a). Intraoperative fluoroscopy is used to avoid iatrogenic damage to the medial cortex and maintain a 1-cm medial bony hinge.
Figure 4.
Distal femoral osteotomy of the left knee. (A) Two guide pins are placed, aiming toward the adductor tubercle and confirmed under fluoroscopy, followed by application of an oscillating saw to score the lateral cortex and straight osteotomes to advance the osteotomy site, anteriorly, posteriorly, and finally across the center of the femur. (B) A spreader device is inserted in the osteotomy site, under fluoroscopic guidance, to carefully distract the medial cortex. (C) A locking plate (ContourLock Femoral Osteotomy Plate; Arthrex) is applied along the lateral cortex with 4 cancellous screws distally and 3 cortical screws proximally (Arthrex).
On the back table, a rongeur is used to remove adherent soft tissue and any remaining cartilage from the remnant diseased native lateral femoral condyle, as any remaining bone will be used as structural bone graft at the femoral osteotomy site. After confirmation of the maintained hinge medially, a spreader device is inserted in the osteotomy site, under fluoroscopic guidance, to carefully distract the medial cortex. In general, an osteotomy opening of 1 mm per degree of valgus correction is desired (Fig. 4b). A bone tamp is used to further secure the graft, and a locking plate (ContourLock Femoral Osteotomy Plate; Arthrex) is applied along the lateral cortex with 4 cancellous screws distally and 3 cortical screws proximally (Arthrex) (Fig. 4 c).
Finally, the tibial tubercle osteotomy is fixed with two 4.5-mm fully threaded cannulated screws (Large Fragment LCP Instrument and Implant Set; Depuy Synthes, Raynham, MA) through previously drilled holes. Final screw placement is confirmed with fluoroscopy. The wound is thoroughly irrigated and closed in a layered fashion.
Rehabilitation
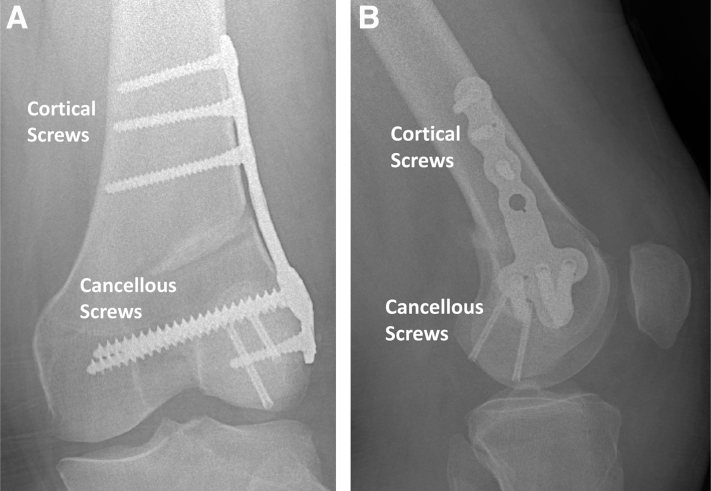
The patient remains non-weightbearing for 12 weeks with the knee in extension in a hinged knee brace. Deep venous thrombosis prophylaxis is given for 4 weeks, and physical therapy begins in the initial perioperative period with a focus initially on quadriceps sets and straight leg raises as well as progressive increases in range of motion. Radiographs should be obtained 2 weeks postoperatively to assess hardware placement (Fig. 5a,b). Repeat CT (Fig. 6) and MRI (Fig. 7) scans are obtained 8 weeks postoperatively to assess graft fixation and hardware placement. 12 weeks postoperatively, the patient will begin weightbearing exercises, with a slow incorporation of closed-chain activities after the compression screws are removed in an arthroscopic procedure.
Figure 5.
Radiographs of the left knee 2 weeks postoperatively. Anteroposterior (A) and lateral (B) radiographs of the lateral opening wedge distal femoral osteotomy with appropriate placement of plating and screws.
Figure 6.
Computed tomography scan of the left knee preoperatively and 8 weeks postoperatively. Coronal preoperative (A), coronal postoperative (B), sagittal preoperative (C), and sagittal postoperative (D) views of the left knee lateral femoral condyle osteochondral allograft transplantation 8 weeks postoperatively showing well-fixed hardware and healing of the large shell graft.
Figure 7.
Magnetic resonance imaging scan of the left knee preoperatively and 8 weeks postoperatively. Coronal preoperative (A), coronal postoperative (B), sagittal preoperative (C), and sagittal postoperative (D) views of the left knee lateral femoral condyle osteochondral allograft transplantation 8 weeks postoperatively showing well-fixed hardware, healing of the large shell graft, and appropriate articular surfaces.
Discussion
Cartilage defects of the knee can be debilitating injuries for athletes, resulting in prolonged pain, diminished function, and difficulty or inability to return to high level of play.8 Small lesions (<2.5 cm2) treated with marrow stimulation procedures (e.g., microfracture) have shown some improvements in pain and function, but these results ultimately deteriorate with time, possibly because of the formation of fibrocartilage rather than hyaline cartilage during the healing process.9, 10, 11, 12, 13, 14 Autologous chondrocyte implantation (ACI) has also demonstrated significant improvements in pain and function, in both adult and adolescent patients.15, 16, 17, 18, 19 A systematic review assessing techniques for managing chondral defects in athletes also reported improved outcomes after ACI and osteochondral autologous transplantation (OAT) compared with microfracture.20 Moreover, overall return to preinjury level of play was seen in 66% of patients, with the slowest rates seen after ACI and fastest rates after OAT. However, OAT procedures are limited by donor site morbidity and challenges when matching available grafts to pre-existing defect size, contour, and cartilage thickness.
Graft healing following OCA is paramount to clinical success and is dependent on the neovascularization of surrounding tissues and cellular repopulation.21 Failure of cartilage healing is typically due to either a loss of the integrity or viability of the cartilage or a lack of graft bone integration into the host.22 Animal studies have noted that OCAs saturated with BMAC ultimately had increase osteoinductive proteins and osteoprogenitor cells within 2 weeks.23 Further, Oladeji et al.24 found improved osseous integration and decreased sclerosis on radiographic imaging obtained 6 months postoperatively for femoral condyle OCAs augmented with BMAC compared with nontreated allografts. Although the literature on the efficacy of BMAC’s ability to improve allograft integration is in relative infancy, there is promise in the potential benefits that may exist to aid healing in the setting of revision cartilage procedures.
Overall, OCA of the knee in young and athletic populations has good outcomes and high rates of return to sport.21,25, 26, 27, 28 Emmerson et al.25 reported on a case series of 66 knees in 64 patients with OCD lesions of the LFC or MFC who underwent fresh OCA with minimum 2-year follow up. They found that mean clinical scores and subjective function significantly improved postoperatively, with 72% of patients reporting “excellent” or “good” clinical scores. McCarthy et al.26 later performed a retrospective review of high-level athletes at the high school, collegiate, and professional levels (mean age 19.2 years) with large (>2.5 cm2) chondral lesions managed with isolated femoral condyle OCA. Of 13 athletes, 77% returned to play at a mean of 7.9 months, with 38% returning to preinjury level of sports. Of note, the majority that did not return to preinjury levels cited graduation from school and the pursuance of other interests. Krych et al.27 reported similarly high return-to-play rates in a group of 43 athletes, with 88% returning in a limited capacity and 79% at preinjury levels at a mean of 9.6 months. They did note that worse return rates were seen in patients ≥25 years of age or with preoperative symptoms >12 months. When evaluating adolescents specifically, Murphy et al.21 found that 89% of patients were extremely satisfied or satisfied and had 90% graft survivorship at 10 years.
Distal femoral osteotomies (DFOs) may be indicated for young, active patients with unicompartmental tibiofemoral arthritis or an isolated symptomatic chondral defect. Moreover, DFOs are commonly performed in conjunction with cartilage procedures to further offload the affected compartment and prevent further arthritic progression. Biomechanical studies reveal lateral opening wedge DFO significantly decreases lateral compartment contact pressures through all degrees of flexion.29,30 In the setting of lateral compartment overload and valgus malalignment, either a lateral opening wedge or medial closing wedge osteotomy may be performed with similar outcomes.31,32 Cameron et al.33 performed a retrospective study of 31 knees that underwent lateral opening wedge DFO for arthritis or a joint preservation procedure (e.g., OCA, meniscal transplantation). While both groups were found to have improved pain and clinical outcome scores, the joint preservation group had notably higher survivorship (92% vs. 74%). A recent systematic review including 7 studies and 149 patients found an overall return-to-sport rate of 70% to 100% from 8.3 to 16.9 months postoperatively; only 41.6% returned to the same or higher level of play.34
In this technique, a combination of a TTO, OCA with BMAC supplementation, and lateral opening wedge DFO were performed for a large LFC OCD lesion that failed previous treatment. Use of a large lateral parapatellar arthrotomy, in combination with TTO, is key to obtaining adequate exposure of the posterior condyle for proper allograft fit. OCA is an appropriate solution to address the resulting large defect and restore the articular surface of the joint. Given the extent of the resection, it may be necessary to partially detach the popliteus. When this occurs, it is important to repair the popliteus before performing the DFO to restore the native knee anatomy. Moreover, the addition of the DFO allows for correction of the valgus deformity and restoration of the mechanical axis; this in conjunction with OCA allows for improved loadbearing through the tibiofemoral joint and thus decreases the risk of progression to osteoarthritis, which is of utmost important in the adolescent patient. It is also important to pay attention to tourniquet time, as leaving the cuff inflated throughout the case can lead to ischemia. It is recommended to deflate for the last third of the procedure duration.
A limitation to this technique is that although large allografts require substantial preoperative planning for adequate allograft acquisition, it may not be feasible to obtain such allografts in areas with more limited resources, thus limiting options for restoring large femoral condyle defects. Moreover, patients must be non-weightbearing for 10 to 12 weeks postoperatively, which is highly limiting, especially for competitive athletes (Tables 1 and 2).
Table 1.
Pearls and Pitfalls
| Pearls |
|
|
|
|
|
| Pitfalls |
|
|
|
Table 2.
Advantages and Limitations
| Advantages |
|
|
|
| Limitations |
|
|
|
|
|
Surgeons faced with young, athletic patients undergoing revision procedures for large OCD lesions with concomitant malalignment can consider this combined, multiprocedural approach to decrease the risk of persistent pain, functional impairment, and early osteoarthritis.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: P.E.G. reports paid consultant/speaker/committee member, CONMED Linvatec. J.C. reports paid consultant, Arthrex, CONMED Linvatec, Ossur, Smith & Nephew; board/committee member, American Orthopaedic Society for Sports Medicine, Arthroscopy Association of North America, International Society of Arthroscopy, Knee Surgery, Orthopaedic Sports Medicine. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Left knee lateral opening wedge distal femoral osteotomy, lateral femoral condyle osteochondral allograft transplantation, and tibial tubercle osteotomy and augmentation with bone marrow aspirate concentrate for failed osteochondral defect fixation in osteochondritis dissecans.
References
- 1.Bruns J., Werner M., Habermann C. Osteochondritis dissecans: Etiology, pathology, and imaging with a special focus on the knee joint. Cartilage. 2018;9:346–362. doi: 10.1177/1947603517715736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heiden J.J., Amirtharaj M.J., Tao M.A. Open treatment for unstable osteochondritis dissecans of the knee: Autologous bone grafting and bioabsorbable fixation. Arthrosc Tech. 2020;9:e1779–e1784. doi: 10.1016/j.eats.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler J.I., Nikizad H., Shea K.G., Jacobs J.C., Jr., Bebchuk J.D., Weiss J.M. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42:320–326. doi: 10.1177/0363546513510390. [DOI] [PubMed] [Google Scholar]
- 4.Schenck R.C., Jr., Goodnight J.M. Osteochondritis dissecans. J Bone Joint Surg Am. 1996;78:439–456. [PubMed] [Google Scholar]
- 5.Edmonds E.W., Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471:1118–1126. doi: 10.1007/s11999-012-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abouassaly M., Peterson D., Salci L., et al. Surgical management of osteochondritis dissecans of the knee in the paediatric population: A systematic review addressing surgical techniques. Knee Surg Sports Traumatol Arthrosc. 2014;22:1216–1224. doi: 10.1007/s00167-013-2531-y. [DOI] [PubMed] [Google Scholar]
- 7.Accadbled F., Vial J., Sales de Gauzy J. Osteochondritis dissecans of the knee. Orthop Traumatol Surg Res. 2018;104(1 suppl):S97–S105. doi: 10.1016/j.otsr.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Flanigan D.C., Harris J.D., Trinh T.Q., Siston R.A., Brophy R.H. Prevalence of chondral defects in athletes’ knees: A systematic review. Med Sci Sports Exerc. 2010;42:1795–1801. doi: 10.1249/MSS.0b013e3181d9eea0. [DOI] [PubMed] [Google Scholar]
- 9.Gudas R., Simonaityte R., Cekanauskas E., Tamosiūnas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29:741–748. doi: 10.1097/BPO.0b013e3181b8f6c7. [DOI] [PubMed] [Google Scholar]
- 10.Kreuz P.C., Steinwachs M.R., Erggelet C., et al. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. doi: 10.1016/j.joca.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Gobbi A., Karnatzikos G., Kumar A. Long-term results after microfracture treatment for full-thickness knee chondral lesions in athletes. Knee Surg Sports Traumatol Arthrosc. 2014;22:1986–1996. doi: 10.1007/s00167-013-2676-8. [DOI] [PubMed] [Google Scholar]
- 12.Steadman J.R., Briggs K.K., Rodrigo J.J., Kocher M.S., Gill T.J., Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy. 2003;19:477–484. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 13.Mithoefer K., Gill T.J., Cole B.J., Williams R.J., Mandelbaum B.R. Clinical outcome and return to competition after microfracture in the athlete’s knee: An evidence-based systematic review. Cartilage. 2010;1:113–120. doi: 10.1177/1947603510366576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gudas R., Gudaite A., Pocius A., et al. Ten-year follow-up of a prospective, randomized clinical study of mosaic osteochondral autologous transplantation versus microfracture for the treatment of osteochondral defects in the knee joint of athletes. Am J Sports Med. 2012;40:2499–2508. doi: 10.1177/0363546512458763. [DOI] [PubMed] [Google Scholar]
- 15.Mithöfer K., Peterson L., Mandelbaum B.R., Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: Functional outcome and return to competition. Am J Sports Med. 2005;33:1639–1646. doi: 10.1177/0363546505275647. [DOI] [PubMed] [Google Scholar]
- 16.Mithöfer K., Minas T., Peterson L., Yeon H., Micheli L.J. Functional outcome of knee articular cartilage repair in adolescent athletes. Am J Sports Med. 2005;33:1147–1153. doi: 10.1177/0363546504274146. [DOI] [PubMed] [Google Scholar]
- 17.Macmull S., Parratt M.T., Bentley G., et al. Autologous chondrocyte implantation in the adolescent knee. Am J Sports Med. 2011;39:1723–1730. doi: 10.1177/0363546511404202. [DOI] [PubMed] [Google Scholar]
- 18.Saris D.B., Vanlauwe J., Victor J., et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 19.Ogura T., Bryant T., Minas T. Long-term outcomes of autologous chondrocyte implantation in adolescent patients. Am J Sports Med. 2017;45:1066–1074. doi: 10.1177/0363546516682492. [DOI] [PubMed] [Google Scholar]
- 20.Harris J.D., Brophy R.H., Siston R.A., Flanigan D.C. Treatment of chondral defects in the athlete’s knee. Arthroscopy. 2010;26:841–852. doi: 10.1016/j.arthro.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Murphy R.T., Pennock A.T., Bugbee W.D. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42:635–640. doi: 10.1177/0363546513516747. [DOI] [PubMed] [Google Scholar]
- 22.Bugbee W.D., Pallante-Kichura A.L., Görtz S., Amiel D., Sah R. Osteochondral allograft transplantation in cartilage repair: Graft storage paradigm, translational models, and clinical applications. J Orthop Res. 2016;34:31–38. doi: 10.1002/jor.22998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoker A.M., Baumann C.A., Stannard J.P., Cook J.L. Bone marrow aspirate concentrate versus platelet rich plasma to enhance osseous integration potential for osteochondral allografts. J Knee Surg. 2018;31:314–320. doi: 10.1055/s-0037-1603800. [DOI] [PubMed] [Google Scholar]
- 24.Oladeji L.O., Stannard J.P., Cook C.R., et al. Effects of autogenous bone marrow aspirate concentrate on radiographic integration of femoral condylar osteochondral allografts. Am J Sports Med. 2017;45:2797–2803. doi: 10.1177/0363546517715725. [DOI] [PubMed] [Google Scholar]
- 25.Emmerson B.C., Görtz S., Jamali A.A., Chung C., Amiel D., Bugbee W.D. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy M.A., Meyer M.A., Weber A.E., et al. Can competitive athletes return to high-level play after osteochondral allograft transplantation of the knee? Arthroscopy. 2017;33:1712–1717. doi: 10.1016/j.arthro.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Krych A.J., Robertson C.M., Williams R.J., 3rd Return to athletic activity after osteochondral allograft transplantation in the knee. Am J Sports Med. 2012;40:1053–1059. doi: 10.1177/0363546511435780. [DOI] [PubMed] [Google Scholar]
- 28.Briggs D.T., Sadr K.N., Pulido P.A., Bugbee W.D. The use of osteochondral allograft transplantation for primary treatment of cartilage lesions in the knee. Cartilage. 2015;6:203–207. doi: 10.1177/1947603515595072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wylie J.D., Scheiderer B., Obopilwe E., et al. The effect of lateral opening wedge distal femoral varus osteotomy on tibiofemoral contact mechanics through knee flexion. Am J Sports Med. 2018;46:3237–3244. doi: 10.1177/0363546518799353. [DOI] [PubMed] [Google Scholar]
- 30.Quirno M., Campbell K.A., Singh B., et al. Distal femoral varus osteotomy for unloading valgus knee malalignment: A biomechanical analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25:863–868. doi: 10.1007/s00167-015-3602-z. [DOI] [PubMed] [Google Scholar]
- 31.Wylie J.D., Jones D.L., Hartley M.K., et al. Distal femoral osteotomy for the valgus knee: Medial closing wedge versus lateral opening wedge: A systematic review. Arthroscopy. 2016;32:2141–2147. doi: 10.1016/j.arthro.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Chahla J., Mitchell J.J., Liechti D.J., et al. Opening- and closing-wedge distal femoral osteotomy: A systematic review of outcomes for isolated lateral compartment osteoarthritis. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967116649901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron J.I., McCauley J.C., Kermanshahi A.Y., Bugbee W.D. Lateral opening-wedge distal femoral osteotomy: Pain relief, functional improvement, and survivorship at 5 Years. Clin Orthop Relat Res. 2015;473:2009–2015. doi: 10.1007/s11999-014-4106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassi J.S., Chan J.P., Johnston T., Wang D. Return to work and sport after distal femoral osteotomy: A systematic review. Sports Health Sep. 2021;6 doi: 10.1177/19417381211041072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left knee lateral opening wedge distal femoral osteotomy, lateral femoral condyle osteochondral allograft transplantation, and tibial tubercle osteotomy and augmentation with bone marrow aspirate concentrate for failed osteochondral defect fixation in osteochondritis dissecans.
Left knee lateral opening wedge distal femoral osteotomy, lateral femoral condyle osteochondral allograft transplantation, and tibial tubercle osteotomy and augmentation with bone marrow aspirate concentrate for failed osteochondral defect fixation in osteochondritis dissecans.