Abstract
Increased disinfection efforts in various parts of China, including Hong Kong, to prevent the spread of the novel coronavirus may lead to elevated concentrations of disinfectants in domestic sewage and surface runoff in Hong Kong, generating large quantities of toxic disinfection byproducts. Our study investigated the presence and distribution of four trihalomethanes (THMs), six haloacetic acids (HAAs), and eight nitrosamines (NAMs) in rivers and seawater in Hong Kong. The concentrations of THMs (mean concentration: 1.6 µg/L [seawater], 3.0 µg/L [river water]), HAAs (mean concentration: 1.4 µg/L [seawater], 1.9 µg/L [river water]), and NAMs (mean concentration: 4.4 ng/L [seawater], 5.6 ng/L [river water]) did not significantly differ between river water and seawater. The total disinfection byproduct content in river water in Hong Kong was similar to that in Wuhan and Beijing (People's Republic of China), and the total THM concentration in seawater was significantly higher than that before the COVID‐19 pandemic. Among the regulated disinfection byproducts, none of the surface water samples exceeded the maximum index values for THM4 (80 μg/L), HAA5 (60 μg/L), and nitrosodimethylamine (100 ng/L) in drinking water. Among the disinfection byproducts detected, bromoform in rivers and seawater poses the highest risk to aquatic organisms, which warrants attention and mitigation efforts. Environ Toxicol Chem 2022;41:2613–2621. © 2022 SETAC
Keywords: Disinfection byproducts, Surface water, Ecological risk
INTRODUCTION
The increased use of disinfectants in homes and high‐risk public places (e.g., schools, health and other care facilities, food services and workplaces) to prevent the spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of the novel coronavirus disease 2019 (COVID‐19), has been observed in China and around the world (Zambrano‐Monserrate et al., 2020). After the COVID‐19 outbreak, the global market for disinfectants is expected to increase from US $660 million in 2019 to US $780 million in 2020 (Subpiramaniyam, 2021). In 2020, China released at least 2000 t of disinfectants in Wuhan alone (Zhang et al., 2020).
Since the COVID‐19 outbreak, most studies have focused on the efficiency of disinfectants in killing viruses. In contrast, few studies have assessed the occurrence, distribution, and potential risk of disinfection byproduct contamination in water caused by increased disinfectant use (Li et al., 2021). The disinfection byproducts can enter sewage treatment plants (STPs) from municipal sewage and enter surface water via surface runoff, potentially posing a threat to aquatic organisms and the ecological environment. Li et al. (2020) investigated the disinfection strategies of more than 50 STPs in East China during the COVID‐19 pandemic and found that some were combining their typical ultraviolet disinfection method with sodium hypochlorite dosing for added protection; some STPs increased the amount of disinfectant used in the treatment process, and the average amount of available chlorine in STP effluent increased from 1.5 to 5 mg/L.
Haloacetic acids (HAAs) and trihalomethanes (THMs) are the most common disinfection byproducts in the world, and n‐nitrosamides (NAMs) are rapidly emerging disinfection byproducts. Compared with HAAs and THMs, NAMs are more toxic to humans (Xu et al., 2022). Weng et al. (2022) demonstrated the cytotoxicity of mono‐HAAs on HEK 293T cells. Cui et al. (2021) showed that THMs and HAAs inhibited the growth of Scenedesmus spp., affected the swimming ability of macroalgae, and led to abnormal development and mortality in zebrafish embryos. Moreover, disinfection byproducts disrupted the relationship between algae and Pseudomonas aeruginosa, which may lead to harmful cyanobacterial blooms in aquatic ecosystems (Cui et al., 2022). Ecological risk assessments have shown that disinfection byproducts can be harmful to aquatic environments (Boudjellaba et al., 2016; Wang et al., 2021b); however, the occurrence and risk of disinfection byproducts in surface water have not been assessed in the context of increased disinfectant use during the COVID‐19 pandemic.
During this pandemic, Hong Kong adopted a number of disinfection measures to reduce the threat of the virus to human life and safety. For example, at the Hong Kong Airport temporary testing center, the clean area was disinfected every 2 h, the patient area was disinfected every hour, and the patients were disinfected with sodium hypochlorite (1000 mg/L) immediately after discharge (Wong et al., 2020). Disinfectants are placed in shopping malls, restaurants, and other public places in Hong Kong for residents' use. These disinfection procedures may result in disinfection byproducts entering natural waters through urban sewage or surface runoff. However, disinfection byproduct pollution may pose safety risks in the water environment in Hong Kong. Therefore, determining the disinfection byproduct pollutants present in Hong Kong's surface water, analyzing disinfection byproduct distribution characteristics before and after the pandemic, and evaluating the potential ecological risks of disinfection byproducts in aquatic environments are essential for protecting human health and aquatic organisms and ecosystems.
MATERIALS AND METHODS
Materials
Four target THMs, namely, chloroform (67‐66‐3), chlorodibromomethane (DBCM; 124‐48‐1), bromodichloromethane (DCBM; 75‐27‐4), and bromoform (75‐25‐2); six target haloacetic acids, namely, chloroacetic acid (MCAA; 79‐11‐8), bromoacetic acid (MBAA; 79‐08‐3), dichloroacetic acid (DCAA; 79‐43‐6), monobromomonochloroacetic acid (BCAA; 5589‐96‐8), dibromoacetic acid (DBAA; 631‐64‐1), and trichloroacetic acid (TCAA, 76‐03‐9); and eight target nitrosamines, namely, N‐nitrosodimethylamine (NDMA; 62‐75‐9), N‐nitrosodiphenylamine (NDPHA; 86‐30‐6), di‐n‐propylnitrosamine (NDPA; 621‐64‐7), N‐nitrosopyrrolidine (NPyr; 930‐55‐2), diethylnitrosamine (NDEA; 55‐18‐5), nitrosodibutylamine (NDBA; 924‐16‐3), nitroso‐methyl ethylamine (NMEA; 10595‐95‐6), and nitroso‐piperidine (NPip; 100‐75‐4), as well as the internal standards NDMA‐d6 and 1,2‐dibromopropane (1,2‐DBPP; 78‐75‐1) were purchased from Chem Service.
Ultrapure water was obtained using a Milli‐Q water purification system (Waters). The chemicals Na2SO4, Na2HPO4, KH2PO4, NH4Cl, NaHCO3, acetone (guaranteed reagent grade), methyl tert‐butyl ether (high‐performance liquid chromatography [HPLC] grade), and dichloromethane (HPLC grade) were purchased from Duksan Pure Chemicals. The chemicals CuSO4·5H2O, Na2S2O3, EDTA‐Na2, formic acid, and methanol (HPLC grade) were purchased from Honeywell. CNW Technology provided the CNWBOND coconut charcoal solid‐phase extraction cartridges (6 ml, 2 g).
Sampling and pretreatment
In September 2021, during the COVID‐19 outbreak, 23 seawater samples and 20 river water samples were collected from across Hong Kong and stored in amber glass bottles. The sampling locations are shown in Figure 1. Detailed information about the sampling sites is shown in the Supporting Information, Table S6. The samples were packed in an icebox (4 °C) for transfer to the laboratory, where they were stored in a refrigerator at 4 °C.
Figure 1.

River and seawater disinfection byproduct sampling sites in Hong Kong.
THMs
Following US Environmental Protection Agency (USEPA) method 551.1 (USEPA, 1990a), the water samples were pretreated via liquid–liquid extraction. One gram of a premixed buffer salt/dechlorination agent mixture (2 g Na2HPO4, 198 g KH2PO4, and 1.2 g NH4Cl) was added to each 60‐ml amber glass sampling bottle before filling the bottles until they just overflowed.
For the liquid–liquid extraction, 50 μl of a 10‐mg/L 1,2‐DBPP solution was added to the sample bottles as an internal standard. Next, 3 ml of methyl tert‐butyl ether and 20 g of Na2SO4 were added to the sample bottles, and the bottles were shaken by hand for 4 min. The bottles were then turned upside down for approximately 2 min to separate the water and methyl tert‐butyl ether. Finally, 1.5 ml of the solvent phase was pipetted from the sample bottles to the injection vials to be tested.
HAAs
Following USEPA method 552.2 (USEPA, 1990b), the water samples were pretreated via liquid–liquid extraction. Before sampling, 6 mg of ammonium chloride was added to the 60‐ml amber glass sample bottles to remove free chlorine from the water samples. The sample bottles were then sealed and well shaken.
For the liquid–liquid extraction, 40 ml of each water sample was added to a separate 60‐ml glass bottle with 20 μl of 10‐mg/L 1,2‐DBPP methanol solution and mixed upside down. At least 2 ml of concentrated sulfuric acid was added to adjust the pH to less than 0.5. Next, 16 g of sodium sulfate was quickly added and shaken for 3–5 min until almost completely dissolved, and then 4.0 ml of methyl tert‐butyl ether was added and shaken for 30 min. The mixture was then allowed to separate for 5 min before the upper methyl tert‐butyl ether portion was transferred to a 15‐ml tapered centrifuge tube, and 1 ml of 10% methanol sulfuric acid was added for methylation. The centrifuge tube was placed in a hot bath at 50 °C for 2 h, and then the lid was removed to allow cooling. Saturated sodium bicarbonate solution (4 ml) was added to the centrifuge tube, and 1.0 ml of the methyl tert‐butyl ether layer was transferred to the injection vial, which was then sealed and stored at −20 °C until subsequent analyses.
NAMs
Following USEPA method 521 (USEPA, 2004), a GF/C filter (Whatman; 1.2 μm) was used to filter 1 L of each water sample. The residual chlorine was removed using Na2S2O3, and the pH of the sample was adjusted to 8.0 with NaHCO3. Twenty nanograms of NDMA‐d6 was added to each sample as the internal standard.
For the solid‐phase extraction, the CNWBOND coconut charcoal solid‐phase extraction column (6 ml, 2 g) was activated with 12 ml of dichloromethane, 12 ml of methanol, and 15 ml of water. After the water sample was loaded, it was drained for 10 min and washed four times with 12 ml of dichloromethane at a flow rate of 1 ml/min. The extract was concentrated to nearly 1 ml and stored at −20 °C for chemical quantitative analysis.
Quantitative analysis
The disinfection byproducts in the samples were analyzed using an Agilent 7890 (B) gas chromatograph, equipped with a 7010 triple quadrupole mass spectrometry detector, 7693 automatic liquid injector, and DB‐1701ms chromatographic column (30 m × 0.25 mm, film thickness 1.0 μm). We used an electron ionization ion source with an electron energy of 70 eV. Details are provided in the Supporting Information, Tables S1–S3. The analysis parameters for each disinfection byproduct type were as follows:
THMs
Injection: 170 °C, split, 1 µL; flow rate, 1.5 ml/min; oven temperature, 32 °C (hold 2 min), ramp 4 °C/min to 37 °C (hold 0 min), ramp 30 °C/min to 127 °C (hold 2 min); source temperature, 200 °C; quadrupole temperature, 150 °C; and transfer line temperature, 200 °C.
HAAs
Injection: 200 °C, splitless, 3 µl; flow rate, 1 ml/min; oven temperature, 35 °C (hold 1 min), ramp 5 °C/min to 75 °C (hold 15 min), ramp 25 °C/min to 100 °C (hold 2 min), ramp 35 °C/min to 135 °C (hold 2 min); source temperature, 230 °C; quadrupole temperature, 150 °C; and transfer line temperature, 280 °C.
NAMs
Injection: 260 °C, splitless, 1 µl; flow rate, 1.2 ml/min; oven temperature, 33 °C (hold 1 min), ramp 35 °C/min to 80 °C (hold 2 min), ramp 10 °C/min to 140 °C, ramp 50 °C/min to 280 °C (hold 2 min); source temperature, 280 °C; quadrupole temperature, 150 °C; and transfer line temperature, 280 °C.
Quality control
Three parallel samples were set at each sampling point. Both NDMA‐d6 and 1,2‐DBPP were used as the internal standards. The mixed NAMs solution of 0.1, 0.5, 1, 2, 5, 10, 25, and 50 μg/L was used to create a linear calibration curve (R 2 > 0.99). For THMs, 25 μl of the mixed THM standard solution (100, 200, 400 µg/L, 1.6, 4, and 10 mg/L) was added to 50 ml of water with 50 μl of the internal standard. The pretreatment steps described in the Sampling and pretreatment (THMs) section were then used to pretreat the standard curve samples together with the collected samples (R 2 > 0.99). The final concentrations of the HAAs standard curve were 0.1, 0.5, 1, 5, 10, 50, and 100 μg/L. The corresponding standard of 25 μl was added to the water, and the sample was pretreated as described in the Sampling and pretreatment (HAAs) section (R 2 > 0.99). Quantitative analysis software (Agilent) was used for the quantification. The analytical samples were labeled with different concentrations of the target chemicals in distilled water as positive controls, and distilled water was used as the blank control. The final concentration was corrected by the blank control. The recovery and detection limits of marine and surface water samples were estimated. The detection limit of NAMs was 0.04–0.46 μg/L, and the recovery rate was 71%–137%. The detection limit of THMs was 0.001–0.055 μg/L, and the recovery rate was 88%–95%. The detection limit of HAAs was 0.02–0.17 μg/L, and the recovery rate was 74%–95%. The details of the limits of detections, blank levels, and method detection limits can be found in the Supporting Information, Table S7.
Ecological risk assessment
The risk quotient (RQ) method was used to assess the ecological risk of disinfection byproducts in Hong Kong aquatic environments, according to the following formula:
where MEC is the measured concentration in the environment, and PNEC is the predicted‐no‐effect concentration of the target compound. The PNECs were mainly obtained from the USEPA ECOTOX database (USEPA, 2022) and are listed in the Supporting Information, Tables S4 and S5. When the RQ is less than 0.01, the ecological risk has no significant effect; 0.01 less than RQ less than 0.10 is low risk; 0.10 less than RQ less than 1.0 is medium risk; and RQ greater than 1.0 is high risk.
Data analysis
The SPSS 21 (IBM) and Origin Pro 2021 (OriginLab) programs were used for data analyses. Origin Pro 2021 was used for difference analyses; p < 0.05 was considered statistically significant. The sampling map was drawn with ArcMap 10.1 (ESRI).
RESULTS AND DISCUSSION
Pollution characteristics of disinfection byproducts
Many countries and regions have imposed restrictions on disinfection byproducts in drinking water, but there are few restrictions for surface water. China's national standards stipulate that chloroform and DCBM in drinking water shall not exceed 0.06 mg/L, and bromoform and DBCM shall not exceed 0.1 mg/L (Ministry of Health of China, 2006). The USEPA limits for total THMs and HAAs in drinking water are 0.08 and 0.06 mg/L, respectively (USEPA, 2018). Among the identified disinfection byproducts, THMs and HAAs are the most common in chlorinated drinking water (Craun & Pegram, 2004). As emerging disinfection byproducts, NAMs are more carcinogenic than THMs and HAAs (Chen et al., 2016). Among the NAMs, NDMA has a high detection frequency and strong teratogenicity and carcinogenicity. Levels of NDMA are regulated by the World Health Organization (WHO) and many countries/regions, with limits of 100 and 10 ng/L established by the WHO and the California Health and Human Services Agency, respectively (Bei et al., 2016; WHO, 2017).
As shown in Table 1 and Figure 2, HAAs and THMs were still the most common disinfection byproducts in Hong Kong. The concentrations of THM4 (total concentration of four targeted THMs) were 955–4680 ng/L in seawater and 672–8090 ng/L in river water; the concentrations of HAA6 (total concentration of six HAAs) were 198–15 100 ng/L in seawater and 88–6570 ng/L in river water; and the concentrations of total NAMs were 1.27–11.1 ng/L in seawater and 0.57–14.0 ng/L in river water. The three types of disinfection byproducts in natural water in Hong Kong did not exceed the international and Chinese standards. Among them, the detection rates of all four THMs were 100% in river water and seawater. Among the HAAs, the river water detection rates were 100% for DCAA, 90% for TCAA and MCAA, 80% for BCAA, and 55% for DBAA; MBAA was not detected. Among the NAMs, the river water detection rates were 100% for NDEA and NDBA, 70% for NDMA, 25% for NMEA, 10% for NPip and NDPA, and 5% for NDPHA; NPyr was not detected. Among the HAAs, the seawater detection rates were generally lower than the river water detection rates, being 100% for MCAA, 39% for BCAA, 17% for DCAA, 13% for TCAA, and 4.4% for DBAA; MBAA was still not detected. Among the NAMs, the seawater detection rates were 100% for NDEA and NDBA, 96% for NDMA, 26% for NDPHA, 13% for NMEA, and 4.4% for NPir and NDPA; NPyr was still not detected. The detection rate of NDMA was higher in seawater than in river water.
Table 1.
Three types of disinfection byproducts in surface water
| N‐nitrosamines (ng/L) | Trihalomethanes (µg/L) | Haloacetic acids (µg/L) | ||||
|---|---|---|---|---|---|---|
| Compound | River water | Seawater | River water | Seawater | River water | Seawater |
| Average | 5.55 ± 4.07 | 4.35 ± 2.95 | 2.97 ± 1.96 | 1.63 ± 0.86 | 1.87 ± 1.96 | 1.41 ± 3.01 |
| Min | 0.57 | 1.27 | 0.67 | 0.96 | 0.08 | 0.20 |
| Max | 14.0 | 11.1 | 8.09 | 4.68 | 6.57 | 15.1 |
| Median | 3.83 | 3.28 | 2.67 | 1.38 | 1.04 | 0.88 |
| MDF (%) | 25.0 | 26.1 | 100 | 100 | 90.0 | 15.2 |
| Detection rate range | 5.0%–100% | ND–100% | 100% | 100% | ND–100% | ND–100% |
MDF = median detection frequency; ND = not detected.
Figure 2.

Percentage contributions to total concentrations of disinfection byproducts in Hong Kong. (A) River water sampling sites. (B) Seawater sampling sites. CF = chloroform; DBCM = chlorodibromomethane; DCBM = bromodichloromethane; BF = bromoform; MCAA = chloroacetic acid; MBAA = bromocetic acid; DCAA = dichloroacetic acid; BCAA = monobromomonchloracetic acid; DBAA = dibromoacetic acid; TCAA = trichloroacetic acid; NDphA = N‐nitrosodiphenylamine; NDMA = N‐nitrosodimethylamine; NDPA = di‐n‐propylnitrosamine; NPyr = N‐nitrosopyrrlidine; NDEA = diethylnitrrosamine; NDBA = nitrosodibutylamine; NMEA = mitroso‐methyl ethylamine; NPip = nitroso‐piperidine; DPHA = dipentaerythrytol hexaacrylate.
As shown in Figure 3, the disinfection byproduct concentrations at S2, S15, and S16 seawater sampling sites were significantly higher than those at other sites. A school, a large community, ecological farms, and religious sites are near point S2. Sites S15 and S16 are the North Point Ferry Pier and Star Ferry Pier, respectively, both of which host high numbers of passengers and are near large commercial shopping malls, hotels, and other establishments. The use of disinfectants in public places likely increased during the COVID‐19 pandemic, resulting in higher disinfection byproduct concentrations in the water nearby public areas.
Figure 3.

Disinfection byproduct concentrations in Hong Kong. (A) Seawater sampling sites. (B) River water sampling sites. (C) Nitrosamines (NAMs) in seawater sampling sites. (D) Nitrosaines (NAMs) in river water sampling sites. HAA = haloacetic acid; THM = trihalomethane. For other abbreviations, see Figure 2 legend.
The highest disinfection byproduct concentrations (HAA6 and THM4) in river water sampling sites were at R5, R7, R14, and R17. Kindergartens, middle schools, and some buildings and gymnasiums are near R5; large shopping malls, playgrounds, parking lots, and ambulance depots are near R7; and police stations, fire stations, many auto parts sales points, and Fan Kam Road are near R14. Hospitals and several schools are near R17 and R14. The nearby sites R15 and R16 had high disinfection byproduct concentrations. These results indicate that the amount of disinfectant used in Hong Kong Kam Tin may be higher than that used in other regions. The highest concentrations of NAMs in river sampling sites were at R7, R12, R16, and R18. There are some residential areas near R7, which is in the same river as R12. Site R12 is near one wetland and one slaughterhouse but is also close to R7 and may be affected by the same upstream pollution source. Site R16 is near a hospital and two nursing homes. Site R18 is near an STP; the high content of nitrosamines may originate from disinfectants used in the treatment process, such as chloramines or ozone (Chen & Valentine, 2008; Maqbool et al., 2021).
Comparison of the occurrence of disinfection byproducts before and after COVID‐19
Luo et al. (2021) summarized the occurrence of THMs and HAAs in surface water in China before the COVID‐19 pandemic, reporting average concentrations ranging from 0.14 to 2.8 μg/L and 0.01 to 3.37 μg/L, respectively. In May and June 2020, Li et al. (2021) detected disinfection byproducts—mainly THMs and HAAs—in domestic sewage, surface water, and drinking water in Beijing and Wuhan. The average concentrations of THM4, HAA5, and NAMs were 1.5, 2.4 μg/L and 9.1 ng/L, respectively, in Beijing surface water and 1.2, 7.6 μg/L, and 8.4 ng/L, respectively, in Wuhan surface water. Wang et al. (2021a) detected average THM and NAM concentrations of 2.2 μg/L and 60.8 ng/L, respectively, in Wuhan surface water in May 2020, which suggests that the significant increase in NAMs may be related to the increased use of disinfectants during the pandemic. In the present study, the average concentrations of THM4, HAA5, and NAMs in river water in Hong Kong were 3.0 and 1.9 μg/L, and 5.6 ng/L, respectively. Except for THMs, the average concentrations of disinfection byproducts in Hong Kong surface water were slightly lower than those found in Beijing and Wuhan surface water after the pandemic. The average concentration of THMs in Hong Kong was slightly higher than that in other regions in China before the pandemic, and the average concentration of HAAs was within the range of average concentrations in various regions in China before the pandemic. In addition, THM concentrations were higher than HAA concentrations before and after the pandemic.
Before the pandemic, Feng et al. (2019) detected four THMs in Hong Kong seawater near Hong Kong Island. Compared with the THM concentrations detected in the same sampling area in the present study (sampling site: S7–16 in Figure 1) during the COVID‐19 pandemic, THM concentrations were lower before the COVID‐19 outbreak in Hong Kong. As shown in Figure 3, the concentrations of chloroform, DCBM, and DBCM were significantly higher after the COVID‐19 pandemic (p < 0.05). Although the p value for bromoform was more than 0.05 (p = 0.57), the overall bromoform concentration was higher than that before the pandemic. As the earliest identified disinfection byproducts, THMs are mainly produced by reactions between chlorinated disinfectants with compounds in water, indicating that the use of conventional disinfectants increased during the pandemic period, resulting in an increase in conventional disinfection byproduct concentrations in water. Disinfectants used in public places and living areas may enter surface water through pathways such as domestic wastewater and surface runoff. A previous study suggested that disinfection in urban centers is the main source of disinfection byproducts in surface water (Scott et al., 2000; Figure 4).
Figure 4.

Trihalomethane (THM) concentrations in seawater near Hong Kong Island before and after the COVID‐19 pandemic: (A) CF; (B) DBCM; (C) DCBM; (D) BF. Column A: data from Feng et al. (2019); column B: data from present study.
Ecological risk assessment
Luo et al. (2021) calculated the ecological risk of THMs and HAAs before the pandemic in China and found that, except for Zhongshan in Guangdong province, the RQ values based on acute toxicity in surface water were less than 0.1 in all other cities, indicating that the ecological risk was low, but clarified that the chronic toxicity of disinfection byproducts to aquatic organisms in surface water could not be ignored. As shown in Figure 5, the RQ values of most disinfection byproducts in the present study were less than 0.01 in river water and seawater, indicating no significant ecological risk. The RQs of MCAA and bromoform in seawater and DCAA, DBCM, and bromoform in river water indicate low ecological risk; bromoform had the highest RQ in both river water and seawater. These findings suggest that the main risk was still the disinfection byproducts produced by common disinfectants. Although the risk was generally low, these pollutants should be carefully monitored to avoid increasing risks.
Figure 5.
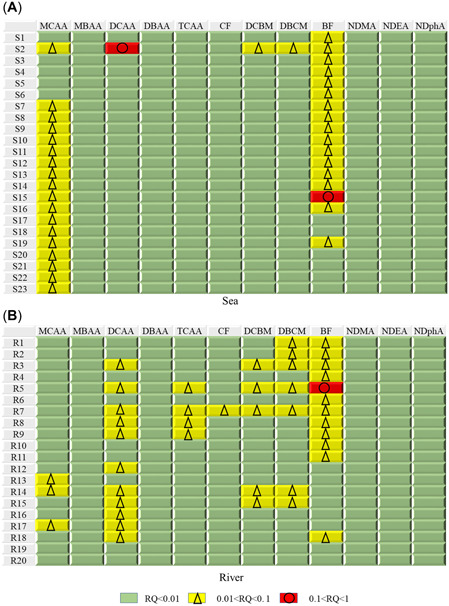
Risk quotients (RQs) of disinfection byproducts in (A) seawater and (B) river water. For abbreviations, see Figure 2 legend
Green algae are often used to assess the ecological risk of pollutants (Andrade et al., 2018; Hela et al., 2005). Li et al. (2019) found that HAAs in STP effluent had a high ecological risk to green algae but a lower risk to fish, whereas NAMs were low risk (RQ less than 0.1). During the COVID‐19 pandemic, Li et al. (2021) conducted an ecological risk assessment of disinfection byproducts in the effluent of domestic STPs and found that most HAAs posed a high risk to green algae, especially MCAA, DCAA, and TCAA (RQ greater than 10). Most THMs and NAMs were low or no risk, similar to the results of the present study. The disinfection byproducts produced by influent disinfection in STPs are discharged into the surface water (Bandala et al., 2021), which may be an important source of disinfection byproducts in surface water. However, STP effluent is generally sufficiently diluted in the receiving water bodies, reducing the disinfection byproduct concentrations in river and seawater (Zhang et al., 2021) and consequently lowering the calculated RQ. The toxicity of chlorinated disinfection byproducts is less than that of brominated disinfection byproducts (Carter & Joll, 2017), and the main risk found in our study was bromoform, a brominated disinfection byproduct. Among the four regulated THMs, bromoform has the strongest chronic toxic effects on the growth, reproduction, and molecular biology of aquatic organisms, and the toxic effects of bromoform on the growth and reproduction of aquatic organisms may be higher than those of the other three disinfection byproducts (Luo et al., 2021), suggesting that bromoform is worthy of further study. In addition, toxicity studies have shown that plants may be more vulnerable to disinfection byproduct genotoxicity than animals (Cortés & Marcos, 2018). To summarize, disinfection byproducts in surface water may pose a threat to ecological health.
CONCLUSIONS
The disinfection byproduct concentrations in rivers and seawater in Hong Kong did not exceed the Chinese and international guidelines for disinfection byproducts in drinking water. However, THM concentrations in seawater samples collected during the COVID‐19 pandemic were significantly higher than those before the pandemic. The disinfection byproduct concentrations in river water in Hong Kong were similar to those in Beijing and Wuhan during the pandemic. Among the surface water sampling sites in Hong Kong, those near public places had higher disinfection byproduct concentrations, suggesting that the disinfection byproduct contamination originated from disinfection in public places, residential buildings, STPs, and other facilities. Overall, the ecological risk of disinfection byproducts in surface water in Hong Kong is low, but bromoform poses a low‐to‐medium ecological risk in rivers and seawater.
Supporting Information
The Supporting Information is available on the Wiley Online Library at https://10.1002/etc.5449.
Author Contributions Statement
Jing Liu: Conceptualization; writing—original draft. Wen‐Jing Deng: Funding acquisition; Supervision; Writing—review & editing. Li‐Xin Hu: Writing—review & editing. Guang‐Guo Ying: Writing—review & editing. Huachang Hong: Writing—review & editing. Eric P. K. Tsang: Writing—review & editing. Damià Barceló: Writing—review & editing.
Supporting information
This article includes online‐only Supporting Information.
Supporting information.
Acknowledgments
The present study was financially supported by the General Research Fund of Hong Kong (grant. 18300919), FLASS Dean's Research Fund (grant 04615), and Internal Research Fund (grant R4175) of The Education University of Hong Kong.
Contributor Information
Wen‐Jing Deng, Email: wdeng@eduhk.hk.
Guang‐Guo Ying, Email: guangguo.ying@m.scnu.edu.cn.
Data Availability Statement
Data, associated metadata, and calculation tools are available in the Supporting Information, and from the corresponding author (wdeng@eduhk.hk; guangguo.ying@m.scnu.edu.cn).
REFERENCES
- Andrade, G. A. , Inês, D. , José, A. , & Fábio, K. (2018). Ecotoxicological effects, water quality standards and risk assessment for the anti‐diabetic metformin. Environmental Pollution, 243, 534–542. 10.1016/j.envpol.2018.09.031 [DOI] [PubMed] [Google Scholar]
- Bandala, E. R. , Kruger, B. R. , Cesarino, I. , Leao, A. L. , Wijesiri, B. , & Goonetilleke, A. (2021). Impacts of COVID‐19 pandemic on the wastewater pathway into surface water: A review. Science of the Total Environment, 774, 145586. 10.1016/j.scitotenv.2021.145586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei, E. , Shu, Y. Y. , Li, S. X. , Liao, X. B. , Wang, J. , Zhang, X. J. , Chen, C. , & Krasner, S. (2016). Occurrence of nitrosamines and their precursors in drinking water systems around mainland China. Water Research, 98, 168–175. 10.1016/j.watres.2016.04.013 [DOI] [PubMed] [Google Scholar]
- Boudjellaba, D. , Dron, J. , Revenko, G. , Démelas, C. , & Boudenne, J. L. (2016). Chlorination by‐product concentration levels in seawater and fish of an industrialised bay (Gulf of Fos, France) exposed to multiple chlorinated effluents. Science of the Total Environment, 541, 391–399. 10.1016/j.scitotenv.2015.09.046 [DOI] [PubMed] [Google Scholar]
- Carter, R. A. A. , & Joll, C. A. (2017). Occurrence and formation of disinfection by‐products in the swimming pool environment: A critical review. Journal of Environmental Sciences, 58, 19–50. 10.1016/j.jes.2017.06.013 [DOI] [PubMed] [Google Scholar]
- Chen, W. H. , Wang, C. Y. , & Huang, T. H. (2016). Formation and fates of nitrosamines and their formation potentials from a surface water source to drinking water treatment plants in Southern Taiwan. Chemosphere, 161, 546–554. 10.1016/j.chemosphere.2016.07.027 [DOI] [PubMed] [Google Scholar]
- Chen, Z. , & Valentine, R. (2008). The influence of the pre‐oxidation of natural organic matter on the formation of N‐nitrosodimethylamine (NDMA). Environmental Science & Technology, 42, 5062–5067. 10.1021/es8006673 [DOI] [PubMed] [Google Scholar]
- Cortés, C. , & Marcos, R. (2018). Genotoxicity of disinfection byproducts and disinfected waters: A review of recent literature. Mutation Research/Genetic Toxicology and Environmental Mutagenesis, 831, 1–12. 10.1016/j.mrgentox.2018.04.005 [DOI] [PubMed] [Google Scholar]
- Craun, G. , & Pegram, R. (2004). Environmental Health Criteria 216: Disinfectants and disinfectant by‐products. World Health Organization. [Google Scholar]
- Cui, H. J. , Chen, B. Y. , Jiang, Y. L. , Tao, Y. , Zhu, X. S. , & Cai, Z. H. (2021). Toxicity of 17 disinfection by‐products to different trophic levels of aquatic organisms: Ecological risks and mechanisms. Environmental Science & Technology, 55(15), 10534–10541. 10.1021/acs.est.0c08796 [DOI] [PubMed] [Google Scholar]
- Cui, H. J. , Zhu, X. S. , Zhu, Y. J. , Huang, Y. X. , & Chen, B. Y. (2022). Ecotoxicological effects of DBPs on freshwater phytoplankton communities in co‐culture systems. Journal of Hazardous Materials, 421, 126679. 10.1016/j.jhazmat.2021.126679 [DOI] [PubMed] [Google Scholar]
- Feng, H. R. , Ruan, Y. F. , Wu, R. B. , Zhang, H. Y. , & Lam, P. K. S. (2019). Occurrence of disinfection by‐products in sewage treatment plants and the marine environment in Hong Kong. Ecotoxicology and Environmental Safety, 181, 404–411. 10.1016/j.ecoenv.2019.06.034 [DOI] [PubMed] [Google Scholar]
- Ministry of Health of China . (2006). National Standard for the People's Republic of China GB‐5749‐2006. Standards for drinking water quality.
- Hela, D. G. , Lambropoulou, D. A. , Konstantinou, I. K. , & Albanis, T. A. (2005). Environmental monitoring and ecological risk assessment for pesticide contamination and effects in Lake Pamvotis, Northwestern Greece. Environmental Toxicology and Chemistry, 24(6), 1548–1556. 10.1897/04-455R.1 [DOI] [PubMed] [Google Scholar]
- Li, J. , Wang, Y. , Xiong, H. S. , Tan, Z. J. , Lv, Y. , Zheng, K. K. , Zou, L. X. , Luo, G. B. , Ye, L. , Zhang, Z. H. , & Wang, M. (2020). Investigation and optimization strategies on the operation of disinfection facilities in municipal WWTPs. China Water & Wastewater, 36(8), 7–19. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=GSPS202008007%26DbName=DKFX2020 [Google Scholar]
- Li, Z. G. , Song, G. F. , Bi, Y. H. , Gao, W. , He, A. , Lu, Y. , Wang, Y. W. , & Jiang, G. B. (2021). Occurrence and distribution of disinfection byproducts in domestic wastewater effluent, tap water, and surface water during the SARS‐CoV‐2 pandemic in China. Environmental Science & Technology, 55(7), 4103–4114. 10.1021/acs.est.0c06856 [DOI] [PubMed] [Google Scholar]
- Li, Z. G. , Liu, X. Y. , Huang, Z. J. , Hu, S. Y. , Wang, J. J. , Qian, Z. Y. , Feng, J. F. , Xian, Q. M. , & Gong, T. T. (2019). Occurrence and ecological risk assessment of disinfection byproducts from chlorination of wastewater effluents in East China. Water Research, 157, 247–257. 10.1016/j.watres.2019.03.072 [DOI] [PubMed] [Google Scholar]
- Luo, Y. , Liu, N. , Sun, S. W. , Hou, S. , Guo, C. S. , & Xu, J. (2021). Occurrence and ecological risk of typical DBPs in Chinese surface water. China Environmental Science 41(4), 1806–1814. 10.3969/j.issn.1000-6923.2021.04.035 [DOI] [Google Scholar]
- Maqbool, T. , Zhang, J. X. , Li, Q. Y. , Qin, Y. L. , Chen, L. , & Zhang, Z. H. (2021). Occurrence and fate of N‐nitrosamines in three full‐scale drinking water treatment systems with different treatment trains. Science of the Total Environment, 783, 146982. 10.1016/j.scitotenv.2021.146982 [DOI] [PubMed] [Google Scholar]
- Scott, B. F. , Mactavish, D. , Spencer, C. , Strachan, W. J. , & Muir, D. G. (2000). Haloacetic acids in Canadian lake waters and precipitation. Environmental Science & Technology, 34, 20. 10.1021/es9908523 [DOI] [Google Scholar]
- Subpiramaniyam, S. (2021). Outdoor disinfectant sprays for the prevention of COVID‐19: Are they safe for the environment? Science of the Total Environment, 759, 144289. 10.1016/j.scitotenv.2020.144289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency . (1990a). Method 551.1: Determination of chlorination disinfection byproducts, chlorinated solvents, and halogenated pesticides/herbicides in drinking water by liquid‐liquid extraction and gas chromatography with electron‐capture detection.
- U.S. Environmental Protection Agency . (1990b). Method 552.2: Determination of haloacetic acids and dalapon in drinking water by liquid‐liquid extraction, derivatization and gas chromatography with electron capture detection.
- U. S. Environmental Protection Agency . (2004). Method 521: Determination of nitrosamines in drinking water by solid phase extraction and capillary column gas chromatography with large volume injection and chemical ionization tandem mass spectrometry (MS/MS).
- U.S. Environmental Protection Agency . (2018). Drinking water standards and health advisories.
- U.S. Environmental Protection Agency . (2022). ECOTOX knowledgebase. http://cfpub.epa.gov/ecotox
- Wang, L. Y. , Zhang, X. , Chen, S. S. , Meng, F. B. , Zhang, D. Y. , Liu, Y. , Li, M. , Liu, X. , Huang, X. , & Qu, J. H. (2021a). Spatial variation of dissolved organic nitrogen in Wuhan surface waters: Correlation with the occurrence of disinfection byproducts during the COVID‐19 pandemic. Water Research, 198, 117138. 10.1016/j.watres.2021.117138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. Q. , Liu, H. H. , Yang, X. H. , & Wang, L. J. (2021b). Aquatic toxicity and aquatic ecological risk assessment of wastewater‐derived halogenated phenolic disinfection byproducts. Science of the Total Environment, 809, 151089. 10.1016/j.scitotenv.2021.151089 [DOI] [PubMed] [Google Scholar]
- Weng, H. , Wang, C. T. , Ye, T. , Xu, Z. Q. , Sun, H. j , Lin, H. J. , Deng, W. J. , Wu, F. Y. , & Hong, H. C. (2022). Precursor characteristics of mono‐HAAs during chlorination and cytotoxicity of mono‐HAAs on HEK‐293T cells. Chemosphere, 301, 134689. 10.1016/j.chemosphere.2022.134689 [DOI] [PubMed] [Google Scholar]
- World Health Organization . (2017). Guidelines for drinking‐water quality: Fourth edition incorporating the first addendum (Licence: CC BY‐NC‐SA 3.0). [PubMed]
- Wong, S. C. , Leung, M. , Lee, L. L. Y. , Chung, K. L. , & Cheng, V. C. C. (2020). Infection control challenge in setting up a temporary test centre at Hong Kong International Airport for rapid diagnosis of COVID‐19 due to SARS‐CoV‐2. Journal of Hospital Infection, 105(3), 571–573. 10.1016/j.jhin.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Z. Q. , Shen, J. , Qu, Y. Q. , Chen, H. F. , Zhou, X. L. , Hong, H. C. , Sun, H. J. , Lin, H. J. , Deng, W. J. , & Xu, F. Y. (2022). Using simple and easy water quality parameters to predict trihalomethane occurrence in tap water. Chemosphere, 286, 131586. 10.1016/j.chemosphere.2021.131586 [DOI] [PubMed] [Google Scholar]
- Zambrano‐Monserrate, M. A. , Ruano, M. A. , & Sanchez‐Alcalde, L. (2020). Indirect effects of COVID‐19 on the environment. Science of the Total Environment, 728, 138813. 10.1016/j.scitotenv.2020.138813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Tang, W. Z. , Chen, Y. S. , & Yin, W. (2020). Disinfection threatens aquatic ecosystems. Science, 368(6487), 146–147. 10.1126/science.abb8905 [DOI] [PubMed] [Google Scholar]
- Zhang, Z. , Zhou, Y. , Han, L. F. , Guo, X. Y. , Wu, Z. H. , Fang, J. Y. , Hou, B. L. , Cai, Y. P. , Jiang, J. , & Yang, Z. F. (2021). Impacts of COVID‐19 pandemic on the aquatic environment associated with disinfection byproducts and pharmaceuticals. Science of the Total Environment, 811, 151409. 10.1016/j.scitotenv.2021.151409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article includes online‐only Supporting Information.
Supporting information.
Data Availability Statement
Data, associated metadata, and calculation tools are available in the Supporting Information, and from the corresponding author (wdeng@eduhk.hk; guangguo.ying@m.scnu.edu.cn).


