Abstract
Objective
Coronavirus disease 2019 (COVID‐19) has rapidly spread worldwide, but there is so far no comprehensive analysis of all known symptoms of the disease. Our study aimed to present a comprehensive picture of the clinical symptoms of COVID‐19 using an evidence map.
Methods
We systematically searched MEDLINE via PubMed, Web of Science, Embase, and Cochrane library from their inception to March 16, 2021. We included systematic reviews reporting the clinical manifestations of COVID‐19 patients. We followed the PRISMA guidelines, and the study selection, data extraction, and quality assessment were done by two individuals independently. We assessed the methodological quality of the studies using AMSTAR. We visually presented the clinical symptoms of COVID‐19 and their prevalence.
Results
A total of 102 systematic reviews were included, of which, 68 studies (66.7%) were of high quality, 19 studies (18.6%) of medium quality, and 15 studies (14.7%) of low quality. We identified a total of 74 symptoms including 17 symptoms of the respiratory system, 21 symptoms of the neurological system, 10 symptoms of the gastrointestinal system, 16 cutaneous symptoms, and 10 ocular symptoms. The most common symptoms were fever (67 studies, ranging 16.3%–91.0%, pooled prevalence: 64.6%, 95%CI, 61.3%–67.9%), cough (68 studies, ranging 30.0%–72.2%, pooled prevalence: 53.6%, 95%CI, 52.1%–55.1%), muscle soreness (56 studies, ranging 3.0%–44.0%, pooled prevalence: 18.7%, 95%CI, 16.3%–21.3%), and fatigue (52 studies, ranging 3.3%‐58.5%, pooled prevalence: 29.4%, 95%CI, 27.5%–31.3%). The prevalence estimates for COVID‐19 symptoms were generally lower in neonates, children and adolescents, and pregnant women than in the general populations.
Conclusion
At least 74 different clinical manifestations are associated with COVID‐19. Fever, cough, muscle soreness, and fatigue are the most common, but attention should also be paid to the rare symptoms that can help in the early diagnosis of the disease.
Keywords: clinical manifestations, COVID‐19, evidence map, SARS‐CoV‐2, systematic review
1. INTRODUCTION
Human coronaviruses have in the past caused widespread outbreaks of serious diseases, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). 1 At the end of 2019, a novel coronavirus was identified as the etiology of a group of cases of pneumonia. The virus spread rapidly, resulting in an epidemic throughout China, followed by an increasing number of cases in other countries throughout the world. In February 2020, the World Health Organization (WHO) named the coronavirus disease 2019 (COVID‐19). 2 The virus that causes COVID‐19 has been given the name severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). On January 30, 2020, the WHO declared the outbreak a Public Health Emergency of International Concern (PHEIC) 3 and on 11 March 2020, a pandemic. 4 As of August 10, 2021, the reported cumulative COVID‐19 death toll surpassed four million lives, and the pace of deaths is accelerating. COVID‐19 pandemic is becoming regular in our lives. 5
Clinical symptoms are the external manifestations of the disease and are important for the diagnosis, treatment, and evaluation of the disease. Pneumonia is the most common manifestation in patients with COVID‐19, characterized primarily by fever, fatigue, dry cough, dyspnea, and other similar symptoms. 6 Some patients also exhibit gastrointestinal symptoms, such as anorexia, nausea, vomiting, and diarrhea. 7 Ocular manifestations have also been reported in some COVID‐19 patients. 8 Since the end of March 2020, skin manifestations and loss of sense of smell and taste have also been reported in patients with COVID‐19. 9 , 10 As of April 20, 2021, several systematic reviews of the symptoms of COVID‐19 have been published. 11 , 12 , 13 , 14 , 15 However, none of these have attempted to describe the full range of clinical manifestations of COVID‐19.
Evidence mapping is a method to summarize the evidence. It consists of a comprehensive search of the relevant research, systematical summarization of the basic characteristics and results of various types of studies, and an accurate visual representation of the evidence, progress, and problems in the field, to provide a comprehensive picture of research in the field and improve the effectiveness and usefulness of research in the field. 16 This study aimed to summarize the existing knowledge on clinical manifestations of COVID‐19 using evidence mapping to present a full picture of the clinical manifestations of COVID‐19 patients to provide a basis for clinical practice.
2. METHODS
2.1. Registration and reporting guideline
This study has been prospectively registered in the PROSPERO, and the registration number is CRD42021251418. We conducted this overview with an evidence mapping study following the guideline of Campbell Collaboration. 17 We used the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines for reporting of methods and findings of this study 18 (Supplementary Material 1).
2.2. Eligibility criteria and literature search
Systematic reviews were included if they met one of the following criteria: (1) systematic reviews with meta‐analyses that pooled the incidence of different clinical manifestations of COVID‐19; (2) systematic review with the proportions of different clinical manifestations of COVID‐19. If the format of Population, Intervention, Comparison, Outcomes, and Study design (PICOS) is used to present our research questions, P is for COVID‐19 patients, I and C are not applicable, and O is different clinical manifestations of COVID‐19 patients, and S is systematic review.
We excluded studies on traditional Chinese medicine; studies focusing on other diseases in patients with COVID‐19. We also excluded the systematic reviews that were not able to extract the incidence of COVID‐19 symptoms and systematic reviews that were not able to access the full text after contacting the corresponding authors by email. The definition of the systematic review was determined according to the criteria of the Cochrane Handbook.
We searched MEDLINE via PubMed, Web of Science, Embase, and Cochrane library on March 16, 2021, with the terms [“2019‐nCoV” OR “novel coronavirus” OR “COVID‐19” OR “SARS‐CoV‐2” OR “2019 novel coronavirus”] AND [“systematic review” OR “meta‐analysis” OR “literature review”] AND [“characteristics” OR “features” OR “manifestations” OR “presentation” OR “symptoms”] published between January 1, 2020 and March 16, 2021 without any language restriction (see Supplementary Material 2 for details of search strategies). We also searched Google Scholar, the WHO database of publications on COVID‐19 (https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/global‐research‐on‐novel‐coronavirus‐2019‐ncov), and the reference lists of the included studies to find reports of additional studies. All searches were conducted independently by two separate reviewers (XL and XZ), and if the number of searches was inconsistent, the two reviewers searched together and determined the results.
2.3. Study selection
Two reviewers (XL and ML) independently screened the titles, abstracts and full texts based on the inclusion and exclusion criteria. Before the screening, the two reviewers (XL and ML) performed a pretest extraction of 100 papers until an agreement on the screening process was reached. Disagreements were resolved by discussion with a third reviewer (YC). If the full text was not available, we contacted the authors to request the full text or further details. All screening was done using EndNote 20 software (Bld16742, Copyright © 1988–2021 Clarivate Analytics) except for full text.
2.4. Data extraction and quality appraisal
Two groups of two reviewers (YL and ML, MR and LW) independently extracted the data. We extracted the following basic information: (1) title, (2) first author and his/her country, (3) journal, (4) the number of included studies, (5) study design of included studies, and (6) sample size; and the following information on the results: manifestations outcomes and related statistical indicators (prevalence, effect size, 95% confidence interval (CI), I 2, P). If essential information was missing, we contacted the author to get the data, or used data conversion to the largest possible extent. Data that could not be obtained were discarded.
We assessed the methodological quality of the included systematic reviews using the “A MeaSurement Tool to Assess systematic Reviews” (AMSTAR) instrument. 19 The AMSTAR score has a total of 11 points, with studies scoring between 9 and 11 being of high quality, studies scoring between 6 and 8 of medium quality, and studies scoring between 0 and 5 of low quality. Quality assessments were done by two independent reviewers (XL and RL) and were determined by consulting a third reviewer (YC) in case of inconsistency.
2.5. Data analysis
We presented the general characteristics of the included studies descriptively. We calculated the ranges for the proportion of COVID‐19 patients having different symptoms. We presented the outcomes visually using a human anatomy diagram and a heat map. The heat map was prepared using Microsoft Excel 2016 software, and the human anatomy diagram was done using the Edraw Software (https://www.edrawsoft.com/). The clinical characteristics of COVID‐19 patients were divided into five parts according to the different systems of the body: (1) respiratory symptoms; (2) neurological symptoms; (3) gastrointestinal symptoms; (4) cutaneous symptoms, and (5) ocular symptoms. We also divided the population into three categories, namely the general population, neonates, children and adolescents, and pregnant women. The pooled prevalence estimate (PPE) will be performed for each symptom using the Comprehensive Meta‐analysis software, and if possible, we will examine the differences in prevalence by country, age, and gender.
3. RESULTS
3.1. Results of study selection
Our initial search revealed 2811 records, 759 of which were excluded as duplicates. After screening the titles and abstracts, 1897 of the remaining studies were excluded because of not related to COVID‐19. We reviewed the full texts of the remaining 155 articles and excluded 53 irrelevant articles, 38 articles that did not pool clinical symptoms, and five articles that only reported on complications. Finally, we identified 102 systematic reviews related to the clinical symptoms of COVID‐19. Figure 1 shows the flow of search and selection. Supplementary Material 3 presents a list of the inclusion and exclusion of systematic reviews.
FIGURE 1.
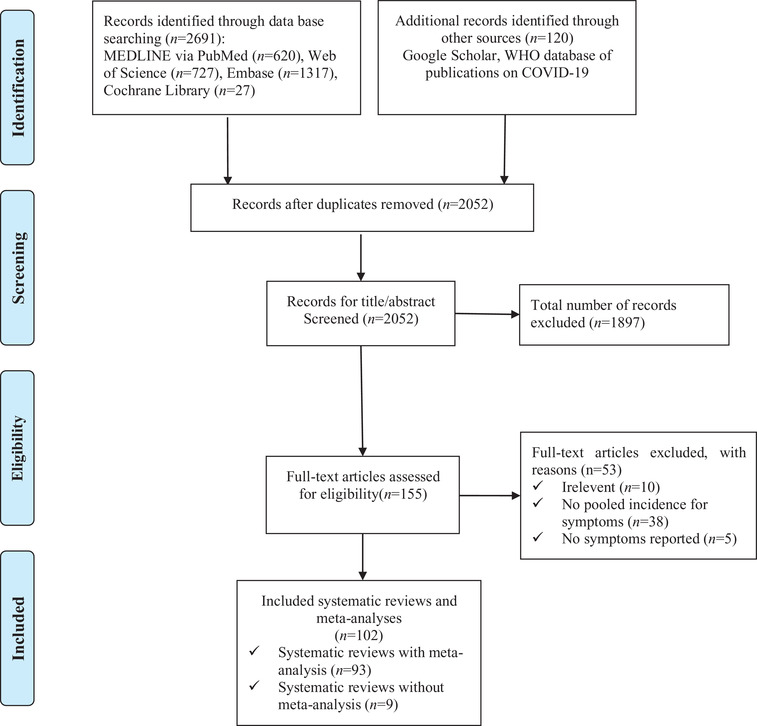
Flow chart of the literature search and selection
3.2. Characteristics of the included studies
Ninety‐three (91.2%) of the 102 studies were systematic reviews with meta‐analyses, the others have calculated percentages of different kinds of symptoms but no meta‐analysis. They were published between March 11, 2020 and March 4, 2021, with 89 (87.3%) of them in 2020. One hundred and two studies were conducted mainly in 26 countries or regions, 28 (27.5%) of the studies were conducted in China; 16 (15.7%) in Iran; 8 (7.8%) in India; 7 (6.9%) in the United States; five (4.9%) in the United Kingdom; four (3.9%) each in Italy and Brazil; three (2.9%) each in Malaysia, Nigeria, and Republic of Korea; two (2.0%) each in Colombia, Nepal, Canada, Egypt, and France; and one (1.0%) each in Singapore, Indonesia, Philippines, Switzerland, Ethiopia, Turkey, Australia, Bangladesh, Peru, Kuwait, and the United Arab Emirates. Most included systematic reviews (n = 74, 72.5%) focused on the general populations of COVID‐19, 15 (14.7%) systematic reviews focused on neonates, children, and adolescents, and 13 (12.8%) were pregnant women. The number of studies included in the systematic reviews varied from 5 to 349, and the sample size included varied from 33 to 280,000 COVID‐19 patients, and the types of studies included were mainly case reports, case series, and other observational studies. Table 1 describes the characteristics of the included studies.
TABLE 1.
Characteristics of the included systematic reviews and meta‐analyses
| Research ID | Published/online date | Country/region of First Author | Patients | Journal title abbreviations | Number of included primary studies | Number of participants | Age of participants (in years; range or mean) | Female |
|---|---|---|---|---|---|---|---|---|
| Hashan et al. | 2021/3/1 | Australia | General populations | EClinicalMedicine | 49 | 25,567 | Mean: 81.5 years | NA |
| Shehab et al. | 2021/3/4 | Kuwait | General populations | BMJ Open Gastroenterol | 158 | 78,798 | Mean: 66.6 years | 45.20% |
| Soltani et al. | 2021/1/12 | Iran | General populations | Rev Neurosci | 14 | 3148 | Ranged from 19 to 95 years | NA |
| Kouhsari et al. | 2020/11/4 | Iran | General populations | Indian J Med Microbiol | 50 | 8815 | Mean: 46 years | 46% |
| Soheili et al. | 2021/2/18 | Iran | Pregnant women | J Matern Fetal Neonatal Med | 11 | 177 | NA | 100% |
| Irfan et al. | 2021/2/16 | Canada | Neonates, children and adolescents | Arch Dis Child | 129 | 10,251 | Mean: 7 years | 44.50% |
| Zhong et al. | 2021/2/5 | China | General populations | Medicine | 40 | 2459 | NA | 37.70% |
| Hassanipour et al. | 2020/12/21 | Iran | Pregnant women | Int J Reprod Biomed | 10 | 135 | Ranged from 22 to 42 years | 100% |
| Xie et al. | 2020/12/4 | China | General populations | Ann Palliat Med | 90 | 16,526 | Ranged from 37 to 68 years | 46.90% |
| Olumade et al. | 2021/2/5 | Nigeria | General populations | J Med Virol | 7 | 4499 | NA | 31.20% |
| Nasiri et al. | 2021/1/20 | Iran | General populations | J Ophthalmic Vis Res | 38 | 8219 | NA | 55.30% |
| Israfil et al. | 2021/1/11 | Bangladesh | General populations | Front Public Health | 34 | 10,889 | Mean 50.6 years | 39.70% |
| Goel et al. | 2021/1/27 | India | General populations | Obstet Gynecol Sci | 7 | 3231 | Ranged from 47 to 62 years | 44.85% |
| Lee et al. | 2020/12/15 | USA | General populations | Dermatol Online J | 71 | 144 | Mean: 45.9 years | 46.50% |
| Nazari et al. | 2021/1/9 | Iran | General populations | Brain Behav | 64 | 11,687 | Mean 48.6 years | 47.60% |
| Jafari et al. | 2020/12/1 | Iran | Pregnant women | Rev Med Virol | 349 | 138,176 | Mean age 51.2 (nonpregnant) Mean age 33 (pregnant) | 100% |
| Khamis et al. | 2020/12/3 | United Arab Emirates | General populations | J Formos Med Assoc | 35 | 10,972 | NA | NA |
| Islam et al. | 2020/11/27 | Malaysia | General populations | Front Neurol | 86 | 14,275 | Ranged from 35.0 ± 8.0 to 70.7 ± 13.5 years | 49.40% |
| Merola et al. | 2020/10/12 | Italy | General populations | Acta Gastroenterol Belg | 33 | 4434 | NA | NA |
| Saniasiaya et al. | 2020/12/15 | Malaysia | General populations | Otolaryngol Head Neck Surg | 59 | 29,349 | Ranged 28.0 ± 16.4 to 66.4 ± 14.9 years | 64.40% |
| Ciaffi et al. | 2020/10/28 | Italy | General populations | BMC Rheumatol | 88 (51 in meta) | NA | NA | NA |
| Silva et al. | 2020/11/25 | Brasil | General populations | Rev Soc Bras Med Trop | 43 | 18,246 | NA | NA |
| Wang et al. | 2020/11/25 | China | General populations | Medicine | 25 | 4881 | NA | NA |
| Li et al. | 2020/11/2 | China | Neonates, children and adolescents | Front Pediatr | 96(54 in meta) | 7004 | NA | NA |
| Novoa et al. | 2021/2/2 | Peru | Pregnant women | Travel Med Infect Dis | 37(4 in meta) | 322 | range 20−45 | 100% |
| Saniasiaya et al. | 2020/12/5 | Malaysia | General populations | Laryngoscope | 83 | 27,492 | NA | NA |
| Karabay et al. | 2020/11/19 | Turkey | Neonates, children and adolescents | J Matern Fetal Neonatal Med | 35 | NA | NA | NA |
| Aggarwal et al. | 2020/11/5 | India | General populations | PLoS One | 16 | 2347 | NA | NA |
| Alimohamadiÿ et al. | 2020/10/6 | Iran | General populations | J Prev Med Hyg | 54 | NA | NA | NA |
| Cagnazzo et al. | 2020/10/30 | France | General populations | J Neurol | 39 | 68,361 | Mean age 64.4 | 49% |
| Yee et al. | 2020/10/22 | Republic of Korea | General populations | Sci Rep | 11 | 9370 | NA | NA |
| Favas et al. | 2020/12/1 | India | General populations | Neurol Sci | 212(74 in meta) | NA | NA | NA |
| Collantes et al. | 2020/7/15 | Philippines | General populations | Can J Neurol Sci | 49 | 6335 | NA | NA |
| Ibekwe et al. | 2020/9/11 | Nigeria | General populations | OTO Open | 32 | 20,451 | NA | NA |
| Amorim et al. | Feb‐21 | Brazil | General populations | J Dent Res | 40 | 10,228 | NA | NA |
| Panda et al. | 2020/9/10 | India | Neonates, children and adolescents | J Trop Pediatr | 26 | 3707 | Range: 0–18 years | NA |
| Allotey et al. | 2020/9/1 | UK | Pregnant women | BMJ | 77 | 96,604 | NA | 100% |
| Ochoa et al. | 2021/1/4 | Colombia | General populations | Am J Epidemiol | 97 | 230,398 | 40 (11) years | 69.98% |
| Hasani et al. | 2020/8/14 | Iran | General populations | Biomed Res Int | 30 | 3420 | NA | NA |
| Khalil et al. | 2020/8/25 | UK | pregnant women | EClinicalMedicine | 86 | NA | NA | 100% |
| Kaur et al. | 2020/7/9 | India | General populations | SN Compr Clin Med | 50 | 6635 | NA | NA |
| Jutzeler et al. | 2020/7/27 | Switzerland | General populations | Travel Med Infect Dis | 148 | 12,149 | Median age: 47 years | 47.20% |
| Kumar et al. | Jun‐20 | India | General populations | Indian J Gastroenterol | 62 | 8301 | 48.7(16.5) | 46% |
| Gao et al. | 2020/8/3 | China | Pregnant women | BMC Infect Dis | 14 | 236 | NA | NA |
| Chen et al. | Feb‐21 | China | General populations | J Neurol | 100 | NA | NA | NA |
| Pormohammad et al. | Oct‐20 | Canada | General populations | Microb Pathog | 80 | 61,742 | NA | NA |
| Zarifian et al. | Jan‐21 | Iran | General populations | J Med Virol | 67 | 13,251 | NA | 53.30% |
| Abdullahi et al. | 2020/6/26 | Nigeria | General populations | Front Neurol | 60 | 11,069 | NA | NA |
| Koh et al. | 2020/6/11 | Singapore | General populations | Front Med | 29 | 578 | NA | NA |
| Meena et al. | 2020/9/15 | India | Neonates, children and adolescents | Indian Pediatrics | 27 | 4857 | 6.4 (3.4) years | 43% |
| Tahvildari et al. | 2020/5/15 | Iran | General populations | Front Med | 80 | 417 | Mean: 49 years | NA |
| Grant et al. | 2020/6/23 | UK | General populations | Plos One | 148 | 24,410 | 49 (11) years | 45.50% |
| Wang et al. | 2020/5/1 | China | Neonates, children and adolescents | Ann Transl Med | 49 | 1667 | NA | 42.70% |
| Ma et al. | 2021/1/1 | China | Neonates, children and adolescents | J Med Virol | 15 | 486 | NA | 40.70% |
| Parasa et al. | 2020/6/1 | USA | General populations | JAMA Netw Open | 29 | 4805 | Mean 52.2 years | 33.20% |
| Wan et al. | 2020/7/1 | China | General populations | Acad Radiol | 14 | 1115 | NA | NA |
| Park et al. | 2020/5/1 | Republic of Korea | General populations | Clin Exp Otorhinolaryngol | 9 | 627 | NA | 45.00% |
| Sultan et al. | 2020/7/1 | USA | General populations | Gastroenterology | 57 | NA | NA | NA |
| Mao et al. | 2020/7/1 | China | General populations | Lancet Gastroenterol Hepatol | 35 | 6686 | NA | NA |
| Hu et al. | 2020/6/1 | China | General populations | J Clin Virol | 21 | 47,344 | NA | 48.40% |
| Chang et al. | 2020/5/1 | Taiwan, China | Neonates, children and adolescents | J Formos Med Assoc | 9 | 93 | NA | 48.40% |
| Zhu et al. | 2020/10/1 | China | General populations | J Med Virol | 38 | 3062 | NA | 43.10% |
| Fu et al. | 2020/6/1 | China | General populations | J Infect | 43 | 3600 | Median: 41 years | 43.50% |
| Cheung et al. | 2020/7/1 | HongKong, China | General populations | Gastroenterology | 69 | 4875 | Median: 45.1 years | 42.70% |
| Cao et al. | 2020/9/1 | China | General populations | J Med Virol | 31 | 46,959 | Median: 46.62 years | 44.40% |
| Morales et al. | 2020/3/11 | Colombia | General populations | Travel Med Infect Dis | 58 | NA | NA | NA |
| Li et al. | 2020/6/1 | China | General populations | J Med Virol | 10 | 1994 | NA | 42.40% |
| Sun et al. | 2020/6/1 | China | General populations | J Med Virol | 10 | 50,466 | NA | 48.00% |
| Daha et al. | 2020/6/1 | Nepal | General populations | Trop Biomed | 40 | 2735 | NA | 45.20% |
| Elshazli et al. | 2021/2/2 | Egypt | General populations | J Med Virol | 125 | 25,252 | Mean 52.1 years | 47.80% |
| Mansourian et al. | 2021/1/9 | Iran | Neonates, children and adolescents | Arch Pediatr | 32 | 759 | NA | 47.40% |
| Badal et al. | 2020/12/8 | USA | Neonates, children and adolescents | J Clin Virol | 20 | 1810 | Median age 8 | 42.74% |
| Chi et al. | 2021/2/1 | China | General populations | Arch Gynecol Obstet | 20 | 386 | NA | NA |
| Sameni et al. | 2020/10/29 | Iran | General populations | Front Med | 43 | 2621 | NA | NA |
| Wong et al. | 2020/11/13 | Hong Kong, China | General populations | Sci Rep | 76 | 11,028 | NA | NA |
| Han et al. | 2020/11/26 | China | Pregnant women | J Perinat Med | 36 | 1103 | NA | 100% |
| Sheleme et al. | 2020/9/10 | Ethiopia | General populations | Infect Dis (Auckl) | 30 | 4829 | Range: 0.25–94 years | 47.40% |
| Bennett et al. | 2020/9/23 | UK | General populations | Int J Clin Pract | 45 | 14,358 | Average age 51 years | 49% |
| Nasiri et al. | 2020/7/21 | Iran | General populations | Front Med | 34 | 5057 | NA | NA |
| Li et al. | Mar‐21 | China | General populations | J Med Virol | 212 | 281,461 | NA | NA |
| Yasuhara et al. | Oct‐20 | USA | Neonates, children and adolescents | Pediatr Pulmonol | 46 | 114 | Range: 0–16 years | NA |
| Ding et al. | 2020/7/3 | China | Neonates, children and adolescents | Front Pediatr | 33 | 396 | Range: 0–17 years | 56.30% |
| Nepal et al. | 2020/7/13 | Nepal | General populations | Crit Care | 37 | NA | NA | NA |
| Matar et al. | 2021/2/1 | USA | Pregnant women | Clin Infect Dis | 24 | 136 | Range: 25–34 | 100% |
| Pinzon et al. | 2020/5/29 | Indonesia | General populations | Front Neurol | 33 | 7559 | NA | NA |
| Mantovani et al. | 2020/6/17 | Italy | Neonates, children and adolescents | Pediatr Res | 19 | 2855 | Mean age 6.9 ± 7.0 years | 49.70% |
| Wang et al. | 2020/10/1 | China | General populations | J Neurol | 41 | NA | NA | NA |
| Kim et al. | 2020/11/1 | Republic of Korea | General populations | Eur Rev Med Pharmacol Sci | 16 | 33 | Median age 66 | 45.50% |
| Makvandiet al. | 2020 | Iran | Pregnant women | Gastroenterol Hepatol Bed Bench | 43 | 374 | NA | 100% |
| Mesquita et al. | 2020/11/26 | Brasil | General populations | Wien Klin Wochenschr | 152 | 41,409 | NA | NA |
| Jindal et al. | 2020/9/30 | India | General populations | J Family Med Prim Care | 44 | 458 | NA | NA |
| Mirza et al. | 2020/11/3 | USA | Neonates, children and adolescents | Int J Dermatol | 86 | 2560 | NA | NA |
| Ibrahim et al. | 2020/10/21 | Egypt | General populations | CNS spectr | 20 | NA | NA | NA |
| Turan et al. | Oct‐20 | UK | Pregnant women | Int J Gynaecol Obstet | 63 | 637 | NA | 100% |
| Zhao et al. | Nov‐20 | China | General populations | J Eur Acad Dermatol Venereol | 44 | 507 | NA | NA |
| Matar et al. | Nov‐20 | France | General populations | J Eur Acad Dermatol Venereol | 56 | 1020 | NA | NA |
| Tsai et al. | 2020/5/19 | Taiwan, China | General populations | Front Neurol | 92 | NA | NA | NA |
| Souza et al. | 2020/8/1 | Brazil | Neonates, children and adolescents | Pediatr Pulmonol | 38 | 1124 | NA | 42.60% |
| Passarelli et al. | 2020/6/1 | Italy | General populations | Am J Dent | 5 | 10,818 | NA | NA |
| Kasraeian et al. | 2020/5/19 | Iran | Pregnant women | J Matern Fetal Neonatal Med | 9 | 87 | Median age: 30 years | 100% |
| Yang et al. | 2020/4/30 | China | Pregnant women | J Matern Fetal Neonatal Med | 18 | 114 | NA | 100% |
| Yang et al. | 2020/5/1 | China | General populations | Int J Infect Dis | 7 | 1576 | Median: 49.6 years | 43.50% |
According to the AMSTAR scores, among the one hundred and two reviews included, 68 studies (66.7%) were of high quality, 19 studies (18.6%) of medium‐quality, and 15 studies (14.7%) of low quality (Supplementary Material 4). The main reasons for low quality include lack of prospective registration, failure to report on conflict of interests, and nonrepeatable data extraction and screening processes, etc.
3.3. Respiratory symptoms
Seventeen different respiratory symptoms were reported in 71 systematic reviews and meta‐analyses. Cough was reported in 40 systematic reviews in the general populations, 13 in neonates, children, and adolescents, and 15 in pregnant women; sore throat was reported in 29 systematic reviews in the general populations, 8 in neonates, children, and adolescents, and 10 in pregnant women; dyspnea was reported in 49 systematic reviews, of which, 9 related to neonates, children, and adolescents, 11 related to pregnant women, and the others were in the general populations. The remaining 14 symptoms are detailed in Figure 2A and Supplementary Material 5.
FIGURE 2.
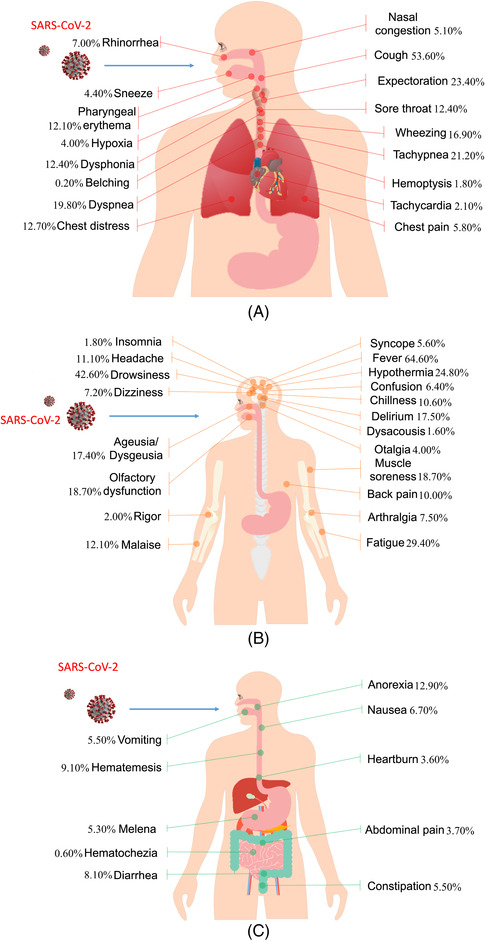
Human anatomy diagram of COVID‐19 manifestations. (A) Respiratory symptoms; (B) neurological symptoms; and (C) gastrointestinal symptoms
The most common symptoms of the respiratory system were cough (PPE = 53.6%, 95%CI, 52.1%−55.1%), sore throat (PPE = 12.4%, 95%CI, 9.8%−15.7%), dyspnea (PPE = 19.8%, 95%CI, 18.2%−21.6%), and expectoration (PPE = 23.4%, 95%CI, 21.6%−25.3%) (Figure 2A). Prevalence of cough was inconsistent across systematic reviews, ranging between 30.0% and 72.2%. The corresponding prevalence estimate for sore throat was 0.8%−32.0%, for dyspnea 1.0%−74.0% and for expectoration 1.5%−41.8%. The prevalence estimates for cough, sore throat, dyspnea and expectoration were lower in neonates, children and adolescents and pregnant women than in the general populations (Table 2, Supplementary Material 5 and 6).
TABLE 2.
Meta‐analyses of symptoms of COVID‐19
| No. | Symptoms | Number of SRs | Pooled prevalence | LCI | UCI | p Value |
|---|---|---|---|---|---|---|
| 1 | Cough | 68 | 53.60% | 52.10% | 55.10% | 0.000 |
| 2 | Fever | 68 | 64.60% | 61.30% | 67.90% | 0.000 |
| 3 | Diarrhea | 63 | 8.10% | 7.30% | 9.10% | 0.000 |
| 4 | Headache | 58 | 11.10% | 9.00% | 13.80% | 0.000 |
| 5 | Muscle soreness | 56 | 18.70% | 16.30% | 21.30% | 0.000 |
| 6 | Fatigue | 52 | 29.40% | 27.50% | 31.30% | 0.000 |
| 7 | Dyspnea | 50 | 19.80% | 18.20% | 21.60% | 0.000 |
| 8 | Sore throat | 47 | 12.40% | 9.80% | 15.70% | 0.000 |
| 9 | Vomiting | 42 | 5.50% | 4.70% | 6.30% | 0.000 |
| 10 | Nausea | 37 | 6.70% | 6.00% | 7.40% | 0.000 |
| 11 | Expectoration | 32 | 23.40% | 21.60% | 25.30% | 0.000 |
| 12 | Dizziness | 28 | 7.20% | 5.30% | 9.70% | 0.000 |
| 13 | Tachypnea | 26 | 21.20% | 19.80% | 22.60% | 0.000 |
| 14 | Abdominal pain | 26 | 3.70% | 2.80% | 4.80% | 0.000 |
| 15 | Rhinorrhea | 24 | 7.00% | 6.10% | 8.00% | 0.000 |
| 16 | Ageusia | 23 | 17.40% | 12.50% | 23.80% | 0.000 |
| 17 | Anorexia | 21 | 12.90% | 10.00% | 16.60% | 0.000 |
| 18 | Anosmia | 20 | 18.70% | 12.20% | 27.40% | 0.000 |
| 19 | Nasal congestion | 19 | 5.10% | 3.90% | 6.80% | 0.000 |
| 20 | Hemoptysis | 18 | 1.80% | 1.20% | 2.80% | 0.000 |
| 21 | Chest pain | 17 | 5.80% | 4.60% | 7.40% | 0.000 |
| 22 | Chest distress | 14 | 12.70% | 8.90% | 17.90% | 0.000 |
| 23 | Chillness | 14 | 10.60% | 8.00% | 13.90% | 0.000 |
| 24 | Malaise | 10 | 12.10% | 7.00% | 19.90% | 0.000 |
| 25 | Confusion | 10 | 6.40% | 4.10% | 9.90% | 0.000 |
| 26 | Arthralgia | 8 | 7.50% | 5.20% | 10.80% | 0.000 |
| 27 | Rash | 8 | 14.00% | 6.80% | 26.60% | 0.000 |
| 28 | Tachycardia | 6 | 2.10% | 1.70% | 2.70% | 0.000 |
| 29 | Chilblains‐like | 5 | 24.60% | 12.20% | 43.30% | 0.010 |
| 30 | Livedo | 5 | 4.60% | 3.30% | 6.50% | 0.000 |
| 31 | Conjunctivitis | 5 | 5.50% | 2.90% | 10.20% | 0.000 |
| 32 | Pharyngeal erythema | 4 | 12.10% | 8.00% | 17.80% | 0.000 |
| 33 | Hypoxia | 4 | 4.00% | 0.40% | 29.50% | 0.007 |
| 34 | Urticaria | 4 | 16.80% | 14.30% | 19.70% | 0.000 |
| 35 | Sneeze | 3 | 4.40% | 0.50% | 29.60% | 0.006 |
| 36 | Rigor | 3 | 2.00% | 0.10% | 37.80% | 0.025 |
| 37 | Hypothermia | 3 | 24.80% | 8.70% | 53.20% | 0.079 |
| 38 | Delirium | 3 | 17.50% | 15.20% | 20.10% | 0.000 |
| 39 | Constipation | 3 | 5.50% | 5.20% | 5.80% | 0.000 |
| 40 | Papulosquamous | 3 | 5.80% | 1.70% | 18.20% | 0.000 |
| 41 | Erythematous | 3 | 33.90% | 21.20% | 49.40% | 0.043 |
| 42 | Pruritic | 3 | 41.30% | 17.20% | 70.30% | 0.569 |
| 43 | Cyanosis | 3 | 2.00% | 0.30% | 11.30% | 0.000 |
| 44 | Conjunctival congestion | 3 | 3.80% | 0.90% | 13.90% | 0.000 |
| 45 | Eye pain | 3 | 6.90% | 1.90% | 22.60% | 0.000 |
| 46 | Blurred vision | 3 | 1.20% | 0.00% | 26.50% | 0.011 |
| 47 | Wheezing | 2 | 16.90% | 15.40% | 18.60% | 0.000 |
| 48 | Chickenpox‐like Vesicles | 2 | 16.20% | 13.50% | 19.40% | 0.000 |
| 49 | Petechia | 2 | 3.50% | 0.90% | 12.50% | 0.000 |
| 50 | Edematous | 2 | 6.90% | 3.70% | 12.30% | 0.000 |
| 51 | Vesicular | 2 | 11.80% | 7.80% | 17.40% | 0.000 |
| 52 | Dry eyes | 2 | 14.50% | 12.20% | 17.20% | 0.000 |
| 53 | Eye itching | 2 | 9.20% | 4.80% | 16.80% | 0.000 |
| 54 | Photophobia | 2 | 4.80% | 2.00% | 11.00% | 0.000 |
| 55 | Chemosis | 2 | 4.50% | 3.90% | 5.30% | 0.000 |
| 56 | Lid edema | 2 | 1.60% | 0.60% | 4.20% | 0.000 |
| 57 | Dysphonia | 1 | 12.40% | 8.30% | 18.10% | 0.000 |
| 58 | Belching | 1 | 0.20% | 0.10% | 0.40% | 0.000 |
| 59 | Dysacousis | 1 | 1.60% | 0.00% | 97.60% | 0.301 |
| 60 | Drowsiness | 1 | 42.60% | 32.70% | 53.20% | 0.169 |
| 61 | Numbness | 1 | 5.80% | 0.20% | 65.40% | 0.110 |
| 62 | Insomnia | 1 | 1.80% | 0.20% | 12.00% | 0.000 |
| 63 | Syncope | 1 | 5.60% | 4.30% | 7.20% | 0.000 |
| 64 | Back pain | 1 | 10.00% | 9.50% | 10.60% | 0.000 |
| 65 | Otalgia | 1 | 4.00% | 1.20% | 12.30% | 0.000 |
| 66 | Heartburn | 1 | 3.60% | 3.40% | 3.80% | 0.000 |
| 67 | Hematemesis | 1 | 9.10% | 8.80% | 9.50% | 0.000 |
| 68 | Melena | 1 | 5.30% | 5.00% | 5.60% | 0.000 |
| 69 | Hematochezia | 1 | 0.60% | 0.50% | 0.70% | 0.000 |
| 70 | Goosebumps | 1 | 13.50% | 11.70% | 15.50% | 0.000 |
| 71 | Pustule | 1 | 1.80% | 0.20% | 12.00% | 0.000 |
| 72 | Scales | 1 | 7.40% | 2.80% | 18.10% | 0.000 |
| 73 | Ulcer | 1 | 1.80% | 0.20% | 12.00% | 0.000 |
| 74 | Tearing | 1 | 12.80% | 10.80% | 15.10% | 0.000 |
SR, systematic review; LCI, lower 95% confidence intervals; UCI, upper 95% confidence intervals.
3.4. Neurological symptoms
Eighty‐eight systematic reviews and meta‐analyses covered a total of 21 different neurological symptoms: fever (67 studies), headache (58 studies), muscle soreness (56 studies), fatigue (52 studies), dizziness (28 studies), ageusia (23 studies), anosmia (20 studies), chillness (14 studies), confusion (10 studies), malaise (10 studies), arthralgia (8 studies), delirium (3 studies), rigor (3 studies), hypothermia (3 studies), dysacousis (1 study), back pain (1 study), drowsiness (1 study), numbness (1 study), otalgia (1 study), insomnia (1 study), and syncope (1 study) (Supplementary Material 5).
Fever (PPE = 64.6%, 95%CI, 9.8%−15.7%), fatigue (PPE = 29.4%, 95%CI, 27.5%−31.3%), headache (PPE = 11.1%, 95%CI, 9.0%−13.8%), and muscle soreness (PPE = 18.7%, 95%CI, 16.3%−21.3%) were the most common (Figure 2B). Prevalence of fever was above 80% in most studies, reaching up to 91.3%; the lowest reported value, 27.6%, was in a study on pregnant women. The prevalence of fatigue ranged between 3.3% and 58.5%, headache ranged between 0.1% and 67.0%, and the prevalence of muscle soreness between 3.0% and 44.0%; the lowest reported prevalence for both conditions was among pregnant women and neonates, children and adolescents. The prevalence of the remaining symptoms is detailed in Table 2 and Supplementary Material 5 and 6.
3.5. Gastrointestinal symptoms
A total of 10 gastrointestinal symptoms were reported in 67 systematic reviews and meta‐analyses. Diarrhea was reported in 63 systematic reviews and meta‐analyses (PPE = 8.1%, 95%CI, 7.3%−9.1%), vomiting in 42, nausea in 37, anorexia in 21, abdominal pain in 26, constipation in 3 studies, and 1 each in heartburn, hematemesis, melena, and hematochezia. The prevalence of diarrhea ranged between 0.1% and 19.6% (Figure 2C). Nausea (1.2%−27.0%) and vomiting (1.2%−20.0%) occurred often together. The prevalence of gastrointestinal symptoms is generally less than 20% and does not differ from the general population in neonates, children and adolescents, or pregnant women (Table 2 and Supplementary Material 5 and 6).
3.6. Cutaneous and ocular symptoms
Thirteen systematic reviews and meta‐analyses reported 16 cutaneous symptoms (Supplementary Material 5). Rash was the most common symptom reported in nine studies, and the prevalence of cutaneous symptoms according to those reviews was generally less than 20% (Figure 3B). The symptoms of the eyes were as low in incidence as those of the cutaneous. A total of 10 ocular symptoms were reported in 8 systematic reviews and meta‐analyses (Figure 3A). The ocular symptoms were relatively rare in neonates, children and adolescents, and pregnant women, and the overall prevalence was low, between 5% and 20% (Table 2 and Supplementary Material 5).
FIGURE 3.
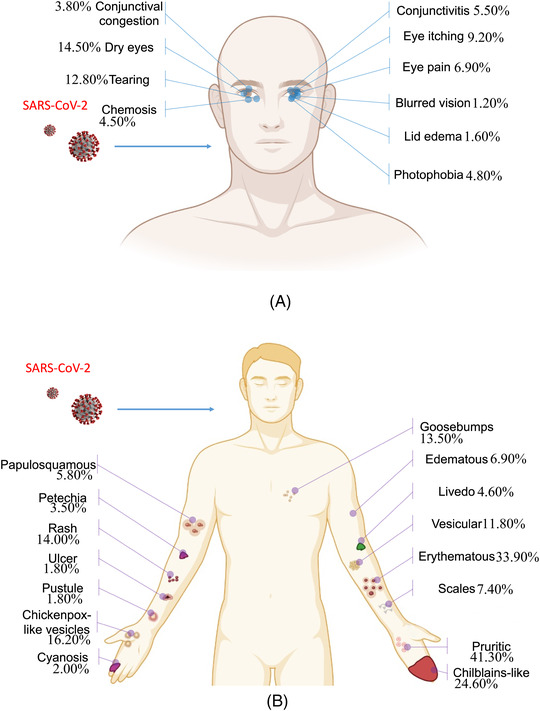
Human anatomy diagram of COVID‐19 manifestations. (A) Ocular symptoms and (B) cutaneous symptoms
4. DISCUSSION
4.1. Principal findings
Our study identified 74 different clinical manifestations of COVID‐19 in 102 systematic reviews and meta‐analyses. The most common respiratory symptoms were cough, sore throat, dyspnea, and expectoration, and the most common symptoms of neurological symptoms were fever, fatigue, headache, and muscle soreness. The gastrointestinal system included diarrhea, nausea, and vomiting, and we also identified some other symptoms such as manifestations of the eyes or skin. The prevalence of the same condition tended to vary broadly across the different systematic reviews and population groups, and lower prevalence of symptoms in pregnant women and neonates, children and adolescents than in the general population.
Clinical symptoms are important for the diagnosis of a disease. There is no doubt that fever, cough, and fatigue are the three most prevalent symptoms of COVID‐19 patients. Many studies have estimated the prevalence of different symptoms of SARS‐CoV‐2 infection. Like in the case of SARS‐CoV and MERS‐CoV, cough and fever are the most common symptoms, which can be caused also by many other causes, such as common flu. 20 , 21 An accurate diagnosis of COVID‐19 therefore often requires a combination of clinical symptoms, laboratory tests and CT findings. Attention in the diagnosis should also be paid to the differentiation of clinical manifestations associated with comorbidities, such as hypertension, diabetes, and coronary heart disease.
The clinical symptoms of COVID‐19 vary across population groups. One systematic review 22 suggested that children appear to have a less severe course and better prognosis than adults, and deaths in children are extremely rare. In addition, the multisystem inflammatory syndrome in children (MIS‐C) should be given more attention when diagnosing children with COVID‐19, in addition to symptoms similar to those of adults. 23 Studies have shown that pregnant women's symptoms are essentially the same as in the general population, but the prevalence was lower. 24 , 25 At the same time, gastrointestinal symptoms, eye symptoms and skin symptoms are relatively less common in pregnant women. 26
The diagnosis of SARS‐CoV‐2 infection in asymptomatic patients requires special attention. Many asymptomatic patients have been reported worldwide. Nishiura et al. 27 estimated the proportion of asymptomatic patients was 30.8% (95% CI, 7.7%−53.8%). Hu et al. found that the course of illness was milder in asymptomatic cases than in other cases. 28 However, the asymptomatic carriers may be a challenge to containment for COVID‐19 transmission. 29 Asymptomatic people can transmit SARS‐CoV‐2 to others for a long time, perhaps more than 14 days. 30 Therefore, it is important to screen asymptomatic SARS‐CoV‐2 carrier populations, when resources are available, to minimize the chance of infection, although it may raise the treatment cost31.
Our study identified some unusual symptoms, such as skin (livedo, cyanosis, edematous, etc.) and eye manifestations (conjunctivitis, blurred vision, eye pain, etc.). However, because such symptoms were rarely reported during the pre‐epidemic period, they can be easily overlooked. Patients with unusual symptoms are not easily screened and diagnosed; therefore, understanding and knowing these unusual symptoms, has important implications for the current improvement in the identification of SARS‐CoV‐2 infections. Besides, as the epidemic grows and some COVID‐19 variant strains emerge, some specific symptoms may appear. However, there is no relevant systematic review yet, and further updating of associated symptoms regarding COVID‐19 variant strains is needed in the future.
4.2. Implications for future research and practice
As the second wave of the outbreak rages on, countries need again to pay attention to finding as many infected patients as early as possible to cut the transmission chains and avoid a new wave of the epidemic. This means that even rare symptoms can be important in the screen and diagnosis. Our study found that many systematic reviews and meta‐analyses of different quality are being conducted for the same symptom, which may result in wasting research on COVID‐19, 32 and before conducting systematic reviews of symptoms for researchers, we recommend retrieval to determine if a systematic review is already available on the PROSPERO website, and if not, it should be registered.
For patients with COVID‐19 in the second wave of the epidemic, our study can provide a full picture of the symptoms map of COVID‐19 to inform the screening and diagnosis of patients. Furthermore, in the context of a global COVID‐19 epidemic, our study could help clinicians or stakeholders to identify COVID‐19 through some rare symptoms.
4.3. Strengths and limitations
To the best of our knowledge, this is the first evidence map to comprehensively review and summarize the clinical symptoms of COVID‐19. We systematically searched the main databases and performed a detailed analysis of the included literature. However, this study also has some limitations. First, because of the substantial overlap between the studies included in the systematic reviews, we have limited confidence in the pooled results of the meta‐analyses. Second, although we systematically searched the literature, there is a possibility that some studies were missed due to the constantly increasing number of COVID‐19 studies. Third, given that the frequency of COVID‐19 symptoms may be varied in different countries or territories due to different sources or genotypes of COVID‐19, we did not perform a subgroup analysis of symptoms in different geographical locations. However, this can provide information for tracing the origin of the SARS‐CoV‐2 virus. To address the above limitations, we believe it is meaningful and necessary to conduct a living systematic review of the symptoms of COVID‐19 patients.
4.4. Conclusion
In conclusion, COVID‐19 is associated with at least 74 different clinical manifestations, the most common of which are fever, cough, muscle soreness, and fatigue. In addition, some symptoms, despite being rare, may be useful in the early diagnosis of COVID‐19 in patients who otherwise have no or only mild symptoms. Future research should pay particular attention to these rare symptoms to help treat the infected patients and control the epidemic.
CONFLICT OF INTEREST
There are no relevant financial or nonfinancial competing interests to report.
Supporting information
Supplementary Material 1 PRISMA checklist
Supplementary Material 2 Search Strategy
Supplementary Material 3 Systematic Reviews and Meta‐analyses included and excluded references of this study
Supplementary Material 4 AMSTAR scores and main conclusions of systematic reviews
Supplementary Material 5 Heat map for different symptoms of COVID‐19
Supplementary Material 6 Subgroup analyses
ACKNOWLEDGMENTS
We thank Xianzhuo Zhang for helping to make the human anatomy diagram.
Luo X, Lv M, Zhang X, et al. Clinical manifestations of COVID‐19: An overview of 102 systematic reviews with evidence mapping. J Evid Based Med. 2022;1‐15. 10.1111/jebm.12483
Xufei Luo and Meng Lv contributed equally to the work.
REFERENCES
- 1. Hui DS, Azhar EI, Memish ZA, et al. Human coronavirus infections—severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and SARS‐CoV‐2. Encyclop Respir Med. 2020:146‐161. doi:10.1016/B978‐0‐12‐801238‐3.11634‐4 [Google Scholar]
- 2. World Health Organization . Naming the coronavirus disease (COVID‐19) and the virus that causes it. [cited 2021 May 17]. Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/technical‐guidance/naming‐the‐coronavirus‐disease‐(covid‐2019)‐and‐the‐virus‐that‐causes‐it
- 3. World Health Organization . COVID‐19 Public Health Emergency of International Concern (PHEIC) Global research and innovation forum. [cited 2021 May 17]. Available from: https://www.who.int/publications/m/item/covid‐19‐public‐health‐emergency‐of‐international‐concern‐(pheic)‐global‐research‐and‐innovation‐forum
- 4. World Health Organization . WHO characterizes COVID‐19 as a pandemic. [cited 2021 May 17]. Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/events‐as‐they‐happen
- 5. World Health Organization . WHO Coronavirus (COVID‐19) Dashboard. [cited 2021 Aug 10]. Available from: https://covid19.who.int/
- 6. Johnson KD, Harris C, Cain JK, et al. Pulmonary and extra‐pulmonary clinical manifestations of COVID‐19. Front Med (Lausanne). 2020;7:526. doi:10.3389/fmed.2020.00526. Published 2020 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abbasinia M, Hormati A, Eshagh Hossaini SK, et al. Clinical manifestations of gastrointestinal symptoms in COVID‐19 patients: an integrative review. Gastroenterol Nurs. 2021;44(1), E1‐E10. doi:10.1097/SGA.0000000000000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nasiri N, Sharifi H, Bazrafshan A, et al. Ocular MANIFESTATIONS of COVID‐19: a systematic review and meta‐analysis. J Ophthalmic Vis Res. 2021;16(1), 103‐112. doi:10.18502/jovr.v16i1.8256. Published 2021 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koyama S, Ueha R, Kondo K. Loss of smell and taste in patients with suspected COVID‐19: analyses of patients' reports on social media. J Med Internet Res. 2021;23(4), e26459. doi:10.2196/26459. Published 2021 Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mercante G, Ferreli F, De Virgilio A, et al. Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 2020;146(8), 723‐728. doi:10.1001/jamaoto.2020.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hashan MR, Smoll N, King C, et al. Epidemiology and clinical features of COVID‐19 outbreaks in aged care facilities: a systematic review and meta‐analysis. EClinicalMedicine. 2021; 33: 100771. doi:10.1016/j.eclinm.2021.100771. PMID: 33681730; PMCID: PMC7917447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shehab M, Alrashed F, Shuaibi S, Alajmi D, Barkun A. Gastroenterological and hepatic manifestations of patients with COVID‐19, prevalence, mortality by country, and intensive care admission rate: systematic review and meta‐analysis. BMJ Open Gastroenterol. 2021;8(1), e000571. doi:10.1136/bmjgast‐2020‐000571. PMID: 33664052; PMCID: PMC7934201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Soltani S, Tabibzadeh A, Zakeri A, et al. COVID‐19 associated central nervous system manifestations, mental and neurological symptoms: a systematic review and meta‐analysis. Rev Neurosci. 2021;32(3), 351‐361. doi:10.1515/revneuro‐2020‐0108. PMID: 33618441. [DOI] [PubMed] [Google Scholar]
- 14. Daha SK, Koirala B, Chapagain D, Lohani P, Acharya S, Sharma P. Clinical features and management of COVID‐19: a systematic review. Trop Biomed. 2020;37(2), 409‐420. PMID: 33612810. [PubMed] [Google Scholar]
- 15. Kouhsari E, Azizian K, Sholeh M, et al. Clinical, epidemiological, laboratory, and radiological characteristics of novel Coronavirus (2019‐nCoV) in retrospective studies: a systemic review and meta‐analysis. Indian J Med Microbiol. 2021;39(1):104‐115. doi:10.1016/j.ijmmb.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miake‐Lye IM, Hempel S, Shanman R, et al. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5:28. doi:10.1186/s13643‐016‐0204‐x. Published 2016 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White H, Albers B, Gaarder M, et al. Guidance for producing a Campbell evidence and gap map. Campb Syst Rev. 2020;16(4):e1125. doi:10.1002/cl2.1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6(7), e1000097. doi:10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shea BJ, Grimshaw JM, Wells GA, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7:10. doi:10.1186/1471‐2288‐7‐10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su YJ, Lai YC. Comparison of clinical characteristics of coronavirus disease (COVID‐19) and severe acute respiratory syndrome (SARS) as experienced in Taiwan. Travel Med Infect Dis. 2020;36:101625. doi:10.1016/j.tmaid.2020.101625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gralinski LE, Menachery VD. Return of the coronavirus: 2019‐nCoV. Viruses. 2020;12(2), 135. doi:10.3390/v12020135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ludvigsson JF. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6), 1088‐1095. doi:10.1111/apa.15270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Girona‐Alarcon M, Bobillo‐Perez S, Sole‐Ribalta A, et al. The different manifestations of COVID‐19 in adults and children: a cohort study in an intensive care unit. BMC Infect Dis. 2021;21(1), 87. doi:10.1186/s12879‐021‐05786‐5. Published 2021 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jafari M, Pormohammad A, Sheikh Neshin SA, et al. Clinical characteristics and outcomes of pregnant women with COVID‐19 and comparison with control patients: a systematic review and meta‐analysis. Rev Med Virol. 2021;31(5), 1‐16. doi:10.1002/rmv.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalil A, Kalafat E, Benlioglu C, et al. SARS‐CoV‐2 infection in pregnancy: a systematic review and meta‐analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:100446. doi:10.1016/j.eclinm.2020.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID‐19 patients with digestive symptoms in Hubei, China: a descriptive, cross‐sectional, multicenter study. Am J Gastroenterol. 2020;115(5), 766‐773. doi:10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID‐19). Int J Infect Dis. 2020;94:154‐155. doi:10.1016/j.ijid.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5), 706‐711. doi:10.1007/s11427‐020‐1661‐4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu X, Yang R. COVID‐19 transmission through asymptomatic carriers is a challenge to containment. Influenza Other Respir Viruses. 2020;14(4), 474‐475. doi:10.1111/irv.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oran DP, Topol EJ. Prevalence of asymptomatic SARS‐CoV‐2 infection: a narrative review. Ann Intern Med. 2020;173(5), 362‐367. doi:10.7326/M20‐3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jethi N, Pandav G, Nagri D, et al. Asymptomatic COVID‐19 patients and possible screening before an emergency aerosol related endodontic protocols in dental clinic—a review. J Family Med Prim Care. 2020;9(9), 4552‐4556. doi:10.4103/jfmpc.jfmpc_796_20. Published 2020 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luo X, Lv M, Wang X, et al. Avoidable waste of research on coronavirus disease 2019 (COVID‐19). Obstet Gynecol. 2020;136(1), 191. doi:10.1097/AOG.0000000000003978 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1 PRISMA checklist
Supplementary Material 2 Search Strategy
Supplementary Material 3 Systematic Reviews and Meta‐analyses included and excluded references of this study
Supplementary Material 4 AMSTAR scores and main conclusions of systematic reviews
Supplementary Material 5 Heat map for different symptoms of COVID‐19
Supplementary Material 6 Subgroup analyses


