Abstract
Knowledge of the immunogenicity of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in liver transplant recipients (LTRs) is mainly limited to messenger RNA (mRNA)‐based types. We aimed to evaluate the humoral response in LTRs and to address the use of different doses of mycophenolate (MMF) on the probability of developing anti‐spike immunoglobulin G (IgG). In this prospective cohort study, SARS‐CoV‐2 anti‐spike IgG, neutralizing antibodies (NAs), and nucleocapsid protein (N) were evaluated in LTRs and healthy volunteers 21–90 days after receiving the second vaccine dose of either ChAdOx1 (AstraZeneca), rAd26‐rAd5 (Sputnik V), inactivated BBIBP‐CorV (Sinopharm), or the heterologous combination rAd26/mRNA‐1273 (Sputnik V/Moderna). We collected information regarding clinical data and vaccine side effects. After excluding three LTRs due to a positive N test, 120 LTRs and 27 controls were analyzed. No significant differences were found among groups. Overall, 24 (89%) controls and 74 (62%) LTRs were positive for anti‐spike IgG (p = 0.007). Among LTRs, those immunized with rAd26/mRNA‐1273 presented significantly higher positive serology and NAs when compared with the homologous regimens (91% vs. 55%, p = 0.001; and 1182 IU/ml vs. 446 IU/ml, p = 0.002; respectively). In the multivariate analysis, humoral response was significantly reduced in LTRs who received higher doses of MMF (odds ratio [OR], 0.1; 95% confidence interval [CI], 0.03–0.3; p < 0.001) and with increased BMI (OR, 0.4; 95% CI, 0.2–0.7; p = 0.005); and it was significantly higher in those immunized with rAd26/mRNA‐1273 (OR, 13.1; 95% CI, 2.3–72.9; p = 0.003). In LTRs anti‐spike IgG concentrations showed a very good correlation with NA titers (R 2 = 0.949; 95% CI, 0.919–0.967; p < 0.001). No serious adverse events were reported in either group. Conclusion: In LTRs, rAd26/mRNA‐1273 was independently associated with higher antibody response. Future studies are necessary to evaluate whether combining different vaccine platforms and MMF reduction may lead to a better booster response.

INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection causing coronavirus disease 2019 (Covid‐19) represents a substantial problem for public health worldwide, mainly due to its high transmission and mortality rates. Factors related to worse prognosis have been extensively described.[ 1 , 2 ] In solid organ transplant (SOT) recipients, the amount of immunosuppression correlates with the severity of varied infectious diseases,[ 3 ] which led to the initial prediction that SOT recipients may be more vulnerable to severe Covid‐19. Different platforms of Covid‐19 vaccines have proved successful in the development of humoral response and consequently in the prevention of severe forms and reduction of mortality in the general population.[ 4 , 5 ] Knowledge on a vaccine‐induced humoral response in SOT recipients is mostly limited and restricted to messenger RNA (mRNA) vaccines. Recent data on the humoral response to mRNA SARS‐CoV‐2 vaccines in SOT recipients indicated the detection of anti‐spike immunoglobulin G (IgG) ranged from 34%–81% after two doses, being significantly lower than in the general population.[ 6 , 7 , 8 , 9 ] Poor response to the vaccine has been associated with the use of mycophenolate (MMF) as well as other risk factors, such as obesity, advanced age, or short interval between doses.[ 7 , 10 , 11 ] The neutralizing antibody (NA) levels have also been shown to be strongly associated with immune protection from severe Covid‐19.[ 12 ] Immunogenicity in SOT recipients after administration of nonreplicating vector‐based and inactivated vaccines is scarce. Boyarsky et al.[ 13 ] evaluated 12 transplant recipients immunized with Ad26.COV.S (Janssen), of which only 17% of patients developed a humoral response. In addition, Prieto et al.[ 14 ] reported only a 36.5% humoral response in 74 liver transplant recipients (LTRs) after immunization with an inactivated virus vaccine (CoronaVac). Different strategies have been proposed to increase vaccine immunogenicity.
In this setting, we aimed to determine the humoral response in LTRs to the nonreplicating vector‐based vaccines ChAdOx1 (AstraZeneca/Oxford‐Covishield) and rAd26‐rAd5 (Sputnik V, Gamaleya Institute), inactivated vaccine BBIBP‐CorV (Sinopharm, Beijing Institute of Biological Products), and the heterologous combination of rAd26/mRNA‐1273 (Sputnik/Moderna), assigned as per national policies. We also assessed the use of different doses of MMF on the probability of developing anti‐spike IgG and the safety of the different SARS‐CoV‐2 vaccines in LTRs.
MATERIALS AND METHODS
Patients
This was a multicenter, prospective, observational study in which consecutive and volunteering LTRs and immunocompetent controls were recruited from August 3 to October 26, 2021. Participants were immunized either with nonreplicating adenovirus‐vector vaccines ChAdOx1 or rAd26‐rAd5, inactivated virus vaccine BBIBP‐CorV, or the heterologous regimen rAd26/mRNA‐1273. Vaccination schemes were determined by the national vaccination plan of the Ministry of Health of Argentina based on the availability of vaccines. The interdose interval varied depending on the availability of application of second doses. All variables, including the presence of side effects, were collected at the time of inclusion. Patients (aged ≥18 years) who had a functioning allograft and completed SARS‐CoV‐2 vaccination schemes were included. People with previous confirmed Covid‐19 infection documented by polymerase chain reaction, human immunodeficiency virus infection, and treatment with anti‐CD20 monoclonal antibody in the past 6 months were excluded. In all participants, the immune response was determined 21 to 90 days after the second vaccination.
Baseline assessments
Clinical data were collected at the time of inclusion and were obtained from patients' medical records and routine blood tests up to 3 months before the date of the second dose. We obtained data related to comorbidities (body mass index [BMI], arterial hypertension, chronic obstructive pulmonary disease, diabetes, and renal function by estimated glomerular filtration rate [eGFR] by modification of diet in renal disease), liver transplantation (type of immunosuppression, history of the use of high‐dose steroids in the last 3 months), and vaccination scheme (type of vaccine, time between doses, and time from second dose to extraction). Safety analysis included a phone interview at the time of inclusion to assess a patient's reported side effects either after the first or the second immunization dose. We used a predefined questionnaire on local and systemic symptoms. Study data were collected and managed using REDCap electronic data capture tools hosted at Austral University Hospital.
Dosage, quantification, and study definitions
SARS‐CoV‐2‐specific IgG antibodies toward the receptor‐binding domain of the SARS‐CoV‐2 spike protein (anti‐spike IgG) were quantified by using an enzyme‐linked immunosorbent assay according to the manufacturer's instructions (Covid‐19 spike 1 and 2 IgG; Dia.Pro Diagnostics, Italy). Antibodies toward the nucleocapsid (N) protein were analyzed by chemiluminescence assay (ALINITY SARS‐COV‐2 IGG; Abbott) in a certified biochemistry testing laboratory to estimate past SARS‐CoV‐2 infection. The results are quantitatively expressed in IU/ml (first World Health Organization International Standard for anti‐SARS‐CoV‐2 immunoglobulin, National Institute for Biological Standards and Control, code 20/136). This assay has a lower limit of detection of 10 IU/ml, and as per test instructions, those samples that presented a value >12 IU/ml in plasma were considered positive. NA titers were determined in all individuals with SARS‐CoV‐2‐positive serology, using an angiotensin‐converting enzyme 2 (ACE2)‐ receptor‐binding domain (RBD) neutralization test assay (ACE2‐RBD Neutralization Assay; Dia.Pro Diagnostics) and are expressed as the inverse of the last dilution that shows neutralizing capacity. According to the product insert, the assay results were classified as the following: low‐neutralizing titers, 1:2 dilutions; medium‐neutralizing titers, from 1:4 to 1:16; and high‐neutralizing titers, when the last dilution with neutralizing capacity was ≥1:32.
Ethical considerations
The study was performed in accordance with the Declaration of Helsinki and the E6 Good Clinical Practice Standards of the International Conference on Harmonization as well as the Guide for Human Health Research (Resolution 1480/11) of the Ministry of Health of Argentina. All personal data were codified in accordance with the Organic Law 25,325 of October 30, 2000, on the Protection of Personal Data in Argentina. All study data were treated anonymously, with restricted access by only authorized personnel for the purposes of the study. Each ethics committee from all participating centers approved the study protocol. The protocol followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.[ 15 ] All authors had access to the study data, reviewed, and approved the final version of this manuscript.
Statistical analysis
Median and interquartile range (IQR) were used to describe quantitative variables and number of cases and percentages for qualitative variables. The differences between qualitative variables were compared using the chi square‐test or Fisher's exact test, when indicated. Quantitative variables were analyzed with nonparametric tests (Mann‐Whitney or Kruskal‐Wallis for unpaired samples). To address the influence of the type of vaccination scheme and the use of MMF, we constructed a logistic regression model adjusting for BMI and time between doses because these two variables have been suggested to influence antibody response. The association is presented as odds ratios (ORs) and 95% confidence intervals (CIs). The correlation between anti‐spike IgG and the titers of NA was analyzed using the Pearson correlation. A nomogram was built to predict the presence of humoral response based on the variables of the established model. All tests were two‐tailed, and p < 0.05 was considered statistically significant. SPSS (version 26) and R (version 3.5.1) were used to perform the statistical analyses.
RESULTS
Patients characteristics
After excluding three LTRs for having a positive N protein IgG serology test, 120 LTRs and 27 immunocompetent controls were analyzed. No significant baseline differences were found among groups (Table 1). The median time from liver transplantation to the first vaccination was 4.9 years (IQR, 2.4–10.1). Nine patients were within the first year after liver transplantation, and no patient had received T‐ or B‐cell depletion therapy for induction or rejection. Calcineurin inhibitors were the main immunosuppressor in 94.7% of patients. Everolimus and sirolimus were used in 18 and five patients, respectively. Forty‐one (34.2%) patients received MMF, and 62 (51.7%) individuals were treated with only one immunosuppressive drug. Two patients received high‐dose steroid treatment for acute cellular rejection within the 3 months before vaccination. All LTRs had stable graft function before vaccination.
TABLE 1.
Baseline characteristics of the liver transplant recipients and the immunocompetent control group
| Variable | Liver transplant recipients | Controls | p value |
|---|---|---|---|
| n = 120 | n = 27 | ||
| Age (years), median (IQR) | 65 (55–71) | 68 (57–75) | 0.198 |
| Female sex, n (%) | 49 (41.2) | 15 (55.6) | 0.174 |
| Body mass index, median (IQR) | 27.7 (24.4–31.2) | 27.2 (24.1–31.2) | 0.907 |
| Comorbidities, n (%) | |||
| Hypertension | 70 (58.3) | 12 (44.4) | 0.189 |
| Diabetes | 43 (35.8) | 5 (18.5) | 0.112 |
| COPD/asthma | 9 (7.5) | 2 (7.4) | 0.897 |
| Days second‐dose antibody testing, median (IQR) | 56 (40–73) | 64 (45–74) | 0.373 |
| Days between vaccine doses, median (IQR) | 85 (59–107) | 96 (62–127) | 0.969 |
| Vaccine, n (%) | |||
| ChAdOx1 (AstraZeneca) | 35 (29.2) | 8 (29.6) | 0.962 |
| BBIBP‐CorV (Sinopharm) | 25 (20.8) | 5 (18.5) | 0.787 |
| rAd26‐rAd5 (Sputnik V) | 37 (30.8) | 6 (22.2) | 0.374 |
| r d26/mRNA‐1273 (Sputnik V/Moderna) | 23 (19.2) | 8 (29.6) | 0.229 |
| Detectable anti‐spike IgG, n (%) | 74 (61.7) | 24 (88.9) | 0.006 |
| Detectable anti‐spike IgG, n (%) | |||
| ChAdOx1(AstraZeneca) | 16 (45.7) | 8 (100) | 0.005 |
| BBIBP‐CorV (Sinopharm) | 12 (48) | 2 (40) | 0.743 |
| rAd26‐rAd5 (Sputnik V) | 25 (67.6) | 6 (100) | 0.100 |
| rAd26/mRNA‐1273 (Sputnik V/Moderna) | 21 (91.3) | 8 (100) | 0.389 |
| Anti‐spike IgG UI/ml, median (IQR) | 153.5 (68.9–706.4) | 523.3 (85.5–1755.9) | 0.009 |
| Anti‐spike IgG UI/ml, median (IQR) | |||
| ChAdOx1 (AstraZeneca) | 133 (68–495) | 86 (71–189) | 0.256 |
| BBIBP‐CorV (Sinopharm) | 80 (36–327) | 23 (15–32) | 0.329 |
| rAd26‐rAd5 (Sputnik V) | 132 (77–706) | 931 (160–1615) | 0.065 |
| rAd26/mRNA‐1273 (Sputnik V/Moderna) | 690 (73–2473) | 2015 (1642–2587) | 0.041 |
| Detectable neutralizing antibody, n (%) | 66 (55) | 22 (81.5) | 0.016 |
| Neutralizing antibody titers, n (%) | |||
| Low titer (1:2) | 6 (9.1) | 0 (0) | |
| Medium titer (1:4–1:16) | 31(47) | 7 (31.8) | |
| High titer (≥1:32) | 29 (43.9) | 15 (68.2) | 0.090 |
Abbreviations: COPD, chronic obstructive pulmonary disease; IgG, immunoglobulin G; IQR, interquartile range.
SARS‐CoV‐2 humoral response in LTRs
The specific anti‐spike IgG concentration and NA titers were significantly lower among LTRs than in immunocompetent controls after receiving both vaccination doses (62% vs. 89%, p = 0.007; median, 153; IQR, 68–706 IU/ml vs. median, 523; IQR, 85–1755 IU/ml; p = 0.009; respectively) (Figure 1A). Detection of anti‐spike IgG and NA in healthy controls and LTRs was significantly higher after immunization with the heterologous combination rAd26/mRNA‐1273 compared to both the adenovirus vector and inactivated vaccines (Table 1). In LTRs, significant differences in humoral response among vaccines were detected (Table 2). The levels of anti‐spike IgG were significantly higher in LTRs vaccinated with the heterologous regimen rAd26/mRNA‐1273 (median, 2015 IU/ml; IQR, 1642–2587) compared to the homologous schemes ChAdOx1 (median, 86 UI/ml; IQR, 71–189), rAd26‐rAd5 (median, 931 IU/ml; IQR, 160–1615), and BBIBP‐CorV (median, 23 IU/ml; IQR, 15–32) (p < 0.001; Figure 1B). Finally, in the LTR group, the median Pearson correlation between the anti‐spike IgG concentration and the NA titers was 0.949 (IQR, 0.919–0.967; p < 0.001) (Figure 2).
FIGURE 1.
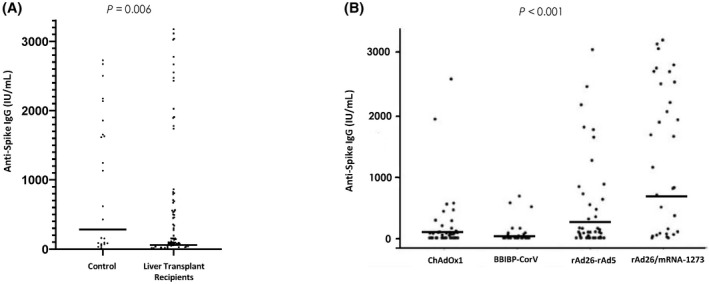
Specific anti‐spike IgG concentration in healthy controls and liver transplant recipients. (A) Differences between liver transplant recipients and the control group were analyzed by the Mann‐Whitney U test. (B) Differences in liver transplant recipients according to vaccine regimens; statistical analysis was performed using the Kruskal‐Wallis test. Data show median (solid horizontal lines) and range. IgG, immunoglobulin G.
TABLE 2.
Detection of anti‐spike IgG serology among liver transplant recipients
| Variable | Detection of IgG anti‐spike | p value | |
|---|---|---|---|
| Negative | Positive | ||
| n = 46 | n = 74 | ||
| Age (years), median (IQR) | 65 (55–72) | 64 (53–70) | 0.851 |
| Female sex, n (%) | 18 (39.1) | 31 (42.5) | 0.719 |
| Body mass index, median (IQR) | 29.7 (25.5–31.3) | 26.6 (24.2–30.9) | 0.067 |
| eGFR by MDRD‐4 mL/minute/1.73 m2, median (IQR) | 68.4 (45.6–86) | 67.6 (50.9–87.1) | 0.855 |
| Hemoglobin (g/dL), median (IQR) | 13.2 (11.8–14.4) | 13.0 (11.8–14.1) | 0.593 |
| Comorbidities, n (%) | |||
| Hypertension | 29 (63) | 41 (55.4) | 0.409 |
| Diabetes | 20 (43.5) | 23 (31.1) | 0.169 |
| COPD/asthma | 2 (4.3) | 7 (9.5) | 0.480 |
| Vaccine, n (%) | |||
| ChAdOx1 (AstraZeneca) | 19 (55) | 16 (45) | 0.020 |
| BBIBP‐CorV (Sinopharm) | 13 (52) | 12 (48) | |
| rAd26‐rAd5 (Sputnik V) | 12 (32.4) | 25 (67.6) | |
| rAd26/mRNA‐1273 (Sputnik V/Moderna) | 2 (8.7) | 21 (91.3) | |
| Immunosuppression, n (%) | |||
| Calcineurin inhibitors | 44 (95.7) | 63 (85.1) | 0.128 |
| Prednisone | 10 (21.7) | 12 (16.2) | 0.447 |
| mTOR | 5 (10.9) | 18 (24.3) | 0.095 |
| Mycophenolate | 25(54.3) | 16 (21.6) | <0.001 |
| High‐dose steroids in the last 3 months, n (%) | 1 (2.2) | 1 (1.4) | 0.732 |
| Time | |||
| Days between second dose and extraction, median (IQR) | 61 (47–76) | 52 (37–68) | 0.279 |
| Days between first and second vaccine dose, median (IQR) | 68 (54–93) | 92 (69–114) | 0.001 |
| Years since transplantation, median (IQR) | 3.5 (1.4–7.7) | 5.6 (3.1–11.1) | 0.091 |
Abbreviations: COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; IgG, immunoglobulin G; IQR, interquartile range; MDRD‐4, modification of diet in renal disease; mTOR, mammalian target of rapamycin.
FIGURE 2.
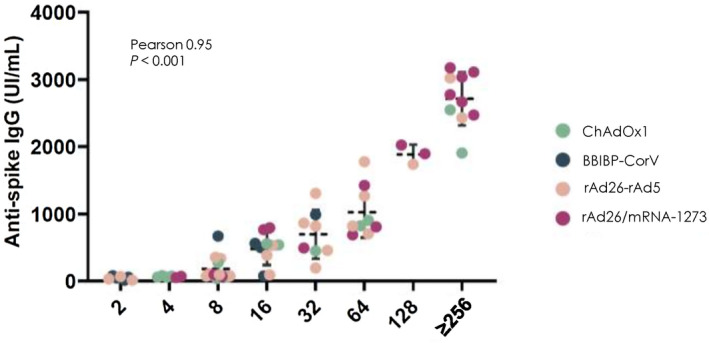
Pearson correlation between anti‐spike IgG concentration (IU/ml) and neutralizing antibodies dilution in liver transplant recipients according to the vaccine regimen used. Dashed horizontal line indicates median. IgG, immunoglobulin G.
Factors associated with humoral response in LTRs
Comparison of the clinical and laboratory data for LTRs with negative and positive anti‐spike IgG serology responses is presented in Table 2. Overall, there were no significant differences in age, sex, BMI, and eGFR. In the univariate analysis, the factors associated with a lack of humoral response were the use of MMF (lower response to higher doses used), the vaccination scheme based on ChAdOx1, and a shorter time between the application of the first and second vaccine dose (Figure 3A–C). In contrast, the use of the rAd26/mRNA‐1273 heterologous scheme was associated with higher rates of humoral response (Figure 3D). In a multivariable analysis, including time between doses, BMI, use of different doses of MMF, and type of vaccination schemes, the presence of a higher BMI and the use of increasing doses of MMF were independently associated with a higher risk of lack of humoral response, while the administration of rAd26/mRNA‐1273 was independently associated with a higher humoral response rate (Table 3). The impact of the type of vaccination scheme as a function of the MMF dose and adjusting for BMI and interdose interval is depicted in Figure 4; this demonstrates the significant impact of the heterologous scheme, even in the presence of high doses of MMF. Finally, for easy clinical use, we added a predictive nomogram to individualize the probability of developing a humoral response according to the different variables analyzed (Figure 5).
FIGURE 3.
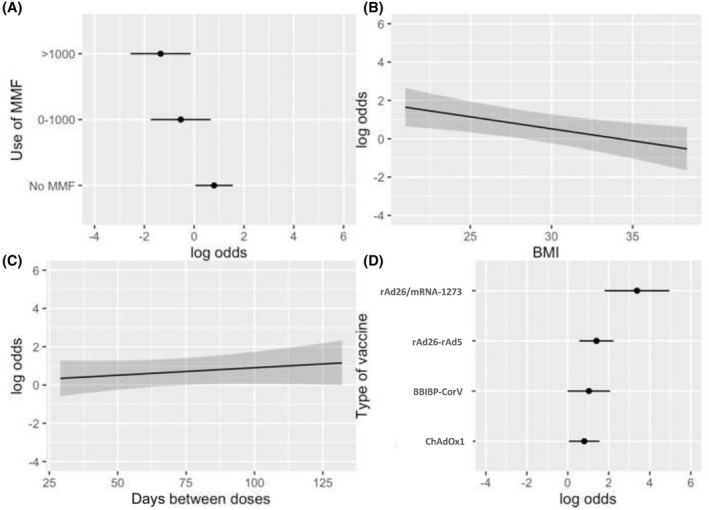
Humoral response rate. Rate according to (A) MMF dose, (B) increasing BMI, (C) days between vaccine doses, and (D) vaccine type. BMI, body mass index; MMF, mycophenolate.
TABLE 3.
Multivariable logistic regression model addressing the influence of the type of vaccination and the use of mycophenolate for developing anti‐spike IgG, adjusted for body mass index and time between doses
| Variable | OR (95% CI) | p value |
|---|---|---|
| Body mass index | 0.426 (0.234–0.771) | 0.005 a |
| Vaccine type | ||
| ChAdOx1 (AstraZeneca) | [Ref.] | [Ref.] |
| BBIBP CorV (Sinopharm) | 1.256 (0.414–3.808) | 0.687 |
| rAd26‐rAd5 (Sputnik V) | 1.814 (0.605–5.432) | 0.287 |
| rAd26/mRNA‐1273 (Sputnik V/Moderna) | 13.138 (2.368–72.891) | 0.003 a |
| Mycophenolate use | ||
| No mycophenolate | [Ref.] | [Ref.] |
| Mycophenolate dose ≤1000 mg | 0.262 (0.083–0.820) | 0.021 a |
| Mycophenolate dose >1000 mg | 0.117 (0.036–0.376) | <0.001 a |
| Time between vaccine doses | 1.502 (0.691–3.265) | 0.304 |
Abbreviations: CI, confidence interval; IgG, immunoglobulin G; OR, odds ratio; Ref., reference.
p value is significant.
FIGURE 4.
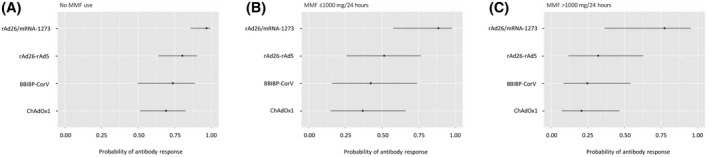
Probability of developing anti‐spike immunoglobulin G based on the dose of MMF and the vaccine type, adjusted for body mass index and time between vaccine doses. (A) No MMF use, (B) MMF dose ≤1000 mg, and (C) MMF dose >1000 mg. MMF, mycophenolate.
FIGURE 5.
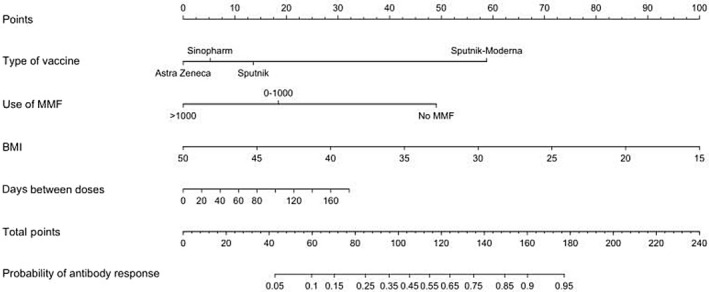
Nomogram to predict the probability of developing an antibody response. Antibody response is based on the variables included in the multivariate analysis (MMF dose, BMI, vaccine regimen, and days between the first and second dose application). Example: AstraZeneca vaccine (0 points), no MMF (50 points), BMI 30 (55 points), and 80 days between the first and second dose (13 points); total 118 points = 0.55 probability of antibody response. BMI, body mass index; MMF, mycophenolate.
Safety
Overall, the different vaccine regimens were well tolerated and no major adverse events occurred in any participant (Table S1). The frequency of any side effect after the first or second dose was similar in both groups (61/120, 51% vs. 17/27, 63% for LTRs and controls, respectively; p = 0.2). The most common systemic side effect in both groups was fatigue, followed by fever and myalgia. During the follow‐up period (more than 90 days after the second dose), no events of graft rejection, allergic reaction, or neurologic events were reported in LTRs.
DISCUSSION
The Covid‐19 pandemic is one of the most significant medical milestones in history, representing a real challenge to adopting the best prevention strategies. One of these challenges is the equitable distribution of vaccines. Considering that most of the low‐ and middle‐income countries do not have access to mRNA vaccines, we believe our assessment of effectiveness and safety of viral‐vector inactivated vaccines and especially the heterologous combination viral vector/mRNA in LTRs is relevant. Compared with subjects who were immunocompetent, we described a significantly lower humoral response to SARS‐CoV‐2 vaccines in LTRs. Interestingly, the regimen rAd26/mRNA‐1273 was significantly associated with higher anti‐spike IgG and NA development compared to viral‐vector and inactivated vaccines, even in the presence of increasing doses of MMF. Overall, vaccines were well tolerated with no serious adverse events in any of the schemes applied.
Several recently published studies reported a humoral response rate ranging from 37% to 81% in LTRs immunized with mRNA vaccines.[ 10 ] In line with these findings, we described a 62% positive‐serologic response rate. However, the heterologous combination rAd26/mRNA‐1273 presented a significantly higher response rate (91%) compared to the different homologous regimens included in our study. These findings are similar to those recently described by Ruether et al.[ 7 ] who reported an 82% seroconversion rate in 11 LTRs who received prime ChAdOx1 and second mRNA vaccination. A robust immune response of heterologous vaccination schedules has been described in immunocompetent individuals.[ 16 , 17 ] Given the ongoing pandemic, different studies have evaluated a third and fourth booster dose, describing an increased humoral response.[ 18 , 19 ] Larger studies with in‐depth analysis are needed to evaluate the best booster option to improve the vaccination response.
The most relevant factors influencing a negative humoral response in our study are consistent with previous reports and include the use of MMF and increased BMI.[ 8 , 9 , 10 , 20 , 21 , 22 ] The effectiveness of the Covid‐19 vaccine depends on multiple factors related to patients, vaccine types, and immunosuppression characteristics. We constructed a nomogram capable of predicting the probability of developing antibody response that could be of relevance in clinical practice. The vaccine regimen and the MMF dose are two of the modifiable variables that could potentially improve humoral response in LTRs. Our study supports the dose‐dependent unfavorable effect of MMF on humoral immune response. Moreover, Kantauskaite et al.[ 23 ] demonstrated a negative correlation between trough MMF concentrations in the blood and antibody titers in kidney transplant recipients. The detrimental effects of an MMF‐containing immunosuppressive regimen in the vaccination‐induced humoral immune response are not just evident after vaccination against SARS‐CoV2. Previous studies also reported a significant attenuated immune response of MMF treatment in SOT recipients after vaccination against Pneumococcus pneumoniae and influenza virus.[ 24 , 25 , 26 , 27 ] MMF inhibits both T‐ and B‐cell proliferation by depleting guanosine nucleotides through inhibition of the enzyme inosine monophosphate dehydrogenase, which is expressed preferentially in activated lymphocytes.[ 28 ] However, there are no sufficient data to recommend universal MMF reduction to increase vaccine response. This decision should be taken on an individual patient basis and balanced with the risk of organ rejection. Based on our findings, we believe that a heterologous vaccine schedule for MMF users may be recommended to ensure a higher humoral immune response. In this sense, our nomogram can be a useful tool to individualize this decision. Further clinical studies are needed to define whether reduction of immunosuppression levels before vaccination is possible and for how long.
The main strengths of our study are (i) the assessment of the humoral immune response, particularly IgG levels and neutralizing capacity of LTRs immunized with different vaccination regimens mostly used in low‐ and middle‐income countries, and (ii) the comparison of these outcomes with a control group in a real‐world setting. Likewise, the possibility of measuring the impact of MMF doses and vaccination schedules on the probability of humoral response describes a point of clear relevance in the current scenario of the Covid‐19 pandemic.
Our study has limitations, particularly related to a relatively small sample size and the absence of vaccine regimens with two doses of mRNA. We were also unable to determine previous SARS‐CoV‐2 infection in subjects that received the inactivated vaccine BBIBP‐CorV, but given the low antibody response detected in these patients, we may infer that they were not previously exposed to the virus. Another limitation is that we did not assess cellular immune response. However, we will continue our study to obtain data about humoral and cellular immune response after a third vaccine dose that has recently been introduced in Argentina as a booster for this population.
To conclude, our study revealed that the heterologous combination viral‐vector/mRNA regimen produces a strikingly high humoral response rate compared to other inactivated and homologous viral‐vector regimens, even in the presence of high doses of MMF where the humoral response of other regimens has been reported as substantially lower. Future studies with larger sample sizes are necessary to confirm whether combining different vaccine platforms may lead to a more comprehensive booster response exceeding that of a series of homologous vaccines. In the meantime, personal protection measures and advocacy for higher vaccination rates are essential in the LTR population.
FUNDING INFORMATION
Argentine Society of Hepatology (SAHE), National Ministry of Health of Argentina, Salud Investiga 2021–2022; Grant Number: 4052/2021.
CONFLICT OF INTEREST
Nothing to report.
Supporting information
Figure S1 Weight of each of the components within the prediction model. Higher X2 and lower p‐value represent a greater contribution of that variable in explaining antibody response.
Table S1. Side effects
Mendizabal M, Ducasa N, Benencio P, Anders M, Cairo F, Barbero M, et al. Heterologous adenovirus‐vector/messenger RNA regimen is associated with improved severe acute respiratory syndrome coronavirus 2 humoral response in liver transplant recipients. Hepatol Commun. 2022;6:2850–2859. 10.1002/hep4.2034
Manuel Mendizabal, Nicolás Ducasa, and Paula Benencio contributed equally to this work as first authors.
Mirna Biglione and Ezequiel Mauro contributed equally to this work as senior authors.
Contributor Information
Manuel Mendizabal, Email: mmendiza@cas.austral.edu.ar.
Ezequiel Mauro, Email: ezequiel.mauro@hiba.org.ar.
REFERENCES
- 1. Mendizabal M, Ridruejo E, Piñero F, Anders M, Padilla M, Toro LG, et al. Comparison of different prognostic scores for patients with cirrhosis hospitalized with SARS‐CoV‐2 infection. Ann Hepatol. 2021;25:100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fishman JA. Infection in solid‐organ transplant recipients. N Engl J Med. 2007;357:2601–14. [DOI] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas S, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid‐ 19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Logunov DY, Dolzhikova IV, Shcheblyakov DV, Tukhvatulin AI, Zubkova OV, Dzharullaeva AS, et al.; Gam‐COVID‐Vac Vaccine Trial Group . Safety and efficacy of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–81. Erratum in: Lancet. 2021;397:670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rabinowich L, Grupper A, Baruch R, Ben‐Yehoyada M, Halperin T, Turner D, et al. Low immunogenicity to SARS‐CoV‐2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, et al. SARS‐CoV2‐specific humoral and T‐cell immune response after second vaccination in liver cirrhosis and transplant patients. Clin Gastroenterol Hepatol. 2022;20:162–72.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guarino M, Cossiga V, Esposito I, Furno A, Morisco F. Effectiveness of SARS‐Cov‐2 vaccination in liver transplanted patients: the debate is open! J Hepatol. 2022;76:237–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, et al. Antibody response to 2‐dose sars‐cov‐2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabinowich L, Shibolet O, Katchman H. Reply to: Effectiveness of SARS‐Cov‐2 vaccination in liver transplanted patients: the debate is open! J Hepatol. 2022;76:239–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cholankeril G, Al‐Hillan A, Tarlow B, Abrams D, Jacobs JS, Flores NP, et al. Clinical factors associated with lack of serological response to SARS‐CoV‐2 mRNA vaccine in liver transplant recipients. Liver Transpl. 2022;28:123–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khoury D, Cromer D, Reynaldi A, Schlub TE, Wheatley AK, Juno JA, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS‐ CoV‐ 2 infection. Nat Med. 2021;27:1205–11. [DOI] [PubMed] [Google Scholar]
- 13. Boyarsky BJ, Chiang TP‐Y, Ou MT, Werbel WA, Massie AB, Segev DL, et al. Antibody response to the Janssen COVID‐19 vaccine in solid organ transplant recipients. Transplantation. 2021;105:e82–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prieto J, Rammauro F, López M, Rey R, Fernández A, Bianchi S, et al. Low immunoglobulin G antibody levels against severe acute respiratory disease coronavirus 2 after two‐dose vaccination among liver transplant recipients. Liver Transpl. 2022;28:891–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Von EE, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt T, Klemis V, Schub D, Mihm J, Hielscher F, Marx S, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV‐19/mRNA vaccination. Nat Med. 2021;27:1530–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borobia AM, Carcas AJ, Pérez‐Olmeda M, Castaño L, Bertran MJ, García‐Pérez J, et al.; CombiVacS Study Group . Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1‐S‐primed participants (CombiVacS): a multicentre, open‐label, randomised, controlled, phase 2 trial. Lancet. 2021;398:121–30. Erratum in: Lancet. 2021:398:582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamar N, Abravanel F, Marion O, Couat C, Izopet J, del Bello A. Three doses of an mRNA covid‐19 vaccine in solid‐organ transplant recipients. N Engl J Med. 2021;385:661–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alejo JL, Mitchell J, Chiang TP, Abedon AT, Boyarsky BJ, Avery RK, et al. Antibody response to a fourth dose of a SARS‐CoV‐2 vaccine in solid organ transplant recipients: a case series. Transplantation. 2021;105:e280–e281. Erratum in: Transplantation. 2022:106:e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall VG, Ferreira VH, Ierullo M, Ku T, Marinelli T, Majchrzak‐Kita B, et al. Humoral and cellular immune response and safety of two‐dose SARS‐CoV‐2 mRNA‐1273 vaccine in solid organ transplant recipients. Am J Transpl. 2021;21:3980–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cuadrado A, Barrio M, Fortea JI, Amigo L, Segundo DS, Rodriguez‐Cundin MP, et al. Antibody response to the messenger RNA‐1273 vaccine (Moderna) in liver transplant recipients. Hepatol Commun. 2022;6:1673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toniutto P, Falleti E, Cmet S, Cussigh A, Veneto L, Bitetto D, et al. Past COVID‐19 and immunosuppressive regimens affect the long‐term response to anti‐SARS‐CoV‐2 vaccination in liver transplant recipients. J Hepatol. 2022;77:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kantauskaite M, Müller L, Kolb T, Fischer S, Hillebrandt J, Ivens K, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS‐CoV‐2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scharpé J, Evenepoel P, Maes B, Bammens B, Claes K, Osterhaus AD, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8:332–7. [DOI] [PubMed] [Google Scholar]
- 25. Mulley WR, Visvanathan K, Hurt AC, Brown FG, Polkinghorne KR, Mastorakos T, et al. Mycophenolate and lower graft function reduce the seroresponse of kidney transplant recipients to pandemic H1N1 vaccination. Kidney Int. 2012;82:212–9. [DOI] [PubMed] [Google Scholar]
- 26. Baluch A, Humar A, Eurich D, Egli A, Liacini A, Hoschler K, et al. Randomized controlled trial of high‐dose intradermal versus standard‐dose intramuscular influenza vaccine in organ transplant recipients. Am J Transplant. 2013;13:1026–33. [DOI] [PubMed] [Google Scholar]
- 27. Kumar D, Rotstein C, Miyata G, Arlen D, Humar A. Randomized, double‐ blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J Infect Dis. 2003;187:1639–45. [DOI] [PubMed] [Google Scholar]
- 28. Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80(Suppl):S181–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Weight of each of the components within the prediction model. Higher X2 and lower p‐value represent a greater contribution of that variable in explaining antibody response.
Table S1. Side effects


