Abstract
Due to the recent coronavirus disease 2019 (COVID‐19) pandemic and emergent administration of various vaccines worldwide, comprehensive studies on the different aspects of vaccines are in demand. This study evaluated antibody response after the second dose of the COVID‐19 vaccine in the Children's Medical Center personnel. The blood samples of 174 healthcare workers were gathered at least 10 days after vaccination. The administered vaccines included Oxford/AstraZeneca, COVAXIN, Sinopharm, and Sputnik V. This study assessed all antibodies employing ELISA methods, including anti‐SARS‐CoV‐2 neutralizing antibody by DiaZist and Pishtazteb kits, anti‐SARS‐CoV‐2‐nucleocapsid by Pishtazteb kit, and anti‐SARS‐CoV‐2‐Spike by Razi kit. The cutoff for the tests' results was calculated according to the instructions of each kit. Totally, 174 individuals with an average age of 40 ± 9 years participated in this study, the proportion of men was 31%, and the frequency of past COVID‐19 infection was 66 (38%). Sixteen (9%) personnel received Oxford/AstraZeneca, 28 (16%) COVAXIN, 29 (17%) Sinopharm, and 101 (58%) Sputnik V. anti‐SARS‐CoV‐2‐nucleocapsid and anti‐SARS‐CoV‐2‐Spike were positive in 37 (21%), and 163 (94%) participants and their mean level were more in adenoviral‐vectored vaccines (p value < 0.0001). Neutralizing antibody was positive in 74% using Pishtazteb kit while 87% using DiaZist kit. All antibodies' levels were significantly higher in those with a past COVID‐19 infection (p value < 0.0001). In conclusion, Oxford/AstraZeneca and Sputnik V had a similar outcome of inducing high levels of anti‐SARS‐Cov‐2‐spike and neutralizing antibodies, which were more than Sinopharm and COVAXIN. The titers of Anti‐SARS‐CoV‐2‐nucleocapsid antibody were low in all of these four vaccines.
Keywords: anti‐spike antibody, COVAXIN, COVID‐19 vaccine, neutralizing antibody, Oxford/AstraZeneca, Sinopharm, Sputnik V
1. INTRODUCTION
It has been more than 2 years since the Coronavirus Disease 2019 (COVID‐19) pandemic caused by the newly emerged Severe Respiratory Distress Syndrome Coronavirus 2 (SARS‐CoV‐2), an airborne infection that can lead to various signs, symptoms, and complications. 1 These symptoms diverge from an asymptomatic carrier to acute respiratory distress syndrome, severe extra‐pulmonary reactions, end‐organ failure, and death. 2 , 3 More than half a billion are diagnosed with COVID‐19, and six million people have died due to this disease worldwide so far. 4 , 5 The SARS‐CoV‐2 whole‐genome study demonstrated that from 5′ terminal to 3′, it comprises a primary translation region containing 14 Open‐reading Frames (ORFs) that code 27 proteins. 6 ORF1a and ORF1b code two polyproteins that divide into 16 nonstructural proteins after proteolysis. 7 ORF1ab is followed by 13 ORFs that code four main structural proteins, including spike (S), envelope (E), membrane (M), nucleocapsid (N), and eight subsidiary proteins. 8 The SARS‐CoV‐2‐S is a crucial protein responsible for binding to the Angiotensin‐Converting Enzyme 2 (ACE2) and entrance to the host cells. The S protein comprises two domains, the S1 part or receptor‐binding domain (RBD) and the S2 part or N‐terminal domain. 9 The neutralizing antibody against RBD blocks the entrance of the virus to the host cell. 10 , 11 It warrants the host's immunity against COVID‐19, albeit the humoral immunity system produces many other antibodies. 12 Both humoral and cellular immunity is crucial to be induced by the vaccine in a brief period to protect the body against adverse complications of the COVID‐19. 9 , 13 Most of the released COVID‐19 vaccines are employed to generate a constant and a proper count of anti‐SARS‐CoV‐2 neutralizing antibodies.
Administration of the COVID‐19 vaccine began in December 2020. Until now, more than 65% of the world population has received at least one dose of the COVID‐19 vaccine. 14 Predictably the vast majority of the unvaccinated population are from low‐income countries.
According to World Health Organization, the number of COVID‐19 vaccines accounts for 349, and 153 of them are in the clinical phase. 15 In total, candidate COVID‐19 vaccines are established based on the 11 major platforms, including protein subunit, replicating viral vector, non‐replicating viral vector, DNA, RNA, virus‐like particles, inactivated virus, viral vector replicating (VVr) + antigen presenting cell, live attenuated virus, VVnr + antigen presenting cell, and bacterial antigen‐spore expression vector. 15 Most doses of vaccines delivered to the world population belong to Sinovac (inactivated virus), Pfizer‐BioNTech (mRNA based), Oxford/AstraZeneca (non‐replicating viral vector), Sinopharm (inactivated virus), Moderna (mRNA based), Johnson & Johnson (nonreplicating viral vector), Sputnik V (nonreplicating viral vector), and COVAXIN (inactivated virus). 16 Adenoviral vectors are affirmed to cause an acceptable, safe, and long‐lasting immune response against the target antigen and the vector's particles. 17 , 18
In Iran, more than 140 000 people have died due to COVID‐19, and more than seven million people have been diagnosed with COVID‐19 by June 2022. 19 Vaccination against COVID‐19 in Iran began on February 9, 2021. The last reports indicate that more than 75% of the Iranian population has received at least one dose of the Coronavirus vaccine, and fully vaccinated population has encompassed 57 million people. 14 By June 2022, the Iranian ministry of health has approved 12 vaccines against COVID‐19 that are based on three different platforms, including protein subunit, nonreplicating viral vector, and inactivated virus. 20 Sputnik V, COVAXIN, Oxford/AstraZeneca, and Sinopharm were approved earlier for emergency use against COVID‐19, comprising the most administered vaccine doses in Iran. 21 , 22 The eight other vaccines were later approved, including COVIran Barekat, FAKHRAVAC, Razi Cov Pars, Soberana 02, Noora Vaccine, Sputnik light, Ad26.COV2.S (Johnson & Johnson), and SpikoGen. 20 Various studies have tried to evaluate attitude and willingness toward the COVID‐19 vaccine. In total, attitude toward vaccination against COVID‐19 among Iranian has been reported positive regardless of the vaccine type, from about 70% acceptance rate to more than 80% acceptance rate, which is slightly more than the global willingness. 23 , 24 , 25 , 26 , 27 Reportedly, misinformation and lack of knowledge contribute to hesitancy and negative attitudes toward COVID‐19 vaccination. 23 , 28 , 29
Due to the extensive mortality of COVID‐19, the health authorities approved emergency administration of the COVID‐19 vaccines worldwide. However, many aspects and features of these vaccines, especially COVAXIN and Sputnik V, are under the shadow of uncertainty.
Consequently, more studies are required to gather comprehensive data and obtain cumulative knowledge about these vaccines. This study has evaluated the anti‐SARS‐CoV‐2‐S antibody and anti‐SARS‐CoV‐2‐N antibody in healthcare workers at Children's Medical Center, a referral hospital in Iran.
2. METHODS
2.1. Sampling and vaccine types
This cross‐sectional study was conducted in the Children's Medical Center of excellence, an Iranian referral hospital, from March to September 2021. The research ethics committee of Tehran University of Medical Sciences approved the project with the ethical code IR.TUMS.CHMC.1400.104 in March 2021. All enrolled individuals signed an informed consent form before the study.
Criteria to include the participants consisted of healthy and nonretired local healthcare workers who had received the second dose of COVID‐19 before the past 10 days or more. Furthermore, negative history of using immunocompromising drugs from the first vaccine dose was essential to enroll in the study. The Children's Medical Center supplied the COVID‐19 vaccine doses from the national health ministry. The COVID‐19 vaccine type administered in the second dose was considered identical to the first COVID‐19 vaccine. These types included Oxford/AstraZeneca (ChAdOx1‐S‐(AZD1222), Covishield, Vaxzevria), Sinopharm (BBIBP‐CorV), Sputnik V (Gam‐COVID‐Vac Adeno‐based, rAd26‐S + rAd5‐S), and COVAXIN (BBV152 vaccine).
Blood sampling that at least 10 days after the second dose was begun in April 2021 and ended in mid‐September 2021. Peripheral venous blood (2.5 ml) was collected from the elbow of healthcare workers. Then, the serums were obtained after centrifugation with high‐speed centrifuge at 8,000 × g for 10 min and were stored in the refrigerator at −20 degrees Celsius. The collected parameters include age, sex, COVID‐19 vaccine type and the time of the second dose, vaccination complications, and previous COVID‐19 infection.
History of COVID‐19 infection before vaccination was considered positive as if the diagnosis had been made by a physician due to one of the following criteria: (1) Positive SARS‐CoV‐2 Real‐Time PCR test. (2) developing COVID‐19‐related signs and symptoms within 14 days after traveling to a high‐risk area or being in close contact with a person with proved COVID‐19 infection. (3) Developing signs and symptoms along with related COVID‐19 involvements in the radiographic study of the chest. 30 , 31 , 32
2.2. Neutralizing antibody
Neutralizing antibody interacts with the RBD of the S glycoprotein on the surface of the SARS‐CoV‐2, which is responsible for entering the host cells by binding to the ACE2 ligand. 33 This experiment implemented the enzyme‐linked immunosorbent assay (ELISA) kits (DiaZist and Pishtazteb) (competitive method) and applied their indicated instruction.
During the antibody titer quantification by Pishtazteb kit, Anti RBD immunoglobulins in the patients' serum and conjugated ACE2 with horseradish peroxidase (HRP) enzyme get simultaneously exposed to the plate coated with RBD Ag. After washing the wells and adding the chromogen substrate, the composed immune complexes in the dish make a bluish color. The stop‐solution changes blue color to yellow with an optimum absorption at the 450 nanometer and luminous intensity that is inversely correlated with the amount of the composing immune complexes in the plate. The intensity of the color formed is proportional to the amount of enzyme present and is inversely related to the amounts of neutralizing antibodies to SARS‐CoV‐2 in the sample.
Following Diazist ELISA kit instructions, the plate is washed five times after adding a buffer to diluted serum. In the next step, the conjugated enzyme is added to the plate, and after 30 min, the plate is rewashed. Dispensing chromogen substrate to the wells is followed by a 15‐min incubation and adding the stop‐solution. The standard curve for this method has a positive decreasing slope, which means the luminous intensity is directly correlated with the concentration of the neutralizing antibody. The cutoff for a positive result is antibody titers equal to or more than 11 AU/ml, and titers lower than 9 AU/ml are considered negative. The amounts between 9 and 11 AU/ml are considered borderline.
2.3. Anti‐SARS‐CoV‐2 S and anti‐SARS‐CoV‐2 N antibodies
A particular ELISA kit (Razi) (Sandwich method) was used to assess the quantity of anti‐SARS‐CoV‐2‐S IgG in the patient's serum. The IgG against SARS‐CoV‐2 in the diluted serum attaches to the S antigens of the bottom of the plate. An anti‐human IgG antibody added to the plate after washing the plate binds to the fragment crystallizable (FC) region of anti‐SARS‐CoV‐2‐S IgG. The luminous intensity of the bluish color formed after adding chromogen‐substrate correlates with the quantity of the composed immune complex.
Qualitative measurement of the Anti‐SARS‐CoV‐2‐N antibody was performed with the Anti‐SARS‐CoV‐2‐N IgG ELISA kit (Pishtazteb) with the similar steps.
2.4. Statistical analysis
After gathering the data, the first step was to statistically check whether the numeric variables have a normal distribution (Shapiro–Wilk test) to determine which statistical test should be implemented (parametric or non‐parametric). Evaluation of correlation between nominal or ordinal variables was performed by Chi‐square test. Parametric and nonparametric assays to compare groups and assess relationships for numeric variables included ANOVA and Mann–Whitney U tests. This study employed the Statistical Package for the Social Sciences (IBM® SPSS® Statistics) software version 26 for statistical analysis. The significance level (p value) for the result to be meaningful was considered lower than 0.05.
3. RESULTS
Totally, 174 healthy healthcare workers enrolled in this study with a mean age of 40 ± 9 years. Fifty‐four of the participants (31%) were males, and 66 (38%) of them reported a history of previously diagnosed COVID‐19 infection. Regarding the frequency of past COVID‐19, there were no statistical difference between the types of vaccines (p value = 0.583). The demographical data of the patients are available in Table 1.
Table 1.
The demographic data of participants regarding vaccine type and in total
| Variables | AstraZeneca | COVAXIN | Sinopharm | Sputnik V | Total | p‐value |
|---|---|---|---|---|---|---|
| Frequency, (%) | 16 (9%) | 28 (16%) | 29 (17%) | 101 (58%) | 174 (100%) | |
| Age, years (mean ± SD) | 32 ± 6 | 44 ± 8 | 42 ± 8 | 39 ± 9 | 40 ± 9 | |
| Days from vaccine to test (mean (min‐max)) | 25 (15–53) | 41 (15–101) | 35 (25–105) | 62 (11–180) | 59 (11–180) | |
| The first and second dose interval, days (mean ± SD) | 92.7 ± 1.9 | 30.3 ± 6 | 29.1 ± 2.3 | 29.7 ± 5 | 35.9 ± 19.4 | |
| Previously diagnosed COVID‐19, (%) | 6 (38%) | 10 (36%) | 8 (28%) | 42 (42%) | 66 (38%) | 0.583 |
Abbreviations: COVID‐19, coronavirus disease 2019; SD, standard deviation.
The first dose of vaccines was administered from January 2021 to July 2021. The administration of the second dose was from February 2021 to August 2021. Meanwhile, the mean interval between the first and the second dose of vaccines has been represented in Table 1.
This study also evaluated the vaccine adverse effects 48–72 h after injection. The most frequent symptoms included myalgia (30%), fever (21%), headache (14%), chills (14%), fatigue (13%), and pain in injection site (7%). The frequency of symptoms is shown in Table 2.
Table 2.
The frequency of different symptoms after vaccination
| AstraZeneca (%) | COVAXIN (%) | Sinopharm (%) | Sputnik V (%) | Total (%) | |
|---|---|---|---|---|---|
| Fever | 12 (75) | 2 (7) | 1 (3.4) | 23 (22) | 36 (20.6) |
| Fatigue | 2 (12.5) | 4 (14) | 0 | 16 (16) | 22 (12.6) |
| Headache | 1 (6.3) | 2 (7) | 4 (13.8) | 17 (17) | 24 (13.7) |
| Myalgia | 8 (50) | 5 (17.8) | 4 (13.8) | 34 (33.6) | 51 (29.3) |
| Chills | 5 (31.3) | 0 | 1 (3.4) | 18 (17.8) | 24 (13.7) |
| Pain in injection site | 0 | 2 (7) | 6 (21.4) | 4 (4) | 12 (6.8) |
| Hypertension | 0 | 1 (3.5) | 0 | 1 (18) | 2 (1.1) |
| Abdominal pain | 0 | 0 | 0 | 1 (18) | 1 (0.5) |
| Vomiting | 0 | 0 | 0 | 1 (18) | 1 (0.5) |
| Back pain | 0 | 0 | 1 (3.4) | 1 (18) | 2 (1.1) |
| Vertigo | 0 | 2 (7) | 0 | 2 (2) | 4 (2.2) |
| Anosmia | 0 | 0 | 0 | 1 (18) | 1 (0.5) |
| Foot pain | 0 | 0 | 0 | 2 (2) | 2 (1.1) |
| Sore throat | 0 | 0 | 1 (3.4) | 1 (18) | 2 (1.1) |
| Ear pain | 0 | 0 | 0 | 1 (18) | 1 (0.5) |
| Nausea | 0 | 1 (3.5) | 2 (7) | 1 (18) | 4 (2.2) |
| Lethargy | 0 | 0 | 0 | 2 (2) | 2 (1.1) |
| Achilles tendonitis | 0 | 0 | 0 | 1 (18) | 1 (0.5) |
| Flushing | 0 | 2 (7) | 0 | 1 (18) | 3 (1.7) |
| Rhinorrhea | 0 | 0 | 1 (3.4) | 0 | 1 (0.5) |
| Post‐nasal drip | 0 | 0 | 1 (3.4) | 0 | 1 (0.5) |
| Hand pain | 0 | 1 (3.5) | 0 | 1 (18) | 2 (1.1) |
| Sweating | 0 | 1 (3.5) | 0 | 1 (18) | 2 (1.1) |
| Arm or shoulder pain | 0 | 0 | 0 | 1 (18) | 1 (0.5) |
| Hip pain | 0 | 0 | 1 (3.4) | 0 | 1 (0.5) |
| Agitation | 0 | 0 | 0 | 1 (18) | 1 (0.5) |
| Menstrual changes | 0 | 0 | 1 (3.4) | 0 | 1 (0.5) |
| Chest pain | 0 | 0 | 1 (3.4) | 0 | 1 (0.5) |
| Abortion | 0 | 1 (3.5) | 0 | 0 | 1 (0.5) |
| Mottling | 0 | 1 (3.5) | 0 | 0 | 1 (0.5) |
In total, 156 participants filled the career field in this study. They were categorized into three groups. Group one included frontline healthcare workers with direct clinical contact with patients, including physicians, registered or practical nurses, and care aids.
Group two were the ones who had contact with clients but were not in clinical or direct contact with patients, including janitors, housekeepers, cleaners, cashiers, and laboratory technicians. The last group was those in administrative departments that were not in contact with clients or had been able to work remotely during the lockdown period. Of 156 individuals, 78 (50%) were from the first group (physicians, nurses, care aids), 43 (27.5%) were from the second group, and finally, 35 (22.5%) were from the administrative departments. This study applied the Kruskal‐Wallis statistical test, and no consistent pattern was found between these three personnel groups regarding the anti‐S, anti‐N, or neutralizing antibody titers.
3.1. Anti‐SARS‐CoV‐2‐N antibody
This study measured the anti‐SARS‐CoV‐2‐N IgG qualitative and quantitative. In total, serum samples of 37 out of 174 participants were positive, 135 (78%) were negative, and 2 (1%) were borderline for the anti‐SARS‐CoV‐2‐N IgG levels.
The quantification of anti‐SARS‐CoV‐2‐N IgG revealed an average level of 0.933 in serum samples of participant. The mean levels of this antibody were 1.08 AU/ml for AstraZeneca, 1.98 AU/ml for COVAXIN, 1.25 AU/ml for Sinopharm, and 0.52 AU/ml for Sputnik V.
The statistical analysis revealed a significant difference between levels of anti‐SARS‐CoV‐2‐N IgG in each group of vaccinations for both quality and quantity titers of antibody (p value < 0.05). In addition, there was no correlation between the levels of anti‐N antibody and the time interval between vaccine and the test (p value = 0.37).
The median and interquartile range (IQR) of anti‐SARS‐CoV‐2‐N antibody for each type of vaccine is shown in Table 3.
Table 3.
The comparison of the anti‐SARS‐CoV‐2 antibodies in each vaccine group
| Variable | AstraZeneca | COVAXIN | Sinopharm | Sputnik V | Total | p value | |
|---|---|---|---|---|---|---|---|
| Anti‐SARS‐CoV‐2 nucleocapsid antibody, (%) | Positive | 4 (25) | 11 (39) | 7 (24) | 15 (15) | 37 (21) | 0.005* |
| Negative | 12 (75) | 17 (61) | 20 (69) | 86 (85) | 135 (78) | ||
| Borderline | 0 | 0 | 2 (7) | 0 | 2 (18) | ||
| Anti‐SARS‐CoV‐2 nucleocapsid antibody, median (IQR) | 0.23 (0.76) | 0.68 (1.57) | 0.47 (0.9) | 0.17 (0.46) | 0.29 (0.65) | 0.000** | |
| Neutralizing antibody (DiaZist), (%) | Positive | 16 (100) | 8 (57) | 16 (80) | 35 (97) | 75 (87) | 0.000* |
| Negative | 0 | 6 (43) | 2 (10) | 1 (3) | 9 (10.5) | ||
| Borderline | 0 | 0 | 2 (10) | 0 | 2 (2) | ||
| Neutralizing antibody titer (DiaZist), median (IQR) | 104.9 (14) | 37.7 (88) | 48.5 (85) | 94.55 (53) | 87.05 (75) | 0.000** | |
| Neutralizing antibody (PishtazTeb), (%) | Positive | 13 (81) | 10 (36) | 20 (69) | 86 (85) | 129 (74) | 0.000* |
| Negative | 3 (19) | 17 (60) | 9 (31) | 15 (15) | 44 (25) | ||
| Borderline | 0 | 1 (4) | 0 | 0 | 1 (0.6) | ||
| Neutralizing antibody titer (PishtazTeb), median (IQR) | 0.1 (0.29) | 0.93 (0.68) | 0.6 (0.97) | 0.09 (0.41) | 0.15 (0.81) | 0.000** | |
| Anti‐SARS‐CoV‐2‐spike antibody, 34 (%) | Positive | 16 (100) | 19 (68) | 27 (93) | 101 (100) | 163 (94) | 0.000* |
| Negative | 0 | 6 (21) | 1 (3.5) | 0 | 7 (4) | ||
| Borderline | 0 | 3 (11) | 1 (3.5) | 0 | 4 (2) | ||
| Anti‐SARS‐CoV‐2‐spike antibody titer, median (IQR) | 7.21 (0.45) | 4.63 (5.99) | 6.35 (3.7) | 7.43 (1.33) | 7.19 (2.39) | 0.000** | |
Note: The instruction of each ELISA kit comes as follows: The anti‐nucleocapsid and anti‐spike antibodies are considered positive with serum levels >1.1 AU/ml, and 0.9–1.1 AU/ml is considered as borderline. Neutralizing antibody measured by DiaZist ELISA kit considered positive if >11 AU/ml. Neutralizing antibody measured by Pishtazteb ELISA kit considered positive if the test result is less than 0.78 OD (i.e., equal to 2.5 µg/ml concentration of neutralizing antibody).
Abbreviations: ELISA, enzyme‐linked immunosorbent assay; IQR, interquartile range; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; OD, optical density.
Pearson Chi‐Square.
Kruskal–Wallis test.
3.2. Anti‐SARS‐CoV‐2 neutralizing antibody
Anti‐SARS‐CoV‐2 neutralizing antibody was examined by two different ELISA kits, DiaZist and Pishtazteb. Pishtazteb ELISA kit results are as follows: 129 (74%) positive, 44 (25%) negative, and 1 (0.6%) borderline for the serum anti‐SARS‐CoV‐2 neutralizing antibody. Neutralizing antibody reported by DiaZist ELISA kit included 75 (87%) positive, 9 (10.5%) negative, and 2 (2%) borderline. The quantification of the neutralizing antibody performed by these two kits is also shown in Table 2. The mean amounts of neutralizing antibody for each vaccine type measured by Pishtazteb kit were 0.45 OD (~8µg/ml) in total, 0.34 OD (~10µg/ml) for AstraZeneca, 0.83 OD (~2µg/ml) for COVAXIN, 0.6 OD (~5µg/ml) for Sinopharm, and 0.31 OD (~10µg/ml) for Sputnik V (p value< 0.0001)
The mean amounts of the Anti‐SARS‐CoV‐2 neutralizing antibody measured by DiaZist ELISA kit were 68.68 AU/ml in total, 96.32 AU/ml for AstraZeneca, 45.77 AU/ml for COVAXIN, 51.84 AU/ml for Sinopharm, and 76.97 AU/ml for Sputnik V (p value< 0.0001).
The median level of the anti‐SARS‐CoV‐2 neutralizing antibody for each vaccine type has been shown in Table 3.
The comparison between the levels of the neutralizing antibody measured by DiaZist ELISA kit is shown in Figure 1, demonstrating significantly higher levels of the neutralizing antibody in Oxford/AstraZeneca vaccine.
Figure 1.
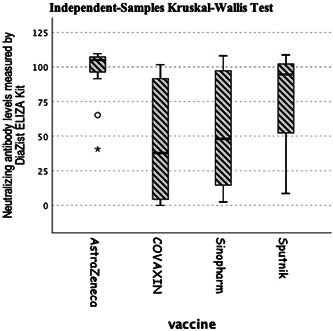
The anti‐SARS‐CoV‐2 neutralizing antibody (DiaZist) in different groups of vaccines. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.3. Anti‐SARS‐CoV‐2‐S antibody
Anti‐SARS‐CoV‐2‐S antibodies evaluated by Razi ELISA kit, were positive in 163 (94%) participants, negative in 7 (4%), and borderline in 4 (2%). The positive results were significantly more frequent than negative levels (p value <0.05) and significantly higher in those who received Sputnik V compared with COVAXIN and Sinopharm (p value< 0.0001) (Figure 2).
Figure 2.
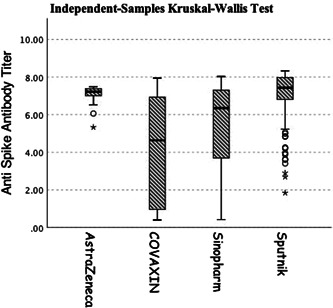
Anti‐SARS‐CoV‐2‐spike antibody between different groups of vaccines. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.4. Previous infection of COVID‐19
This study found a significant correlation between previously diagnosed COVID‐19 and the levels of anti‐S, anti‐N, and neutralizing antibodies (p value < 0.0001) (Figure 3).
Figure 3.
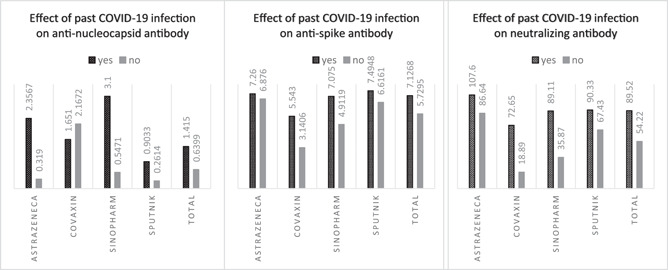
The effect of past COVID‐19 infection on titers of anti‐SARS‐CoV‐2 antibodies. COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
4. DISCUSSION
Due to the extensive and rapid dissemination of COVID‐19 leading to a hazardous mortality and morbidity rate, emergent administration of vaccines was crucial. However, since the number of COVID‐19 vaccines approved to be used is vast and various and released in a short period of time, comprehensive information about all aspects of these vaccines is not yet to be thoroughly available in the literature. 35
Regarding the risk of COVID‐19, the healthcare workers are highly exposed to patients with SARS‐CoV‐2 infection; therefore, early receiving of the COVID‐19 vaccine was a priority for this group. 36 , 37
In the Children's Medical Center, four types of vaccines were delivered to healthcare workers: Oxford/AstraZeneca and Sputnik V, based on the nonreplicating viral vector, and COVAXIN and Sinopharm, which are based on the inactivated virus. 38 , 39
In this study, the absence of anti‐SARS‐CoV‐2‐N antibodies was more prevalent than the presence of anti‐SARS‐CoV‐2‐N antibodies; however, this difference was not significant (p value > 0.05). The absence of anti‐SARS‐CoV‐2‐N antibodies was seen in 85%, 75%, 69%, and 61% of vaccinated individuals with Sputnik V, Oxford/AstraZeneca, Sinopharm, and COVAXIN.
This study examined the anti‐SARS‐CoV‐2‐neutralizing antibody with ELISA assay by Pishtazteb and DiaZist kits which both are made in Iran. The frequency of positive levels of neutralizing antibody measured by DiaZist and Pishtazteb was 87% versus 74% in total, 100% versus 81% for AstraZeneca, 97% versus 85% for Sputnik V, 80% versus 69% for Sinopharm, 57% versus 36% for COVAXIN. These differences demonstrate a higher sensitivity and specificity of the DiaZist anti‐SARS‐CoV‐2‐neutralizing antibody ELIZA kit compared with the Pishtazteb kit.
Both kits represented higher positive levels of neutralizing antibodies in Oxford/AstraZeneca and Sputnik V than inactivated virus vaccines, including COVAXIN and Sinopharm, similar to previous clinical trials that examined the neutralizing antibody in adenoviral vector vaccines and whole‐cell based vaccines. 38 However, the differences in the quantity of antibodies titers between the two studies may be due to differences in sample size and various factors.
Anti‐SARS‐CoV‐2‐S antibody was positive in 100% of viral‐vector‐based vaccines (Oxford/AstraZeneca and Sputnik V), while it was found in 80% of cases vaccinated with inactivated virus vaccines (93% in Sinopharm and 69% in COVAXIN). Therefore, it could be assumed that adenoviral‐vector‐based vaccines were more successful than inactivated‐virus‐based vaccines at induction of the immune system against SARS‐CoV‐2‐S antigen.
The previous studies reported a 90% increase in anti‐SARS‐CoV‐2 antibodies, including anti‐S and neutralizing antibodies for adenoviral‐based vaccines, which appeared 14 days after the first dose of vaccine and reached the optimum level 14 days after the second dose. The mentioned study also indicated a 90%–100% increase for Sinopharm and COVAXIN. However, it is in contrast to this study regarding antibody levels in the COVAXIN vaccine, which may be because of the small sample size of the COVAXIN‐vaccinated group in this study. 38
This study also evaluated the vaccine side effects 48 to 72 after the vaccination in participants. The most common side effects after vaccination included myalgia, fever, headache, chills, fatigue, and pain in the injection site. In a recent study by Coggins et al., the adverse effects of COVID‐19 vaccination involved fever, pain in the injection site, headache, chills, and myalgia. 40 In another study in Ethiopia, they studied the adverse effects of the Oxford/AstraZeneca COVID‐19 vaccine. Fatigue, headache, myalgia, and fever were the most frequent symptoms. 41 Almufty et al. reported fatigue, injection‐site reaction, fever, myalgia, headache, and chills as the most common side effects among 1012 participants who received the COVID‐19 vaccine. They also indicated that fever, fatigue, and headache were more common in Oxford/AstraZeneca compared with Sinopharm. 42 The diversity between the other studies and this study regarding the frequency of various side effects can be due to differences in delivered vaccines, sample sizes, study location, and other various factors that may influence the generalizability of the studies. 43 , 44 This study figured out that fever was more common in Oxford/AstraZeneca and Sputnik V, the adenoviral‐vector‐based vaccines, compared with COVAXIN and Sinopharm (p value < 0.0001). Moreover, chills and myalgia were also more common in participants who received Sputnik V compared with other vaccines (p value = 0.006 for chills and p value = 0.025 for myalgia).
The titers of anti‐SARS‐CoV‐2 antibodies measured in this study (anti‐S, anti‐N, and neutralizing antibodies) were significantly higher in the participants with a past history of COVID‐19 infection than in others. However, the titer of neutralizing antibody measured by Pishtazteb showed a different result and was lower in the participants with a history of COVID‐19 infection. That is because of the different approach of the Pishtazteb neutralizing antibody kit (less than 0.78 OD is considered positive, that is, equal to 2.5 µg/ml of neutralizing antibody concentration) and its lower sensitivity and specificity. A previous study in France reported a remarkable increase in anti‐SARS‐CoV‐2 antibodies in patients with COVID‐19 after 2 weeks. 45 A cohort study focused on the antibody response after the second dose of COVID‐19 vaccination with or without history of COVID‐19 infection remarked the significance role of previous COVID‐19 infection on impowered immune response and antibody levels after vaccination. 46 Moreover, in case of anti‐S titers after vaccination, Angyal et al. and Bilgin et al. reported that anti‐S antibody titers were significantly higher in COVID‐19‐experienced individuals after the first and second dose of vaccine. 47 , 48 This increased response may be due to the fact that vaccine elicits stronger B cell immunity in patients with a history of COVID‐19.
4.1. Limitations
This study evaluated and compared the healthcare workers' immune response to different COVID‐19 vaccines at Children's Medical Center. However, conducting multicentral studies with bigger sample size is highly recommended. Moreover, this study was a cross‐sectional experiment that requires an additional follow‐up to assess these vaccines' long‐term and chronic effects and side effects.
5. CONCLUSION
Regarding the results of this study, the vaccines based on the adenoviral‐vector platform have a better function to induce an immune response against SARS‐CoV‐2 than the vaccines based on the inactivated virus. Oxford/AstraZeneca and Sputnik V had a similar outcome of inducing high levels of anti‐SARS‐CoV‐2‐S antibody and anti‐SARS‐CoV‐2 neutralizing antibody, which were more than Sinopharm and COVAXIN. The titers of anti‐SARS‐CoV‐2‐N antibody were low in all of these four vaccines.
The adverse effects of the vaccination are common, and according to the vaccine type and other factors may differ. Critical adverse complications, including abortion and hypertension, occurred in a negligible frequency (1.7%) that needs to be studied extensively.
AUTHOR CONTRIBUTIONS
Babak Pourakbari, Shima Mahmoudi, and Setareh Mamishi participated in the research design. Babak Pourakbari and Shima Mahmoudi had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Reihaneh H. Hosseinpour Sadeghi performed experimental tests. Mona Mirbeyk wrote the first draft of manuscript. Babak Pourakbari, Shima Mahmoudi, Mona Mirbeyk, Nima Rezaei, Raheleh Ghasemi, and Fatemeh Esfandiari contributed to data acquisition and data interpretation. Shima Mahmoudi contributed to the statistical analysis and revising of the manuscript. All authors reviewed and approved the final version.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
All participants gave written informed consent, and the study was carried out following the guidelines of the Declaration of Helsinki.
ACKNOWLEDGMENTS
This study was supported by a grant (grant number: 1400‐1‐149‐53183) from Tehran University of Medical Sciences to Professor Setareh Mamishi.
Pourakbari B, Mirbeyk M, Mahmoudi S, et al. Evaluation of response to different COVID‐19 vaccines in vaccinated healthcare workers in a single center in Iran. J Med Virol. 2022;1‐9. 10.1002/jmv.28029
Contributor Information
Shima Mahmoudi, Email: sh-mahmoudi@sina.tums.ac.ir.
Setareh Mamishi, Email: smamishi@sina.tums.ac.ir.
DATA AVAILABILITY STATEMENT
All analysis is available from the corresponding author on reasonable request. All the remaining data are included in this article.
REFERENCES
- 1. Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al‐Nasser AD. SARS‐CoV‐2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO. WHO Coronavirus (COVID‐19) Dashboard: World Health Organization (WHO) , 2022. Available from https://covid19.who.int/
- 5.Coronaviridae—Positive Sense RNA Viruses—Positive Sense RNA Viruses. 2011. https://talk.ictvonline.org/ictv-reports/ictv_9th_report/positive-sense-rna-viruses-2011/w/posrna_viruses/222/coronaviridae
- 6. Wu A, Peng Y, Huang B, et al. Genome composition and divergence of the novel coronavirus (2019‐nCoV) originating in China. Cell Host Microbe. 2020;27(3):325‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim D, Lee J‐Y, Yang J‐S, Kim JW, Kim VN, Chang H. The architecture of SARS‐CoV‐2 transcriptome. Cell. 2020;181(4):914‐21.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS‐CoV‐2 and other human coronaviruses. Trends Immunol. 2020;41(5):355‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Secchi M, Bazzigaluppi E, Brigatti C, et al. COVID‐19 survival associates with the immunoglobulin response to the SARS‐CoV‐2 spike receptor binding domain. J Clin Invest. 2020;130(12):6366‐6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen P, Nirula A, Heller B, et al. SARS‐CoV‐2 neutralizing antibody LY‐CoV555 in outpatients with Covid‐19. N Engl J Med. 2020;384(3):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rauch S, Jasny E, Schmidt KE, Petsch B. New vaccine technologies to combat outbreak situations. Front Immunol. 2018;9:1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ritchie H, Mathieu E, Rodés‐Guirao L, et al. Coronavirus pandemic (COVID‐19). Our World in Data . 2020;2022.
- 15. World Health Organization . COVID‐19 vaccine tracker and landscape. 2022. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 16. Dolgin E. Omicron thwarts some of the world's most‐used COVID vaccines: Nature, 2022. https://www.nature.com/articles/d41586-022-00079-6 [DOI] [PubMed]
- 17. Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector‐based heterologous prime‐boost COVID‐19 vaccine in two formulations: two open, non‐randomised phase 1/2 studies from russia. Lancet. 2020;396(10255):887‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dolzhikova IV, Tokarskaya EA, Dzharullaeva AS, et al. Virus‐Vectored ebola vaccines. Acta Naturae. 2017;lKg 9(3):4‐11. [PMC free article] [PubMed] [Google Scholar]
- 19. WHO COVID‐19 Dashboard. Geneva: World Health Organization , 2020. https://covid19.who.int/ © World Health Organization 2020, All rights reserved. https://covid19.who.int/region/emro/country/ir
- 20. Basta NE, Moodie EMM, on behalf of the VIPER (Vaccines, Infectious disease Prevention, and Epidemiology Research) Group COVID‐19 Vaccine Development and Approvals Tracker Team. COVID‐19 Vaccine Development and Approvals Tracker. 2020. https://covid19.trackvaccines.org/country/iran-islamic-republic-of/
- 21. ReliefWeb . Red Crescent‐imported Covid‐19 Vaccines exceed 80m doses, 2021. https://reliefweb.int/report/iran-islamic-republic/red-crescent-imported-covid-19-vaccines-exceed-80m-doses
- 22. Mallapaty S. Iran hopes to defeat COVID with home‐grown crop of vaccines. Nature. 2021;475(2021):596. [DOI] [PubMed] [Google Scholar]
- 23. Nakhostin‐Ansari A, Zimet GD, Khonji MS, et al. Acceptance or rejection of the COVID‐19 vaccine: a study on Iranian People's opinions toward the COVID‐19 vaccine. Vaccines (Basel). 2022;10(5):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Omidvar S, Firouzbakht M. Acceptance of COVID‐19 vaccine and determinant factors in the Iranian population: a web‐based study. BMC Health Serv Res. 2022;22(1):652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ezati Rad R, Kahnouji K, Mohseni S, et al. Predicting the COVID‐19 vaccine receive intention based on the theory of reasoned action in the south of Iran. BMC Public Health. 2022;22(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khankeh HR, Farrokhi M, Khanjani MS, et al. The barriers, challenges, and strategies of COVID‐19 (SARS‐CoV‐2) vaccine acceptance: a concurrent mixed‐method study in Tehran city, Iran. Vaccines (Basel). 2021;9(11):1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kazeminia M, Afshar ZM, Rajati M, Saeedi A, Rajati F. Evaluation of the acceptance rate of Covid‐19 vaccine and its associated factors: a systematic review and meta‐analysis. J Prev. 2022;43(4):421‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dror AA, Eisenbach N, Taiber S, et al. Vaccine hesitancy: the next challenge in the fight against COVID‐19. Eur J Epidemiol. 2020;35(8):775‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ullah I, Khan KS, Tahir MJ, Ahmed A, Harapan H. Myths and conspiracy theories on vaccines and COVID‐19: potential effect on global vaccine refusals. Vacunas. 2021;22(2):93‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mirbeyk M, Saghazadeh A, Rezaei N. Geriatrics and COVID‐19. In: Rezaei N, ed. Coronavirus Disease ‐ COVID‐19. Springer International Publishing; 2021:209‐222. [DOI] [PubMed] [Google Scholar]
- 31. Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID‐19. J Korean Med Sci. 2020;35(13):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang K, Kang S, Tian R, Zhang X, Zhang X, Wang Y. Imaging manifestations and diagnostic value of chest CT of coronavirus disease 2019 (COVID‐19) in the Xiaogan area. Clin Radiol. 2020;75(5):341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dispinseri S, Secchi M, Pirillo MF, et al. Neutralizing antibody responses to SARS‐CoV‐2 in symptomatic COVID‐19 is persistent and critical for survival. Nat Commun. 2021;12(1):2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the lombardy region, Italy. JAMA. 2020;323(16):1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jafari Baghiabadi S, Farshid R. Studying of research related to COVID‐19 vaccine in Iran and the world: a thematic analysis and scientific collaborations . Iran‐J‐Med‐Microbiol 2021;15(4):414‐457. [Google Scholar]
- 36. Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID‐19 among front‐line health‐care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5(9):e475‐e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Núñez López C, de Abreu JMG, Pérez‐Blanco V, de Miguel Buckley R, Romero Gómez MP, Díaz‐Menéndez M. [Not available]. Enferm Infecc Microbiol Clin (Engl Ed) . 2021.
- 38. Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine‐induced protection against COVID‐19 in humans. Nat Rev Immunol. 2021;21(8):475‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones I, Roy P. Sputnik V COVID‐19 vaccine candidate appears safe and effective. Lancet. 2021;397(10275):642‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coggins SAA, Laing ED, Olsen CH, et al. Adverse effects and antibody titers in response to the BNT162b2 mRNA COVID‐19 vaccine in a prospective study of healthcare workers. medRxiv . 2021, 9(1). 10.1093/ofid/ofab575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Solomon Y, Eshete T, Mekasha B, Assefa W. COVID‐19 vaccine: side effects after the first dose of the Oxford AstraZeneca vaccine among health professionals in Low‐Income country: Ethiopia. J Multidiscip Healthc. 2021;14:2577‐2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross‐sectional study. Diabetes Metab Syndr. 2021;15(5):102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faber J, Fonseca LM. How sample size influences research outcomes. Dental Press J Orthod. 2014;19(4):27‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Canlas FQ, Nair S, Paat ID. Exploring COVID‐19 vaccine side effects: A correlational study using python. Procedia Comput Sci. 2022;201:752‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brochot E, Demey B, Touzé A, et al. Anti‐spike, anti‐nucleocapsid and neutralizing antibodies in SARS‐CoV‐2 inpatients and asymptomatic individuals. Front Microbiol. 2020;11:584251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buonfrate D, Piubelli C, Gobbi F, et al. Antibody response induced by the BNT162b2 mRNA COVID‐19 vaccine in a cohort of health‐care workers, with or without prior SARS‐CoV‐2 infection: a prospective study. Clin Microbiol Infect. 2021;27(12):1845‐1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Angyal A, Longet S, Moore SC, et al. T‐cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS‐CoV‐2‐naive UK health‐care workers: a multicentre prospective cohort study. Lancet Microbe. 2022;3(1):e21‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bilgin H, Marku M, Yilmaz SS, et al. The effect of immunization with inactivated SARS‐CoV‐2 vaccine (CoronaVac) and/or SARS‐CoV‐2 infection on antibody levels, plasmablasts, long‐lived‐plasma‐cells, and IFN‐γ release by natural killer cells. Vaccine. 2022;40(18):2619‐2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All analysis is available from the corresponding author on reasonable request. All the remaining data are included in this article.


