Abstract
Lung ultrasound has the potential to enable standardized follow‐up without radiation exposure and with lower associated costs in comparison to CT scans. It is a valuable tool to follow up on patients after a COVID‐19 infection and evaluate if there is pulmonary fibrosis developing. Echocardiography, including strain imaging, is a proven tool to assess various causes of dyspnea and adds valuable information in the context of long COVID care. Including two‐dimensional (2D) strain imaging, a better comprehension of myocardial damage in post‐COVID syndrome can be made. Especially 2D strain imaging (left and the right ventricular strain) can provide information about prognosis.
Keywords: COVID‐19, lung ultrasound, echocardiography, rehabilitation
Abbreviations
- 2‐ChV
apical two‐chamber view
- 4‐ChV
apical four‐chamber view
- CT
computerized tomography
- GLS
global longitudinal peak systolic strain
- ICU
intensive care unit
- LA
left atrium
- LUS
lung ultrasound
- LV
left ventricle
- MCpEF
chronic myocarditis with preserved left ventricular ejection fraction
- PLAX
parasternal long axis view
- PSAX
parasternal short axis view
- RV
right ventricle
- RVF
right ventricular function
Introduction
The ongoing COVID‐19 pandemic is a global crisis holding the world hostage and is continuing to present an immense burden on healthcare workers. 1 Millions of people have been infected, with numbers still rising, and variants of the virus are still being discovered. With widespread vaccination, this pandemic might come to an end. 2 , 3 Nevertheless, follow‐up after COVID‐19 still presents some difficulties. Some patients still suffer from symptoms such as dyspnea after acute COVID‐19. In this consensus document, the usefulness of lung ultrasound (LUS) in post‐COVID‐19 patients is underlined.
Furthermore, the combination of LUS and cardiac imaging, especially echocardiography with newer parameters, such as strain imaging, is discussed. One main aim was to illustrate how ultrasound can be easily implemented understandably. LUS can identify persistent reverberation artifacts after a COVID‐19 infection, commonly known as B‐lines. 4 Echocardiography can show deterioration in regional and global strain imaging. 5 , 6 Informed consent of all published material was obtained.
Long COVID and post‐COVID syndrome
There is yet a lack of data on the rehabilitation of COVID‐19 patients after a critical or severe course of the disease. At the same time, there is concern about the difficulties that the tremendous need for rehabilitation after critical and severe COVID‐19 disease presents. 7 , 8 A recent study by Sonnweber et al. showed that post‐COVID‐19 patients suffered from persistent symptoms, such as dyspnea and impairment in lung function testing. 5 This study indicated that follow‐up care of patients after COVID‐19 with persistent clinical symptoms should include serial echocardiographic scans, serial lung function testing, and imaging of the lung with computerized tomography (CT) scans. 5
As the number of symptomatic patients after an acute COVID‐19 infection increased, the terms “long COVID” (symptoms after 4 weeks of acute infection) and “post‐COVID syndrome” (symptoms after 12 weeks of acute infection) were introduced and are now used to describe more than 100 different persistent symptoms after acute infection. 9 , 10 , 11 In a meta‐analysis, Lopez‐Leon et al identified symptoms significantly associated with long COVID, including fatigue (58%), headache (44%), and dyspnea (24%). Pulmonary fibrosis (5%), reduced pulmonary capacity (10%), palpitations (11%), increase in heart rate (11%), chest pain (16%), and myocarditis (1%) were also reported. A pathological chest CT was observed in imaging 35% of patients 60 to 100 days after initial presentation. 9 Pathological chest radiographs were found in almost two‐thirds of hospitalized but non‐critical COVID‐19 patients. 12 Even bronchoscopically verified organizing pneumonia following COVID‐19 pneumonia was described. 13 Regarding elevated laboratory parameters, NT‐pro‐BNP was elevated in 11% of diagnosed long COVID patients. 9
Lung Ultrasound for COVID‐19 Pneumonia
Artifacts in LUS are created by air and, in the case of reverberation artifacts, by air and fluid mismatch. 14 Fluid in the interstitium of the lung and the partial filling of alveolar spaces create vertical artifacts arising from a smooth pleural line with a bilateral symmetric distribution. Such lines are called B‐lines. 14 In the differential diagnosis of B‐lines, echocardiography and scans of the inferior vena cava can be used to verify a cardiogenic cause of pulmonary edema. 14
LUS can be of considerable value for predicting the clinical course and outcome in patients with COVID‐19. 15 Typical findings in patients with COVID‐19 pneumonia are bilateral areas of fragmentation of the pleural line with reverberation artifacts. 16 In a 2020 publication of the world federation of ultrasound in medicine and biology, a differentiation of reverberation artifacts depending on the origin was proposed. 14 Artifacts that arise from a smooth pleural line and denote pulmonary edema should be called “B‐lines.” In contrast, artifacts arising from a fragmented pleural line are described as “comet tails.” 14 To avoid confusion and as reverberation artifacts in COVID‐19 are called B‐lines, B‐lines will stay as the nomenclature in this publication with the note that comet tails are a more detailed term to differentiate reverberation artifacts. 14 , 17 , 18 , 19 , 20 Reverberation artifacts in the case of sub‐pleural consolidations and a reduction or absence of pleural sliding can be seen, as well. 21 Reverberation artifacts arising from a fragmented pleural line indicate diffuse alveolar damage, diffuse parenchymatous lung diseases, and inflammatory diseases such as COVID‐19. 14 , 16
Consolidations can be visualized using ultrasound. Hypoechoic areas with small hyperechoic (bronchograms) or hypoechoic (fluid bronchograms) structures within them, as well as a “tissue‐like appearance” or hepatization of the lungs, are described as consolidations in lung ultrasound. 22 , 23 , 24
Pleural effusions in post‐COVID‐19 patients indicate a bacterial superinfection (small and localized effusions) or other differential diagnoses, such as left heart failure leading to elevated filling pressures, right heart failure, and loss of protein in kidney failure, or liver disease. 16 , 25
12‐Zone LUS Scanning Protocol for Long COVID
Follow‐up examinations, such as those in a rehabilitative setting, for COVID‐19 patients should be consistent with previous scans, and scanning must be performed thoroughly to identify changes in the LUS in detail. Patients with persistent symptoms, such as dyspnea, should be imaged. 5 A reduction in B‐line artifacts, a reduction in the size of consolidations, and a reduction or disappearance of pleural effusions in the case of complications (bacterial pneumonia, right and left heart failure) can be seen in LUS and should be documented. 26 Chest radiographs are an alternative and cheap diagnostic tool for bacterial or viral pneumonia in general and the follow‐up of COVID‐19. 27 , 28 Chest radiographs are widely available but have a lower sensitivity in detecting COVID‐19 pneumonia compared to LUS. 28
Overall, no international consensus exists on which transducer should be used for COVID‐19 in which exact situation. The usage of a convex transducer with a low frequency or a linear transducer for better evaluation of the pleural line is recommended. 19 , 20 , 29 , 30 In the author's opinion, in order to detect optimally and follow up on ultrasound artifacts associated with COVID‐19 pneumonia, a specific lung preset with a low mechanical index, single‐focal point modality, and without harmonic imaging or other cosmetic filters should be chosen. 17 The focal zone should be placed at the area of the pleural line.
In the literature, several protocols are described, and no standardized international consensus has yet been achieved. 17 , 20 , 29 , 30 The authors' agreement regarding feasibility is a 12‐zone scanning protocol described in the literature. 20 , 29 Starting with zone one in a longitudinal view, followed by a transverse view, a scan of all intercostal spaces should be performed in a supine patient. Posterior zones should always be included in follow‐up examinations and performed in an upright position. A linear transducer with a specific preset should be used whenever possible. Depth settings should be adapted to patient size but ought to be in the range of 4 to 8 cm. In cases of larger, persistent pleural effusions or obese patients, a convex transducer should be selected. 30 The depth setting should be adapted to patients' sizes and will be in the range of 8 to 15 cm for optimal detection below fatty tissue. A 12‐zone scanning protocol with pathologies as seen in critically ill, severely ill, and post‐COVID‐19 patients is presented in figures one and two (Figures 1 and 2). 31
Figure 1.
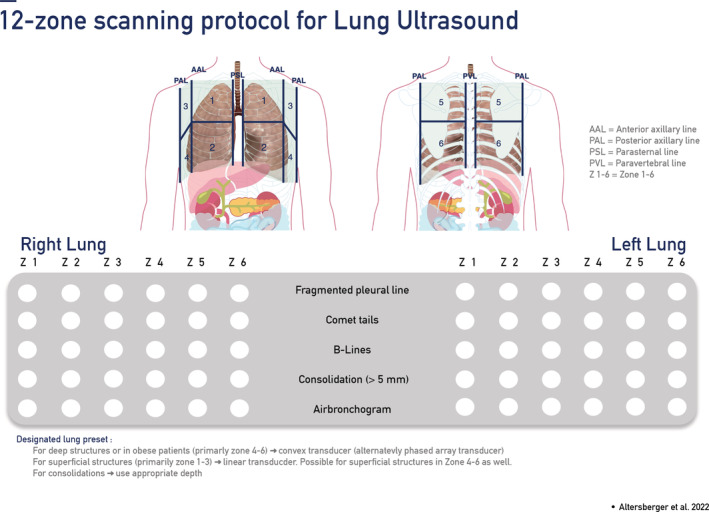
12‐Zone scanning protocol for lung ultrasound.
Figure 2.
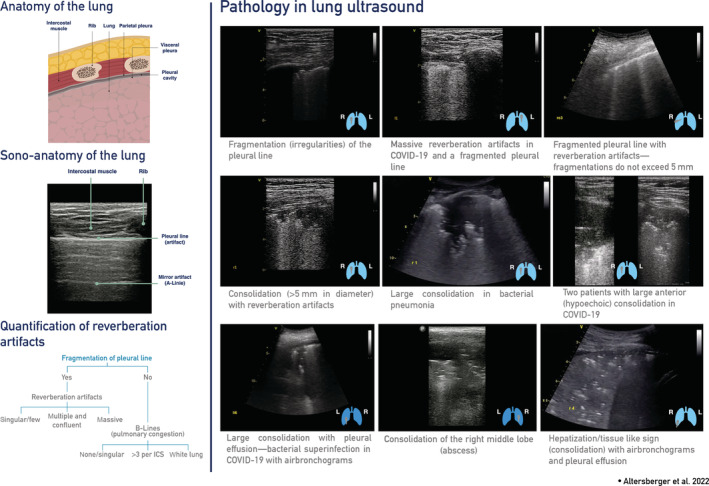
LUS pathologies seen in critical, severely ill, and post COVID‐19 scans with a suggestion to quantify reverberation artifacts.
Findings are comparable with CT‐Imaging (Video S1—LUS and CT imaging, from the ICU to the rehabilitation—left: after acute infection with a ground‐glass pattern, consolidation and pleural effusion/corresponding ultrasound image of zone one, right hemithorax, lateral; right: CT and corresponding lung ultrasound four months later). 20 It has to be noted that ultrasound evaluation of the phrenic nerve and the diaphragm may be an additional tool in LUS to diagnose unilateral or bilateral paralysis of the diaphragm. The longitudinal assessment of respiratory patterns in patients with lung disease can be monitored, and in a standardized setting, monitoring is possible. 32
Comprehensive Echocardiography in Long COVID
In follow‐up during rehabilitation after COVID‐19 with cardiac involvement, a comprehensive TTE exam should be performed only if a clinical benefit is expected. 33 , 34 In a post‐COVID‐19 setting, patients often present with symptoms of potential cardiac involvement such as palpitations and chest pain. 35 In a UK cohort, it was displayed that after a COVID‐19 infection, the risk for death and readmission to the hospital is increased compared to matched controls. 36 In a median follow‐up of 140 days of 47780 individuals, 29.4% were readmitted to the hospital, and 12.3% died after discharge after an acute COVID‐19 infection. The risk for major adverse cardiovascular events was approximately threefold higher than in the matched cohort. 36 Cases of myocarditis were even reported in asymptomatic or mild COVID‐19 diseases. 37
Echocardiography is the key investigation tool for assessing cardiac function and provides information on cardiac chamber size, wall thickness, regional wall motion abnormalities, pericardial effusion, valvular pathology, stiffness of the LV, and pulmonary hypertension. 38 After assessing the clinical status, the electrocardiogram, blood tests (NT‐proBNP), echocardiography, and lung ultrasound can diagnose and classify patients with heart failure. 38 Furthermore, in patients with suspected cardiomyopathy, echocardiography is the first‐line diagnostic tool recommended by the European Society of echocardiography. 38
In the author's opinion, patients after COVID‐19 who suffered from cardiac injury, myocarditis, acute coronary syndromes, pericarditis, stress‐induced cardiomyopathy, worsening cardiovascular disease, persistent dyspnea with the clinical suspicion of cardiomyopathy, and thoracic pain should be evaluated with echocardiography concordant to the indications for echocardiography in existing guidelines. 38 , 39 , 40 , 41 , 42
A comprehensive TTE protocol, as recommended by the American Society of Echocardiography and the European Society of Echocardiography, with applying routine measurements. 38 , 43 , 44
As there are no specific recommendations for post‐COVID syndrome patients, a diagnostic workup as provided by the European Society of Echocardiography and the American Society of Echocardiography might be appropriate to apply. 38 , 43 , 44
A comprehensive exam starts with a parasternal long‐axis view (PLAX), applying color Doppler and measuring the left ventricular end‐diastolic diameter, the thickness of the interventricular septum, and the posterior wall. 44 In a parasternal short axis view (PSAX), the aortic valve should be visualized at the basis of the heart. The basal segments of the left ventricle (LV) and the opening of the mitral valve is the second view in a PSAX achieved by tilting the transducer. The mid‐ and apical segments can be visualized with further tilting. In a PSAX, radial left ventricular function can be visually assessed. 43
There is a special focus on regional wall motion abnormalities in the apical views. The apical four‐chamber view (4‐ChV), the apical two‐chamber‐view (2‐ChV), and the apical long‐axis view have to be visualized. 43 Diastolic dysfunction should be assessed according to current guidelines. 45
A specific focus in post‐COVID‐19 patients should be on the right heart as it can be involved in case of complications such as pulmonary embolism and may also provide prognostic information. 41 , 46 , 47 An assessment of right ventricular function (RVF) and size should be performed. Additionally, systolic pulmonary artery pressure should be estimated by measuring the peak velocity of the tricuspid regurgitation signal. 48 Moreover, the pulmonary acceleration time and the size of the pulmonic trunk can be assessed. 43 , 44 , 48 To complete the evaluation of the right ventricle (RV) and longitudinal RVF, the strain of the free RV wall in a focused view on the RV should be performed 43 , 44 (Video S2: RV strain in a focused apical 4‐ChV of the RV).
Schneider et al. reported that a ratio of pulmonary trunk diameter to ascending aorta diameter >1 provides additional information on pulmonary hypertension and should be taken into account in the context of routine assessment of pulmonary hypertension in echocardiography. 49
Subcostal views, such as the subcostal 4‐ChV, are essential for detecting pericardial effusions. 50 Additionally, the inferior vena cava should be scanned to estimate right atrial pressure. 44
In echocardiography protocols in follow‐up settings after COVID‐19, strain imaging should be included. Changes in regional and global longitudinal systolic strain (GLS) should be reported 6 , 43 (Video S3: Strain imaging in post‐COVID‐19 patients with reduction in basal regional strain).
As there is evidence that diastolic function after an acute inflammation with COVID‐19 is impaired, the routine evaluation should also include diastolic parameters. 33 , 34 , 43 , 45 In recent literature, left atrial (LA) strain is used to determine filling pressures; it might give valuable information in the future, but, as of now, LA strain did not show to correlate with prognosis in acute COVID‐19 disease. 46 , 51 Dilatation of the LA is an important marker of prognosis of patients with prior cardiovascular diseases. 52 , 53 , 54
In our experience, a thorough evaluation of all heart chambers is necessary to detect potential cardiac complications, such as myocarditis, pericarditis, and acute coronary syndromes, which occur in acute and long COVID patients, and to identify the actual underlying cause of dyspnea 35 , 36 , 37 (Video S4: Strain imaging in a post‐COVID‐19 patient after an acute coronary syndrome with a reduction in GLS and regional basal and mid‐segmental strain).
Patients with a prolonged course of COVID‐19 disease may be facing symptoms such as dyspnea, fatigue, and palpitations. Pathological changes can be found in pulmonary function test, lung imaging, and echocardiography. 9 , 13 , 55 , 56 , 57 Significant pathological residuals of clinical, functional, and morphological changes are often found in post‐COVID‐19 patients. Sonnweber et al. conclude that follow‐up care should be considered in patients with persistent symptoms, including serial measures of pulmonary function testing, echocardiography, and CT‐imaging. 5
In the case of pulmonary imaging, we recommend a 12‐zone scanning protocol in LUS to visualize residual changes such as reverberation artifacts and the reduction thereof over time. 20 LUS can be a low‐cost, easy‐to‐use, widely available, and radiation‐sparing alternative to serial chest radiographs or CT scans for tracking clinical improvement. CT scans are costly and should be reserved for unclear cases. 20 , 30 Chest radiographs have lower sensitivity than LUS but are widely available, cheap, and standardized. 27 , 28 , 30 LUS might serve as an early tool for detecting long‐term complications such as pulmonary fibrosis. 18 , 58 If pleural effusions are present in post‐COVID‐19 patients, differential diagnoses such as left and right heart failure, kidney disease, or liver disease will have to be considered. 20
To detect cardiac complications and possible residual findings in strain imaging, comprehensive transthoracic echocardiography, including strain imaging, should be considered for implementation during rehabilitation after COVID‐19 disease. In the authors' opinion, for patients with clinical findings which are suggestive of cardiomyopathy or elevated cardiac markers such as NT‐proBNP, a comprehensive TTE should be performed as the risk of cardiac complications after COVID‐19 is increased compared to controls. 5 , 6 , 36 , 38 , 43 In particular, in myocarditis or myocardial injury cases, echocardiography, including strain imaging, should be applied. 6 , 46 , 47 , 59 Given that myocarditis is a cause of sudden cardiac death, cardiac MRI should be considered the gold standard. Still, optimal resource management should be taken into consideration, and echocardiography might serve as the first screening tool. 60 However, the combination of imaging modalities has an even higher accuracy than cardiac MRI or speckle tracking echocardiography alone for detecting the entity of chronic myocarditis with preserved left ventricular ejection fraction (MCpEF). 59
A GLS of −13.8 in critically ill COVID‐19 patients was defined as a predictor of mortality. 61 A reduction in GLS can be seen when the EF is still normal and can be considered an early marker of myocardial damage. 61
RV strain is a marker of poor prognosis (values >−23%) in critical COVID‐19 and is considered to be a potential marker for long‐term prognosis in follow‐up in long COVID patients. 47
In the case of the post‐COVID syndrome, there are yet no international recommendations for performing echocardiography. In a large UK cohort with a median follow‐up of 140 days, cardiac complications were described as occurring substantially higher than in a cohort that never had COVID‐19. 36 Sequels of COVID‐19 described in a meta‐analysis in a median time to assessment of 41 days included a reduction in ejection fraction in 23 of 811 participants and diastolic dysfunction in 80 of 811 participants. 35 Overall, the included studies did show an increased risk of cardiovascular sequels after acute COVID‐19 disease with a higher rate of heart failure, myocarditis, pericarditis, myocardial infarction, and arrhythmias. 35
Conclusion
The usage of ultrasound should be encouraged not only in the context of this pandemic but also in future challenges. 29 Advanced techniques, such as 2D strain imaging of the cardiac chambers, can identify subclinical myocardial damage and are mentioned more and more in current guidelines. Still, there is a lack of data on the outcome for COVID‐19 patients. Lung ultrasound and echocardiography hold great potential in the aftermath of COVID‐19 disease. Still, more literature is needed in the context of the post‐COVID syndrome to evaluate which patient population profits the most from standardized ultrasound exams.
Conflict of Interest
All authors have read the manuscript and approved its submission. The authors declare that there is no conflict of interest.
Supporting information
Video S1 LUS and CT imaging, from the ICU to the rehabilitation—left: after acute infection with ground‐glass pattern, consolidation and pleural effusion/corresponding ultrasound image of zone one, right hemithorax, lateral; right: CT and corresponding lung ultrasound 4 months later.
Video S2 RV strain in a focused apical 4‐ChV of the RV with normal (−23.6%) and hyperdynamic longitudinal RVF (−33.3%)
Video S3 Strain imaging of the LV in post‐COVID‐19 patients with reduction in regional basal strain with normal GLS (GLS range −19.2% to −22.1%)
Video S4 Strain imaging in a post‐COVID‐19 patient after an acute coronary syndrome with a reduction in GLS (−14.1%) and regional strain
References
- 1. Schneider M, Altersberger M, Binder C, Hengstenberg C, Binder T. The COVID‐19 burden for health care professionals: results of a global survey. Eur J Intern Med 2020; 83:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontanet A, Autran B, Lina B, Kieny MP, Karim SSA, Sridhar D. SARS‐CoV‐2 variants and ending the COVID‐19 pandemic. Lancet 2021; 397:952–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conti P, Caraffa A, Gallenga CE, et al. The British variant of the new coronavirus‐19 (Sars‐Cov‐2) should not create a vaccine problem. J Biol Regul Homeost Agents 2021; 35:1–4. [PubMed] [Google Scholar]
- 4. Demi L, Mento F, Di Sabatino A, et al. Lung ultrasound in COVID‐19 and post‐COVID‐19 patients, an evidence‐based approach. J Ultrasound Med 2021; 9999:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID‐19 ‐ an observational prospective multi‐center trial. Eur Respir J 2020; 57:2003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stöbe S, Richter S, Seige M, Stehr S, Laufs U, Hagendorff A. Echocardiographic characteristics of patients with SARS‐CoV‐2 infection. Clin Res Cardiol 2020; 109:1549–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Demeco A, Marotta N, Barletta M, et al. Rehabilitation of patients post‐COVID‐19 infection: a literature review. J Int Med Res 2020; 48:300060520948382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wade DT. Rehabilitation after COVID‐19: an evidence‐based approach. Clin Med 2020; 20:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. López‐León S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. SSRN Electron J 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Raveendran A V, Jayadevan R, Sashidharan S. Since January 2020 Elsevier has created a COVID‐19 resource centre with free information in English and Mandarin on the novel coronavirus COVID‐19. The COVID‐19 resource centre is hosted on Elsevier Connect, the company's public news and information. 2020.
- 11. Hayes LD, Ingram J, Sculthorpe NF. More than 100 persistent symptoms of SARS‐CoV‐2 (long COVID): a scoping review. Front Med 2021; 8:750378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Miao ZY, Min SY, Bin SW, et al. Follow‐up study of the pulmonary function and related physiological characteristics of COVID‐19 survivors three months after recovery. EClinicalMedicine 2020; 25:100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funk GC, Nell C, Pokieser W, Thaler B, Rainer G, Valipour A. Organizing pneumonia following Covid19 pneumonia. Wien Klin Wochenschr 2021; 133:979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mathis G, Horn R, Morf S, et al. WFUMB position paper on reverberation artefacts in lung ultrasound: B‐lines or comet‐tails? Med Ultrason 2020; 23:70–73. [DOI] [PubMed] [Google Scholar]
- 15. Lichter Y, Topilsky Y, Taieb P, et al. Lung ultrasound predicts clinical course and outcomes in COVID‐19 patients. Intensive Care Med 2020; 46:1873–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID‐19 pandemic? J Ultrasound Med 2020; 39:1459–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for COVID‐19 patients; a simple, quantitative, reproducible method. J Ultrasound Med 2020; 39:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tung‐Chen Y, Martí de Gracia M, Parra‐Gordo ML, Díez‐Tascón A, Agudo‐Fernández S, Ossaba‐Vélez S. Usefulness of lung ultrasound follow‐up in patients who have recovered from coronavirus disease 2019. J Ultrasound Med 2021; 40:1971–1974. [DOI] [PubMed] [Google Scholar]
- 19. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38:577–591. [DOI] [PubMed] [Google Scholar]
- 20. Altersberger M, Schneider M, Schiller M, et al. Point of care echocardiography and lung ultrasound in critically ill patients with COVID‐19. Wien Klin Wochenschr 2021; 133:1298–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Volpicelli G, Lamorte A, Villén T. What's new in lung ultrasound during the COVID‐19 pandemic. Intensive Care Med 2020; 46:1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gehmacher O, Mathis G, Kopf A, Scheier M. Ultrasound imaging of pneumonia. Ultrasound Med Biol 1995; 21:1119–22. [DOI] [PubMed] [Google Scholar]
- 23. Buda N, Kosiak W, Wełnicki M, et al. Recommendations for lung ultrasound in internal medicine. Diagnostics 2020; 10:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayo PH, Copetti R, Feller‐Kopman D, et al. Thoracic ultrasonography: a narrative review. Intensive Care Med 2019; 45:1200–1211. [DOI] [PubMed] [Google Scholar]
- 25. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tung‐Chen Y. Lung ultrasound in the monitoring of COVID‐19 infection. Clin Med 2020; 20:e62–e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rubin GD, Haramati LB, Kanne JP, et al. The role of chest imaging in patient management during the COVID‐19 pandemic: a multinational consensus statement from the Fleischner society. Radiology 2020; 158:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pare JR, Camelo I, Mayo KC, et al. Point‐of‐care lung ultrasound is more sensitive than chest radiograph for evaluation of COVID‐19. West J Emerg Med 2020; 21:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hussain A, Via G, Melniker L, et al. Multi‐organ point‐of‐care ultrasound for COVID‐19 (PoCUS4COVID): international expert consensus. Crit Care 2020; 24:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gargani L, Soliman‐Aboumarie H, Volpicelli G, Corradi F, Pastore MC, Cameli M. Why, when, and how to use lung ultrasound during the COVID‐19 pandemic: enthusiasm and caution. Eur Heart J Cardiovasc Imaging 2020; 21:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kiefl D, Eisenmann S, Michels G, et al. German recommendations on lung and thoracic ultrasonography in patients with COVID‐19. Med Klin Intensivmed Notfmed 2020; 115:654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel Z, Franz CK, Bharat A, et al. Diaphragm and phrenic nerve ultrasound in COVID‐19 patients and beyond: imaging technique, findings, and clinical applications. J Ultrasound Med 2021; 41:285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID‐19. Eur Heart J Cardiovasc Imaging 2020; 21:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tudoran C, Tudoran M, Pop GN, et al. Associations between the severity of the post‐acute COVID‐19 syndrome and echocardiographic abnormalities in previously healthy outpatients following infection with SARS‐CoV‐2. Biology (Basel) 2021; 10:469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramadan MS, Bertolino L, Zampino R. Since January 2020 Elsevier has created a COVID‐19 resource centre with free information in English and Mandarin on the novel coronavirus COVID‐ 19. The COVID‐19 resource centre is hosted on Elsevier Connect, the company's public news and information. 2020.
- 36. Ayoubkhani D, Khunti K, Nafilyan V, et al. Post‐covid syndrome in individuals admitted to hospital with covid‐19: retrospective cohort study. BMJ 2021; 31:372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low‐risk individuals with post‐COVID‐19 syndrome: a prospective, community‐based study. BMJ Open 2021; 11:48391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 39. Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. Eur Heart J 2018; 39:119–177. [DOI] [PubMed] [Google Scholar]
- 40. Neumann FJ, Sechtem U, Banning AP, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020; 41:407–477. [DOI] [PubMed] [Google Scholar]
- 41. Konstantinides SV, Meyer G, Bueno H, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J 2020; 41:543–603. [DOI] [PubMed] [Google Scholar]
- 42. Collet JP, Thiele H, Barbato E, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Eur Heart J 2021; 42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 43. Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019; 32:1–64. [DOI] [PubMed] [Google Scholar]
- 44. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults. Eur Heart J Cardiovasc Imaging 2015; 28:1‐39.e14. [DOI] [PubMed] [Google Scholar]
- 45. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29:277–314. [DOI] [PubMed] [Google Scholar]
- 46. Xie Y, Wang L, Li M, et al. Biventricular longitudinal strain predict mortality in COVID‐19 patients. Front Cardiovasc Med 2021; 7:632434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID‐19. JACC Cardiovasc Imaging 2020; 13:2287–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Parasuraman S, Walker S, Loudon BL, et al. Assessment of pulmonary artery pressure by echocardiography: a comprehensive review. Int J Cardiol Heart Vasc 2016; 12:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneider M, Ran H, Pistritto AM, et al. Pulmonary artery to ascending aorta ratio by echocardiography: a strong predictor for presence and severity of pulmonary hypertension. PLoS One 2020; 15:e0235716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases. Eur Heart J 2015; 36:2921–2964. [DOI] [PubMed] [Google Scholar]
- 51. Singh A, Addetia K, Maffessanti F, Mor‐Avi V, Lang RM. LA strain for categorization of LV diastolic dysfunction. JACC Cardiovasc Imaging 2017; 10:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frydas A, Morris DA, Belyavskiy E, et al. Left atrial strain as sensitive marker of left ventricular diastolic dysfunction in heart failure. ESC Heart Fail 2020; 7:1956–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cameli M, Incampo E, Mondillo S. Left atrial deformation: useful index for early detection of cardiac damage in chronic mitral regurgitation. IJC Heart Vasc 2017; 17:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carpenito M, Fanti D, Mega S, et al. The central role of left atrium in heart failure. Front Cardiovasc Med 2021; 8:704762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garg P, Arora U, Kumar A, Wig N. The “post‐COVID” syndrome: how deep is the damage? J Med Virol 2021; 93:673–674. 10.1002/jmv.26465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID‐19 infection: a cross‐sectional evaluation. J Med Virol 2021; 93:1013–1022. [DOI] [PubMed] [Google Scholar]
- 57. Desai AD, Boursiquot BC, Melki L, Wan EY. Management of Arrhythmias Associated with COVID‐19. Curr Cardiol Rep 2021; 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hernández‐píriz A, Tung‐chen Y, Jiménez‐virumbrales D, et al. Importance of lung ultrasound follow‐up in patients who had recovered from coronavirus disease 2019: results from a prospective study. J Clin Med 2021; 10:3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kasner M, Aleksandrov A, Escher F, et al. Multimodality imaging approach in the diagnosis of chronic myocarditis with preserved left ventricular ejection fraction (MCpEF): the role of 2D speckle‐tracking echocardiography. Int J Cardiol 2017; 243:374–378. [DOI] [PubMed] [Google Scholar]
- 60. Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol 2021; 6:945–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Park J, Kim Y, Pereira J, et al. Understanding the role of left and right ventricular strain assessment in patients hospitalized with COVID‐19. Am Hear J Plus Cardiol Res Pract 2021; 6:100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 LUS and CT imaging, from the ICU to the rehabilitation—left: after acute infection with ground‐glass pattern, consolidation and pleural effusion/corresponding ultrasound image of zone one, right hemithorax, lateral; right: CT and corresponding lung ultrasound 4 months later.
Video S2 RV strain in a focused apical 4‐ChV of the RV with normal (−23.6%) and hyperdynamic longitudinal RVF (−33.3%)
Video S3 Strain imaging of the LV in post‐COVID‐19 patients with reduction in regional basal strain with normal GLS (GLS range −19.2% to −22.1%)
Video S4 Strain imaging in a post‐COVID‐19 patient after an acute coronary syndrome with a reduction in GLS (−14.1%) and regional strain


