Abstract
The Matrix Metalloproteinases are important regulators of bone metabolism and can influence bone mass and bone remodeling. We investigate the role of Matrix Metalloproteinase 3 (MMP3) on bone in mice, by using Mmp3 knockout (Mmp3 KO) in the context of estrogen deficiency, and in human, by analyzing the association of promoter polymorphism with bone mineral density in postmenopausal women and with MMP3 expression. We presented evidence in this paper that Mmp3 KO significantly increases trabecular bone mass and trabecular number and does not affect cortical bone thickness. We also found that Mmp3 KO protects from the deleterious effects of ovariectomy on bone mineral density in mice by preventing deterioration of bone microarchitecture. The effect of Mmp3 KO does not involve bone formation parameters but instead acts by inhibition of bone resorption, leading to a reduced bone loss associated to ovariectomy. By studying a human cohort, we found that a polymorphism located in the promoter of the human MMP3 gene is associated with bone mineral density in postmenopausal women and found that MMP3 rs632478 promoter variants are associated with change in promoter activity in transfection experiments. In conclusion MMP3, although weakly expressed in bone cells, could be one of the important regulators of sex hormone action in bone and whose activity could be targeted for therapeutic applications such as in Osteoporosis.
Keywords: bone mineral density, bone resorption, estrogen deficiency, matrix metalloproteinase 3, osteoporosis, SNP
Abbreviations
- 3D
Three‐dimensional
- ADAM
A disintegrin and metalloproteinase
- ADAMTS
A disintegrin and metalloproteinase with thrombospondin motifs
- BA
Bone area
- BMD
Bone mineral density
- BV/TV
Bone volume over trabecular volume
- Crt.Th
Cortical thickness
- DEXA
Dual‐energy x‐ray absorptiometry
- ECM
Extracellular matrix
- KO
Knockout
- MAF
Minor allele frequency
- MAR
Matrix apposition rate
- MicroCT
micro‐computed tomography
- MMP
Matrix Metalloproteinase
- OVX
Ovariectomized
- ROI
Region of interest
- Runx2
Runt‐related transcription factor 2
- SNP
Single nucleotide polymorphism
- Tb.N
Trabecular number
- Tb.Sp
Trabecular separation
- Tb.Th
Trabecular thickness
- TIMP
Tissue Inhibitors of MMPs
1. INTRODUCTION
The Matrix Metalloproteinase (MMP) family consists of numerous homologous proteins that require protease‐mediated activation of the proenzyme. They are capable of degrading collagens and most, if not all, other extracellular matrix (ECM) components (for review 1 , 2 , 3 ). MMPs are important regulators of bone metabolism and can influence bone mass and bone remodeling (for review 4 , 5 , 6 , 7 , 8 , 9 ). The role of MMPs has been investigated by generating various Mmp knockout (KO) mice and among them several have evidenced a bone phenotype (for review, 4 , 10 ). For example, lower bone mineral density and bone mass have been found in Mmp2 KO in long bones 11 , 12 and vertebrae, 11 and in Mmp14 KO mice 13 , 14 respectively. However, a higher bone mineral density has been found in the Mmp2 KO at the calvaria leading to sclerosis at older age. 11 The trabecular bone network has been also affected, with the Mmp13 15 and Mmp2 16 KOs showing increased trabecular bone while Mmp9 KO has negative effects on trabecular architecture. 16 Invalidation of Mmp9 and Mmp14 genes have evidently reduced size of the skeleton that is due to aberrant growth plate formation accompanied with concomitant troubles in vascularization of the forming bone. 13 , 14 , 15 , 17 , 18 A worse growth phenotype, leading to dwarfism has been found in the double Mmp13/Mmp9 15 and in the Mmp14 KO model. 14 In a study of Mmp2 KO at the cell level, it has been evidenced a decrease in osteoblast and osteoclast number in vivo, and a decrease in their proliferation in vitro. 12
The phenotypes found in mouse KO models of these MMPs often resemble those found with corresponding human mutations. This is the case with homozygous human mutation of MMP2 19 with abnormal craniofacial development, bone and joint growth and low BMD. In a human mutation of MMP14 20 there is a crippling osteolysis although not as severe as the mouse KO phenotype. In human metaphyseal anadysplasia mutations has been found in either MMP9 or MMP13, resembling the mouse KO phenotypes that cause severe distortion of the metaphyseal growth plate. 21
Among inhibitors of the catalytic activity of MMPs, the tissue inhibitors of MMPs (TIMPs) are the most widely expressed. The TIMP family comprises four members, TIMP1 to TIMP4, having similarities of action but showing large differences in their primary sequence (for review 4 , 22 ). TIMPs are the major natural inhibitors of the MMPs and of several other disintegrin proteases of the “A Disintegrin And Metalloproteinase” (ADAM) and “A Disintegrin And Metalloproteinase with Thrombospondin Motifs” (ADAMTS) families (for review, 22 ). By their interplay with matrix proteases, TIMPs play majors roles in extracellular matrix (ECM) and tissue remodeling. 23 In fact, it is the MMP/TIMP ratio that is a major determinant of the extent of ECM protein degradation and tissue remodeling. Therefore, a change in either TIMP or MMP levels could alter the MMP/TIMP ratio and cause a specific change in MMP activity (for review, 3 ).
The role of TIMPs has been investigated in bone. They have been shown in vitro to control activity of osteoblasts 24 and osteoclasts. 25 The role of TIMPs has also been studied through the use of genetically engineered mouse models, although individual TIMPs deletion has induced no evident bone phenotype (for review, 4 ). Overexpression of Timp1 in cells of the osteoblastic lineage has induced an increase in trabecular bone volume and a decrease in bone turnover. 26 Furthermore, this model of Timp1 overexpression has been used to demonstrate that increase in Timp1 expression can be used to control bone resorption and potentiate the anabolic PTH treatment, 27 and to reduce the loss of bone mass induced by ovariectomy 28 or by overexpression of Runx2 in osteoblast. 29 To date, the MMPs which are inhibited by TIMP1 in this model and which could be responsible for these effects remain to be identified. Mmp3 is one of the candidates.
Although being documented for its expression in bone, little is known about MMP3 in bone physiology. MMP3 (also known as Stromelysin‐1) is a matrix metalloproteinase able to cut numerous collagens and other matrix proteins and having the ability to activate several MMPs, including itself (for review, 9 ). In skeleton, MMP3 has been studied mostly in cartilage biology, because of its involvement in the etiology of arthritis 30 (for review, 31 ). MMP3 has been located first in vitro, in mouse 32 and human 33 osteoblasts. It is almost a decade later that it has been described in vivo, in developing human bone. 34
Our focus was to investigate the role of MMP3 on bone. For this purpose, we studied the function of MMP3 in adult mice and the impact of its invalidation on bone using Mmp3 KO mice in the context of estrogen deficiency. We also investigated the role of MMP3 in human by analyzing the association of promoter polymorphism with bone mineral density in postmenopausal women and with MMP3 expression.
2. MATERIALS AND METHODS
2.1. Animals
Mmp3 KO mice were kindly provided by Dr Zena Werb (University of California, San Francisco [UCSF]) and were made as previously described. 35 Mice were housed under controlled conditions at 24°C on a 12‐h light/12‐h dark cycle. Heterozygous animals (inbred FVB/N strain) were bred to generate Mmp3 KO mice and their wild‐type littermates. Ovariectomy experiments were done in 3‐month‐old Mmp3 KO female mice and their wild‐type littermates. Animals of each genotype were subdivided in two groups, and were either sham‐operated (Sham) or ovariectomized (OVX), housed for 30 days and sacrificed. Prior to sacrifice, and to evaluate the dynamic bone formation parameters (see section 2.4), two fluorochromes were injected to mice (intraperitoneally), first oxytetracycline 5 days before necropsy (20 mg/kg; Pfizer), then calcein 1 day before necropsy (20 mg/kg; Sigma‐Aldrich). Urinary samples were collected for deoxypyridinoline (DPD) assay before surgery and before animals were sacrificed. Accurate DPD measurement requires overnight fasting before collecting urine. DPD was measured with the IMMULITE Pyrilinks‐D in vitro diagnostic kit (Siemens Healthcare SAS). To correct for flow variations, DPD results were normalized to the urinary creatinine concentration (ADVIA System, Siemens Healthcare SAS). After sacrifice, femurs were collected from all animals (OVX and Sham) and were cleaned from soft tissues and processed. Right femur was used for micro‐computed tomography (microCT) analysis while left femur was embedded without demineralization in methyl methacrylate and sliced.
All animal procedures have been approved by the Ethical Committee Lariboisiere‐Villemin (approval CRRALV/ 2011‐03‐03).
2.2. Dual‐Energy x‐ray Absorptiometry
Dual‐energy x‐ray absorptiometry (DEXA) analysis of all animals was performed to determine the bone mineral density in a selected region of interest (ROI). The measurement was performed on the animals under anesthesia with a PIXImus instrument (software version 1.44; PIXImus, Lunar, France) using ultrahigh resolution mode (resolution of 0.18 × 0.18 mm). The coefficient of variation was less than 2% for all primary measured parameters (Bone mineral content [BMC] and ROI bone area [BA]) and a phantom was scanned daily to monitor measurement stability. BMC and BA were measured at the femur and the L4–L6 vertebrae just before and 1 month after ovariectomy in order to express the results as fold change (% of baseline). The femoral and vertebral bone mineral density (BMD) were calculated from these two measured parameters.
2.3. Micro‐computed tomography
Mice were killed 1 month after surgery and right femurs were harvested for bone morphometric analysis. Femurs were analyzed with high‐resolution micro‐computed tomography (micro‐CT) using a Skyscan 1172 scanner (Skyscan). These qualitative and quantitative imaging analyses were mainly focused on the distal metaphysis of the femurs. The radiographic projections (n = 210) were acquired at 80 kV and 100 mA with an exposure time of 100 ms and a 0.5‐mm aluminum filter. Ten frames were averaged for each rotation increment of 0.5° to increase the signal‐to‐noise ratio. Three‐dimensional (3D) images were reconstructed with a mean voxel size of 6 μm, using the manufacturer's reconstruction software NRecon version 1.6.2.0 (Skyscan, Bruker microCT). Cortical bone thickness (Crt.Th3D) was analyzed in the midshaft of the femur over 300 μm at 4 mm from the growth plate cartilage. Trabecular bone was analyzed at the distal femoral metaphysis. Trabecular bone volume (BV/TV3D), trabecular number (Tb.N3D), trabecular separation (Tb.Sp3D), and trabecular thickness (Tb.Th3D) were quantified using the resident software CTAn version 1.10.1.0 (Skyscan, Bruker microCT).
2.4. Histology and histomorphometry
The left femur from each animal was excised at sacrifice and was stored in 70% ethanol at 4°C before being trimmed. The distal halves of the bones were post‐fixed in 70% ethanol, dehydrated in xylene at 4°C, and then embedded without demineralization in methyl methacrylate. Five micrometer‐thick coronal sections were cut parallel to the long axis of the femur, using an SM2500S microtome (Leica, Germany). Two nonconsecutive sections were stained for tartrate‐resistant acid phosphatase (TRACP) activity detection using naphthol ASTR phosphate (Sigma, St Louis, France) as substrate, and then counterstained with toluidine blue pH 4.3. Other sections were stained with aniline blue to evaluate the morphometric parameters. The bone volume over trabecular volume (BV/TV2D), trabecular number (Tb.N2D), trabecular separation (Tb.Sp2D), trabecular thickness (Tb.Th2D), and dialysis cortical thickness (Crt.Th2D) were measured using a Bonolab/histolab software package version 1.0 developed for bone histomorphometry (Microvision, Evry, France).
Two 12‐μm thick unstained sections were taken for measurement of the dynamic parameters under UV light.
The matrix apposition rate (MAR) was measured using the Microvision image analyzer by a semiautomatic method using tetracycline and calcein double‐labeled bone surfaces. The mineralizing surfaces (MS/BS) were measured in the same areas using the objective eyepiece Leitzintegrateplatte II and the bone formation rate was calculated using the two measured parameters. All the histomorphometric parameters were recorded in compliance with the recommendation of the American Society for Bone and Mineral Research Histomorphometry Nomenclature Committee. 36
2.5. Culture and differentiation of primary osteoblasts
Primary osteoblasts were isolated from calvariae of 2‐ to 4‐day‐old WT and Mmp3 KO mouse pups. Briefly, calvariae were digested for 15 min with 0.2% type IV collagenase (Sigma‐Aldrich) in phosphate‐buffered saline (PBS) with EDTA in order to remove fibroblasts, and then for 45 minutes with 0.2% type IV collagenase in PBS for osteoblastic cells release. The osteoblastic cells were expanded for 5–6 days in minimum essential medium‐alpha containing 10% fetal calf serum and plated at a density of 2.5 × 10+4 cells/cm2. For the determination of alkaline phosphatase activity, cells were plated at 4 × 10+4 cells per well in 24‐well plates. For RNA extraction and the evaluation of mineralization, cells were plated at 2 × 10+5 cells per well in 6‐well plates. The culture medium was supplemented with 50 μM ascorbic acid and 10 mM beta‐glycerophosphate, which was replaced every 2–3 days. Nodules of mineralized extracellular matrix were labeled by Alizarin red staining (Sigma‐Aldrich) following fixation of the cells with paraformaldehyde 4% 15 min at 4°C. The mineralized nodules were then quantified by a colorimetric assay adapted from Wu et al. 37 The stained cells were incubated with a 10% acetic acid solution for 30 min with agitation to elute all calcium‐bound stain. Optical density of the resulting solution was measured at 450 nm.
2.6. Patients
For the BMD association study, 501 Caucasian women were recruited, investigated at a single clinical center (Hôpital Lariboisière) and monitored to evaluate determinant factors for bone loss (Cohorte ViggOs). We included women between 50 and 80 years of age who agreed with blood DNA sampling. Individuals who had been treated with corticosteroids for more than 3 months, or with bisphosphonates, fluoride or any other bone‐acting drugs were excluded.
All 501 women were interviewed to evaluate risk factors for osteoporosis (personal and familial history of fractures, physical exercise, and nutritional habits). This population is described in Table 1. BMD was measured in a Lunar DPX device and expressed as Z‐score as there was a large range in age in the studied population.
TABLE 1.
Description of the cohort (n = 501)
| Mean | SD | |
|---|---|---|
| Age (years) | 63 | 9.9 |
| Height (cm) | 159 | 6.3 |
| Body mass (kg) | 59.8 | 9.3 |
| Body mass index (kg/m2) | 23.8 | 3.3 |
| Hormone replacement therapy duration (years) | 8.4 | 5.9 |
2.7. Single nucleotide polymorphism (SNP) genotyping
For determination of the major haplotypes in the MMP3 promoter and gene we have extracted the genotype of all population from European ancestry in the 1000 genome project database (CEU, TSI, IBS, FIN, and GBR; www.ncbi.nlm.nih.gov/variation/tools/1000genomes/; genomic DNA from 374 individuals representing 748 alleles) and found seven polymorphisms with a minor allele frequency (MAF) over 1%. SNP genotyping were performed on a LC480 apparatus (Roche Diagnostics) using the Roche probe master mix. The rs632478 SNP located in the MMP3 promoter region was assessed using a dual fluorescent probe hybridization system followed by melting curve analysis. PCR primers and probes are summarized in Table 2. For rs632478 analysis, probes and specific primers were designed by TibMolBiol®. The PCR conditions were as follows: an initial denaturing step at 95°C for 10 min; then 40 denaturing cycles at 95°C for 10 s, annealing for 15 s at 58°C, and extension at 72°C for 20 s. The melting curves were performed after denaturing at 95°C (for 1 min), followed by rapid cooling to 37°C and then a continuous increase in temperature up to 70°C (five fluorescence acquisitions per degree Celsius). All curves were analyzed using LC480 Genotyping software 3.5.
TABLE 2.
Primer sequences used for qPCR Amplification of human and mouse genes
| Gene | Human primers | Mouse primers |
|---|---|---|
| MMP3/Mmp3 | 5′‐caaaggatacaacagggacc‐3′ | 5′‐ttgttctttgatgcagtcagc‐3′ |
| 5′‐gtgagtgatagagtgggtac‐3′ | 5′‐gatttgcgccaaaagtgc‐3′ | |
| B‐Actin/B‐Actin | 5′‐gagaagagctacgagctgcctg‐3′ | 5′‐ gctctggctcctagcaccat‐3′ |
| 5′‐ggtagtttcgtggatgccaca‐3′ | 5′‐gccaccgatccacacagagta‐3′ | |
| PPIA/Ppia | 5′‐gtcaaccccaccgtgttctt‐3′ | 5′‐cagctgtttgcagacaaagttc‐3′ |
| 5′‐ctgctgtctttgggaccttgt‐3′ | 5′‐ccctggcacatgaatcctgg‐3′ | |
| GAPDH/Gapdh | 5′‐cttccaggagcgagatccc‐3’ | 5’‐acacattgggggtaggaaca‐3’ |
| 5’‐gaagacgccagtggactcca‐3’ | 5’‐aactttggcattgtggaagg‐3’ |
2.8. Luciferase activity assay
MC3T3‐E1 preosteoblastic cells (CRL‐2593, Subclone 4) were cultured in α‐MEM medium supplemented with glutamine, penicillin/streptomycin, 10% fetal calf serum and incubated at 37°C, 5% CO2 atmosphere. Cells (seeded at 8.10+5 cells/well in 12‐well plates) were transfected 48 h after plating using the lipofectamine kit. Renilla control plasmid of 40 ng and 1 μg of pGL4.10 reporter vector containing 2.0 kb or 1.5 kb of human MMP3 promoter containing different sets of SNPs (haplotypes) were transfected per well. Luciferase and Renilla light emitting activities were measured 48 h following transfection by using the Dual Luciferase Reporter Assay System (Promega).
2.9. RNA extraction from mouse samples
Total RNA was extracted from mouse long bones that were crushed directly in TRIzol™ using a Polytron® and total RNA was prepared according to the manufacturer's instructions.
2.10. Total RNA and genomic DNA extraction from human bone samples
Tibial plateaus were obtained in collaboration with the Department of Orthopedic Surgery (Hospital Lariboisiere) from patients undergoing knee replacement surgery due to osteoarthritis. This procedure has been approved by the local Ethics Committee (#06NICB of CCP Ile de France IV). For polymorphism studies, the patients were only females (n = 31) between 52 and 86 years old and were processed as described previously. 38 Briefly, trabecular bone “carrot” samples were obtained from the inner part of the tibial plateau using a 2‐mm diameter biopsy trocar, and frozen in liquid nitrogen. Only tibial plateaus containing trabecular bone of apparent good quality and remote from the areas affected by osteoarthritis were selected. After crushing the trabecular samples in liquid nitrogen using a mortar and a pestle, total RNA was extracted using the TRIzol™ procedure (Invitrogen). Genomic DNA was extracted following the same crushing procedure, but using a QIAamp DNA Mini extraction kit from Qiagen.
2.11. Reverse transcription and Real‐Time quantitative PCR
For each sample, 1 μg of total RNA was reverse transcribed using the Verso cDNA kit (Abge) with a mix of random primers and oligo dT in accordance with the manufacturer instructions. The final reaction (20 μl) was diluted in water to a final volume of 400 μl and 5 μl of it was used for each quantitative PCR reaction. Target genes and reference genes were subjected to real‐time quantitative PCR using the ABsolute™ Blue QPCR SYBR® Green master mix (Abgene) in triplicate. Oligonucleotides (Eurogentec) were designed for specifically amplifying mRNA sequences by targeting different exons, or straddling 2 exons. PCR products were between 80 and 120 bp in size, and the oligonucleotides had a melting temperature of around 60°C (primers for human genes in Table 3). After each real‐time PCR using SYBR® Green, a fusion curve was plotted to confirm the absence of unwanted PCR products. For the human bone samples, the choice of three reference genes and the quality assessment of RNA samples were described previously. 38
TABLE 3.
PCR primers and probes used for the detection of rs632478 MMP3 promoter polymorphism
| SNP | Primers for PCR | Probes |
|---|---|---|
| rs632478 |
F 5’‐caacccaaagggaatatactt‐3’ S 5′‐tttcacagaccctctttgttc‐3′ A 5′‐aagcagttttattttaagaaacaca‐3′ R 5′‐aaaatcagaattgtgcagaact‐3′ |
agtggctatcacctgtgtgg‐FL a |
FL: Fluorescein
LC640: LightCycler Red 640
P: Phosphate
2.12. Statistical analysis
The results are expressed as means ± Standard deviation (SD).
First the potential outliers were identified and remove from the data sets before statistical analysis. The data were subjected to the Shapiro–Wilk normality test. For parameters that showed homogeneity, analysis of variance was performed using two‐way ANOVA. Where significant overall differences were detected by analysis of variance, a post hoc Tukey's comparisons test was used to compare differences between the different genotypes or treatments. When data did not pass the control of normality, the nonparametric Kruskal–Wallis test and the Dunn posttest were applied.
Statistical analysis of the transfection data and of the MMP3 expression in WT mice (two groups comparisons) was performed using unpaired t‐test.
The statistical analysis program used was Prism version 9.3.0 (GraphPad Software). For each test, p < 0.05 was considered significant.
Variation of Z‐score according to MMP3 polymorphisms was analyzed using ANOVA to compare multiple groups, followed by a post hoc test in case of a significant effect. The post hoc test consisted of a pairwise comparison by using the Tukey's test. The statistical program used was R version 3.1.0 (R Core Team, R Foundation for Statistical Computing, 2014 (https://www.R‐project.org).
3. RESULTS
We have used Mmp3 KO mice in order to study the role of MMP3 in adult bone and to investigate the effect of Mmp3 deletion in the bone loss induced by ovariectomy.
3.1. Mmp3 KO prevents the deleterious effects of ovariectomy on bone mineral density in mice
First, we have shown that ovariectomy increases Mmp3 mRNA expression in wild‐type mice long bone cells (Figure S1). We then evaluated the effect of Mmp3 KO on bone mass in mice and the role played by ovariectomy. We noticed that Mmp3 KO has no effect on the baseline femoral and vertebral BMD. Indeed, femoral and vertebral BMD were not significantly different in wild‐type and Mmp3 KO mice before and 1 month after sham operation (respectively Figure 1A, B, Sham mice) indicating that the lack of MMP3 has no effect on femoral and vertebral BMD. We also observed that BMD was significantly decreased in wild‐type OVX mice compared to their sham‐operated counterpart. However, there was no significant effect of ovariectomy in femurs and vertebrae of Mmp3 KO mice (Figure 1B). Then, by analyzing change of BMD from baseline on live animals, a significant decrease in bone mass was seen in wild‐type mice between ovariectomized versus sham‐operated (Sham) animals but was not observed in ovariectomized Mmp3 KO females compared to their Sham counterpart (Figure 1C). In femurs, but not vertebrae, the change in BMD after ovariectomy was significantly greater in wild‐type than in Mmp3 KO mice. These data also showed that gain in BMD was higher in vertebrae than in femurs mice during the 1‐month period, regardless of the phenotype (wild‐type and Mmp3 KO).
FIGURE 1.

Bone mineral density (BMD) analysis of femur and vertebrae from wild‐type and Mmp3 KO mice after ovariectomy and sham‐surgery. (A) BMD was measured by DEXA before (A) and 30 days after ovariectomy or sham‐surgery (B). Change in BMD is expressed in % of baseline (C). BMD is expressed in g/cm2. All values are expressed as mean ± SD. Significance was determined using ANOVA and post hoc Tukey's tests. (**, p < 0.005), n = 7–9 animals per experimental groups
3.2. Mmp3 KO prevents deterioration of bone microarchitecture in OVX mice
Compared to wild‐type, Mmp3 KO mice did not exhibit any apparent modification of the macroscopic bone structure of the femur either by microCT or histological analyses (Figure 2A, B respectively). The 2D and 3D analyses confirmed in wild‐type mice a significant decrease of bone volume over tissue volume (BV/TV) and of trabecular number (Tb.N) and a significant increase in trabecular spacing (Tb.Sp) in those femurs after ovariectomy (Figure 2C). Only trabecular thickness (Tb.Th) was unaffected by ovariectomy in wild‐type mice. A closest look at a fine microCT 3D analysis of the trabecular structure has shown that BV/TV and Tb.N were significantly higher in Mmp3 KO mice than in wild‐type mice (Figure 2C). An apparent lack of effect induced in Mmp3 KO mice by ovariectomy was observed by 2D and 3D analyses showing no significant change in any trabecular parameters (Figure 2C). This was corroborated by 3D microCT analysis showing significant differences in trabecular parameters (BV/TV, Tb.N, and Tb.Sp) between wild‐type and Mmp3 KO mice that have been ovariectomized (Figure 2C).
FIGURE 2.

MicroCT and histology analyses of mouse femur in 4‐month‐old wild‐type and Mmp3 KO mice after ovariectomy or sham‐surgery. Two‐dimensional (2D) microCT (A) and histological (Toluidine blue staining) (B) pictures are representative images of the distal femur (coronal sections). (C) Microarchitecture parameters were measured by histomorphometry on 2D histological sections (left panel) and by 3D microCT (right panel). Bone volume per tissue volume (BV/TV, %), trabecular thickness (Tb.Th, μm), trabecular number (Tb.N, 1/μm), and trabecular spacing (Tb.Sp, μm) are presented as mean ± SD. Significance was determined using ANOVA and post hoc Tukey's tests. (*p < 0.05; **p < 0.005; ***p < 0.0005; ****p < 0.0001), n = 5–7 animals per experimental group for histological analysis and n = 8–10 for microCT analysis
Otherwise, no significant change in the cortical thickness (Crt.Th) of the femur diaphysis has been found between wild‐type and Mmp3 KO mice or in both after ovariectomy (Figure S2).
3.3. Mmp3 KO in mice does not affect bone formation parameters
Evaluation of static and dynamic bone parameters by histology revealed that neither ovariectomy nor genotype seems to affect bone formation since Tb.Th, MS/BS, MAR, and BFR/BS parameters were not significantly different in sham‐operated versus ovariectomized wild‐type or Mmp3 KO mice (Figure 2C and Figure S3A–C).
Moreover, primary osteoblasts isolated from calvariae of wild‐type or Mmp3 KO exhibited similar kinetics of ALP activity (Figure S3D) and thus similar ability to form mineralized modules up to 20 days in culture as confirmed by a colorimetric assay of alizarin staining (Figure S3E, F, respectively).
3.4. Mmp3 KO inhibits bone resorption and reduces the bone loss associated to ovariectomy
DPD/creatinine determination was used to illustrate bone resorption activity in our mice. DPD/creatinine was determined in wild‐type and Mmp3 KO at 3 months of age before ovariectomy and sham operation and at 4 months of age in operated animals (Figure 3A). These results indicate that Mmp3 KO mice have significantly less DPD excretion compared to wild‐type mice a both ages and that this difference seems to increase over time. In our wild‐type mice, the bone loss associated to ovariectomy (Figure 1A, B) is primarily due to an increase in bone resorption, as evidenced by an increase in urinary DPD excretion (Figure 3B). However, this increase in DPD excretion is not observed in Mmp3 KO mice after ovariectomy (Figure 3B) suggesting in part a reduced bone resorption activity in these OVX mice. Interestingly when analyzing the osteoclast parameters on bone sections by histomorphometry, we showed that osteoclast surface over bone surface (Oc.S/BS) and number of osteoclast over trabecular volume (N.Oc/TV) were not modified in wild‐type OVX mice compared to the Sham controls but were significantly increased in Mmp3 KO OVX mice compared to their Sham controls (Figure 3C, D). These results suggest an increase in osteoclast differentiation to compensate the inhibition of bone resorption observed in the absence of MMP3 expression.
FIGURE 3.

Bone resorption parameters measured after ovariectomy or sham‐surgery in wild‐type and Mmp3 KO mice. (A) Urinary deoxypyridinoline cross‐links level (DPD) normalized by the amount of urinary creatinine (DPD/creatinine) was measured before (3‐month‐old [3‐mo] mice) and 30 days after surgery (4‐month‐old [4‐mo] mice) in the Sham groups (n = 6–8 animals per experimental groups). (B) DPD/creatinine was also measured in all groups before sacrifice at 4 months of age. Osteoclast parameters were determined by histomorphometry after tartrate‐resistant acid phosphatase staining of femur coronal sections. Osteoclast surfaces per bone surface (Oc.S/BS, C) and osteoclast number per tissue volume (N.Oc/TV, D) were quantified (n = 3–4 animals per experimental groups). All values are expressed as mean ± SD. Significance was determined using the nonparametric Kruskal–Wallis test and the Dunn posttest (## p < 0.005) or using ANOVA and post hoc Tukey's tests. (*p < 0.05; **p < 0.005; ***p < 0.0005)
3.5. A polymorphism located in the promoter of the human MMP3 gene is associated with bone mineral density in postmenopausal women
Since lack of MMP3 is able to prevent ovariectomy‐induced bone loss in mice, we have investigated the possible role played by MMP3 polymorphisms in humans and especially in postmenopausal woman. Thus, we have looked for single nucleotide polymorphisms (SNPs) located in the promoter of MMP3 gene and selected one of them, rs632478 (G/T variant [C or A in this orientation]), because it is in complete linkage disequilibrium with rs3025058 (equivalent to rs35068180) in the cohorts with European ancestry studied in the 1000genome project, a SNP known as 5A/6A polymorphism and known to affect MMP3 gene expression. This latter SNP has been intensively investigated for its associations with various human diseases (for review 39 ), with MMP3 mRNA expression (e.g., 40 ) and MMP3 promoter activity. 40 , 41
We have determined rs632478 variants with Z‐scores at lumbar spine and at femoral neck in a cohort of 501 postmenopausal women (Figure 4A, B). The Z‐score associated to the G/G appeared to be significantly different from those of the T/T genotype in both lumbar spine and femoral neck, G/G being associated to lower Z‐score values. In addition, MMP3 mRNA expression is the lowest for G/G genotypes, slightly higher for T/T genotypes and intermediate for G/T, in pieces of human bone obtained from tibial plateaus after knee replacement surgery, that are looking undamaged (Figure 4C).
FIGURE 4.
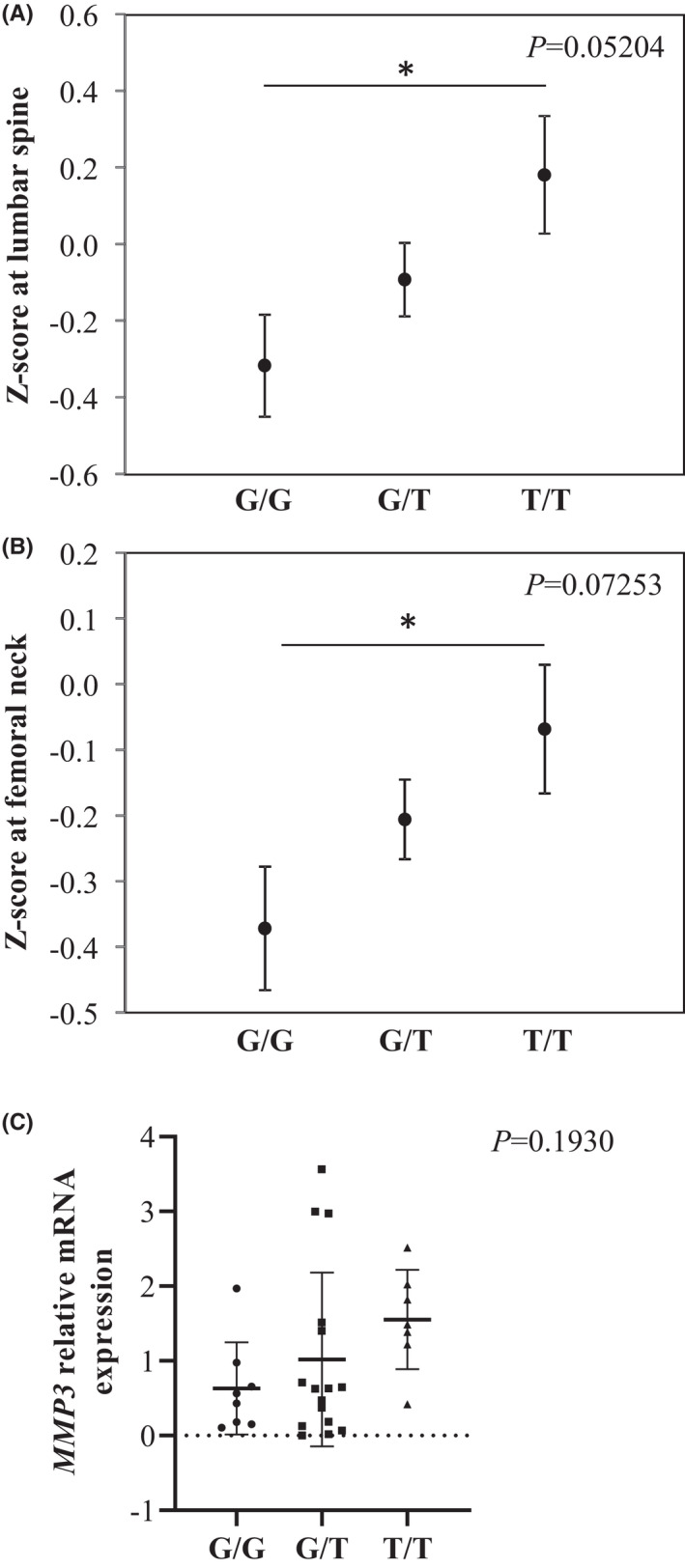
Effect of human MMP3 promoter polymorphism rs632478 on BMD and MMP3 expression. Women BMD was expressed as Z‐score at femoral neck (A) and at L2‐L4 lumbar spine (B), and compared between the different rs632478 genotypes. Z‐scores at femoral neck (n = 487; G/G n = 124, G/T n = 261, T/T n = 102) and at lumbar spine (n = 485; G/G n = 123, G/T n = 260, T/T n = 102) were measured in a cohort of French women with osteoporosis. Values are expressed as mean ± SD. (C) Relative expression of MMP3 mRNA was measured in cortical bone of human tibial plateau and was presented by class of genotype (G/G n = 8; G/T n = 16; T/T n = 7; n = 31) (C). Values are expressed as mean ± SD (*P < 0.05). Significance was determined using ANOVA and post hoc Tukey's tests. Variance p values are indicated on each panel
3.6. MMP3 rs632478 promoter variants are associated with change in promoter activity in transfection experiments
We have looked for SNP in the promoter region of MMP3 within 2 kb 5′ of the transcriptional start site and containing rs3025058 (5A/6A). We have determined seven SNPs with a MAF over 1% in populations of European ancestry and six major haplotypes (Figure 5A) are fully representative of all 748 analyzed alleles (see Materials and Methods). We therefore assumed that rs3022058, rs632478, and rs617819 are at least very close to full linkage disequilibrium. Interestingly the rs679620 SNP, located early in the coding region (Lys45Glu) and abundantly studied for MMP3 genotype variation related to several diseases, is in full linkage disequilibrium and belongs to the same haploblock that these latter promoter SNPs.
FIGURE 5.

Effect of SNP variation on the promoter activity of the human MMP3 gene. A, Scheme representing the SNP variations with frequency over 1% found in the 2.0 kb region of the promoter located 5′ of the Human MMP3 gene. (A) Various combinations of haplotypes were determined. SNP rs679620 (Lys45Glu) located in the MMP3 gene coding sequence is also indicated. Percentage of the MAF was estimated for each SNP in a subpopulation of the 1000genome project with European ancestry. (B) Fragments of 2.0 and 1.5 kb of the MMP3 promoter region, both containing the rs632478 G/T variant were cloned in front of the luciferase reporter cDNA sequence in a pGL4.10 reporter plasmid. The plasmids were co‐transfected into MC3T3‐E1 cells along with a construct expressing constitutively the Renilla reporter gene as internal control. Promoter activity is expressed as the ratio of luciferase activity over Renilla activity. All values are expressed as mean ± SD and reported to the expression of the rs632478 C promoter variant. Significance was determined using unpaired t‐ test. (**p < 0.005; ***p < 0.0005) (n = 4 independent transfection experiments performed in triplicate)
In order to study a possible role of SNP rs3022058 (5A/6A) or rs632478 on MMP3 promoter activity, we have made two luciferase reporter constructs containing 2 kb of the MMP3 promoter with variations corresponding to SNPs rs3022058, rs632478, and rs617819. We have chosen to perform transfection experiments with these constructs in osteoblasts, cells that are mostly expressing MMP3 in bone. After transfection into MC3T3‐E1 cells, luciferase activity was significantly higher (Figure 5B) with the 5A(−)‐G‐A‐A‐C‐G‐T than with the 6A(T)‐G‐C‐A‐C‐C‐T haplotype. By transfecting a shorter promoter fragment (−1.5 kb) having lost two distal SNPs including rs3025058 (5A/6A), we obtained a milder effect (22.6% vs. 50.6%) but still significant, suggesting that both 5A/6A and the remaining rs632478 and rs617819 polymorphisms play a role in the variation of MMP3 promoter activity. Variation of the rs617819 polymorphism alone has no effects on the MMP3 promoter activity (data not shown).
4. DISCUSSION
The cellular and tissue functions of MMPs are mainly mediated by their own proteinase activity. Moreover, MMPs and TIMPs expression are also interconnected to regulate locally tissue/matrices degradation and remodeling, and subsequently cell behavior. In bone tissue, these enzymes are required for the degradation of matrix and unmineralized osteoid that line the bone surface to facilitate cells attachment and migration. MMPs expression is non‐cell specific and one MMP can be expressed by different cell types within the same tissue. These multiple patterns of expression render Mmp KO phenotype quite complex to analyze. In bone MMP3 has been described to be expressed mostly in osteoblasts, fibroblasts, and osteocytes, the most abundant cells in bone tissue. 34 , 42 The function of cell‐specific MMP3 is difficult to evaluate as no cell‐specific Mmp3 conditional KO model has been developed to date. The understanding of MMPs function in vivo is also closely related to their substrate nature. MMPs can cleave indeed a wide range of non‐collagenous proteins and activate many latent growth factors that affect the fate of surrounding cells. It is known that individual MMPs share substrates possibly leading to functional redundancies. Thus, we cannot exclude that the mild bone phenotype observed in Mmp3 KO mice may be the consequence of this overlapping MMPs substrate register, and that some aspects of MMP3 function in bone are not apparent in Mmp3 KO mice. We presented evidence in this paper that Mmp3 KO significantly increases trabecular bone mass and trabecular number. Further evaluations indicated that these increases were due to decreased bone resorption rather than increase in bone formation, although cells of the osteoblast lineage are the main cells expressing MMP3 in bone. These data also showed that Mmp3 depletion, similarly to the inhibition of MMPs elicited by TIMP1 overexpression in osteoblasts, 28 abolish osteoclast resorption induced by ovariectomy and reduced the response of osteoblast to estrogen deficiency.
The involvement of MMP3 in different steps leading to bone formation was suggested in previous publications and may depend on the stage of development in humans and rodents. In the nineties, the presence of MMP3 in osteoblasts and osteocytes in the human neonatal rib was demonstrated and located especially in the osteogenic fronts suggesting that MMP3 may be required for connective tissue degradation to allow cellular migration and bone growth. 34 The presence of MMP3 in human bone was confirmed in calvaria samples of fetuses by Zeitler in 2004. 43 In addition, Zhao et al. 44 showed in vitro an increase in osteoblast differentiation of mouse bone marrow stem cells upon depletion of Mmp3 by transfection with Lentivirus vectors encoding shRNA targeting Mmp3 or by treatment with MMP3‐specific inhibitor 5 but no in vivo evidence has confirmed these in vitro data. In our study in adult mouse females, no global effect on bone formation parameters in vivo and on osteoblast differentiation in vitro has been observed in Mmp3 KO adult mice compared to WT mice. This does not exclude that MMP3 could affect earlier stages of bone growth. It is not surprising since Mmp null mutants exhibited mostly mild phenotypes resulting from protease redundancy and compensation, and controlled balance between MMPs and TIMPs expressions. 42 As an example, Stickens et al. presented evidence that the endochondral bone phenotype observed in Mmp13‐deficient mice progressed with age. 15 Mmp2 deficiency also results in transient and long‐lasting bone remodeling defects. 12
Osteoclasts as well as macrophages, also produce MMPs, MMP9 being the most abundantly expressed. Several MMPs have been shown to affect bone resorption mainly through the regulation of osteoclast migration and progenitor cells fusion. However, MMP3 expression in osteoclast has been found lacking to date. 45 , 46 , 47 Thus, the impact of MMP3 depletion we observed on bone resorption in Mmp3 KO mice may be independent of its expression in osteoclasts. This effect of Mmp3 deficiency on bone resorption was mainly illustrated by a strong decrease in DPD/creatinine ratio in Mmp3 KO compared to WT mice illustrating a strong decrease in global osteoclasts activity. This decrease is probably due to the reduction of individual osteoclast activity, as the surface of resorbing osteoclast (Oc.S/BS) and their number (N.Oc/TV) are not affected by Mmp3 deletion. MMP3 like the other MMPs secreted by neighborhood cells (osteoblasts and others) can indirectly affect osteoclasts and bone resorption in Mmp3 KO mice. MMP3 induced by mechanical stimulation of osteoblasts is one of the candidate key enzymes in the processing of collagen on bone surface, which is necessary for osteoclast recruitment and bone resorption. 48 MMP3 is able to activate MMP1 to generate a mature enzyme that has 10‐fold more collagenase activity than the proenzyme. In addition, exogenous MMP3 has been demonstrated to stimulate macrophage secretion of PGE2 and to increase MMP9 expression in vitro, both required for macrophage activity. 49 RAW264.7 monocyte/macrophage cell line knockdown for Mmp3 also exhibited impaired osteoclast differentiation. Moreover, decreased osteoclast differentiation was observed in RAW264.7 cells treated with the MMP3‐specific inhibitor. 43
MMPs have been shown to be important regulators of bone remodeling especially in situations in which bone remodeling is accelerated such as osteoporosis induced by ovariectomy. In general, higher expression of MMPs was observed in osteoporotic bone tissues compared to normal bones. This was described for many of them including MMP1, MMP2, and MMP9, in osteoporotic patients. 50 Changes in the activity of MMP2, MMP9, and MMP13 have been also shown to participate in the deleterious effects of ovariectomy on bone tissue in rodents. 51 , 52 Furthermore, deletion of both Mmp9 and Mmp14 in mice has been suggested to protect against ovariectomy‐induced bone loss. 47 In ovariectomized Mmp3 KO mice, although the resorption activity was decreased, an increase in osteoclast number and surfaces relative to wild type ovariectomized mice has been noticed. This was also observed in mice KO for Mmp9 and Mmp14 in which osteoclast numbers were increased whereas bone erosion surfaces were decreased compared to wild type mice, resulting in an osteopetrotic‐like phenotype. 47 Several reports also referred to the involvement of upregulation of Mmp13 in the osteoporosis induced by ovariectomy. Li et al. 53 stated that the increased level of Mmp13 after ovariectomy is positively correlated with increased bone turnover parameters and negatively correlated with bone volume.
Although mostly associated to increased bone resorption, upregulation of Mmp3 has also been shown to partially mediate the bone‐protective effects of E2 through induction of osteoclast apoptosis. 54 Conversely, we presented evidence here that absence of Mmp3 in mice protects against bone loss induced by ovariectomy. Indeed, the hallmarks of estrogen deficiency such as increased bone resorption and decreased femoral bone mineral density and trabecular bone volume are absent in ovariectomized Mmp3 KO mice. Moreover, we have shown in this work that ovariectomy increases significantly MMP3 expression in bone that confirms increased Mmp3 expression, both in bone lining osteoblasts 55 and in primary osteoblasts differentiated from bone marrow precursor cells 28 observed in ovariectomized mice. Increased MMP3 expression could enhance MMP9, secreted as a zymogen, activation through the proteolytic removal of its pro‐domain 45 and could affect matrix composition through its proteinase activity that cleaves proteoglycans, laminins, gelatin, casein, and multiple non‐matrix substrates. We cannot exclude that modifications of the extracellular matrix composition may thereby modulate cell behavior including osteoclasts or be more prone to resorption by activated osteoclasts. Furthermore, in other skeletal tissues, Mmp3 mRNA levels decreased under 17b‐E2 treatments such as in intervertebral disc 56 and nucleus pulposus. 57 Interestingly, MMP3 levels have been shown to vary in women endometrium, with its expression increasing when estrogen levels drop. 58 This could be linked with an increase in human bone resorption markers at the same cycling period 59 where levels of deoxypyridinoline are inversely correlated with estrogen levels. The possible role of MMP3 change of expression upon estrogen levels led us to test in a cohort of postmenopausal women the possible association of MMP3 SNPs with bone loss.
The great influence of the mouse genetic background on bone phenotype and metabolism is well known, some being more prone to lose bone after ovariectomy. There are only few papers comparing FVB mice to mice of other strains. Adebayo et al. 60 consider that FVB mice exhibit a higher bone mass compared to C57BL/6. On the other end, Zanotti et al. 61 showed interestingly that sex and genetic background affect osteoblastic differentiation potential. They showed in particular that the sex effect on osteoblastic gene expression and on ALP activity, is milder in FVB mice than in C57BL/6 mice. This observation may in part explain the absence of effect induced by ovariectomy on bone formation parameters in both WT and Mmp3 KO mice.
In the field of MMPs, association studies with diseases have been mainly tested with promoter SNPs due to their potential role in the change of gene expression. Consequently, a 5A/6A promoter SNP of the MMP3 gene has been intensively studied for its association with human diseases in which matrix metalloproteases might be involved. 39 Meta‐analysis has shown this SNP robustness mainly in several heart and blood circulation diseases such as atherosclerosis, 62 myocardial infarction, 63 coronary artery, 64 aortic aneurism, 65 and abdominal aortic aneurism 53 with the 5A allele always showing an increased risk of the disease. Interestingly the rs632478 SNP is in very strong linkage disequilibrium with the 5A/6A SNP in cohort of European descent (but not in cohorts of Asian or African origin as evidenced with data from the 1000genome project). The rs632478 T‐allele (corresponding to the 5A allele) is associated with the higher BMD (lumbar spine and femoral neck) in our cohort of postmenopausal women, and therefore is possibly protecting against estrogen deficiency‐associated bone loss. Interestingly, the 5A SNP has been associated with a lower MMP3 expression in human serum in several studies studying cohorts of European ancestry. 66 , 67 , 68 , 69 It is possible that none of the cell types used in those studies represent major sources of MMP3 found in blood.
In apparent contradiction with these results, we have found in transfection experiments in MC3T3 osteoblastic cells that a promoter with the 5A allele has a higher activity than its counterpart with the 6A allele. Interestingly, 5A/6A promoter constructs have been used in transfection experiments in the past and have shown a similar effect. 41 , 70 , 71 Therefore, it is very possible that the use of relatively short constructs in transfection does not reflect the MMP3 promoter activity in the genomic context. In addition, Medley et al. have shown in 40 skin pieces that the 5A/5A genotype is associated with higher MMP3 mRNA and protein expression.
In conclusion, we have shown in this paper that Mmp3 deletion has a protective effect on bone loss induced by ovariectomy in mice. We have found that this effect was essentially mediated by inhibition of bone resorption. Most importantly we have presented evidence that polymorphisms that affect MMP3 expression are also linked to modification of Z‐score in osteoporotic women. MMP3, although weakly expressed in bone cells, could be one of the important regulators of sex hormone action in bone and whose activity could be targeted for therapeutic applications.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
V. Geoffroy designed and performed research; F. Jehan, M. Zarka, and G. de la Houssaye performed research; V. Geoffroy, F. Jehan, G. de la Houssaye, J. Veziers, and A. Ostertag analyzed data: M. Cohen‐Solal provided the cohort of Human patients; F. Jehan and V. Geoffroy wrote the paper.
Supporting information
Figure S1–S3
ACKNOWLEDGMENTS
The authors thank Zena Werb for providing the Mmp3 knockout model. The authors also thank Pascale Chantrenne for taking care of mice, Caroline Marty for advises in histomorphometric analysis, SC3M imaging facility of the SFR Santé F Bonamy (UMS INSERM 016/CNRS 3556) (Nantes, France) for help in microCT analyses, and Marie‐Christine de Vernejoul for contribution in the genetic access to the cohort of the osteoporotic patients.
Jehan F, Zarka M, de la Houssaye G, et al. New insights into the role of matrix metalloproteinase 3 (MMP3) in bone. FASEB BioAdvances. 2022;4:524‐538. doi: 10.1096/fba.2021-00092
Frédéric Jehan, Mylene Zarka and Guillaume de la Houssaye should be considered joint first authors.
Funding information
This work was supported by Inserm (Institut National de la Santé et de la Recherche Médicale) and AP‐HP (Contrat d'interface CHU‐Lariboisière hospital to VG).
REFERENCES
- 1. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hardy E, Fernandez‐Patron C. Destroy to rebuild: the connection between bone tissue remodeling and matrix metalloproteinases. Front Physiol. 2020;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ortega N, Behonick D, Stickens D, Werb Z. How proteases regulate bone morphogenesis. Ann N Y Acad Sci. 2003;995:109‐116. [DOI] [PubMed] [Google Scholar]
- 6. Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14:86‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Krane SM, Inada M. Matrix metalloproteinases and bone. Bone. 2008;43:7‐18. [DOI] [PubMed] [Google Scholar]
- 8. Paiva KB, Granjeiro JM. Bone tissue remodeling and development: focus on matrix metalloproteinase functions. Arch Biochem Biophys. 2014;561:74‐87. [DOI] [PubMed] [Google Scholar]
- 9. Tokuhara CK, Santesso MR, Oliveira GSN, et al. Updating the role of matrix metalloproteinases in mineralized tissue and related diseases. J Appl Oral Sci. 2019;27:e20180596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aiken A, Khokha R. Unraveling metalloproteinase function in skeletal biology and disease using genetically altered mice. Biochim Biophys Acta. 2010;1803:121‐132. [DOI] [PubMed] [Google Scholar]
- 11. Inoue K, Mikuni‐Takagaki Y, Oikawa K, et al. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem. 2006;281:33814‐33824. [DOI] [PubMed] [Google Scholar]
- 12. Mosig RA, Dowling O, DiFeo A, et al. Loss of MMP‐2 disrupts skeletal and craniofacial development and results in decreased bone mineralization, joint erosion and defects in osteoblast and osteoclast growth. Hum Mol Genet. 2007;16:1113‐1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmbeck K, Bianco P, Caterina J, et al. MT1‐MMP‐deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81‐92. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane‐type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97:4052‐4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stickens D, Behonick DJ, Ortega N, et al. Altered endochondral bone development in matrix metalloproteinase 13‐deficient mice. Development. 2004;131:5883‐5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nyman JS, Lynch CC, Perrien DS, et al. Differential effects between the loss of MMP‐2 and MMP‐9 on structural and tissue‐level properties of bone. J Bone Miner Res. 2011;26:1252‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vu TH, Shipley JM, Bergers G, et al. MMP‐9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93:411‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inada M, Wang Y, Byrne MH, et al. Critical roles for collagenase‐3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci U S A. 2004;101:17192‐17197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martignetti JA, Aqeel AA, Sewairi WA, et al. Mutation of the matrix metalloproteinase 2 gene (MMP2) causes a multicentric osteolysis and arthritis syndrome. Nat Genet. 2001;28:261‐265. [DOI] [PubMed] [Google Scholar]
- 20. Evans BR, Mosig RA, Lobl M, et al. Mutation of membrane type‐1 metalloproteinase, MT1‐MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am J Hum Genet. 2012;91:572‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lausch E, Keppler R, Hilbert K, et al. Mutations in MMP9 and MMP13 determine the mode of inheritance and the clinical spectrum of metaphyseal anadysplasia. Am J Hum Genet. 2009;85:168‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page‐McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen Y, Winkler IG, Barbier V, Sims NA, Hendy J, Lévesque JP. Tissue inhibitor of metalloproteinase‐3 (TIMP‐3) regulates hematopoiesis and bone formation in vivo. PLoS One. 2010;5:e13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sobue T, Hakeda Y, Kobayashi Y, et al. Tissue inhibitor of metalloproteinases 1 and 2 directly stimulate the bone‐resorbing activity of isolated mature osteoclasts. J Bone Miner Res. 2001;16:2205‐2214. [DOI] [PubMed] [Google Scholar]
- 26. Geoffroy V, Marty‐Morieux C, Le Goupil N, et al. In vivo inhibition of osteoblastic metalloproteinases leads to increased trabecular bone mass. J Bone Miner Res. 2004;19:811‐822. [DOI] [PubMed] [Google Scholar]
- 27. Merciris D, Schiltz C, Legoupil N, Marty‐Morieux C, de Vernejoul MC, Geoffroy V. Over‐expression of TIMP‐1 in osteoblasts increases the anabolic response to PTH. Bone. 2007;40:75‐83. [DOI] [PubMed] [Google Scholar]
- 28. Schiltz C, Marty C, de Vernejoul MC, Geoffroy V. Inhibition of osteoblastic metalloproteinases in mice prevents bone loss induced by oestrogen deficiency. J Cell Biochem. 2008;104:1803‐1817. [DOI] [PubMed] [Google Scholar]
- 29. Schiltz C, Prouillet C, Marty C, et al. Bone loss induced by Runx2 over‐expression in mice is blunted by osteoblastic over‐expression of TIMP‐1. J Cell Physiol. 2010;222:219‐229. [DOI] [PubMed] [Google Scholar]
- 30. Clements KM, Price JS, Chambers MG, Visco DM, Poole AR, Mason RM. Gene deletion of either interleukin‐1beta, interleukin‐1beta‐converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 2003;48:3452‐3463. [DOI] [PubMed] [Google Scholar]
- 31. Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529‐543. [DOI] [PubMed] [Google Scholar]
- 32. Thomson BM, Atkinson SJ, McGarrity AM, Hembry RM, Reynolds JJ, Meikle MC. Type I collagen degradation by mouse calvarial osteoblasts stimulated with 1,25‐dihydroxyvitamin D‐3: evidence for a plasminogen‐plasmin‐metalloproteinase activation cascade. Biochim Biophys Acta. 1989;1014:125‐132. [DOI] [PubMed] [Google Scholar]
- 33. Meikle MC, Bord S, Hembry RM, Compston J, Croucher PI, Reynolds JJ. Human osteoblasts in culture synthesize collagenase and other matrix metalloproteinases in response to osteotropic hormones and cytokines. J Cell Sci. 1992;103:1093‐1099. [DOI] [PubMed] [Google Scholar]
- 34. Bord S, Horner A, Hembry RM, Compston JE. Stromelysin‐1 (MMP‐3) and stromelysin‐2 (MMP‐10) expression in developing human bone: potential roles in skeletal development. Bone. 1998;23:7‐12. [DOI] [PubMed] [Google Scholar]
- 35. Mudgett JS, Hutchinson NI, Chartrain NA, et al. Susceptibility of stromelysin 1‐deficient mice to collagen‐induced arthritis and cartilage destruction. Arthritis Rheum. 1998;41:110‐121. [DOI] [PubMed] [Google Scholar]
- 36. Parfitt AM, Drezner MK, Glorieux FH, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res. 1987;2:595‐610. [DOI] [PubMed] [Google Scholar]
- 37. Wu J, Jia Q, He W, et al. Conditioned medium from periapical follicle cells induces the odontogenic differentiation of stem cells from the apical papilla in vitro. J Endod. 2013;39:1015‐1022. [DOI] [PubMed] [Google Scholar]
- 38. Lhaneche L, Hald JD, Domingues A, et al. Variations of SOST mRNA expression in human bone are associated with DNA polymorphism and DNA methylation in the SOST gene. Bone. 2016;92:107‐115. [DOI] [PubMed] [Google Scholar]
- 39. Munhoz FB, Godoy‐Santos AL, Santos MC. MMP‐3 polymorphism: genetic marker in pathological processes (review). Mol Med Rep. 2010;3:735‐740. [DOI] [PubMed] [Google Scholar]
- 40. Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ. Matrix metalloproteinase‐3 genotype contributes to age‐related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92:1254‐1261. [DOI] [PubMed] [Google Scholar]
- 41. Beyzade S, Zhang S, Wong YK, Day IN, Eriksson P, Ye S. Influences of matrix metalloproteinase‐3 gene variation on extent of coronary atherosclerosis and risk of myocardial infarction. J Am Coll Cardiol. 2003;41:2130‐2137. [DOI] [PubMed] [Google Scholar]
- 42. Bord S, Horner A, Hembry RM, Reynolds JJ, Compston JE. Distribution of matrix metalloproteinases and their inhibitor, TIMP‐1, in developing human osteophytic bone. J Anat. 1997;191:39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeitler P, Pahnke J, Marx A. Expression of stromelysin‐1 (MMP‐3), gelatinase B (MMP‐9), and plasminogen activator system during fetal calvarial development. Histopathol. 2004;44:360‐366. [DOI] [PubMed] [Google Scholar]
- 44. Zhao J, Zhou X, Tang Q, et al. BMAL1 deficiency contributes to mandibular dysplasia by upregulating MMP3. Stem Cell Reports. 2018;10:180‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Löffek S, Schilling O, Franzke CW. Series "matrix metalloproteinases in lung health and disease": biological role of matrix metalloproteinases: a critical balance. Eur Respir J. 2011;38:191‐208. [DOI] [PubMed] [Google Scholar]
- 46. Andersen TL, del Carmen Ovejero M, Kirkegaard T, Lenhard T, Foged NT, Delaissé JM. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone. 2004;35:1107‐1119. [DOI] [PubMed] [Google Scholar]
- 47. Zhu L, Tang Y, Li XY, et al. Osteoclast‐mediated bone resorption is controlled by a compensatory network of secreted and membrane‐tethered metalloproteinases. Sci Transl Med. 2020;12(529):eaaw6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Onodera S, Sasaki S, Ohshima S, et al. Transgenic mice overexpressing macrophage migration inhibitory factor (MIF) exhibit high‐turnover osteoporosis. J Bone Miner Res. 2006;21:876‐885. [DOI] [PubMed] [Google Scholar]
- 49. Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)‐1 and MMP‐3 induce macrophage MMP‐9: evidence for the role of TNF‐alpha and cyclooxygenase‐2. J Immunol. 2009;183:8119‐2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pavia KBS, Granjeiro JM. Matrix metalloproteinases in bone resorption, remodeling, and repair. Prog Mol Biol Transl Sci 2017;148:203–303. [DOI] [PubMed] [Google Scholar]
- 51. Zheng X, Zhang Y, Guo S, Zhang W, Wang J, Lin Y. Dynamic expression of matrix metalloproteinases 2, 9 and 13 in ovariectomy‐induced osteoporosis rats. Exp Ther Med. 2018;16:1807‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shiguemoto GE, Prestes J, Leite RD, et al. Effects of resistance training on matrix metalloproteinase‐2 activity and biomechanical and physical properties of bone in ovariectomized and intact rats. Scand J Med Sci Sports. 2012;22:607‐617. [DOI] [PubMed] [Google Scholar]
- 53. Li T, Lv Z, Jing JJ, Yang J, Yuan Y. Matrix metalloproteinase family polymorphisms and the risk of aortic aneurysmal diseases: a systematic review and meta‐analysis. Clin Genet. 2018;93:15‐32. [DOI] [PubMed] [Google Scholar]
- 54. Garcia AJ, Tom C, Guemes M, et al. ERalpha signaling regulates MMP3 expression to induce FasL cleavage and osteoclast apoptosis. J Bone Miner Res. 2013;28:283‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Breckon JJ, Papaioannou S, Kon LW, et al. Stromelysin (MMP‐3) synthesis is up‐regulated in estrogen‐deficient mouse osteoblasts in vivo and in vitro. J Bone Miner Res. 1999;14:1880‐1890. [DOI] [PubMed] [Google Scholar]
- 56. Liu S, Yang SD, Huo XW, Yang DL, Ma L, Ding WY. 17beta‐estradiol inhibits intervertebral disc degeneration by down‐regulating MMP‐3 and MMP‐13 and up‐regulating type II collagen in a rat model. Artif Cells Nanomed Biotechnol. 2018;46:S182‐S191. [DOI] [PubMed] [Google Scholar]
- 57. Ao P, Huang W, Li J, et al. 17beta‐estradiol protects nucleus pulposus cells from serum deprivation‐induced apoptosis and regulates expression of MMP‐3 and MMP‐13 through promotion of autophagy. Biochem Biophys Res Commun. 2018;503:791‐797. [DOI] [PubMed] [Google Scholar]
- 58. Vassilev V, Pretto CM, Cornet PB, et al. Response of matrix metalloproteinases and tissue inhibitors of metalloproteinases messenger ribonucleic acids to ovarian steroids in human endometrial explants mimics their gene‐ and phase‐specific differential control in vivo. J Clin Endocrinol Metab. 2005;90:5848‐5857. [DOI] [PubMed] [Google Scholar]
- 59. Chiu KM, Ju J, Mayes D, Bacchetti P, Weitz S, Arnaud CD. Changes in bone resorption during the menstrual cycle. J Bone Miner Res. 1999;14:609‐615. [DOI] [PubMed] [Google Scholar]
- 60. Adebayo OO, Ko FC, Wan PT, et al. Role of subchondral bone properties and changes in development of load‐induced osteoarthritis in mice. Osteoarthr Cartil. 2017;25(12):2108‐2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zanotti S, Kalajzic I, Aguila HL, Canalis E. Sex and genetic factors determine osteoblastic differentiation potential of murine bone marrow stromal cells. PLoS One. 2014;9(1):e86757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abilleira S, Bevan S, Markus HS. The role of genetic variants of matrix metalloproteinases in coronary and carotid atherosclerosis. J Med Genet. 2006;43:897‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang J, Xu D, Wu X, et al. Polymorphisms of matrix metalloproteinases in myocardial infarction: a meta‐analysis. Heart. 2011;97:1542‐1546. [DOI] [PubMed] [Google Scholar]
- 64. Niu W, Qi Y. Matrix metalloproteinase family gene polymorphisms and risk for coronary artery disease: systematic review and meta‐analysis. Heart. 2012;98:1483‐1491. [DOI] [PubMed] [Google Scholar]
- 65. Morris DR, Biros E, Cronin O, Kuivaniemi H, Golledge J. The association of genetic variants of matrix metalloproteinases with abdominal aortic aneurysm: a systematic review and meta‐analysis. Heart. 2014;100:295‐302. [DOI] [PubMed] [Google Scholar]
- 66. Samnegård A, Silveira A, Lundman P, et al. Serum matrix metalloproteinase‐3 concentration is influenced by MMP‐3 ‐1612 5A/6A promoter genotype and associated with myocardial infarction. J Intern Med. 2005;258:411‐419. [DOI] [PubMed] [Google Scholar]
- 67. White AJ, Duffy SJ, Walton AS, et al. Matrix metalloproteinase‐3 and coronary remodelling: implications for unstable coronary disease. Cardiovasc Res. 2007;75:813‐820. [DOI] [PubMed] [Google Scholar]
- 68. Chen Y, Nixon NB, Dawes PT, Mattey DL. Influence of variations across the MMP‐1 and ‐3 genes on the serum levels of MMP‐1 and ‐3 and disease activity in rheumatoid arthritis. Genes Immun. 2012;13:29‐37. [DOI] [PubMed] [Google Scholar]
- 69. Collazos J, Asensi V, Martin G, Montes AH, Suárez‐Zarracina T, Valle‐Garay E. The effect of gender and genetic polymorphisms on matrix metalloprotease (MMP) and tissue inhibitor (TIMP) plasma levels in different infectious and non‐infectious conditions. Clin Exp Immunol. 2015;182:213‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries SE, Henney AM. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin‐1 promoter which results in reduced gene expression. J Biol Chem. 1996;271:13055‐13060. [DOI] [PubMed] [Google Scholar]
- 71. Souslova V, Townsend PA, Mann J, et al. Allele‐specific regulation of matrix metalloproteinase‐3 gene by transcription factor NFkappaB. PLoS One. 2010;5:e9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1–S3


