Abstract
Ovarian carcinosarcoma (OCS) is a rare and lethal gynecological cancer. The present study reports on the case of a 61-year-old post-menopausal female with abdominal distension who was detected to have a large OCS. The patient underwent cytoreductive surgery, including sub-extensive hysterectomy, bilateral adnexectomy, sigmoid colon and partial rectal resection, and lymph node dissection. Postoperative pathology of the bilateral adnexal masses revealed carcinosarcoma. The main components of the carcinoma included serous carcinoma and a small amount of squamous cell carcinoma. The sarcoma components mainly contained fibrosarcoma, as well as a small amount of chondrosarcoma and rhabdomyosarcoma. Infiltrating cells in cancer tissues or metastasis were observed in the serosal surface, muscular and subserosal layers of the uterus, as well as the sigmoid colon and part of the rectum. The patient was diagnosed postoperatively with International Federation of Gynecology and Obstetrics stage IIIC ovarian carcinosarcoma and T3cN1M0 based on the TNM system. The patient then received six cycles of combination chemotherapy using carboplatin, paclitaxel plus bevacizumab. As severe myelosuppression occurred during and after chemotherapy, and bevacizumab was expensive, bevacizumab therapy was not maintained after chemotherapy. However, following chemotherapy, the patient received niraparib oral maintenance therapy. At 6 months after the sixth chemotherapy, cancer antigen 125 levels dropped to 4.55 U/ml (within normal range). Short-term follow-up of 6 months after the end of chemotherapy indicated that the patient had a remission prognosis based on the ultrasonography, computed tomography, magnetic resonance imaging examinations and serum tumor marker levels. The present study indicated that combined chemotherapy and targeted therapy after cytoreductive surgery may be a promising way for the treatment of OCS.
Keywords: ovarian carcinosarcoma, ovarian cancer, surgery, chemoradiotherapy, adjuvant therapy
Introduction
Ovarian carcinosarcoma (OCS), also known as malignant mixed Müllerian tumor (MMMT), is a rare malignant tumor that postmenopausal females are prone to develop, accounting for 1-4% of ovarian tumors (1). Most patients are diagnosed in the advanced stage with poor prognosis and most of them had elevated cancer antigen (CA)125 levels. Imaging findings usually indicate pelvic masses, mostly with ascites. OCS consists of malignant epithelial components and sarcoma components. According to the sarcoma components, it may be classified as homologous (the sarcoma component was derived from the stromal component of the primary tumor site, e.g., endometrial stromal sarcoma) or heterologous (the sarcoma component was not derived from the interstitial component of the primary tumor site, such as rhabdomyosarcoma component, osteosarcoma component) (2). Immunohistochemical (IHC) analysis of markers including cytokeratin (CK), cytokeratin pan monoclonal antibody (MNF116), epithelial membrane antigen, vimentin, S100, chromogranin, synaptophysin, desmin, myogenin (MYF4) and p53 were used to suggest the presence of the heterologous component (3). The current treatment methods mainly include ovarian tumor cytoreduction surgery, followed by platinum-based chemotherapy. OCS is rare and patients with advanced OCS are prone to metastases. Cytoreductive surgery may only remove local lesions. Chemotherapy and radiotherapy are not able to completely eliminate cancer cells; in addition, they weaken the patients and decrease their immune function. Targeted drugs would only kill tumor cells with gene targets. For those tumor cells without gene targets, targeted drugs are generally ineffective. It was reported that patients with OCS had an obviously increased risk of death compared with those with epithelial ovarian cancer (4). The majority of patients relapsed within one year following treatment and survived nearly two years after the initial diagnosis (4,5). Due to its rarity, there is a lack of large-scale, prospective studies for this disease and there is still no consensus on a standardized diagnosis and treatment strategy for OCS. The following report presents a case of OCS treated with optimal cytoreductive surgery and chemotherapy.
Patients and methods
Methods
For the examination of the present case, the ARIETTA 70 ultrasound machine (Hitachi, Ltd.) with a 2-10 Hz resolution transvaginal ultrasound probe (C41V1) was used. Blood flow signals, including resistive index (RI), peak systolic velocity (PSV) and end-diastolic velocity (EDV) were detected by color Doppler flow imaging (CDFI). The patient underwent MRI scanning on a 1.5 T Philips Achieva MRI system (Philips Healthcare). The parameters of the echo planar imaging diffusion-weighted imaging (DWI) sequence [echo time (TE), 60 msec; repetition time (TR), 1,118 msec; multiband factor, 2; b-values, 0 and 600 sec/mm2; acquisition time, 1:04 for a single b-value). Fat-suppressed gradient-echo enhanced T1-weighted imaging (T1WI) were obtained with the following settings: TE, 7 msec; TR, 497 msec; flip angle, 10; matrix size, 256x256; field of view (FOV), 400 mm; and slice thickness, 5 mm. Images were available in the transverse, coronal and sagittal planes. Intravenous contrast medium (Omniscan; GE Healthcare) was administered at a dose of 0.2 ml/kg.
In the present study, 4 ml of venous blood was collected from the patient and centrifuged at 1,509.3 x g at 25˚C for 6 min to separate serum. Serum tumor marker levels of CA125, alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), CA199 and CA153 were measured by DXI-800 autoanalysers (Beckman Coulter, Inc.).
The excised specimens were fixed using neutral-buffered 10% formalin, dehydrated in a series of ethanols and embedded in paraffin. Serial sections of 4-µm thickness were made and then subjected to hematoxylin-eosin staining. IHC staining was performed following the EnVision two-step l protocol (6,7). 3-3'diaminobenzidine was used for colour development. These steps were performed with a Ventana automated immunostainer (Ventana Medical Systems, Inc.) using an UltraView Universal DAB Detection Kit (Ventana Medical Systems, Inc.). Primary antibodies applied in the IHC analysis were mainly as follows: Monoclonal mouse anti-human cytokeratin (CK) (AE1/AE3), mouse anti-human tumor protein p53 monoclonal antibody (DO-7), rabbit polyclonal anti-human S100 protein, monoclonal mouse anti-vimentin (V9; all from Dako; Agilent Technologies, Inc.), mouse anti-human tumor protein P40 monoclonal antibody (cat. no. 66622-1-Ig) and MYOD1 rabbit polyclonal antibody (cat. no. 18943-1-AP; both from ProteinTech Group, Inc.). The dilution ratio of CK, p53, S100 and P40 antibodies was 1:300, 1:800, 1:1,000 and 1:100, respectively. The different secondary antibodies for IHC were examined through the Ventana automated immunostainer (Ventana Medical Systems, Inc.). The ready-to-use secondary antibody was included in the kit. In addition, a systematic review focusing on the treatment of this disease was performed. The elaborate literature search was undertaken through the PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/) and Web of Science (https://webofscience.com/WOS) database to identify the studies examining OCS treatment options from database inception to September, 2021, using the following key words: Ovarian carcinosarcoma, malignant mixed Mullerian tumor, carcinosarcoma of the ovary, cytoreduction, chemotherapy, radiotherapy and targeted therapy in various combinations. The inclusion criteria were as follows: Patients with OCS, MMMT or carcinosarcoma of the ovary, who received one or more treatments of cytoreduction, chemotherapy, radiotherapy and targeted therapy. Those studies that did not include patients with OCS were excluded. Other potential studies were retrieved by reviewing the reference lists within publications.
Case presentation
A 61-year-old post-menopausal female complained of abdominal distension for one week and presented at Ningbo Women and Children's Hospital (Ningbo, China). The patient had no history of vaginal bleeding after menopause. The patient had received radical mastectomy for left breast cancer at our hospital 10 years previously and had overgone laparoscopic cholecystectomy 7 years ago. Approval for the present study was acquired from the institutional review board of Ningbo Women and Children's Hospital (Ningbo, China) and informed consent was obtained from the patient.
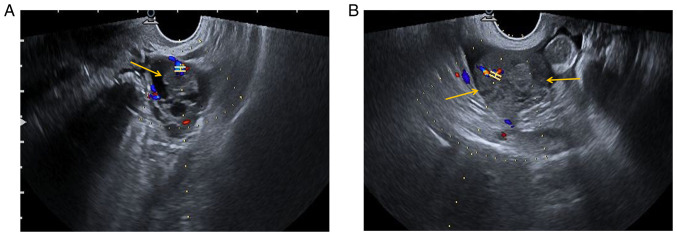
Preoperative ultrasonography (US) examination revealed a mixed cystic-solid sonographic appearance of 37x26x35 mm in the left adnexal area and the solid area was 17x14x22 mm (yellow arrow). In the left adnexal area, CDFI indicated the following: RI, 0.68; PSV, 12.9 cm/sec; and EDV, 4.1 cm/sec (Fig. 1A). In the right adnexal area, a low-echo area of 54x26x41 mm with an irregular shape (yellow arrows) was present and CDFI indicated the following: RI, 0.48; PSV, 22.9 cm/sec; and EDV, 11.8 cm/sec (Fig. 1B). A certain amount of pelvic fluid collection and a small amount of effusion in the uterine cavity were detected.
Figure 1.
Preoperative US findings. (A) Preoperative US examination indicated a mixed cystic-solid area of 37x26x35 mm in the left adnexal region, and the solid area was ~17x14x22 mm (yellow arrow). CDFI of the left adnexal area indicated the following: RI, 0.68; PSV, 12.9 cm/sec; and EDV4.1 cm/sec. (B) A low echo area of 54x26x41 mm with an irregular shape was observed in the right adnexal area (yellow arrows). CDFI indicated the following: RI, 0.48; PSV, 22.9 cm/sec; and EDV, 11.8 cm/sec. H-shaped structure indicates the sample gate. The color represents the direction of blood flow. Red indicates blood flow towards the probe and blue means blood flow away from the probe. A certain amount of pelvic fluid collection and a small amount of effusion in the uterine cavity were observed. US, ultrasonography; RI, resistive index; PSV, peak systolic velocity; EDV, end-diastolic velocity; CDFI, color Doppler flow imaging.
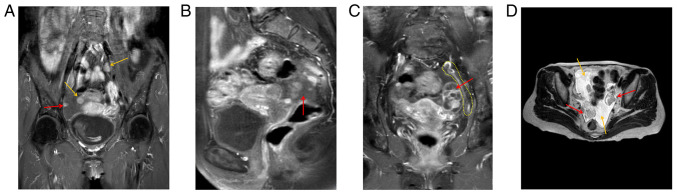
Preoperative MRI indicated two enlarged lymph nodes in the pelvic cavity (yellow arrows), and peritoneal lesions (red arrow) (Fig. 2A). The right adnexal area was irregular in shape, 27x43x28 mm in size, and DWI indicated high signal intensity, with uneven enhancement of the right lesion (red arrow) (Fig. 2B). An elliptical cystic solid lesion was present in the left adnexal area of the pelvis, the size of which was 31x26x40 mm (red arrow) and the peritoneum was thickened (yellow dotted line) (Fig. 2C). T2WI indicated two lesions (red arrows) in the left and right adnexa area and a certain amount of pelvic fluid collection (yellow arrows) (Fig. 2D).
Figure 2.
Preoperative MRI findings. (A) Preoperative MRI indicated two enlarged lymph nodes in the pelvic cavity (yellow arrows) and peritoneal lesions (red arrow). (B) The right adnexal area was irregular in shape and 27x43x28 mm in size, and diffusion-weighted imaging indicated a high signal intensity, with uneven enhancement of the right lesion (red arrow). (C) There was an elliptical cystic solid lesion of 31x26x40 mm (red arrow) in the left adnexal area of the pelvis and peritoneum exhibited thickening (yellow dotted line). (D) T2-weighted MRI indicated two lesions (red arrows) in the left and right adnexa area and a certain amount of pelvic fluid collection (yellow arrows).
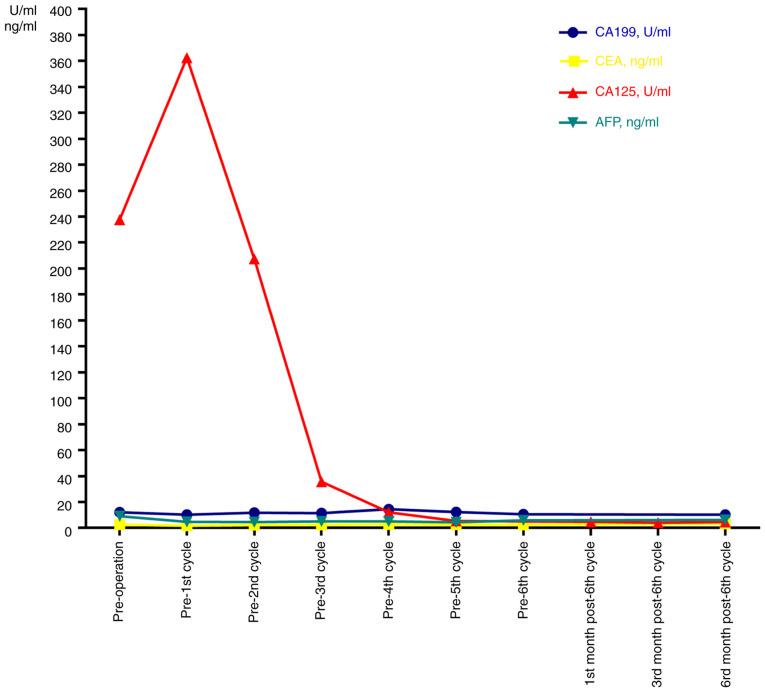
In the present case, homologous recombination DNA repair deficiency (HRD) was positive, while negative results were obtained in BRCA1/2 mutation testing. This suggested cells with HRD may be more sensitive to platinum drugs and poly ADP-ribose polymerase inhibitors (8). Preoperative CA125 levels were elevated above the normal range (237.70 U/ml; normal, 0-35 U/ml) and AFP levels were slightly beyond the normal range (9.13 ng/ml; normal, 0-9 ng/ml). CEA, CA199 and CA153 levels were in the normal ranges (CEA, 0-5 ng/ml; CA199, 0-35 U/ml; CA153, 0-31.3 U/ml) (Fig. 3).
Figure 3.
Serum tumor marker levels in the patient with ovarian carcinosarcoma prior to and after treatment. AFP, alpha-fetoprotein.
The patient underwent an exploratory laparotomy. Tumor cells were detected in 300 ml of ascites. After separation of the adhesions, the left infundibulopelvic ligament, the proper ovarian ligament, isthmus of the left fallopian tube and left ovary were removed. Part of the ovarian lesions and mesenteric neoplasms were used for fast frozen pathology and the results suggested poorly differentiated cancer. The patient then underwent cytoreductive surgery, including sub-extensive hysterectomy, bilateral adnexectomy, sigmoid colon and partial rectal resection, as well as lymph node dissection.
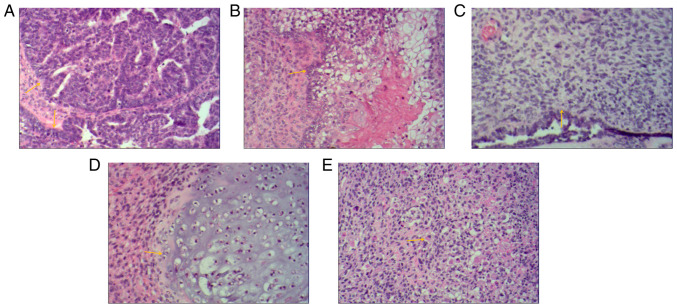
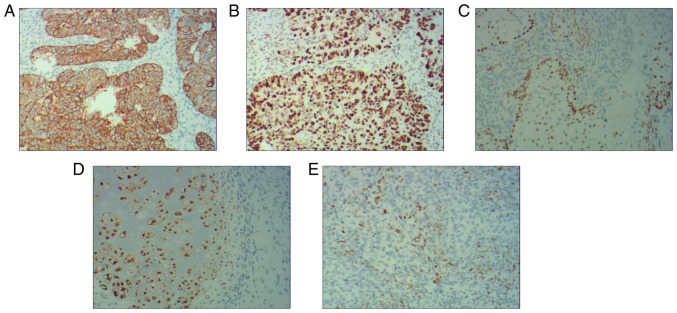
Postoperative pathology of the bilateral adnexal masses confirmed carcinosarcoma. The main carcinomatous components included high-grade serous carcinoma. Tumor cells were arranged in glandular, papillary and solid patterns. The ducts were slit-like or irregular, differentiation was poor and cells were arranged in sheets. The cells had obvious atypia, with large hyperchromatic pleomorphic nuclei, in which prominent nucleoli and numerous mitotic figures were present (yellow arrows) (Fig. 4A). Carcinomatous components included a small amount of squamous cell carcinoma. Tumor cells were arranged in nested, expansile, polygonal, paving stone-like patterns. There were intercellular bridges and intracellular keratinization may be seen in central cells. Nuclei are large, hyperchromatic or vacuolated with visible nucleoli and mitoses (yellow arrow) (Fig. 4B). Sarcomatous components contained fibrosarcoma. The tumor cells were spindle-shaped, with a cord-like distribution. Nuclei were hyperchromatic and mitoses were seen (yellow arrow) (Fig. 4C). Sarcomatous components contained a small amount of chondrosarcoma. Chondrosarcoma was observed to be scattered in the cartilage lobules. The chondrocytes were located in the cartilage lacuna, with large hyperchromatic pleomorphic nuclei, in which binucleated cells and mitotic figures were present (yellow arrow) (Fig. 4D). Sarcomatous components also contained a small proportion of rhabdomyosarcoma. The tumor cells were scattered in round and oval shapes. The cytoplasm was abundant and eosinophilic. The nuclei were eccentric and vacuolated with obvious nucleoli (yellow arrow) (Fig. 4E). Infiltrating cells in cancer tissues or metastasis were observed on the serosal surface, muscular and subserosal layers of the uterus, as well as sigmoid colon and part of the rectum. The final diagnosis of OCS was obtained by histopathological examination of the resected specimen. The biphasic component was further clarified by IHC analysis.
Figure 4.
Histological images of bilateral adnexal masses. Extensive metastases were observed throughout the pelvis and abdomen. Histological images of bilateral adnexal masses revealed the carcinosarcoma and sarcomatous elements of ovarian carcinosarcoma. (A) The main carcinomatous components included high-grade serous carcinoma. Tumor cells were arranged in glandular, papillary and solid patterns. The ducts were slit-like or irregular and poorly differentiated, and cells were arranged in sheets. The cells had obvious atypia, with large hyperchromatic pleomorphic nuclei, in which prominent nucleoli and numerous mitotic figures were present (yellow arrows). (B) Carcinomatous components included a small amount of squamous cell carcinoma. Tumor cells were arranged in nested, expansile, polygonal, paving stone-like patterns. Intercellular bridges were present and intracellular keratinization was observed in central cells. Nuclei were large, hyperchromatic or vacuolated with visible nucleoli and mitoses (yellow arrow). (C) Sarcomatous components contained fibrosarcoma. The tumor cells were spindle-shaped, with a cord-like distribution. Nuclei were hyperchromatic and mitotic bodies were present (yellow arrow). (D) Sarcomatous components contained a small amount of chondrosarcoma. Scattered chondrosarcoma was present in the cartilage lobules. The chondrocytes were located in the cartilage lacuna, with large hyperchromatic pleomorphic nuclei, in which binucleated cells and mitotic figures were present (yellow arrow). (E) Sarcomatous components also contained a small amount of rhabdomyosarcoma. The tumor cells were scattered in round and oval shapes. The cytoplasm was abundant and eosinophilic. Nuclei were eccentric and vacuolated with obvious nucleoli (yellow arrow) (H&E; magnification, x100).
The IHC results indicated that the serous carcinoma component was positive for cytokeratin (Fig. 5A) and P53 (Fig. 5B), squamous cell carcinoma was positive for P40 (Fig. 5C), chondrosarcoma was positive for S100 (Fig. 5D) and rhabdomyosarcoma was positive for MyoD1 (Fig. 5E). The patient was diagnosed postoperatively with International Federation of Gynecology and Obstetrics stage IIIC OCS and T3cN1M0 based on the TNM system.
Figure 5.
Immunohistochemical results of bilateral adnexal masses. The results indicated that the serous carcinoma component was (A) positive for cytokeratin and (B) P53, (C) squamous cell carcinoma was positive for P40, and (D) chondrosarcoma was positive for S100 and (E) rhabdomyosarcoma was positive for MyoD1 (magnification, x100).
After the surgery, the patient was given 6 cycles of chemotherapy consisting of carboplatin (60 to 75 mg/m2) plus paclitaxel (135-175 mg/m2) on day 1 in all cycles, plus bevacizumab (7.5 mg/kg), on day 1 in cycles 3-6. The dose of carboplatin was calculated using the Calvert formula: Dose (mg)=area under curve (AUC; min x mg/ml) [glomerular filtration rate (ml/min) + 25 ml/min] (9); the AUC was 5 mg/ml/min. The HRD result was positive. The patient should have received bevacizumab plus niraparib maintenance therapy after chemotherapy. As severe myelosuppression occurred during and after chemotherapy and bevacizumab was expensive, bevacizumab therapy was not continued after chemotherapy, but oral maintenance therapy with niraparib was given. Initially, the patient received oral niraparib 0.1 g twice daily for half a month. However, the patient then discontinued niraparib for one month due to severe myelosuppression. Subsequently, niraparib was reduced to 0.1 g orally once daily until now.
Follow-up on the 19th day after surgery indicated that the CA125 levels had increased to 362.4 U/ml. After 6 cycles of chemotherapy, the CA125 levels exhibited a gradual reduction. At six months after the sixth chemotherapy, the CA125 levels dropped to 4.55 U/ml, which was in the normal range (Fig. 3). At the six-month follow-up, no tumor recurrence or metastasis was detected in the breast bilateral axillary lymph nodes or in the pelvic cavity on US. No tumor recurrence or metastasis was detected on chest CT and pelvic enhanced MRI.
Discussion
Physicians usually find it difficult to make a specific diagnosis of OCS prior to surgery. OCS is frequently asymptomatic in the early stage and initial signs/symptoms/complaints are usually of a gastrointestinal nature, including abdominal distension, abdominal pain and abdominal lumps at the advanced stage. In the present case, the final diagnosis of OCS was obtained by histopathological examination of the resected specimen. The biphasic component was further clarified by IHC analysis. The present study indicated that the serous carcinoma component was positive for CK and P53, squamous cell carcinoma was positive for P40, chondrosarcoma was positive for S100 and rhabdomyosarcoma was positive for MyoD1. The findings of the present study were similar to those of previous studies (3,10,11). In a study of 8 cases with OCS (3), the carcinomatous components exhibited positive staining for epithelial markers, including cytokeratin MNF116. All sarcomatous components had positive staining for vimentin and all rhabdomyosarcomas were positive for myogenic markers, including MYF4. All chondrosarcomas were positive for S100. All tumors displayed were positive for p53(3). Pankaj et al (10) indicated that CK and vimentin were positive, indicating the likely presence of carcinosarcoma and epithelioid sarcoma. Another study reported that tumor cells were positive for the skeletal muscle marker myoD1 for rhabdomyosarcomas (11). The above IHC results suggested that these indicators may be of great significance for the diagnosis of OCS and further large-scale studies will be required to confirm these findings in the future.
Regarding imaging characteristics of OCS, a previous study reported that US displayed large solid tumors with irregular edges and uneven echo of solid tissues with cystic areas (12). MRI displayed a multicystic tumor of a multilobulated shape located in the ovary. T1WI indicated iso-signal and slightly low signal intensity, while T2WI displayed high signal intensity. When cystic necrosis is combined in the mass, it displays with a low signal on T1WI and a high signal on T2WI. When bleeding is present, the T1WI and T2WI has high signal intensity and the solid part of the tumor is obviously enhanced (13).
In terms of the possible pathogenesis, three different hypotheses have been made to explain the origin of carcinosarcoma tissue. First, the collision theory revealed that the malignant epithelial component and the sarcoma component are derived from two different types of stem cell. According to the combination theory, both the malignant epithelial component and the sarcoma component came from pluripotent stem cells that have divergent differentiation. Finally, the conversion theory suggested that the sarcoma component of the tumor resulted from the evolution of the carcinomatous component of the tumor (14,15).
Regarding the therapeutic strategy, as OCS is rare, it is difficult to perform prospective clinical studies on treatment. Small case series studies reported on the efficacy of OCS treatment (16,17), but no detailed guidelines for the diagnosis and treatment of OCS currently exist. At present, the treatment of OCS mainly includes one therapy alone or a combination of two or more therapies of debulking surgery, chemotherapy, radiotherapy and targeted therapies (17-19).
In terms of surgical treatment, a comprehensive staging operation should be performed in the early stage of the tumor and cytoreductive surgery for advanced tumors is still the most important treatment method. There are numerous factors that may affect the prognosis of patients with OCS, such as residual disease after surgery, disease stage (20,21) and p53 overexpression (22).
Most of the retrospective studies indicated that there may be better survival after optimal debulking surgery. Satisfactory cytoreductive surgery is usually defined as the diameter of the largest residual lesion being >1 cm after surgery (23). It was reported that cytoreductive surgery for tumors with residual tumor burden ≤1 cm was able to improve the prognosis (16). For patients with OCS of all stages, patients with no visible disease had an overall survival (OS) of 57 months, vs. 32 months in those with ≤1 cm of residual disease and those with >1 cm of residual disease. Furthermore, another analysis of patients with stage 3 OCS was performed. It was reported that patients with no visible tumor residue had a significantly longer median OS (57 months) than those with ≤1 cm of residual tumor (median OS of 31 months) and those with >1 cm of residual tumor (median OS of 3 months) (23). Another study of 70 patients with OCS indicated that patients with residual lesions <2 cm after initial surgery had a longer survival time than those with ≥2 cm residual tumor (24). Wang et al (25) analyzed a total of 363 females with OCS and obtained no statistically significant difference in OS between lymph node dissection and no lymph node dissection for early-stage disease (hazard ratio, 0.88; 95% CI: 0.56-1.38), indicating that early lymph node dissection may not be associated with prognosis. However, since only a small number of studies have explored early lymph node dissection for OCS, further research is required.
In addition, preoperative CA125 levels were raised (>35 U/ml) in 93% of patients with OCS. The preoperative mean CA125 level was 447 U/ml. Preoperative CA125 <75 U/ml may be associated with a favorable survival prognosis (P=0.01) (16), which may also be an indicator for the efficacy evaluation and follow-up of patients with OCS. This was similar to the observation in the present study.
In terms of chemotherapy, as OCS is highly malignant, there is a high risk of local and distant recurrences, even for early-stage patients, and thus, adjuvant systemic therapy is generally considered. However, due to the lack of large-scale clinical studies, no consensus has been reached regarding the first-line chemotherapy regimen for OCS. The latest National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Ovarian Cancer suggest paclitaxel/carboplatin as the first choice for chemotherapy of carcinosarcoma (26). The chemotherapy regimens mainly refer to the treatment of epithelial ovarian cancer, including carboplatin/paclitaxel (27), ifosfamide/cisplatin (28) and ifosfamide/paclitaxel (29). A study by Tate Thigpen et al (30) reported on 136 patients with OCS who received cisplatin (50 mg/m2) every 3 weeks until disease progression or unacceptable toxicity occurred. The median progression-free survival was 5.2 months. The overall median survival time was 11.7 months. In 27 patients with OCS using the combination therapy of radiotherapy, melphalan or chemotherapy after surgical cytoreduction, the four patients in Stages I or II were disease-free after at least 5 years. The median survival time and 5-year survival rate for patients with Stage III or IV OCS was 18 months and 8%, respectively (31).
In the Surveillance, Epidemiology and End Results database 1,763 patients with OCS were registered between 1988 and 2007; they had poor prognosis and it was reported that the five-year survival rate for these patients for each stage was low (Stage I, 65.2%; Stage II, 34.6%; Stage IIIC, 18.2%; and Stage IV, 11.2%) (32). However, these studies did not account for chemotherapy application and comorbidities. For 2,886 female patients with chemotherapy for OCS in the National Cancer Data Base between 2003 and 2011, it was indicated that the five-year survival rate for the entire population was 26.63%. However, with the increase in the stage, the survival prognosis also deteriorated (5-year survival rate for Stage I, 59.07%; Stage II, 46.78%; Stage III, 20.14%; and Stage IV, 13.68%) (19). Thus, these studies may suggest that platinum-based combination chemotherapy followed by surgery is associated with better survival.
Adjuvant radiotherapy may have a better effect in terms of local control. The survival rate of patients with combined radiotherapy and chemotherapy after surgery may be higher than that of patients with radiotherapy or chemotherapy alone. Adjuvant radiotherapy (external beam irradiation and/or vaginal brachytherapy) was not able to improve OS but it may decrease local recurrences (33). A previous study reported a case of recurrence of OCS with a large pelvic mass (14 cm) after surgery. After chemotherapy combined with lattice radiation therapy, the tumor size was significantly reduced with favorable clinical and imaging-based follow-up prognosis for >4 years (34).
Although cytoreductive surgery is the most important treatment for OCS, due to the high recurrence rate and the poor survival, it is necessary to determine effective adjuvant therapies. In recent years, targeted therapy has gradually been regarded as an important way to cause tumor cell death whilst minimizing injury to healthy cells. Numerous studies have explored molecular biomarkers that may be utilized for targeted therapy. Numerous studies suggested that human EGFR-2 (HER2)/neu may be a potential target for immunotherapy in gynecological carcinosarcomas. In OCS refractory to salvage chemotherapy, HER2/neu expression was detected in two primary OCS cell lines, which identified the amplification of the c-erbB2 gene by fluorescent in situ hybridization analysis and high sensitivity to antibody-dependent cell-mediated cytotoxicity (mean killing rate, 45.6%; range, 32.3-72.6%) (35). SYD985 is a novel duocarmycin-based HER2-targeting antibody-drug conjugate, which caused efficient bystander killing of HER2/neu 0/1+ tumor cells mixed with HER2/neu 3+ cells, suggesting antitumor activity in OCS with HER2/neu expression (36).
In addition, a study by Zhu et al (18) estimated the expression of programmed death ligand 1 (PD-L1) and intratumoral CD8+ T lymphocytes from 19 OCS cases undergoing primary surgery and determined that positive tumoral CD8+ T lymphocytes and mesenchymal PD-L1-negative expression was associated with favorable survival in OCS. Solitomab is an epithelial cell-adhesion molecule (EpCAM)/CD3 bispecific antibody construct, which is highly active against OCS cell lines in vitro. Ex vivo incubation of autologous tumor-associated-T cells with EpCAM-expressing malignant cells in pleural effusion with solitomab resulted in a significant increase in T-cell proliferation of both CD4+ and CD8+ T cells, as well as a reduction in the number of viable OCS cells in the exudate (37). HRS7 is a humanized monoclonal anti-trophoblast cell-surface antigen-2 (Trop-2) antibody, which may represent a potential effective treatment option for patients with OCS with treatment-refractory carcinosarcomas overexpressing human Trop-2(17), which was associated with increased tumor invasiveness and reduced OS of patients with carcinomas (38). Thus, targeted therapy focusing on inhibiting molecular abnormalities or pathways may be an attractive and novel approach for residual or drug-resistant OCS of refractory chemotherapy.
The present study has certain limitations. The sample size of the present study was only one. Furthermore, the removed part of the ovarian lesions and mesenteric neoplasms was not photographed. Molecular characterization of OCS may indicate both targetable alterations and predictive biomarkers of response and there was a lack of detailed molecular analysis of OCS.
In conclusion, there are still no specific clinical manifestations and serological detection indicators for OCS. The present study showcased that in patients with OCS undergoing cytoreduction surgery, adjuvant platinum-based chemotherapy and targeted therapy may be effective. Owing to the rarity of OCS, in the future, more studies will be required to explore better treatment options to improve the prognosis.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JF designed and performed the study. She treated the patient, wrote the manuscript, performed the literature search and made critical revisions. The author has read and approved the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
As it is a case report, ethics approval is not applicable.
Patient consent for publication
Written informed consent to publish the case, including images and clinical details, was obtained from the patient.
Competing interests
The author declares that she has no competing interests.
References
- 1.del Carmen MG, Birrer M, Schorge JO. Carcinosarcoma of the ovary: A review of the literature. Gynecol Oncol. 2012;125:271–277. doi: 10.1016/j.ygyno.2011.12.418. [DOI] [PubMed] [Google Scholar]
- 2.Le T, Krepart GV, Lotocki RJ, Heywood MS. Malignant mixed mesodermal ovarian tumor treatment and prognosis: A 20-year experience. Gynecol Oncol. 1997;65:237–240. doi: 10.1006/gyno.1997.4625. [DOI] [PubMed] [Google Scholar]
- 3.Trento M, Munari G, Carraro V, Lanza C, Salmaso R, Pizzi S, Santoro L, Chiarelli S, Dal Santo L, Nardelli GB, et al. Mutational and immunophenotypic profiling of a series of 8 Tubo-ovarian carcinosarcomas revealed a monoclonal origin of the disease. Int J Gynecol Pathol. 2020;39:305–312. doi: 10.1097/PGP.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 4.Barnholtz-Sloan JS, Morris R, Malone JM Jr, Munkarah AR. Survival of women diagnosed with malignant, mixed mullerian tumors of the ovary (OMMMT) Gynecol Oncol. 2004;93:506–512. doi: 10.1016/j.ygyno.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Duska LR, Garrett A, Eltabbakh GH, Oliva E, Penson R, Fuller AF. Paclitaxel and platinum chemotherapy for malignant mixed müllerian tumors of the ovary. Gynecol Oncol. 2002;85:459–463. doi: 10.1007/s00432-020-03234-6. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Li XM, Chen SG, Deng J, Lei Y, Li WJ, Zhang HZ, Zhang H, Li D, Xie P. Application of antibodies against Borna disease virus phosphoprotein and nucleoprotein on paraffin sections. Mol Med Rep. 2018;17:5416–5422. doi: 10.3892/mmr.2018.8467. [DOI] [PubMed] [Google Scholar]
- 7.Pinheiro C, Roque R, Adriano A, Mendes P, Praça M, Reis I, Pereira T, Srebotnik Kirbis I, André S. Optimization of immunocytochemistry in cytology: comparison of two protocols for fixation and preservation on cytospin and smear preparations. Cytopathology. 2015;26:38–43. doi: 10.1111/cyt.12156. [DOI] [PubMed] [Google Scholar]
- 8.Stover EH, Fuh K, Konstantinopoulos PA, Matulonis UA, Liu JF. Clinical assays for assessment of homologous recombination DNA repair deficiency. Gynecol Oncol. 2020;159:887–898. doi: 10.1016/j.ygyno.2020.09.029. [DOI] [PubMed] [Google Scholar]
- 9.van Warmerdam LJ, Rodenhuis S, ten Bokkel Huinink WW, Maes RA, Beijnen JH. The use of the Calvert formula to determine the optimal carboplatin dosage. J Cancer Res Clin Oncol. 1995;121:478–486. doi: 10.1007/BF01218365. [DOI] [PubMed] [Google Scholar]
- 10.Pankaj S, Nazneen S, Kumari A, Kumari S, Choudhary V, Roy VK. A rare tumor of the ovary: carcinosarcoma report and review of literature. J Obstet Gynaecol India. 2016;66:648–650. doi: 10.1007/s13224-015-0788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCluggage WG, Lioe TF, McClelland HR, Lamki H. Rhabdomyosarcoma of the uterus: report of two cases, including one of the spindle cell variant. Int J Gynecol Cancer. 2002;12:128–132. doi: 10.1046/j.1525-1438.2002.01069.x. [DOI] [PubMed] [Google Scholar]
- 12.Ciccarone F, Biscione A, Moro F, Fischerova D, Savelli L, Munaretto M, Jokubkiene L, Sladkevicius P, Chiappa V, Fruscio R, et al. Imaging in gynecological disease: clinical and ultrasound characteristics of ovarian carcinosarcomas. Ultrasound Obstet Gynecol. 2022;59:241–247. doi: 10.1002/uog.23733. [DOI] [PubMed] [Google Scholar]
- 13.Saida T, Mori K, Tanaka YO, Sakai M, Amano T, Kikuchi S, Masuoka S, Yoshida M, Masumoto T, Satoh T, et al. Carcinosarcoma of the ovary: MR and clinical findings compared with high-grade serous carcinoma. Jpn J Radiol. 2021;39:357–366. doi: 10.1007/s11604-020-01072-7. [DOI] [PubMed] [Google Scholar]
- 14.Dasgupta S, Bose D, Bhattacharyya NK, Biswas PK. Carcinosarcoma of ovary with its various immunohistochemical expression: Report of a rare case. J Cancer Res Ther. 2015;11(1022) doi: 10.4103/0973-1482.147390. [DOI] [PubMed] [Google Scholar]
- 15.Carnevali IW, Cimetti L, Sahnane N, Libera L, Cavallero A, Formenti G, Riva C, Tibiletti MG. Two cases of carcinosarcomas of the ovary involved in hereditary cancer syndromes. Int J Gynecol Pathol. 2017;36:64–70. doi: 10.1097/PGP.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 16.Sood AK, Sorosky JI, Gelder MS, Buller RE, Anderson B, Wilkinson EJ, Benda JA, Morgan LS. Primary ovarian sarcoma: Analysis of prognostic variables and the role of surgical cytoreduction. Cancer. 1998;82:1731–1737. [PubMed] [Google Scholar]
- 17.Raji R, Guzzo F, Carrara L, Varughese J, Cocco E, Bellone S, Betti M, Todeschini P, Gasparrini S, Ratner E, et al. Uterine and ovarian carcinosarcomas overexpressing Trop-2 are sensitive to hRS7, a humanized anti-Trop-2 antibody. J Exp Clin Cancer Res. 2011;30(106) doi: 10.1186/1756-9966-30-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Wen H, Ju X, Bi R, Zuo W, Wu X. Clinical significance of programmed death Ligand-1 and intra-tumoral CD8+ T lymphocytes in ovarian carcinosarcoma. PLoS One. 2017;12(e0170879) doi: 10.1371/journal.pone.0170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rauh-Hain JA, Gonzalez R, Bregar AJ, Clemmer J, Hernández-Blanquisett A, Clark RM, Schorge JO, Del Carmen MG. Patterns of care, predictors and outcomes of chemotherapy for ovarian carcinosarcoma: A national cancer database analysis. Gynecol Oncol. 2016;142:38–43. doi: 10.1016/j.ygyno.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Paulsson G, Andersson S, Sorbe B. A population-based series of ovarian carcinosarcomas with long-term follow-up. Anticancer Res. 2013;33:1003–1008. [PubMed] [Google Scholar]
- 21.Ariyoshi K, Kawauchi S, Kaku T, Nakano H, Tsuneyoshi M. Prognostic factors in ovarian carcinosarcoma: A clinicopathological and immunohistochemical analysis of 23 cases. Histopathology. 2000;37:427–436. doi: 10.1046/j.1365-2559.2000.01015.x. [DOI] [PubMed] [Google Scholar]
- 22.Zorzou MP, Markaki S, Rodolakis A, Kastritis E, Bozas G, Dimopoulos MA, Papadimitriou CA. Clinicopathological features of ovarian carcinosarcomas: A single institution experience. Gynecol Oncol. 2005;96:136–142. doi: 10.1016/j.ygyno.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 23.Doo DW, Erickson BK, Arend RC, Conner MG, Huh WK, Leath CA III. Radical surgical cytoreduction in the treatment of ovarian carcinosarcoma. Gynecol Oncol. 2014;133:234–237. doi: 10.1016/j.ygyno.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown E, Stewart M, Rye T, Al-Nafussi A, Williams AR, Bradburn M, Smyth J, Gabra H. Carcinosarcoma of the ovary: 19 years of prospective data from a single center. Cancer. 2004;100:2148–2153. doi: 10.1002/cncr.20256. [DOI] [PubMed] [Google Scholar]
- 25.Wang WP, Li N, Zhang YY, Gao YT, Sun YC, Ge L, Wu LY. Prognostic significance of lymph node metastasis and lymphadenectomy in early-stage ovarian carcinosarcoma. Cancer Manag Res. 2018;10:1959–1968. doi: 10.2147/CMAR.S166524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Chen LM, Cristea M, DeRosa M, Eisenhauer EL, et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:191–226. doi: 10.6004/jnccn.2021.0007. [DOI] [PubMed] [Google Scholar]
- 27.Rutledge TL, Gold MA, McMeekin DS, Huh WK, Powell MA, Lewin SN, Mutch DG, Johnson GA, Walker JL, Mannel RS. Carcinosarcoma of the ovary-a case series. Gynecol Oncol. 2006;100:128–132. doi: 10.1016/j.ygyno.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 28.Silasi DA, Illuzzi JL, Kelly MG, Rutherford TJ, Mor G, Azodi M, Schwartz PE. Carcinosarcoma of the ovary. Int J Gynecol Cancer. 2008;18:22–29. doi: 10.1111/j.1525-1438.2007.00948.x. [DOI] [PubMed] [Google Scholar]
- 29.Brackmann M, Stasenko M, Uppal S, Erba J, Reynolds RK, McLean K. Comparison of first-line chemotherapy regimens for ovarian carcinosarcoma: A single institution case series and review of the literature. BMC Cancer. 2018;18(172) doi: 10.1186/s12885-018-4082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tate Thigpen J, Blessing JA, DeGeest K, Look KY, Homesley HD. Cisplatin as initial chemotherapy in ovarian carcinosarcomas: A gynecologic oncology group study. Gynecol Oncol. 2004;93:336–339. doi: 10.1016/j.ygyno.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Muntz HG, Jones MA, Goff BA, Fuller AF Jr, Nikrui N, Rice LW, Tarraza HM. Malignant mixed müllerian tumors of the ovary: experience with surgical cytoreduction and combination chemotherapy. Cancer. 1995;76:1209–1213. doi: 10.1002/1097-0142(19951001)76:7<1209::aid-cncr2820760717>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.George EM, Herzog TJ, Neugut AI, Lu YS, Burke WM, Lewin SN, Hershman DL, Wright JD. Carcinosarcoma of the ovary: natural history, patterns of treatment, and outcome. Gynecol Oncol. 2013;131:42–45. doi: 10.1016/j.ygyno.2013.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berton-Rigaud D, Devouassoux-Shisheboran M, Ledermann JA, Leitao MM, Powell MA, Poveda A, Beale P, Glasspool RM, Creutzberg CL, Harter P, et al. Gynecologic cancer InterGroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. Int J Gynecol Cancer. 2014;24 (9 Suppl 3):S55–S60. doi: 10.1097/IGC.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 34.Blanco Suarez JM, Amendola BE, Perez N, Amendola M, Wu X. The use of lattice radiation therapy (LRT) in the treatment of bulky tumors: A case report of a large metastatic mixed mullerian ovarian tumor. Cureus. 2015;7(e389) doi: 10.7759/cureus.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guzzo F, Bellone S, Buza N, Hui P, Carrara L, Varughese J, Cocco E, Betti M, Todeschini P, Gasparrini S, et al. HER2/neu as a potential target for immunotherapy in gynecologic carcinosarcomas. Int J Gynecol Pathol. 2012;31:211–221. doi: 10.1097/PGP.0b013e31823bb24d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menderes G, Bonazzoli E, Bellone S, Black J, Predolini F, Pettinella F, Masserdotti A, Zammataro L, Altwerger G, Buza N, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows antitumor activity in uterine and ovarian carcinosarcoma with HER2/Neu expression. Clin Cancer Res. 2017;23:5836–5845. doi: 10.1158/1078-0432.CCR-16-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari F, Bellone S, Black J, Schwab CL, Lopez S, Cocco E, Bonazzoli E, Predolini F, Menderes G, Litkouhi B, et al. Solitomab, an EpCAM/CD3 bispecific antibody construct (BiTE®), is highly active against primary uterine and ovarian carcinosarcoma cell lines in vitro. J Exp Clin Cancer Res. 2015;34(123) doi: 10.1186/s13046-015-0241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cubas R, Zhang S, Li M, Chen C, Yao Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9(253) doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.