Key Points
Question
What are the factors associated with pregnancy-related attacks in neuromyelitis optica spectrum disorder?
Findings
This systematic review and meta-analysis of 15 studies with 443 patients found that receiving immunosuppressive treatment during pregnancy and older age at conception were associated with lower risk of pregnancy-related attacks. Annualized relapse rate was elevated especially during the first trimester after delivery while the Expanded Disability Status Scale score worsened during pregnancy and the postpartum period.
Meaning
These findings suggest that receiving immunosuppressive treatment during pregnancy and older age at conception were associated with protection against pregnancy-related neuromyelitis optica spectrum disorder attacks, which mostly occurred in the first trimester of the postpartum period.
This systematic review and meta-analysis assesses factors associated with pregnancy-related be increased in the postpartum period of neuromyelitis attacks, annualized relapse rate, and Expanded Disability Status Scale score in each phase of pregnancy among patients with NMOSD.
Abstract
Importance
Risk of relapse may be increased in the postpartum period of neuromyelitis optica spectrum disorder (NMOSD). Information regarding factors associated with pregnancy-related attacks is still lacking.
Objectives
To identify factors associated with pregnancy-related NMOSD attacks, investigate the integrated annualized relapse rate (ARR) and Expanded Disability Status Scale (EDSS) score in each phase of pregnancy, and summarize pregnancy outcomes and complications in patients with NMOSD.
Data Sources
An electronic search was performed in the MEDLINE, PubMed in-process and non-MEDLINE, EMBASE, Web of Science, and Cochrane databases using the OvidSP search platform, updated through December 30, 2021.
Study Selection
All published and unpublished studies in English were considered, covering all patients with NMOSD with an informative pregnancy.
Data Extraction and Synthesis
Two independent reviewers extracted the published data with a standardized procedure following MOOSE and PRISMA guidelines. The end points were calculated with the DerSimonian and Laird inverse variance (for random effects) method.
Main Outcomes and Measures
The primary outcome was the rate of pregnancies with pregnancy-related NMOSD attacks, measured by risk ratios (RRs). The mean differences (MDs) in ARR and EDSS scores between each phase of pregnancy, pregnancy outcomes, and complications were defined as the secondary outcomes.
Results
A total of 15 studies were analyzed, including 443 patients with NMOSD with 639 informative pregnancies. Patients receiving immunosuppressive treatment during pregnancy (RR, 0.43; 95% CI, 0.32-0.57; P < .001) and with older age at conception (RR, 0.67; 95% CI, 0.47-0.95; P = .02) had lower rates of pregnancy with pregnancy-related attacks. The increase in the ARR was highest in the first trimester after delivery compared with before pregnancy (MD, 1.28; 95% CI, 0.94-1.62; P < .001). The EDSS scores increased significantly both during pregnancy (MD, 0.44; 95% CI, 0.20-0.69; P < .001) and in the postpartum period (MD, 0.88; 95% CI, 0.51-1.26; P < .001) compared with before pregnancy.
Conclusions and Relevance
This systematic review and meta-analysis found that receiving immunosuppressive treatment during pregnancy and older age at conception were associated with reduced risk of pregnancy-related NMOSD attacks, which mostly occurred in the first trimester of the postpartum period, although more high-quality prospective studies are needed.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune inflammatory disease of the central nervous system and predominantly involves the optic nerve and spinal cord, causing blindness and paralysis.1 The major pathogenic antibody of NMOSD is aquaporin-4 antibody (AQP4-Ab), while myelin oligodendrocyte glycoprotein antibody (MOG-Ab) is found in some patients with NMOSD who do not have AQP4-Ab.2,3
NMOSD principally affects women, many of whom develop disease activity during childbearing age. Previous studies have reported that the annualized relapse rate (ARR) among patients with NMOSD with AQP4-Ab increases especially in the first 3 months postpartum, and pregnancy outcomes include miscarriage and preeclampsia.4,5,6 Pregnancy-related NMOSD attacks have also been demonstrated in patients with MOG-Ab, most of which emerged in the postpartum period.7 Consequently, a worsening Expanded Disability Status Scale (EDSS) score has been reported during pregnancy and the postpartum period.7,8,9 Immunotherapy is associated with decreased risk of pregnancy-related NMOSD attacks.10 However, information regarding factors associated with pregnancy-related NMOSD attacks is still lacking, with only 1 systematic review without quantitative analysis and 1 meta-analysis focusing on immunosuppressive treatment retrieved.11,12 In this study, we aimed to identify the factors associated with pregnancy-related NMOSD attacks, investigate the integrated ARR and EDSS scores in each phase of pregnancy, and summarize pregnancy outcomes and complications in patients with NMOSD.
Methods
We registered the systematic review protocol with the International Prospective Register of Systematic Reviews (PROSPERO).13 It was registered prior to conducting the review and is available to the public. This study followed the guidelines of Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline and Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.14,15
Search Strategy
Two reviewers (L.W. and M.S.) independently conducted literature searches in MEDLINE, PubMed in-process, and non-MEDLINE, EMBASE, Web of Science, and Cochrane databases through the OvidSP search platform. We combined relevant terms into a search strategy applied for these databases (eMethods in the Supplement). All published and unpublished studies in English were considered, updated through December 30, 2021, covering all patients with NMOSD with an informative pregnancy.
Inclusion and Exclusion Criteria
Inclusion criteria were all relevant studies referred to the 2006 revised criteria for neuromyelitis optica or 2015 diagnostic criteria for NMOSD,16,17 from prospective cohort studies to retrospective studies. Considering that the AQP4-Ab positivity rate varied in each study, patients with MOG-Ab or seronegative status were also included. Exclusion criteria were case reports or series (ie, fewer than 10 patients), cohort studies without sufficient data, reviews, and animal studies. Two of us (L.W. and M.S.) read the studies thoroughly to evaluate appropriateness for inclusion in the systematic review. Any disagreement was arbitrated by a third investigator (Z. Z.).
Data Extraction and Outcome Measures
We extracted accessible information regarding study year, design, and demographic and clinical characteristics of participants, as well as pregnancy outcomes and complications. Demographic and clinical features were the number of informative patients or pregnancies, AQP4-Ab serostatus, number of informative patients or pregnancies after disease onset, age at NMOSD onset, age at conception, and number of pregnancy-related NMOSD attacks. Patients who became pregnant after disease onset and had a relapse during pregnancy or within 1 year of the postpartum period were defined as having a pregnancy-related NMOSD attack. Pregnancy outcomes and complications included the numbers of term deliveries, premature deliveries, abortions (including spontaneous abortions and elective abortions), preeclampsia, and neonatal complications. Two of us (L.W. and M.S.) extracted the published data with a standardized procedure, and another reviewer (Z.Z.) rechecked the data. The corresponding authors of the included studies were contacted to acquire the unpublished data if needed. Any contradictory data were reexamined and discussed to reach consensus. In this meta-analysis, the primary outcome was the rate of pregnancies with pregnancy-related NMOSD attacks. The differences in ARR and EDSS scores between each phase of pregnancy, pregnancy outcomes, and complications were defined as the secondary outcomes.
For ARR, pregnancy phases were divided into 12 to 0 months before pregnancy, months 0 to 3 of pregnancy (T1), months 3 to 6 of pregnancy (T2), months 6 to 9 of pregnancy (T3), months 0 to 3 of the postpartum period (PP1), months 3 to 6 of the postpartum period (PP2), and months 6 to 12 of the postpartum period (PP3). For EDSS score, the pregnancy phases were divided into before pregnancy, the period during pregnancy, and months 0 to 12 of the postpartum period.
Quality Appraisal: Risk of Bias
Using the Newcastle-Ottawa Scale specific for cohort design,18 2 reviewers (L. W. and M. S.) independently evaluated the risk of bias covering selection, comparability, and outcome, with the total score ranging from 0 to 9 stars. A score of 6 or higher corresponds to low risk of bias. A funnel plot was used to assess publication bias.
Statistical Analysis
Data analysis was conducted using Stata statistical software version 13.0 (StataCorp), while figures were constructed with Prism graphic software version 6 (GraphPad Software). The presence of heterogeneity was evaluated by the I2 test. Subgroup analysis and meta-regression analysis were conducted to explore the high heterogeneity (I2 >50% and P < .10). For dichotomous covariates (immunosuppressive treatment during pregnancy, age at conception [<32 vs ≥32years], AQP4-Ab, EDSS score at conception, coexisting autoimmune disease, and relapse during the year before pregnancy), risk ratios (RRs) and 95% CIs were calculated with the DerSimonian and Laird inverse variance (for random effects) method. For continuous covariates (rate of immunosuppressive treatment during pregnancy, age at conception, AQP4-Ab positivity rate, age at disease onset, and time interval from disease onset to conception), odds ratios (ORs) with 95% CIs were calculated by means of restricted maximum likelihood in meta-regression analysis. Necessary interaction analysis was also performed. For integrated ARR and EDSS scores at each phase, as well as their changing values compared with before pregnancy, mean differences (MDs) with 95% CIs were calculated with a random-effects meta-analysis model using the DerSimonian and Laird inverse variance method. The weight of each included study was calculated simultaneously to interpret the findings cautiously. The standard deviations of changing values in ARR and EDSS scores between before pregnancy and other phases were calculated using an imputed correlation coefficient.19 Publication bias was calculated with the Egger regression intercept test. Statistical significance was set at a 2-sided P < .05. Data were analyzed from January 15 to 30, 2022.
Results
Description of Included Studies
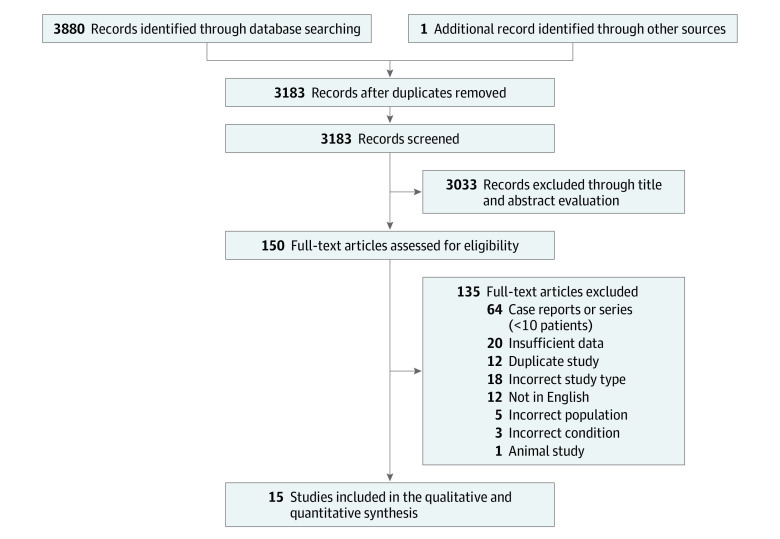
A total of 3880 studies were identified in the database search, as well as 1 additional record identified through other sources, of which 3183 remained after removing duplicates. Following the first round of screening, 3033 studies were found not to be related to NMOSD and pregnancy. The remaining 150 full-text articles were sourced, and a further 135 were excluded for reasons identified in Figure 1. Ultimately, 15 studies were included in the systematic review.4,5,6,7,8,9,12,20,21,22,23,24,25,26,27 The demographic and clinical characteristics of the informative patients and pregnancies in NMOSD from 15 studies are summarized in Table 1. In total, 443 patients with NMOSD with 639 informative pregnancies were included, with the AQP4-Ab positivity rate ranging from 48.3% to 100%.
Figure 1. Flowchart of the Literature Search and Study Selection.
Table 1. Demographic and Clinical Characteristics of the Informative Patients and Pregnancies in Neuromyelitis Optica Spectrum Disorder From 15 Studies.
| Source | Type | Region | Informative patients/pregnancies, No. | AQP4-Ab positivity rate, % | Patients with pregnancies/pregnancies after disease onset, No. | Age at onset, mean (SD), y | Age at conception, mean (SD), y | Pregnancy-related attacks, No. |
|---|---|---|---|---|---|---|---|---|
| Bourre et al,8 2012 | Retrospective | France | 20/25 | 53.3 | NA/13 | 24.6 (7.7) | 25.6 (4.3) | NA |
| Kim et al,4 2012 | Retrospective | Korea | 40/54 | 100 | 26/40 | 25.4 (6.1) | 29.9 (4.8) | 34 |
| Fragoso et al,9 2013 | Retrospective | Brazil | 17/17 | NA | 17/17 | 26 | 28.2 (6.1) | NA |
| Nour et al,5 2016 | Retrospective | International | NA/27 | 100 | NA/22 | NA | 28.5 (5.5) | NA |
| Shimizu et al,6 2016 | Retrospective | Japan | 22/24 | 100 | 11/12 | 25.3 (5.8) | 32.3 (5.4) | 11 |
| Huang et al,21 2017 | Retrospective | China | 55/63 | 92.3 | NA/30 | 25.6 (5.4) | 25.8 (3.5) | 46 |
| Klawiter et al,22 2017 | Retrospective | International | NA/65 | 80.6 | 31/46 | 28.3 (5.7) | NA | 25 |
| Shi et al,20 2017 | Retrospective | China | 16/22 | 81.3 | 16/22 | 22.9 (4.3) | 28.1 (3.9) | 21 |
| Salvador et al,23 2019 | Retrospective | Brazil | 19/30 | 63.2 | 10/21 | NA | NA | 33 |
| Ashtari et al,25 2020 | Retrospective | Denmark | 11/20 | 100 | 11/20 | 27.6 (10.7) | NA | 15 |
| Kim et al,24 2020 | Retrospective | Korea | 26/33 | 97 | 26/33 | 25 (6.7) | 32 (5.2) | 17 |
| Wang et al,7 2020 | Retrospective | China | 110/136 | 75.5 | 60/76 | 23.8 (6.3) | 28.1 (4.5) | 69 |
| Collongues et al,27 2021 | Retrospective | International | 58/89 | 48.3 | NA/67 | 27 (7.0) | 30 (4.9) | 40 |
| Deng et al,12 2021 | Retrospective | China | 33/34 | 100 | 22/22 | 27.7 | 27.8 (4.8) | 32 |
| Kümpfel et al,26 2021 | Retrospective | Germany | 16/NA | NA | 12/13 | NA | NA | 1 |
Abbreviations: AQP4-Ab, anti-aquaporin-4 antibody; NA, not available.
The rate of pregnancies with pregnancy-related NMOSD attacks ranged from 7.7% to 80.8% in 11 studies.4,6,7,12,20,22,23,24,25,26,27 However, the heterogeneity was high (I2 = 87.5%; P < .001) (eFigure 1 in the Supplement). Subgroup analysis and meta-regression analysis were then conducted to find the associated factors (Table 2).
Table 2. Factors Associated With Pregnancy-Related Neuromyelitis Optica Spectrum Disorder Attacks.
| Factor | No. | Effect size (95% CI) | P value | |||
|---|---|---|---|---|---|---|
| Studies | Pregnancies | Events | RR | OR | ||
| With immunosuppressive treatment during pregnancy | 7 | 274 | 159 | 0.43 (0.32-0.57) | NA | <.001 |
| Rate of immunosuppressive treatment during pregnancy | 8 | 287 | 160 | NA | 0.46 (0.29-0.72) | .006 |
| Older age at conception (≥32 y) | 5 | 210 | 113 | 0.67 (0.47-0.95) | NA | .02 |
| Age at conception | 7 | 270 | 158 | NA | 0.98 (0.89-1.08) | .60 |
| With AQP4-Ab | 4 | 198 | 104 | 1.23 (0.83-1.82) | NA | .30 |
| AQP4-Ab positivity rate | 10 | 354 | 215 | NA | 1.004 (0.999-1.008) | .13 |
| High EDSS score at conception (≥4) | 4 | 152 | 89 | 1.36 (0.99-1.87) | NA | .06 |
| With coexisting autoimmune disease | 5 | 232 | 128 | 1.25 (0.99-1.57) | NA | .06 |
| With relapse during the year before pregnancy | 4 | 188 | 96 | 2.16 (0.73-6.41) | NA | .17 |
| Age at disease onset | 9 | 324 | 193 | NA | 1.00 (0.93-1.07) | .99 |
| Time interval from disease onset to conception | 6 | 236 | 134 | NA | 1.01 (0.87-1.18) | .81 |
Abbreviations: AQP4-Ab, anti-aquaporin-4 antibody; EDSS, Expanded Disability Status Scale; RR, risk ratio; OR, odds ratio.
Factors Associated With Pregnancy-Related NMOSD Attacks
Immunosuppressive Treatment During Pregnancy
In the included studies, patients received immunosuppressive treatment before and during pregnancy, regardless of the duration and kind, including glatiramer acetate and interferon therapy in 1 study.23 There were 7 studies6,7,12,20,23,24,27 with 274 informative pregnancies incorporated in the subgroup analysis. The rate of pregnancy-related NMOSD attacks in the group receiving immunosuppressive treatment during pregnancy was significantly lower than that in the group without immunosuppressive treatment during pregnancy (RR, 0.43; 95% CI, 0.32-0.57; P < .001) (eFigure 2 in the Supplement). Eight studies6,7,12,20,23,24,26,27 with 287 informative pregnancies were included in the meta-regression analysis. A negative association was observed between the rate of immunosuppressive treatment during pregnancy and the rate of pregnancies with pregnancy-related NMOSD attacks (OR, 0.46; 95% CI, 0.29-0.72; P = .006).
Age at Conception
We included 5 studies6,7,20,24,27 with 210 informative pregnancies in the age at conception subgroup analysis. Patients were divided into the group with older age at conception (≥32 years) or younger age at conception (<32 years).5,7 The rate of pregnancy-related NMOSD attacks in the group with older age at conception was significantly lower than that in the group with younger age at conception (RR, 0.67; 95% CI, 0.47-0.95; P = .02) (eFigure 2 in the Supplement). Meta-regression analysis was also conducted in 7 studies4,6,7,12,20,24,27 with 270 informative pregnancies. For age at conception, the difference was not statistically significant in the rate of pregnancies with pregnancy-related NMOSD attacks (OR, 0.98; 95% CI, 0.89-1.08; P = .60).
AQP4-Ab
There were 4 studies7,20,24,27 with 198 informative pregnancies included in the AQP4-Ab subgroup analysis. The incidence of pregnancy-related NMOSD attacks was not different between the group with AQP4-Ab and the group without AQP4-Ab (including patients with MOG-Ab and seronegative status) (RR, 1.23; 95% CI, 0.83-1.82; P = .30) (eFigure 2 in the Supplement). Meta-regression analysis was also conducted with in 10 studies4,6,7,12,20,22,23,24,25,27 with 354 informative pregnancies. Presence of AQP4-Ab was not significantly associated with the rate of pregnancies with pregnancy-related NMOSD attacks (OR, 1.004; 95% CI, 0.999-1.008; P = .13).
EDSS Score at Conception
For EDSS score at conception, 4 studies7,20,23,24 with 152 informative pregnancies were included in the EDSS score subgroup analysis. Patients were dichotomized as high EDSS score (≥4) or low EDSS score (<4).23 There was no statistically significant difference in rate of pregnancies with pregnancy-related NMOSD attacks between the group with high EDSS score vs the group with low EDSS score (RR, 1.36; 95% CI, 0.99-1.87; P = .06) (eFigure 2 in the Supplement).
Coexisting Autoimmune Disease
We incorporated 5 studies7,12,20,24,27 with 232 informative pregnancies in the coexisting autoimmune disease subgroup analysis. We did not observe a statistically significant difference in rate of pregnancies with pregnancy-related NMOSD attacks in the group with coexisting autoimmune disease compared with the group without coexisting autoimmune disease (RR, 1.25; 95% CI, 0.99-1.57; P = .06) (eFigure 2 in the Supplement).
Relapse During the Year Before Pregnancy
The relapse during the year before pregnancy subgroup analysis included 4 studies6,7,24,27 with 188 informative pregnancies. There was no significant difference in the rate of pregnancies with pregnancy-related NMOSD attacks between the group with relapse during the year before pregnancy and the group without relapse during the year before pregnancy (RR, 2.16; 95% CI, 0.73-6.41; P = .17) (eFigure 2 in the Supplement).
Age at Disease Onset
We included 9 studies4,6,7,12,20,22,24,25,27 with 324 informative pregnancies in the meta-regression analysis of age at disease onset. However, age at disease onset was not observed to be associated with the rate of pregnancies with pregnancy-related NMOSD attacks (OR, 1.00; 95% CI, 0.93-1.07; P = .99).
Time Interval From Disease Onset to Conception
We incorporated 6 studies4,6,7,20,24,27 with 236 informative pregnancies in the meta-regression analysis of time interval from disease onset to conception. However, time interval from disease onset to conception was not found to be significantly associated with the rate of pregnancies with pregnancy-related NMOSD attacks (OR, 1.01; 95% CI, 0.87-1.18; P = .81).
Interaction Analysis
We conducted an analyses assessing potential interaction between associated factors on pregnancy-related attacks (eTable 1 in the Supplement). We did not observe any statistically significant differences.
ARR at Each Phase
The quantitative analysis of ARR at each phase before, during, and after pregnancy included 12 studies.4,5,6,7,8,9,12,20,21,22,23,27 The integrated ARRs at each phase are shown in Figure 2A. The highest ARR was 1.86 (95% CI, 1.47-2.25) in PP1, while the lowest ARR was 0.36 (95% CI, 0.03-0.70) in T3. The difference in ARR was statistically significant between before pregnancy and PP1 (MD, 1.28; 95% CI, 0.94-1.62; P < .001) (eFigure 3 in the Supplement). However, the differences in ARR were not statistically significant between before pregnancy and the other phases of pregnancy (T1: MD, –0.17; 95% CI, –0.35 to 0.02; P = .08; T2: MD, –0.12; 95% CI, –0.31 to 0.07; P = .21; T3: MD, –0.20; 95% CI, –0.41 to 0.01; P = .06) or postpartum (PP2: MD, 0.27; 95% CI, –0.01 to 0.54; P = .06; PP3: MD, −0.03; 95% CI, –0.20 to 0.15; P = .78) (eFigure 3 in the Supplement). Subgroup analyses based on antibody status are presented in the eAppendix and eFigures 4 through 7 in the Supplement.
Figure 2. Integrated Annualized Relapse Rate (ARR) and Expanded Disability Status Scale (EDSS) Score at Each Phase.
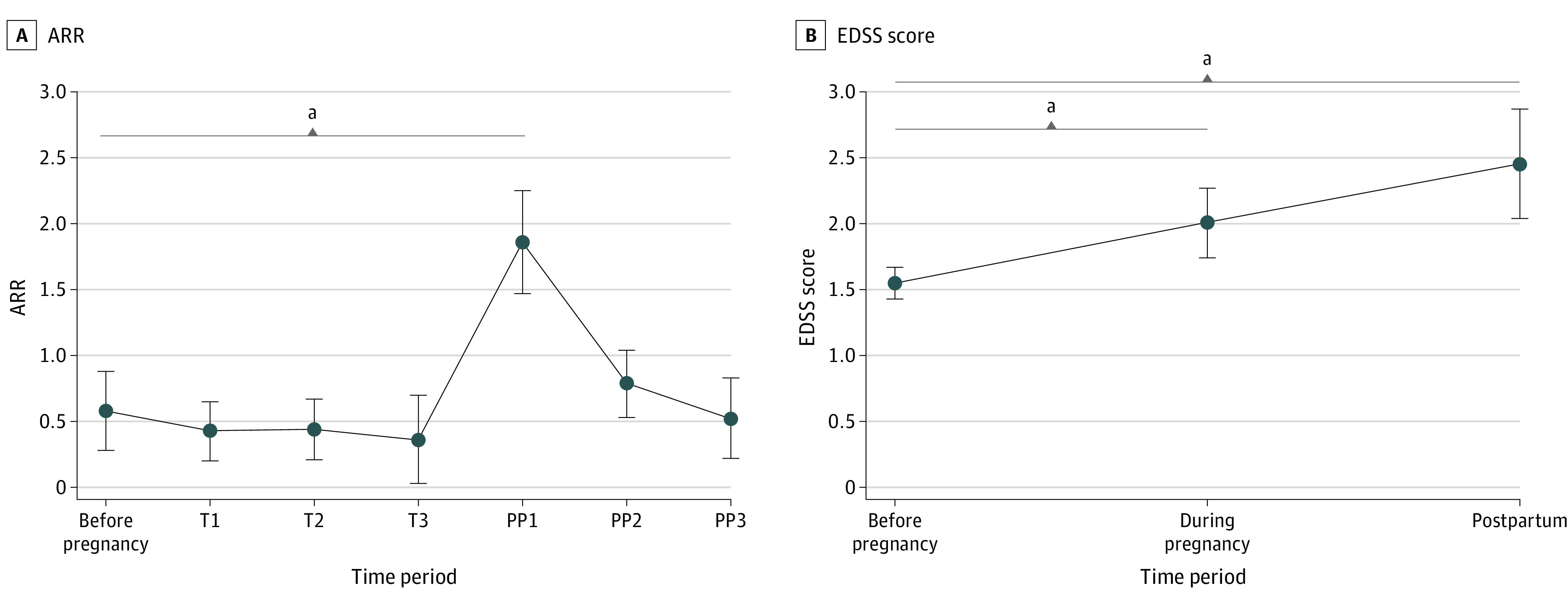
Whiskers indicate 95% CIs. T1 indicates months 0 to 3 of pregnancy; T2, months 3 to 6 of pregnancy; T3, months 6 to 9 of pregnancy; PP1, months 0 to 3 of the postpartum period; PP2, months 3 to 6 of the postpartum period; PP3, months 6 to 12 of the postpartum period. Before pregnancy includes: 12 to 0 months before pregnancy and postpartum, months 0 to 12 after pregnancy.
aP < .01.
EDSS Score at Each Phase
The EDSS score quantitative analysis included 6 studies.7,8,9,20,21,24 Integrated EDSS scores at each phase are presented in Figure 2B. Compared with the EDSS score before pregnancy, the EDSS scores increased significantly during pregnancy (MD, 0.44; 95% CI, 0.20-0.69; P < .001) and postpartum (MD, 0.88; 95% CI, 0.51-1.26; P < .001) (eFigure 8 in the Supplement). Subgroup analyses according to antibody status are presented in the eAppendix, eFigure 4, and eFigure 8 in the Supplement.
Pregnancy Outcomes and Complications
Pregnancy outcomes and complications of the informative pregnancies in NMOSD from 15 studies are summarized in Table 3. Among 619 informative pregnancies, we recorded 396 term deliveries (64.0%), 30 premature deliveries (4.8%), 140 abortions (22.6%; including 50 spontaneous abortions [8.1%] and 90 elective abortions [14.5%]), and 17 patients who developed preeclampsia (2.7%). There were also 33 births (5.3%) with neonatal complications, including various health issues. Factors associated with spontaneous abortions or neonatal complications are presented in the eAppendix, eFigure 9 and eTable 2 in the Supplement.
Table 3. Pregnancy Outcomes and Complications of the Informative Pregnancies in Neuromyelitis Optica Spectrum Disorder From 15 Studies.
| Source | No. | Neonatal complications (No. affected) | ||||||
|---|---|---|---|---|---|---|---|---|
| Informative pregnancies | Term deliveries | Premature deliveries | Abortions | Spontaneous abortions | Elective abortions | Preeclampsia | ||
| Bourre et al,8 2012 | 25 | NA | NA | NA | NA | NA | 0 | NA |
| Kim et al,4 2012 | 40 | 25 | 1 | 14 | 1 | 13 | 1 | Birth defect (1) |
| Fragoso et al,9 2013 | 17 | 15 | 1 | 1 | 1 | 0 | 1 | NA |
| Nour et al,5 2016 | 27 | NA | NA | NA | NA | NA | 1 | Hydrocephalus (1) |
| Shimizu et al,6 2016 | 24 | 20 | 1 | 2 | 0 | 2 | 1 | Low birth weight (1); stillbirth (1) |
| Huang et al,21 2017 | 63 | 42 | 4 | 17 | 7 | 10 | 0 | Low birth weight (8); hydrocephalus (1) |
| Klawiter et al,22 2017 | 46 | 30 | 4 | 12 | 10 | 2 | 3 | Congenital anomalies (1); other health issues (4) |
| Shi et al,20 2017 | 22 | 15 | 1 | 6 | 2 | 4 | 0 | Low birth weight (1) |
| Salvador et al,23 2019 | 30 | 20 | 5 | 5 | 3 | 2 | NA | Cerebral ischemia (1) |
| Ashtari et al,25 2020 | 20 | 14 | 0 | 6 | 4 | 2 | 1 | Hypoxia and seizure (1); low Apgar score (1) |
| Kim et al,24 2020 | 33 | 24 | 0 | 9 | 6 | 3 | 0 | Low birth weight (1) |
| Wang et al,7 2020 | 136 | 84 | 6 | 46 | 4 | 42 | 0 | Low birth weight (4); undeveloped external ear (1); dacryocyst obstruction (1); scoliosis (1) |
| Collongues et al,27 2021 | 89 | 76 | 0 | 13 | 10 | 3 | 3 | NA |
| Deng et al,12 2021 | 34 | 20 | 6 | 8 | 1 | 7 | 6 | Low birth weight (1); splenomegaly (1); thrombocytopenia (1) |
| Kümpfel et al,26 2021 | 13 | 11 | 1 | 1 | 1 | 0 | 0 | NA |
| Total, No. (%) | 619 | 396 (64.0) | 30 (4.8) | 140 (22.6) | 50 (8.1) | 90 (14.5) | 17 (2.7) | 33 (5.3) |
Abbreviation: NA, not available.
Risk of Bias Assessment
The Newcastle-Ottawa Scale for quality appraisal of the included studies is presented in eTable 3 in the Supplement. The total score ranged from 6 to 9, indicating a high quality of the included studies. Egger regression intercept test showed that the publication bias was not statistically significant (eFigure 10 in the Supplement).
Discussion
In this systematic review and meta-analysis, we identified factors associated with pregnancy-related NMOSD attacks, investigated the integrated ARR and EDSS scores in each phase of pregnancy, and summarized pregnancy outcomes and complications. We found that receiving immunosuppressive treatment during pregnancy and older age at conception were associated with lower risk of pregnancy-related NMOSD attacks. Furthermore, compared with the prepregnancy period, ARR was elevated especially during the initial 3 months after delivery, while the EDSS score worsened during pregnancy and the postpartum period. Additionally, several pregnancy outcomes and complications were observed.
Our findings indicated that receiving immunosuppressive treatment during pregnancy was associated with lower rate of pregnancy-related NMOSD attacks. Pregnancies in patients with NMOSD should be regarded as high risk. Generally, mycophenolate mofetil, methotrexate, and mitoxantrone should be stopped before conception, and safer treatment options, like azathioprine and monoclonal antibodies, are recommended.10 It has been reported that using any immunosuppressive treatment, especially rituximab, before pregnancy could significantly reduce the rate of pregnancy-related NMOSD attacks.24 A study by Kümpfel et al26 reported that only 8.3% of patients with NMOSD receiving anti-CD20 therapy experienced an NMOSD relapse postpartum. Using interferon treatment could increase the relapse rate in NMOSD.28 Therefore, choosing appropriate immunosuppressive treatment during pregnancy to avoid pregnancy-related NMOSD attacks is of utmost importance.
We also found that age at conception (≥32 years) was associated with a lower rate of pregnancy-related NMOSD attacks, which may demonstrate the different disease activity at different ages. A study by Kunchok et al29 reported that older age at disease onset could lower the risk of relapse. Therefore, it is age at conception, rather than age at onset, that may be associated with pregnancy-related NMOSD attacks.
In patients with NMOSD with pregnancy-related attacks, we found elevated ARR during the first 3 months after delivery, and lower ARR during pregnancy in subgroup analyses. The results were consistent with the previous observations of multiple sclerosis (MS).30,31 Some studies only included patients with NMOSD with pregnancy-related attacks, which may overestimate the actual ARR during pregnancy. Even so, the statistics of ARR in most of the studies were homogenous. Discrepancies did exist for various races or ethnicities and rates of immunosuppressive treatment during pregnancy, as well as antibody status. The subgroup analysis found ARR during pregnancy was significantly lower compared with before pregnancy regardless of the antibody status. This suggests that immunological tolerance may play a role during pregnancy in patients with NMOSD, like those with MS. We also found that the EDSS score worsened during pregnancy and the postpartum period, similar to what is observed in patients with MS,30,31 as disability was also relapse-dependent in NMOSD. In subgroup analysis, we found EDSS score during pregnancy and the postpartum period were numerically higher in patients with AQP4-Ab than those without AQP4-Ab, which could be explained by the different pathogenesis of the disease entity.
A high rate of abortion was found in patients with NMOSD, including spontaneous and elective abortions. In this meta-analysis, the rate of spontaneous abortion was 8.1%, higher than the general population in China (2.8%) but lower than rates reported by Cohain et al (43%) and Gunnarsdottir et al (13.5%).32,33,34 We thought the included Chinese studies may miss spontaneous abortions that occurred early. A study by Nour et al5 reported that older age at conception was associated with higher odds of miscarriage in NMOSD; however the risk of miscarriage directly increases with age as well as parity. We speculate that it is age at conception that plays a major role in miscarriage rather than the disease entity. Therefore rate of spontaneous abortions was not higher in patients with NMOSD than the general population. Clinical and experimental studies have demonstrated that AQP4 is expressed in the placenta of humans and animals and have correlated AQP4-mediated placental inflammation with fetal death.35 Although the difference between AQP4-Ab positivity rate and the rate of pregnancies with spontaneous abortions was not statistically significant, more evidence is needed to explore the interaction.
Limitations
This study has some limitations. The primary limitation was the retrospective nature and limited sample size of the included studies. Furthermore, some studies did not provide rigorous inclusion criteria and available comparability. Estimates calculated from imputations may affect the accuracy of conclusions to some extent.
Conclusions
The findings of this systematic review and meta-analysis suggest that receiving immunosuppressive treatment during pregnancy and older age at conception were associated with protection against pregnancy-related NMOSD attacks. NMOSD attacks mostly occurred in the first 3 months of the postpartum period, although high-quality prospective studies are needed.
eMethods. Search Strategy for Neuromyelitis Optica
eAppendix. ARR at Each Phase, EDSS Score at Each Phase, and Pregnancy Outcomes and Complications
eFigure 1. Forest Plot of Rates of Pregnancy With Pregnancy-Related Attacks in Patients With NMOSD
eFigure 2. Forest Plot of Factors Associated With Pregnancy-Related Attacks in Patients With NMOSD
eFigure 3. Forest Plot of Differences in ARR of Patients With NMOSD Between Before Pregnancy and Other Phases
eFigure 4. Integrated ARR and EDSS Score in Patients With NMOSD With Different Antibody Status at Each Phase
eFigure 5. Forest Plot of Differences in ARR of Patients With AQP4-Ab Between Before Pregnancy and Other Phases
eFigure 6. Forest Plot of Differences in ARR of Patients With MOG-Ab Between Before Pregnancy and Other Phases
eFigure 7. Forest Plot of Differences in ARR of Patients Who Were Seronegative Between Before Pregnancy and Other Phases
eFigure 8. Forest Plot of Differences in EDSS Score of Patients With NMOSD in Each Phase
eFigure 9. Forest Plot of Immunosuppressive Treatment During Pregnancy on Spontaneous Abortions, or Neonatal Complications in Patients With NMOSD
eFigure 10. Funnel Plot of Publication Bias
eTable 1. Interaction Between Associated Factors on Pregnancy-Related NMOSD Attacks
eTable 2. Factors Associated With Spontaneous Abortions, or Neonatal Complications
eTable 3. The Newcastle-Ottawa Scale for Quality Appraisal of the Included Studies
References
- 1.Jarius S, Paul F, Weinshenker BG, Levy M, Kim HJ, Wildemann B. Neuromyelitis optica. Nat Rev Dis Primers. 2020;6(1):85. doi: 10.1038/s41572-020-0214-9 [DOI] [PubMed] [Google Scholar]
- 2.Jarius S, Wildemann B. Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature. Brain Pathol. 2013;23(6):661-683. doi: 10.1111/bpa.12084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reindl M, Waters P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. 2019;15(2):89-102. doi: 10.1038/s41582-018-0112-x [DOI] [PubMed] [Google Scholar]
- 4.Kim W, Kim SH, Nakashima I, et al. Influence of pregnancy on neuromyelitis optica spectrum disorder. Neurology. 2012;78(16):1264-1267. doi: 10.1212/WNL.0b013e318250d812 [DOI] [PubMed] [Google Scholar]
- 5.Nour MM, Nakashima I, Coutinho E, et al. Pregnancy outcomes in aquaporin-4-positive neuromyelitis optica spectrum disorder. Neurology. 2016;86(1):79-87. doi: 10.1212/WNL.0000000000002208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimizu Y, Fujihara K, Ohashi T, et al. Pregnancy-related relapse risk factors in women with anti-AQP4 antibody positivity and neuromyelitis optica spectrum disorder. Mult Scler. 2016;22(11):1413-1420. doi: 10.1177/1352458515583376 [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Zhou L, ZhangBao J, et al. Neuromyelitis optica spectrum disorder: pregnancy-related attack and predictive risk factors. J Neurol Neurosurg Psychiatry. 2020;92(1):53-61. doi: 10.1136/jnnp-2020-323982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourre B, Marignier R, Zéphir H, et al. ; NOMADMUS Study Group . Neuromyelitis optica and pregnancy. Neurology. 2012;78(12):875-879. doi: 10.1212/WNL.0b013e31824c466f [DOI] [PubMed] [Google Scholar]
- 9.Fragoso YD, Adoni T, Bichuetti DB, et al. Neuromyelitis optica and pregnancy. J Neurol. 2013;260(10):2614-2619. doi: 10.1007/s00415-013-7031-y [DOI] [PubMed] [Google Scholar]
- 10.Mao-Draayer Y, Thiel S, Mills EA, et al. Neuromyelitis optica spectrum disorders and pregnancy: therapeutic considerations. Nat Rev Neurol. 2020;16(3):154-170. doi: 10.1038/s41582-020-0313-y [DOI] [PubMed] [Google Scholar]
- 11.D’Souza R, Wuebbolt D, Andrejevic K, et al. Pregnancy and neuromyelitis optica spectrum disorder—reciprocal effects and practical recommendations: a systematic review. Front Neurol. 2020;11:544434. doi: 10.3389/fneur.2020.544434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng S, Lei Q, Lu W. Pregnancy-related attack in neuromyelitis optica spectrum disorder with AQP4-IgG: a single-center study and meta-analysis. Front Immunol. 2022;12:800666. doi: 10.3389/fimmu.2021.800666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PROSPERO . Predictors of pregnancy-related relapse in neuromyelitis optica spectrum disorder: a systematic review and meta-analysis. Accessed January 3, 2022. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=301542
- 14.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 16.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66(10):1485-1489. doi: 10.1212/01.wnl.0000216139.44259.74 [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Banwell B, Bennett JL, et al. ; International Panel for NMO Diagnosis . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177-189. doi: 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Accessed January 10, 2022. https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp
- 19.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2022. [Google Scholar]
- 20.Shi B, Zhao M, Geng T, Qiao L, Zhao Y, Zhao X. Effectiveness and safety of immunosuppressive therapy in neuromyelitis optica spectrum disorder during pregnancy. J Neurol Sci. 2017;377:72-76. doi: 10.1016/j.jns.2017.03.051 [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Wang Y, Zhou Y, et al. Pregnancy in neuromyelitis optica spectrum disorder: a multicenter study from South China. J Neurol Sci. 2017;372:152-156. doi: 10.1016/j.jns.2016.11.054 [DOI] [PubMed] [Google Scholar]
- 22.Klawiter EC, Bove R, Elsone L, et al. High risk of postpartum relapses in neuromyelitis optica spectrum disorder. Neurology. 2017;89(22):2238-2244. doi: 10.1212/WNL.0000000000004681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salvador NRS, Brito MNG, Alvarenga MP, Alvarenga RMP. Neuromyelitis optica and pregnancy-puerperal cycle. Mult Scler Relat Disord. 2019;34:59-62. doi: 10.1016/j.msard.2019.05.007 [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Huh SY, Jang H, et al. Outcome of pregnancies after onset of the neuromyelitis optica spectrum disorder. Eur J Neurol. 2020;27(8):1546-1555. doi: 10.1111/ene.14274 [DOI] [PubMed] [Google Scholar]
- 25.Ashtari F, Mehdipour R, Shaygannejad V, Asgari N. Pre-pregnancy, obstetric and delivery status in women with neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2020;44:102252. doi: 10.1016/j.msard.2020.102252 [DOI] [PubMed] [Google Scholar]
- 26.Kümpfel T, Thiel S, Meinl I, et al. Anti-CD20 therapies and pregnancy in neuroimmunologic disorders: a cohort study from Germany. Neurol Neuroimmunol Neuroinflamm. 2020;8(1):e913. doi: 10.1212/NXI.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collongues N, Alves Do Rego C, Bourre B, et al. Pregnancy in patients with AQP4-Ab, MOG-Ab, or double-negative neuromyelitis optica disorder. Neurology. 2021;96(15):e2006-e2015. doi: 10.1212/WNL.0000000000011744 [DOI] [PubMed] [Google Scholar]
- 28.Palace J, Leite MI, Nairne A, Vincent A. Interferon beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol. 2010;67(8):1016-1017. doi: 10.1001/archneurol.2010.188 [DOI] [PubMed] [Google Scholar]
- 29.Kunchok A, Malpas C, Nytrova P, et al. Clinical and therapeutic predictors of disease outcomes in AQP4-IgG+ neuromyelitis optica spectrum disorder. Mult Scler Relat Disord. 2020;38:101868. doi: 10.1016/j.msard.2019.101868 [DOI] [PubMed] [Google Scholar]
- 30.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group . Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339(5):285-291. doi: 10.1056/NEJM199807303390501 [DOI] [PubMed] [Google Scholar]
- 31.Vukusic S, Hutchinson M, Hours M, et al. ; Pregnancy In Multiple Sclerosis Group . Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(Pt 6):1353-1360. doi: 10.1093/brain/awh152 [DOI] [PubMed] [Google Scholar]
- 32.Wei Y, Xu Q, Yang H, et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: a population-based cohort study in China. PLoS Med. 2019;16(10):e1002926. doi: 10.1371/journal.pmed.1002926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohain JS, Buxbaum RE, Mankuta D. Spontaneous first trimester miscarriage rates per woman among parous women with 1 or more pregnancies of 24 weeks or more. BMC Pregnancy Childbirth. 2017;17(1):437. doi: 10.1186/s12884-017-1620-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gunnarsdottir J, Stephansson O, Cnattingius S, Akerud H, Wikström AK. Risk of placental dysfunction disorders after prior miscarriages: a population-based study. Am J Obstet Gynecol. 2014;211(1):34.e1-34.e8. doi: 10.1016/j.ajog.2014.01.041 [DOI] [PubMed] [Google Scholar]
- 35.Saadoun S, Waters P, Leite MI, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol. 2013;191(6):2999-3005. doi: 10.4049/jimmunol.1301483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy for Neuromyelitis Optica
eAppendix. ARR at Each Phase, EDSS Score at Each Phase, and Pregnancy Outcomes and Complications
eFigure 1. Forest Plot of Rates of Pregnancy With Pregnancy-Related Attacks in Patients With NMOSD
eFigure 2. Forest Plot of Factors Associated With Pregnancy-Related Attacks in Patients With NMOSD
eFigure 3. Forest Plot of Differences in ARR of Patients With NMOSD Between Before Pregnancy and Other Phases
eFigure 4. Integrated ARR and EDSS Score in Patients With NMOSD With Different Antibody Status at Each Phase
eFigure 5. Forest Plot of Differences in ARR of Patients With AQP4-Ab Between Before Pregnancy and Other Phases
eFigure 6. Forest Plot of Differences in ARR of Patients With MOG-Ab Between Before Pregnancy and Other Phases
eFigure 7. Forest Plot of Differences in ARR of Patients Who Were Seronegative Between Before Pregnancy and Other Phases
eFigure 8. Forest Plot of Differences in EDSS Score of Patients With NMOSD in Each Phase
eFigure 9. Forest Plot of Immunosuppressive Treatment During Pregnancy on Spontaneous Abortions, or Neonatal Complications in Patients With NMOSD
eFigure 10. Funnel Plot of Publication Bias
eTable 1. Interaction Between Associated Factors on Pregnancy-Related NMOSD Attacks
eTable 2. Factors Associated With Spontaneous Abortions, or Neonatal Complications
eTable 3. The Newcastle-Ottawa Scale for Quality Appraisal of the Included Studies