1. Introduction
Vaccination against SARS-Cov-2 infection has significantly reduced the incidence of severe cases. However, safety concerns have increased as possibly related side effects have been described. It is postulated that vaccination could induce different autoimmune diseases, probably due to cross-reactivity between SARS-Cov-2 proteins and human proteins [1]. To date, more than 20 cases of post-vaccination autoimmune hepatitis (AIH) have been described in the literature.
AIH is a chronic inflammatory disease of the liver of unknown aetiology, in which there is a loss of tolerance to the hepatocytes leading to parenchymal destruction. It is characterised by the presence of circulating autoantibodies, hypergammaglobulinaemia, certain histological alterations such as interface hepatitis and response to immunosuppressive therapy. The clinical picture is non-specific and variable between patients, ranging from asymptomatic hepatitis to fulminant hepatic failure [2].
2. Methods
The aim of this study was to collect cases of AIH that were detected after vaccination for SARS-Cov-2 in our hospital, and to analyse their characteristics and the possible causality of the vaccine. Cases of probable or definite autoimmune hepatitis according to the simplified criteria of the International Autoimmune Hepatitis Group that presented less than 90 days after COVID-19 vaccination were collected.
Their medical records were analysed and the following data were collected: age, sex, type of vaccine, latency (time in days from vaccination to detection of transaminase alterations), transaminase and bilirubin levels at diagnosis, liver biopsy, screening for liver disease (autoantibodies, immunoglobulins and viral serology) and HLA, as well as the treatment received and the response to it.
A literature review of the subject was also carried out using the search terms “Autoimmune hepatitis” and “Covid vaccine” in PubMed, finding 20 articles that reported cases of post-vaccine autoimmune hepatitis. Table 1 shows the main characteristics of the cases described so far in the literature, in order to compare them with those of our patients.
Table 1.
Post-vaccine autoimmune hepatitis cases reported in the literature.
| Study | Sex and age | Vaccine | Latency (days) | GOT/GPT at diagnosis (U/L) | Bilirubin at diagnosis (mg/dl) | Antibodies | IgG | Biopsy compatible with HAI |
|---|---|---|---|---|---|---|---|---|
| Bril et al [5] | Female, 35 | Pfizer (1st dose) | 13 | 754/2001 | 4,8 | ANA Ds-DNA |
N | Yes |
| Londoño et al [6] | Female, 41 | Moderna (2nd dose) | 7 | 993/1312 | 2,3 | ANA, ASMA, SLA, LC1 | ↑ | Yes |
| Clayton-Chubb et al [7] | Male, 36 | AstraZeneca (1st dose) | 26 | 633/1774 | 1 | ANA | N | Yes |
| Tan et al [8] | Female, 56 | Moderna (1st dose) | 35 | 1124/1701 | 6 | ANA, ASMA | ↑ | Yes |
| McShane et al [9] | Female, 71 | Moderna (unknown) | 4 | ?/1067 | 12,1 | ASMA | ↑ | Yes |
| Lodato et al [10] | Female, 43 | Pfizer (1st dose) | 15 | 51/52 | 17,5 | – | N | Yes |
| Vuille-Lessard et al [11] | Female, 76 | Moderna (1st dose) | 3 | 811/579 | 6,5 | ANA, ASMA, AAA, p-ANCA | ↑ | Yes |
| Rela et al [12] | Female, 38 | AstraZeneca | 14 | 1101/1025 | 2,86 | ANA | ↑ | Yes |
| Male, 62 | AstraZeneca | 16 | 1361/1094 | 19,2 | – | – | Yes | |
| Tun et al [13] | Male, 47 | Moderna (1st dose) | 3 | --/1048 | 19 | negative | ↑ | Yes |
| Palla et al [14] | Female, 40 | Pfizer (2nd dose) | 30 | x4 ULN | – | ANA | ↑ | Yes |
| Rocco et al [15] | Female, 80 | Pfizer (2nd dose) | 7 | 1401/1186 | 10,5 | ANA | ↑ | Yes |
| Ghielmetti M et al [16] | Male, 63 | Moderna (1st dose) | 7 | 1127/1038 | 20,8 | ANA, atypical AMA | ↑ | Yes |
| Avci et al [17] | Female, 61 | Pfizer (1st dose) | 30 | 455/913 | 11,8 | ANA, ASMA | ↑ | Yes |
| Garrido et al [18] | Female, 65 | Moderna (1st dose) | 14 | 1056/1092 | 1,14 | ANA | ↑ | Yes |
| Goulas A et al [19] | Female, 52 | Moderna (1st dose) | 14 | 350/936 | 9,06 | ANA, ASMA | ↑ | Yes |
| Camacho-Domínguez et al [20] | Male, 79 | AstraZeneca (1st dose) | 27 | 2003/1994 | 11,9 | ANA, ASMA | ↑ | Yes |
| Erard et al [21] | Female, 80 | Pfizer (2nd dose) | 10 | 583/541 | 4,6 | ANA | ↑ | Yes |
| Female, 73 | Moderna (1st dose) | 21 | 1163/1027 | 19,5 | ANA | ↑ | Yes | |
| Female, 68 | AstraZeneca (1st dose) | 20 | 2314/2029 | 44 | ANA | ↑ | Yes | |
| Fimiano et al. [22] | Female, 63 | Pfizer (2nd dose) | 54 | 1625/1778 | 18,6 | Ac antitiroglobulina | ↑ | Yes |
| Ghorbani et al. [23] | Male, 62 | Sinopharm (2nd dose) | 3 | 435/722 | 8 | – | – | Yes |
3. Results
Since the start of vaccination, 5 cases of altered liver biochemistry less than 90 days after the first or second dose of the vaccine have been detected in our centre. One of the cases has already been presented in the journal Gastroenterology and Hepatology by Torrente et al. [3] and has been included together with the other new cases. In total, 4 of the patients were women and 1 was a man, with a median age of 62 years. 3 patients received the vaccine from Pfizer and 2 from Astrazeneca. The median time from the last dose of vaccine to detection of liver function test (LFT) abnormalities was 19 days. No patient showed evidence of liver failure at baseline or until treatment, although there was one case of severe acute hepatitis with a total bilirubin level of 14 mg/dl during follow-up that required hospitalisation. A liver disease screening study showed autoimmunity (positive ANA) and elevated IgG in all patients, with no other alterations of interest. A liver biopsy was also performed in 4 cases, and all of them were compatible with AIH. The histological changes are described in further detail in Table 2 and the Batts-Ludwig system was used to define the fibrosis stage and the acute or chronic phenotype of the hepatitis.
Table 2.
Clinical, histological and laboratory characteristics of the cases detected in our centre.
| Patient number | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sex | Female | Male | Female | Female | Female |
| Age (years) | 47 | 72 | 62 | 72 | 59 |
| Comorbidities | Hypothyroidism | 30 g/day alcohol consumption, ischemic heart disease | Celiac disease | No comorbidities | Hypothyroidism |
| Vaccine | Astrazeneca (1st dose) | Pfizer (2nd dose) | Astrazeneca (2nd dose) | Pfizer (2nd dose) | Pfizer (1st dose) |
| Latency (days) | 24 | 46 | 4 | 14 | 9 |
| ALT (U/L) | 353 | 248 | 655 | 866 | 1799 |
| AST (U/L) | 241 | 204 | 498 | 570 | 1292 |
| Total bilirubin (mg/dl) | 0.4 | 12.3 | 2.5 | 2.3 | 1.3 |
| IgG (mg/dl) | 2016 | 1670 | 1684 | 1916 | 1978 |
| Autoantibodies tested | ANA+ 1/320 (AC-21 Cytoplasmic reticular/AMA) AMA (−) LKM (−) ASMA (−) M2-3E (−) Sp-100 (−) gp-210 (−) LKM1 (−) LC1 (−) SLA/LP (−) AMA-M2 (−) |
ANA+ 1/1280 (AC-1 homogeneous) AMA (−) LKM (−) ASMA (−) M2-3E (−) Sp-100 (−) gp-210 (−) LKM1 (−) LC1 (−) SLA/LP (−) AMA-M2 (−) |
ANA+ 1/160 (AC-4 fine granular) ENA+ (SS-A+, Ro-52+, SS-B+) AMA (−) LKM (−) ASMA (−) M2-3E (−) Sp-100 (−) gp-210 (−) LKM1 (−) LC1 (−) SLA/LP (−) AMA-M2 (−) |
ANA+ 1/320 (AC-1 homogeneous) AMA (−) LKM (−) ASMA (−) M2-3E (−) Sp-100 (−) gp-210 (−) LKM1 (−) LC1 (−) SLA/LP (−) AMA-M2 (−) |
ANA+ (AC-2, 4, 5 nuclear speckled) AMA (−) LKM (−) ASMA (−) M2-3E (−) Sp-100 (−) gp-210 (−) LKM1 (−) LC1 (−) SLA/LP (−) AMA-M2 (−) |
| Liver biopsy | Mixed portal infiltrate composed mainly of lymphocytes and plasma cells with disruption of the limiting plate, emperipolesis and pseudo-rosette formation. Light-moderate lobular activity. Batts-Ludwig: grade 3, stage 1 |
Moderate interface activity with lymphocyte and plasma cell infiltrate and presence of eosinophils and neutrophils. Moderate-severe lobular activity. Batts-Ludwig: grade 4, stage 2 |
Marked interface activity with disruption of the limiting plate and inflammatory infiltrate composed mainly of lymphocytes and plasma cells, with scattered eosinophils and neutrophils. Moderate-severe lobular activity. Batts-Ludwig: grade 3, stage 2 |
Moderate interface hepatitis with dominant lymphocyte and plasma cell infiltrate and scattered eosinophils. Light lobular activity. Batts-Ludwig: grade 3, stage 2 |
Not performed. |
| HLA | HLA-DRB1 *03:01 *04:03 | HLA-DRB1*04 | HLA-DRB1 *03:01 *07:01 | HLA-DR1 *03:01 *07:01 | HLA-DRB1 *01:03 *11:04 (not susceptibility) |
| AIH Group simplified criteria | 8 | 7 | 8 | 8 | / |
| Exclusion of other causes (DILI, viral) | IgM for HAV, HBV, HCV, HEV, HSV1-2, VZV, CMV, EBV, parvovirus negative. No other drugs |
IgM for HAV, HBV, HCV, HEV, CMV, EBV, parvovirus negative. No other drugs |
IgM for HAV, HBV, HCV, HEV, HSV1-2, VZV, CMV, EBV, parvovirus negative. No other drugs |
IgM for HAV, HBV, HCV, CMV, EBV negative. No other drugs |
IgM for HAV, HBV, HCV, HEV, HSV1-2, VZV, CMV, EBV, parvovirus negative. No other drugs |
| CIOMS-RUCAM related to vaccine | 2 | 3 | 3 | 3 | 6 |
| Treatment (dose of steroids and azathioprine) | Prednisone 20 mg Azathioprine 50 mg |
Prednisone 50 mg (taper 10 mg per week) Azathioprine 50 mg (18 days after initiation of prednisone) |
Prednisone 40 mg (slow taper) Azathioprine 50 mg (14 days after initiation of prednisone) |
Prednisone 50 mg (taper 10 mg per week) No initiation of azathioprine due to endometrial cancer diagnosis |
No treatment received due to spontaneous improvement |
| Time to transaminase normalization | 3 months | 5 weeks | 5 months | 5 months | 5 months |
| New vaccine type (on or off IS?) | 2nd dose of Astrazeneca 3 weeks after diagnosis (on IS treatment) without a worsening in transaminases. 3rd dose of Moderna 8 months after diagnosis (on IS treatment) without a worsening in transaminases. |
3rd dose of Moderna 8 months after diagnosis (on prednisone 2,5 mg only, azathioprine had been removed due to gastrointestinal symptoms) without a worsening in transaminases | 3rd dose Pfizer 5 months after diagnosis (on prednisone 5 mg and azathioprine 100 mg) without a worsening in transaminases | Patient refused 3rd dose | 2nd dose of Pfizer 7 months after the previous one without a worsening in transaminases. 3rd dose of Pfizer 11 months after the previous one without a worsening in transaminases |
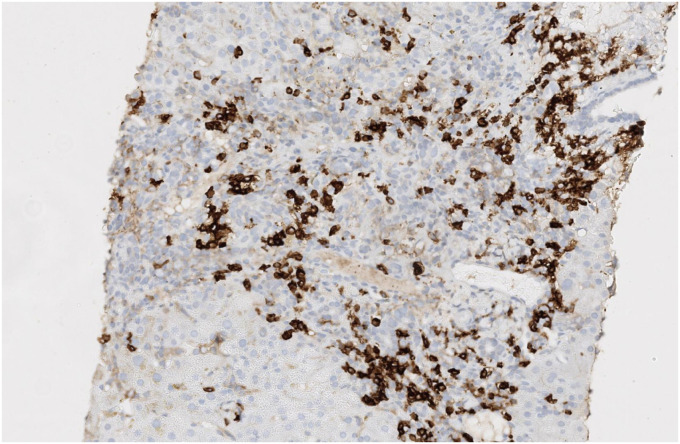
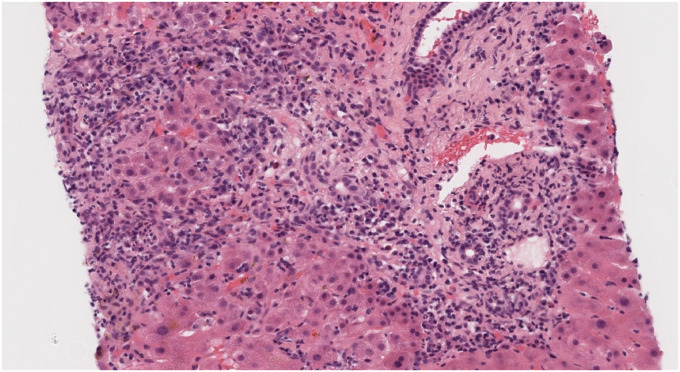
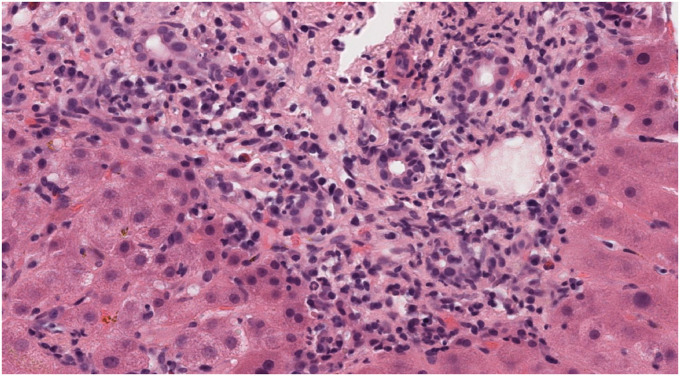
Fig. 1, Fig. 2, Fig. 3: hepatic biopsy sample of patient number 4, showing a portal space with a mixed inflammatory infiltrate with many plasmatic cells (CD38+) as well as some lymphocytes, polymorphonuclear cells and eosinophils. There is moderate interface hepatitis with light lobular activity and fibroinflammatory expansion.
Fig. 1.
Immunohistochemistry staining with CD38 shows many CD38+ plasma cells. These cells are one of the most characteristic histological findings in AIH.
Fig. 2.
Portal tract showing mild ductular proliferation accompanied by this prominent mixed inflammatory cell infiltrate. The inflammation is located mainly in the perifery of the portal tract where interface and lobular can be observed. Portal fibroinflammatory expansion can be easily recognized.
Fig. 3.
Higher magnification of the portal tract, where inflammatory damage can be observed. Lymphocytes, neutrophils and many plasma cells (CD38+, figure 1 caption) with scattered eosinophils can be recognized. A few neutrophils may be permeating ductal epithelium.
Four of the patients were prescribed systemic corticosteroids after the biopsy, and three of them were subsequently prescribed azathioprine as maintenance therapy (in one patient it was contraindicated due to a concomitant diagnosis of endometrial neoplasia). All of them showed a normalization of transaminases with treatment. The last patient was not administered corticosteroids due to spontaneous improvement of transaminases.
Three of the five patients had HLA-DRB1*03:01 and one had HLA-DRB1*04, all of which are predisposing genetic factors for the development of autoimmune hepatitis. However, the fifth patient was positive for HLA-DRB1 *01:03, *11:04, not of susceptibility for AIH.
The score was calculated based on the simplified diagnostic criteria of the International Autoimmune Hepatitis Group, with three of the patients scoring 8 points and one 7 points (definite autoimmune hepatitis). The score could not be calculated for the fifth patient because they did not undergo biopsy.
Of note, the first patient had been under study since 2018 for mild hypertransaminasemia with positive ANA and a predisposing HLA for AIH, but no diagnosis had been made until the time of vaccination.
The second patient had a high alcohol consumption of about 39 g per day. However, no signs of alcoholic liver disease were observed in the liver biopsy.
Four of the patients received a new dose of the vaccine at a later date and none of them showed a worsening in transaminases.
4. Discussion
Although controversial, it is postulated that vaccination could be the cause of autoimmunity, or that it could rather play a role as a trigger for latent disease.
Four of our cases have been diagnosed with AIH, all of them with a temporal relationship with onset of the alterations less than 90 days after vaccination. In addition, at least 20 case reports of probable AIH after vaccination against SARS-COV2 have been described in the literature, with similar characteristics to the cases presented in this study. Moreover, a retrospective multicenter study that included 87 patients with liver injury following Covid-19 vaccination has recently been published. Of all the patients included, 34 were classified as probable/definite AIH according to the simplified criteria. Overall, 46 patients received corticosteroid therapy, more often being patients with more severe liver injury and features of immune-mediated hepatitis. All patients showed resolution of liver injury with treatment except for one patient who had to undergo liver transplantation. During follow-up steroid therapy was withdrawn in 12 patients without a relapse afterwards [4].
However, at present it is not possible to say with certainty whether the vaccine is the cause of the onset of AIH or whether it is a casual association in predisposed patients. In favour of the latter point is the fact that three of our patients already had alterations in the FFP prior to vaccination, without it having been possible, so far, to reach a diagnosis of AIH.
On the other hand, it is worth mentioning that at the present time and with the data available to us, it is not possible to know with certainty whether we are dealing with AIH or DI-AIH (drug induced autoimmune hepatitis) as both have similar clinical, analytical and histological characteristics. One of the main differences between the two entities is that in DI-HAI immunosuppressive treatment can be withdrawn. It is therefore not possible to know whether these patients will need long-term treatment.
In conclusion, after assessing both our cases and those described in the literature, it seems possible to establish a causal relationship between the vaccine and AIH. However, it is necessary to continue to study new cases in order to have more evidence to support this hypothesis.
Author statement
Arantzazu Izagirre: Conceptualization, Writing - Review & Editing, Writing - Original Draft; Teresa Arzallus: Conceptualization, Writing - Review & Editing, Writing - Original Draft; Maddi Garmendia: Investigation, Resources; Silvia Torrente: Conceptualization; Agustin Castiella: Supervision; Eva Maria Zapata: Supervision.
References
- 1.Vojdani A., Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020 Aug 1;217 doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.EASL clinical practice guidelines: autoimmune hepatitis. J. Hepatol. 2015 Oct 1;63(4):971–1004. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Torrente S., Castiella A., Garmendia M., Zapata E. Probable autoimmune hepatitis reactivated after COVID-19 vaccination. Gastroenterol. Hepatol. 2021 Oct 28;(1):115–116. doi: 10.1016/j.gastrohep.2021.10.002. S0210-5705(21)00302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Efe C., Kulkarni A.V., Terziroli Beretta-Piccoli B., Magro B., Friedrich Stättermayer A., Cengiz M., et al. Liver injury after SARS-CoV-2 vaccination Features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology [Internet]. [cited 2022 Jul 5];n/a(n/a) https://onlinelibrary.wiley.com/doi/abs/10.1002/hep.32572 Available from: [DOI] [PMC free article] [PubMed]
- 5.Bril F., Al Diffalha S., Dean M., Fettig D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J. Hepatol. 2021 Jul;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Londoño M.C., Gratacós-Ginès J., Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination – still casualty? J. Hepatol. 2021 Nov;75(5):1248–1249. doi: 10.1016/j.jhep.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clayton-Chubb D., Schneider D., Freeman E., Kemp W., Roberts S.K. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J. Hepatol. 2021 Nov;75(5):1249–1250. doi: 10.1016/j.jhep.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan C.K., Wong Y.J., Wang L.M., Ang T.L., Kumar R. Autoimmune hepatitis following COVID-19 vaccination: true causality or mere association? J. Hepatol. 2021 Nov;75(5):1250–1252. doi: 10.1016/j.jhep.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McShane C., Kiat C., Rigby J., Crosbie Ó. The mRNA COVID-19 vaccine – a rare trigger of autoimmune hepatitis? J. Hepatol. 2021 Nov;75(5):1252–1254. doi: 10.1016/j.jhep.2021.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodato F., Larocca A., D'Errico A., Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: coincidence, autoimmunity or drug-related liver injury. J. Hepatol. 2021 Nov;75(5):1254–1256. doi: 10.1016/j.jhep.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vuille-Lessard É, Montani M., Bosch J., Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J. Autoimmun. 2021 Sep 1;123 doi: 10.1016/j.jaut.2021.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rela M., Jothimani D., Vij M., Rajakumar A., Rammohan A. Auto-immune hepatitis following COVID vaccination. J. Autoimmun. 2021 Sep 1;123 doi: 10.1016/j.jaut.2021.102688. [DOI] [PubMed] [Google Scholar]
- 13.Zin Tun G.S., Gleeson D., Al-Joudeh A., Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J. Hepatol. 2021 Oct;76:747–749. doi: 10.1016/j.jhep.2021.09.031. S0168827821020936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palla P., Vergadis C., Sakellariou S., Androutsakos T. Letter to the editor: autoimmune hepatitis after COVID-19 vaccination: a rare adverse effect? Hepatology. 2022;75(2):489–490. doi: 10.1002/hep.32156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocco A., Sgamato C., Compare D., Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: may not be a casuality. J. Hepatol. 2021 Sep;75(3):728–729. doi: 10.1016/j.jhep.2021.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghielmetti M., Schaufelberger H.D., Mieli-Vergani G., Cerny A., Dayer E., Vergani D., et al. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: a novel clinical entity? J. Autoimmun. 2021 Sep 1;123 doi: 10.1016/j.jaut.2021.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avci E., Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: new-onset or flare-up? J. Autoimmun. 2021 Dec 1;125 doi: 10.1016/j.jaut.2021.102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrido I., Lopes S., Simões M.S., Liberal R., Lopes J., Carneiro F., et al. Autoimmune hepatitis after COVID-19 vaccine – more than a coincidence. J. Autoimmun. 2021 Dec 1;125 doi: 10.1016/j.jaut.2021.102741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulas A., Kafiri G., Kranidioti H., Manolakopoulos S. A typical autoimmune hepatitis (AIH) case following Covid-19 mRNA vaccination. More than a coincidence? Liver Int. 2022;42(1):254–255. doi: 10.1111/liv.15092. [DOI] [PubMed] [Google Scholar]
- 20.Camacho-Domínguez L., Rodríguez Y., Polo F., Restrepo Gutierrez J.C., Zapata E., Rojas M., et al. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. J Transl Autoimmun. 2022 Jan 4;5 doi: 10.1016/j.jtauto.2022.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erard D., Villeret F., Lavrut P.M., Dumortier J. Autoimmune hepatitis developing after COVID 19 vaccine: presumed guilty? Clin Res Hepatol Gastroenterol. 2021 Dec 14 doi: 10.1016/j.clinre.2021.101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fimiano F., D'Amato D., Gambella A., Marzano A., Saracco G.M., Morgando A. Autoimmune hepatitis or drug-induced autoimmune hepatitis following Covid-19 vaccination? Liver Int. 2022;42(5):1204–1205. doi: 10.1111/liv.15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghorbani H., Rouhi T., Vosough Z., Shokri-shirvani J. Drug-induced hepatitis after Sinopharm COVID-19 vaccination: a case study of a 62-year-old patient. Int J Surg Case Rep. 2022 Mar 9;93 doi: 10.1016/j.ijscr.2022.106926. [DOI] [PMC free article] [PubMed] [Google Scholar]