Abstract
Purpose
Coronavirus disease 2019 (COVID-19) has been a constant health threat since its emergence. Amongst risk factors proposed, a diagnosis of cancer has been worrisome. We report the impact of cancer and other risk factors in US Veterans receiving care at Veterans Administration (VA) Hospitals, their adjusted odds ratio (aOR) for infection and death, and report on the impact of vaccines on the incidence and severity of COVID-19 infections in Veterans without/with cancer.
Methods
We conducted a cohort study of US Veterans without/with cancer by mining VA COVID-19 Shared Data Resource (CSDR) data using the VA Informatics and Computing Infrastructure (VINCI). Our observation period includes index dates from 14DEC2020 to 25JAN2022, encompassing both the delta and omicron waves in the US.
Results
We identified 915,928 Veterans, 24% of whom were African Americans who had undergone COVID testing–688,541 were and 227,387 were not vaccinated. 157,072 had a cancer diagnosis in the preceding two years. Age emerged as the major risk factor, with gender, BMI, and (Elixhauser) comorbidity contributing less. Among veterans with solid tumors other than lung cancer, risks of infection and death within 60 days were comparable to Veterans without cancer. However, those with hematologic malignancies fared worse. Vaccination was highly effective across all cancer cohorts; the respective rates of infection and death after infection were 8% and 5% among the vaccinated compared to 47% and 10% in the unvaccinated. Amongst vaccinated, increased risk of infection was noted in both, Veterans with hematologic malignancy treated with chemotherapy (HR, 2.993, P < 0.0001) or targeted therapies (HR, 1.781, P < 0.0001), and in solid tumors treated with either chemotherapy (HR 2.328, 95%CI 2.075–2.611, P < 0.0001) or targeted therapies (HR 1.328, P < 0.0001) when compared to those not on treatment.
Conclusions
Risk for COVID-19 infection and death from infection vary based on cancer type and therapies administered. Importantly and encouragingly, the duration of protection from infection following vaccination in Veterans with a diagnosis of cancer was remarkably like those without a cancer diagnosis. Veterans with hematologic malignancies are especially vulnerable, with lower vaccine effectiveness (VE).
Keywords: Covid-19 vaccine effectiveness, Cancer, Hematologic malignancies, Solid tumors, Chemotherapy, Immunotherapy
Introduction
As of May 2022, more than 500 million cases and over 6 million deaths have occurred worldwide from coronavirus disease 2019 (COVID-19) [1]. Understanding the vulnerability of the host to COVID-19 is vital as their risk will likely remain unchanged even as new variants of concern (VOC) emerge. Older age, male sex, non-Caucasian race, abnormal body mass index (BMI), cumulative smoking exposure, and the number of comorbid conditions have been identified as patient characteristics associated with increased risk of severe COVID-19 infection and/or worse COVID-19 outcomes [2,3].
Registration studies reported 91% and 93% vaccine effectiveness for the two mRNA vaccines, BNT162b2 and mRNA 1273, respectively, and 67% for the viral vector Ad26.COV2.S [4], [5], [6]. However, real-world studies have since reported declining vaccine protection from infections over time and recommended booster doses to maintain efficacy against emerging VOC [7], [8], [9], [10], [11].
Patients diagnosed with cancer have been identified as an “at-risk” population [12,13], with one meta-analysis reporting a pooled mortality estimate of 30% amongst hospitalized patients with cancer and COVID-19 [14]. The increased vulnerability to COVID-19 likely reflects a complex interplay of older age, comorbid conditions, health status, the activity of cancer and its effects on immunity, and a potential impact of anticancer therapies [15], [16], [17], [18]. Additionally, there is evidence of inferior antibody production after COVID-19 infection and vaccinations in patients with cancer, although vulnerability is not uniform across all cancers [19], [20], [21], [22]. Longitudinal data on vaccine effectiveness over time during VOC outbreaks stratified by cancer type could help individualize patients’ risk and guide the implementation of mitigation strategies.
The US Veterans Health Administration (VHA), the largest integrated health care system in the United States, with 171 VA Medical Centres and 1283 outpatient suites, is an egalitarian health system where Veterans receive equal care, decreasing the impact of socioeconomic factors. Importantly, most Veterans obtain all healthcare exclusively at VA Medical Centres. The VHA has the largest and oldest medical record system. Data is collected and stored on the VA corporate data warehouse (CDW) and made available to researchers via an informatics infrastructure.
Our objectives in this cohort study were to 1) explore cancer as an independent risk factor for COVID-19 infection and related outcomes, 2) report COVID-19 vaccine effectiveness in Veterans with various cancers and a matched cohort of Veterans without cancer, and 3) discern the effects of cancer type and anticancer drugs on infection, mortality, and vaccine effectiveness.
Methods
Patient population
We conducted a cohort study of US Veterans by mining data from VA Corporate Data Warehouse (CDW) using the VA Informatics and Computing Infrastructure (VINCI). The James J Peters VA Medical Centre, Bronx, New York Institutional Review Board approved this study.
The VA National Surveillance Tool (VA-NST) is the authoritative data source of all positive/negative COVID-19 data. Data made available through the VA COVID-19 Shared Data Resource (CSDR) is updated hourly, which has allowed us to perform the analysis prospectively. CSDR contains information on all Veterans with positive/negative COVID-19 real-time polymerase chain reaction (RT-PCR) test within the VA and uses natural language processing to extract data from notes in Veterans with a positive test elsewhere. Our observation period covers December 14, 2020, the start date of vaccination at VA facilities (22), to January 25, 2022, the data extraction date and Veterans ≥18 years.
Definitions
Index date is the date when a Veteran had a first positive/first negative COVID test in that hierarchical order or inpatient admission date closest to the first positive/first negative test in the 15 days prior. Index date for Veterans with multiple positive tests is the date of first positive test. All Veterans were included, regardless of reason for testing–screening, symptoms, travel, or preprocedure. RT-PCR was standard test at most centres. Third dose of mRNA vaccines and second dose of viral vector vaccine were considered “booster” doses.
We identified Veterans with an active diagnosis of new or previously established cancer in the 2 years preceding the index date. Hematologic malignancies included leukemia, lymphoma, multiple myeloma, myelodysplastic syndrome, and myelofibrosis, with the rest as solid tumors. We harvested data regarding 82 anticancer drugs Veterans received at the time of/or preceding the index date within the observation period. We categorized drugs into chemotherapy, targeted therapy, and endocrine therapy (Supplemental Table 1).
Outcomes
COVID infection and all-cause mortality within 60 days after testing positive for COVID-19 were collected.
Statistical analyses
Using descriptive analysis, absolute and relative frequencies of categorical demographic and comorbidity characteristics were presented separately in the unvaccinated and vaccinated cohorts. Chi-Square tests were used to compare differences between cohorts. Matching between the cancer cohort and noncancer control used a 1:1 ratio and age ±5 years, same gender, race, BMI, Elixhauser index categories, and vaccine status. For each predictor, a univariate logistic regression model was tested first to compare risks amongst other groups to the selected reference group for various comparisons. A final multivariate model including all variables with significant association with outcomes and the covariates of time and location was built to obtain the adjusted odds ratios (aORs). Vaccine effectiveness was studied using conditional logistic regression models for binary outcomes of COVID infection and death within 60 days. Bonferroni correction adjusted for multiple comparisons, and only tests with P< 0.001 were considered significant. Vaccine effectiveness was defined as (1-OR)·100%. Kaplan-Meier method was used for time-to-event analyses employing SAS, version 9.4, and R, version 3.6.1. Detailed methods, statistical analyses and directed acyclic graph (DAG) are described in Supplementary Appendix.
Results
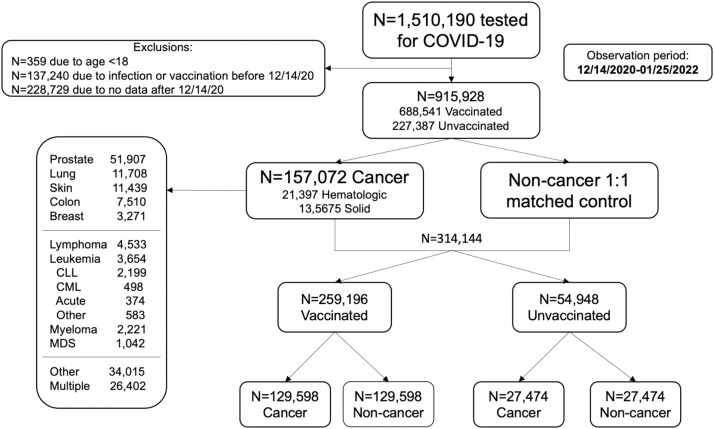
We identified 915,928 Veterans, including 688,541 who were and 227,387 who were not vaccinated and had undergone COVID testing between 14DEC2020 and 25JAN2022 after appropriate exclusions detailed in Fig. 1 . To understand the cancer cohort, we first analyzed the noncancer cohort to establish references for comparison (Supplemental Fig. 1A/B). Forest plots summarize the relative risks of defined patient characteristics. The risk of COVID-19 infection and death within 60 days were chosen as metrics less subject to bias. The results underscore the enormous importance of age on mortality, a risk modestly mitigated by vaccination. Additionally, females fared better than males with a lower risk of infection and of death within 60 days if infected. Infections were less likely in rural settings, but likelihood of dying the same. Higher BMI was associated with a higher risk of infection and death within 60 days for the severely obese, risks mitigated by vaccination. Smoking was protective of infection but not of death. Finally, the risk of a higher Elixhauser index on death within 60 days was mitigated by vaccination. To ensure results had not been biased by excluding the 137,240 Veterans who had a previous COVID-19 infection or vaccination and the 228,729 without data after 14DEC2020, we conducted sensitivity analyses including these Veterans and observed similar results (Supplemental Tables 3A/3B).
Fig. 1.
Flow diagram.
We subsequently assembled a cohort of 157,072 Veterans diagnosed with cancer, including 135,675 and 21,397 Veterans with solid and hematologic malignancies, respectively. We then created the matched “noncancer” control totalling 314,144 Veterans, including 259,196, and 54,948 who were/were not vaccinated. The higher percentage of vaccinated amongst the matched 314,144 Veterans (82.5% v 75.2% of the 915,928 Veteran cohort) reflects a comparator driven by the cancer cohort comprised of Veterans more likely vaccinated (Supplemental Table 2).
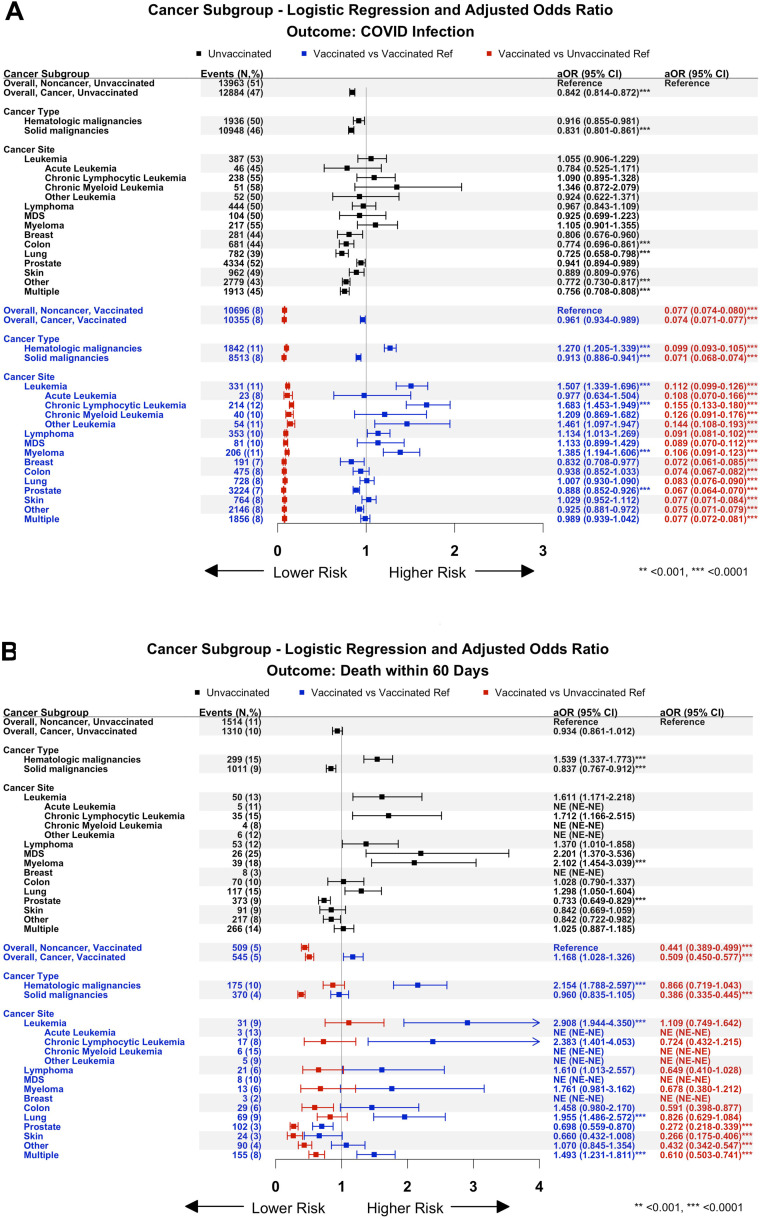
The forest plots in Fig. 2 show the number of events in parentheses and aORs (infection/2A or death within 60 days of infection/2B) for all 157,072 Veterans without and 157,072 with a cancer diagnosis, those with a hematologic or solid malignancy, and cohorts of individual malignancies. Three comparisons are presented. The first 2 comparisons examine the risk relative to a reference cohort of Veterans likewise vaccinated/unvaccinated to understand the impact of the underlying malignancy independent of vaccination. The first comparison, depicted as black symbols, summarises results in unvaccinated Veterans with a cancer diagnosis, using as reference a cohort of unvaccinated Veterans without cancer. The second comparison, depicted as blue symbols, presents results in vaccinated Veterans with a cancer diagnosis using as reference a cohort of vaccinated Veterans without cancer. This comparison allows one to examine the impact of their cancer on vaccine effectiveness compared to vaccinated Veterans without cancer. The third comparison, depicted as red symbols, aligns with the corresponding blue symbols, and provides results in vaccinated Veterans without/with a cancer diagnosis compared to unvaccinated Veterans without cancer. This allows one to assess the benefit of vaccination across different cohorts.
Fig. 2.
Adjusted odds ratios for infection (2A) and death within 60 days of infection (2B) presented as forest plots. The vertical line at 1 represents the risk of infection or death within 60 days of infection for the respective reference cohort. The data is shown as the means with their respective confidence intervals. Movement to the right occurs when the risk is increased, while movement to the left represents a reduction in the risk. See text for a description of the black, blue, and red symbols. Both unvaccinated and vaccinated Veterans with hematologic malignancies are seen to be at greater risk than those with solid tumors for COVID-19 infection (2A) and except for lung cancer and “multiple” cancers, at more risk for death within 60 days (2B). As regards solid tumors, multiple refers to Veterans with more than one cancer diagnosis with prostate and lung cancer often one of those.
Starting in Fig. 2A, comparing outcomes to results in Veterans without cancer who were not vaccinated, an “apparent protection of cancer” is seen in Veterans with solid tumor diagnoses who were not vaccinated, with lower aORs for infection (black symbols, all solid tumor cohorts), but not in those with hematologic malignancies. A higher risk of infection among vaccinated Veterans is seen in those with hematologic malignancies (blue symbols). Still, when compared to unvaccinated controls (red symbols), there is a meaningful reduction in the aORs. As regards death within 60 days after infection, shown in Fig. 2B, amongst the unvaccinated (black symbols), Veterans with hematologic malignancies fared worse (aOR, 1.539, P< 0.0001) than those with solid tumors (aOR, 0.837, P< 0.0001), a differential that persists even amongst vaccinated Veterans (blue symbols, aOR of 2.154, P< 0.0001, for those with hematologic malignancies versus a statistically insignificant aOR of 0.960 for those with a solid tumor diagnosis). Among Veterans with hematologic malignancies, vaccination effectively reduced infection incidence (Fig. 2A, red symbols). Still, its impact on death within 60 days of infection was blunted (aOR, 2.154 amongst vaccinated Veterans, blue symbols) and did not provide meaningful protection compared to the unvaccinated noncancer reference (aOR 0.866, not significant, red symbols). Furthermore, amongst vaccinated Veterans with solid tumor diagnoses, the risk of death within 60 days of COVID infection was significantly greater in Veterans with a diagnosis of lung cancer (aOR, 1.955, P< 0.0001) or multiple cancers (aOR, 1.493, P< 0.0001), despite having similar risks of infection when compared to vaccinated Veterans without cancer (Fig. 2A/B, blue symbols, with vaccinated non-cancer as reference). Importantly, in Veterans diagnosed with lung cancer, vaccination did not protect from death when compared to the overall unvaccinated non-cancer cohort (Fig. 2B, red symbols). Note here that while small numbers in some cohorts limit confidence in statistical validity of results, for most cohorts, there is confidence in their validity (see figure legend and Supplemental Methods).
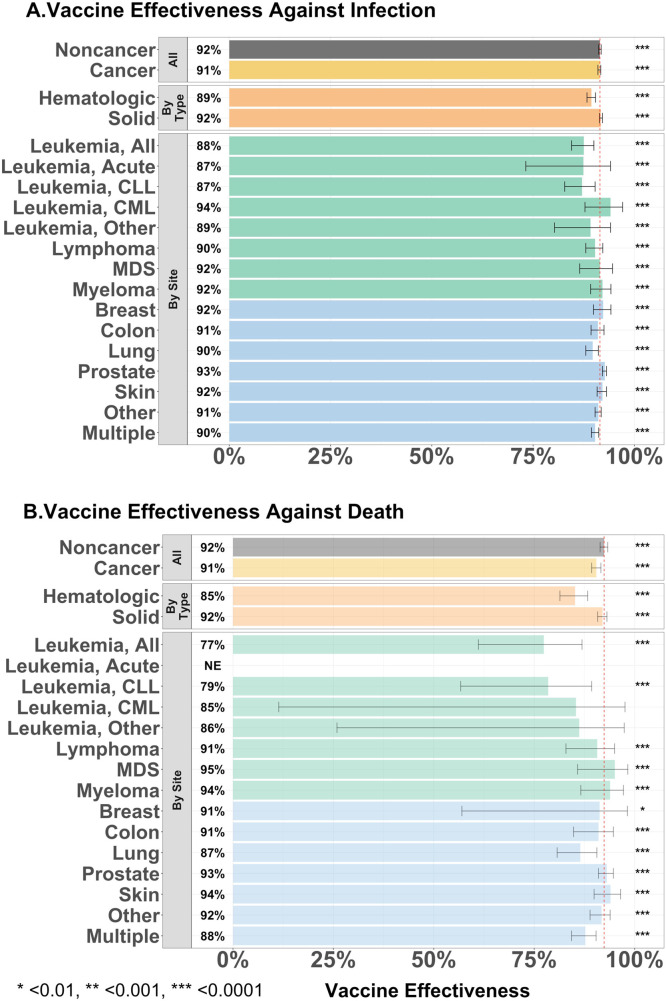
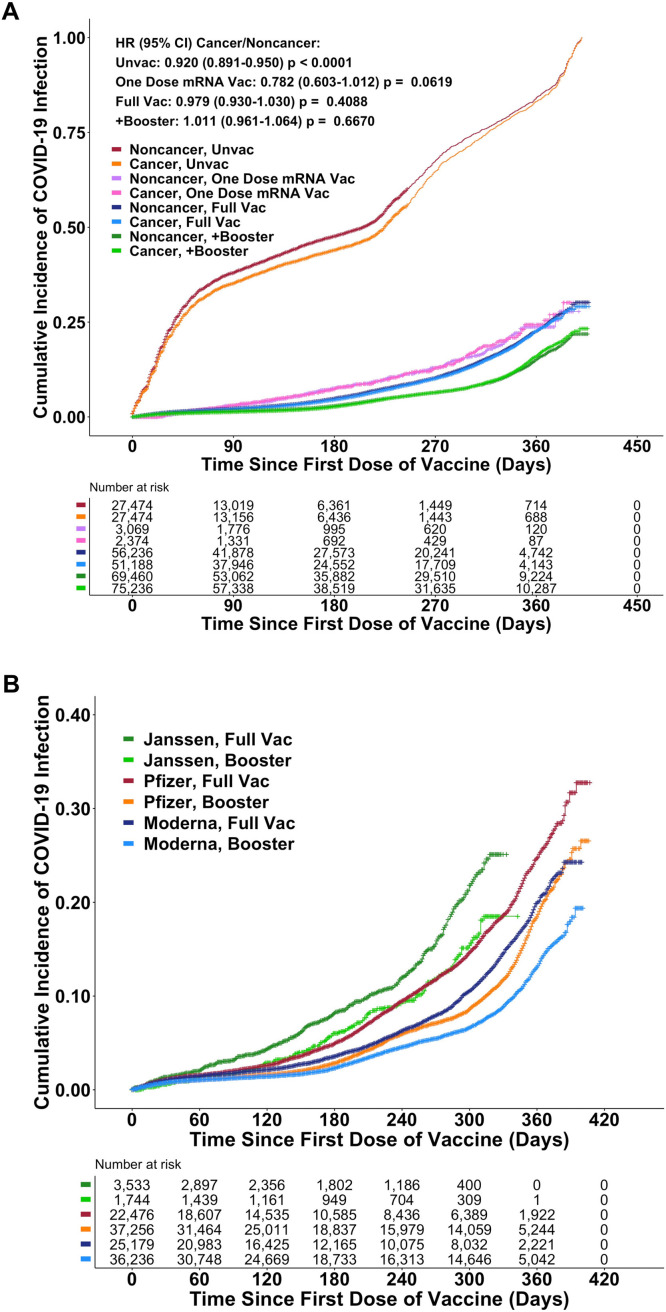
Fig. 3A/B presents estimates of vaccine efficacy in reducing risk of infection from and death due to COVID-19, which was 92% in the control cohort. The vaccine efficacy to prevent infection in Veterans with a hematologic or solid tumor malignancy was 89% and 92%, respectively, and is further broken down into disease-based cohorts. Vaccine efficacy to prevent death of 85% further demonstrates poorer outcomes for Veterans with hematologic malignancies. We examine vaccine efficacy over time in Fig. 4A/B. Fig. 4A documenting the beneficial impact of vaccination without/with the addition of the booster, shows comparability of results in Veterans without/with a diagnosis of cancer and in the unvaccinated again shows the “apparent protective effect of a cancer diagnosis.” Surprising efficacy is seen with a single dose of an mRNA vaccine. Fig. 4B compares the impact of boosters across the three common brands. An increase in the pace of infection (decline in vaccine efficacy) begins sooner with the Janssen vaccine and about 6 months after start of vaccination with the Pfizer-BioNTech and Moderna products with boosters moving curves to the right without meaningful impact on the eventual rates of rise (infection).
Fig. 3.
Estimates of vaccine efficacy in Veterans without/with a diagnosis of cancer as regards infection (3A) and death within 60 days of infection (3B). The data is plotted for all Veterans without/with a cancer diagnosis and with a cancer diagnosis by hematologic or solid tumor malignancy and across individual malignancies. Compared to Veterans without a diagnosis of cancer, vaccine efficacy amongst Veterans with a diagnosis of cancer is comparable in preventing infection and death within 60 days of infection, except for preventing death in Veterans with diagnoses of leukemia. Asterisks coincide with P-value of difference between the cohort of vaccinated compared the corresponding unvaccinated in each group.
Fig. 4.
Kaplan-Meier plots of cumulative COVID-19 infection over time. Fig. 4A demonstrates comparable impact of vaccination and of boosting in Veterans without/with a diagnosis of cancer. Comparable results with a single dose of the mRNA vaccines are observed in the group of Veterans who received only one dose of either mRNA vaccine [Full dose v One dose: HR, 0.827, 95%CI, 0.728–0.939, P = 0.0033]. Hazard ratios for combined Noncancer + Cancer (95%CI): One dose vs. Unvaccinated: 0.260 (0.229–0.295). Full dose vs. Unvaccinated: 0.220 (0.213–0.228). Full dose + booster v Unvaccinated: 0.141 (0.141–0.150). The analysis excludes 733 Veterans who died and 262 who were infected before the 2nd dose could be administered. See Supplementary Fig. 2 for discussion and alternate plots. Fig. 4B documents an increasing rate of infection that begins sooner with the Janssen vaccine and at 6–8 months after completing both vaccinations with the Pfizer-BioNTech and Moderna mRNA products. For the 3 vaccinations, the administration of a booster delays the rise of the infections but does not meaningfully change the qualitative shape of the rising curve consistent with transient increases in the level of immunity but not a change in immune competence and its durability.
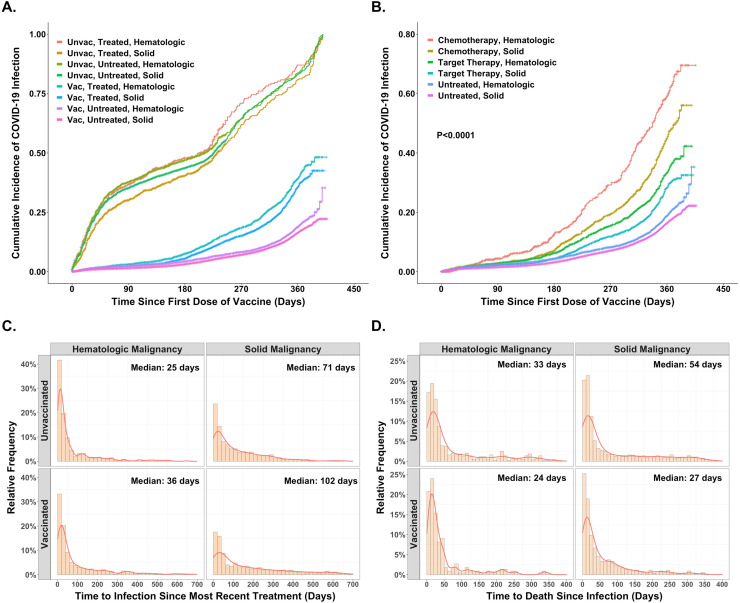
Turning to the impact of cancer therapies, we identified 19,307 Veterans on active treatment before the index date with 4,374, 9,747, and 9,106 Veterans receiving chemotherapy, targeted and endocrine therapies, respectively. Fig. 5 and Supplemental Fig. 3 provide insights into the impact of cancer treatment in both unvaccinated and vaccinated Veterans. With a relatively high 47% incidence of COVID-19 infection in unvaccinated Veterans with a diagnosis of cancer, neither Veterans with a solid tumor diagnosis (HR 0.931, 95%CI, 0.845–1.027, P= 0.1537) nor those with a hematologic malignancy (HR 1.009, 95%CI 0.873–1.166, P= 0.9030) experienced a further increase in infection risk with the administration of anticancer therapies as shown in Fig. 5A. However, in vaccinated Veterans, a different picture emerges. While at 8%, the overall incidence of infection in vaccinated Veterans is much lower, as shown in Fig. 5A, compared to Veterans not undergoing active treatment (untreated in Fig. 5A), the cumulative incidence of infection over time rose more rapidly in those treated with chemotherapy and/or targeted therapy in either hematologic malignancies (HR, 2.064, 95%CI, 1.825–2.333), P< 0.0001) or solid tumors (HR, 1.759, 95%CI, 1.608–1.923, P< 0.0001). Furthermore, as shown in Fig. 5B, amongst vaccinated Veterans with a hematologic malignancy, an increase in the risk of infection with treatment was observed with the administration of chemotherapy (HR, 2.993, 95%CI, 2.484–3.607, P< 0.0001) or targeted therapies (HR, 1.781, 95%CI, 1.546–2.051, P< 0.0001), with augmentation of infections also recorded for Veterans with a diagnosis of a solid tumor treated with either chemotherapy (HR 2.328, 95%CI 2.075–2.611, P< 0.0001) or targeted therapies (HR 1.328, 95%CI 1.166–1.513, P< 0.0001). These observations emerge when comparing outcomes amongst vaccinated Veterans but note that vaccination was very effective in reducing the incidence of infections compared to unvaccinated Veterans (red font in Supplementary Fig. 3A). And while Fig. 5A/B depicts outcomes over the entire observation period, Fig. 5C/D illustrates the interval of vulnerability following treatment administration for those receiving anticancer therapies. 5C displays the occurrence of infections over time amongst Veterans receiving anticancer therapies. Day zero is the last day of therapy administration before the infection in these graphics. While the vulnerable period after a treatment likely varies depending on disease and treatment, one can confidently assume it encompasses the first 30 days, extending to 45 days, 60 days, or even longer after some therapies. With the Y-axis in 5C recording the percent of infections in successive 20-day periods (bars along X-axis), one can see that compared to Veterans with a solid tumor diagnosis undergoing systemic therapy, Veterans with hematologic malignancies receiving therapy have a higher percentage of infections occurring closer to the administration of their treatment–median times to infection of 25–36 days v 71–102 days, P< 0.01 for those with diagnoses of solid tumors, suggesting a larger percentage of infections in those with hematologic malignancies can be ascribed to recent treatment. Peaks of death after infection followed (median 24–33 days v 27–54 days), again supporting a proximate infection as an event likely responsible for their deaths (5D).
Fig. 5.
A and B compares the (cumulative) probability of infection in those who did or did not receive chemotherapy and/or targeted therapy for their cancer during the period of observation. Fig. 5A looks at the impact of vaccination status on the probability of infection presented according to a diagnosis of either a hematologic malignancy or a solid tumor. Fig. 5B compares the results in those treated with either chemotherapy or targeted therapies to those not treated according to their diagnosis of either a hematologic malignancy or a solid tumor. In both figures the order of the legend tracks with the curves from top to bottom. A total of 157,072 Veterans with a diagnosis of cancer were evaluated, with 19,307 having received one of the 82 therapies identified in Supplementary Table 1. Figs. 5C and D present distribution plots looking at the occurrence of infection or death following infection. The data are shown for those who had not been vaccinated separately from those who were vaccinated and presented separately for those with a diagnosis of either a hematologic malignancy or a solid tumor. The X-axis is time after the receipt of therapy and the Y-axis the percent of all those treated in whom infection or death was recorded in successive 20-day time intervals–each bar comprises 20 days. Fig. 5C shows the fraction of infections occurring closer in time to and more likely impacted by treatment was higher in the unvaccinated and those with hematologic malignancies. Fig. 5D peaks of death 4 weeks following infection consistent with most deaths more likely caused by the infection than as a result of the underlying disease.
Discussion
We present an extensive analysis of over one million Veterans who had COVID-19 testing at a VHA facility where they also received medical care. This well-annotated real-world cohort includes 157,072 Veterans with a diagnosis of a new or established cancer within the two years preceding the index test date matched to 157,072 Veterans without a cancer diagnosis. This allowed us to perform conditional logistic regression analyses to ascertain vulnerability to infection, death within 60 days of infection, and the efficacy of COVID-19 vaccination across a diverse group of cancers.
This analysis with a cut-off date of 25JAN2022 captures the delta and omicron waves in the United States [23] and updates an earlier unpublished analysis with a cut-off date of 25SEP2021. The high infectivity of the delta and omicron variants is captured in the current update, with overall rates of infection in the current analysis more than double those in the previous analysis at 51% compared to 21% in unvaccinated Veterans and 8% compared to 3% in those vaccinated, percentages emulated amongst Veterans with a diagnosis of cancer.
Age, a recognized vulnerability factor since the outset of the pandemic, emerges as the most important of all factors analyzed with aORs for death within 60 days rising with increasing age (Supplemental Fig. 1B). The reason(s) for increasing mortality with age remain(s) unclear but is unlikely due solely/primarily to an aging innate or adaptive immune system given very modest aORs for infection (as opposed to death) in unvaccinated Veterans of 1.11 and 1.53 for those 60–70 years of age and over 80, respectively, and values for vaccine effectiveness of 90%–95% consistent with original reports for the 2 commercially available RNA vaccines in volunteers of all ages. Additionally, while co-morbidities are often discussed as risk factors, aORs ranging from 1.6–3.9 for death within 60 days are dwarfed by the age vulnerability. Whether due to more severe cytokine storms, epigenetic changes or other factors, age remains an important area of research [24,25].
Turning to Veterans with a diagnosis of cancer, a more nuanced picture emerges (Fig. 2A/B). In unvaccinated Veterans, lower aORs for infection in those with compared to those without cancer, unlikely to reflect a protective effect of cancer, but rather more attention to personal health practices such as mask-wearing, handwashing, and social distancing by those with a cancer diagnosis [26]. Furthermore, although vaccines were very effective in preventing infection in Veterans with diverse cancers (red symbols in Fig. 2A), they were less effective in Veterans with some hematologic malignancies (blue symbols in Fig. 2A). Among vaccinated Veterans, higher aORs for infection than the matched control without a cancer diagnosis were found in those with hematologic malignancies–with diagnoses of chronic lymphocytic leukemia, other leukemia, lymphoma, and myeloma primarily responsible for the difference. This may eventually be explained by a poorer antibody response due to inherent impairment of humoral and cellular immunity or possibly drugs used in treatment [27], [28], [29], [30], [31]. Looking at the 60-day mortality endpoint (Fig. 2B), we found significantly higher aORs for death consistent with reduced vaccine effectiveness amongst Veterans with hematologic malignancies, with results possibly driven by those with chronic lymphocytic leukemia and myeloma. Amongst those with solid tumors, we found higher aORs for death only in Veterans with lung or multiple cancers (often including lung cancer as one diagnosis)
While recognizing differences amongst Veterans with different cancer diagnoses, it was gratifying to see (1) vaccine efficacy comparable to that of Veterans without cancer when comparing the entire cancer cohort; (2) the ability of a booster to delay rises in the incidence of infections; and (3) the almost certain benefit of personal hygiene practices amongst unvaccinated Veterans with cancer (Figs. 3 and 4). Importantly, the efficacy of vaccination in preventing death of individuals with a diagnosis of cancer from COVID-19, a metric that includes vaccine efficacy in preventing infection and death if infection acquired, is exceptionally high.
Finally, looking at the impact of treatment on COVID-19 infection and mortality in Veterans with cancer, the data demonstrate a complex picture driven primarily by higher cumulative rates of infection, with rises in death rates in those recently treated. The observation of incidence peaks soon after treatment, especially in unvaccinated Veterans and those with hematologic malignancies that then decline over time likely describe an acute/subacute treatment-prompted rise in infections followed by a gradual return to a lower number/rates of infections occurring in those more ill, with more active cancers that would have required treatment and during the pandemic may have found themselves in more vulnerable situations. This is supported by the data in both Fig. 5 and Supplemental Fig. 3, with the latter summarizing aORs confining the period of acquired infection to within 60 days of treatment, a period more likely impacted by recent therapies, with the contribution of additional factors such as disease activity and overall well-being contributing to the Kaplan Meier plots in Fig. 5. These analyses can discern differentials between Veterans with solid and hematologic malignancies, the latter more vulnerable after recent therapies. These observations concur with others in our analyses and the literature reporting greater vulnerability for those with hematologic malignancies [14,17,32]. Specifically, we found higher aORs for infection in Veterans with either hematologic malignancies or solid tumors treated with chemotherapy or targeted therapies–which in those with hematologic malignancies included proteasome inhibitors, anti-CD20, and anti-CD38 antibodies–with adverse outcomes blunted by vaccination. These results implicate use of these therapies as possibly more detrimental [27,[33], [34], [35], [36]. For several cohorts, statistically valid aORs for mortality implicate recently administered therapies as causative or strong contributors to the infection and resultant death. However, we advise caution in interpreting results in smaller cohorts as the data was further subdivided.
As with all similar observational analyses, limitations inherent in analyzing any extensive data set apply to our study. Estimation of “vaccine efficacy” is encumbered by the same variables encumbering all such analyses: 1) receipt of vaccine was voluntary, 2) despite our efforts to control for variables, the vaccinated population cannot be considered directly comparable to the unvaccinated population, and 3) “vaccine efficacy” here, as in every analysis, likely reflects not just efficacy of the vaccine but includes some contribution from health practices, and hence “vaccine efficacy” is likely somewhat overestimated. In assessing the impact of therapies administered, we cannot exclude the existence of differences between those treated for their cancer and those not treated–either more “healthy” or more ill. Finally, with large numbers, statistical validity achieved for some outcomes may not be clinically meaningful.
Important attributes include the large amount of data analyzed from Veterans with extensive follow-up and for which much information not prospectively gathered was harvested without bias. Also importantly, care was administered in the most egalitarian health care system in US, minimizing the impact of many variables, especially access to health care. While we did not obtain cause-specific mortality, peaks of death 4 weeks following infection ( Fig. 5D) are consistent with most deaths more likely caused by the infection than as a result of the underlying disease. Finally, the Veterans included represent a real-world patient population with many co-morbidities, allowing these variables to be explored in depth during a period that included both the delta and omicron “waves.”
In conclusion, we report one of the largest datasets on COVID-19 infection and death within 60 days of infection in Veterans without/with a cancer diagnosis. Age, gender, BMI, and the Elixhauser comorbidity index had measurable impacts on the aORs for infection and/or death. However, a more nuanced influence of a cancer diagnosis emerged. While vaccine effectiveness in preventing COVID-19 infections was comparable amongst Veterans without/with a cancer diagnosis, aORs for infection and death varied across different cancers, with efficacy reduced in those with hematologic malignancies. The impact of ongoing therapy varies, precluding a single guideline. Still, data suggest that administration of chemotherapy and some targeted therapies increases risks of infection and death from infection in those diagnosed with hematologic malignancies and solid tumors, underscoring the need to monitor those receiving treatment carefully and consider COVID pre-exposure prophylaxis before starting therapy. Notably, the similarity between this and a previous analysis we completed before the omicron and delta waves suggests host factors are more important than viral differences, and confidently expecting the host will not change meaningfully, the present observations can inform approaches to future variants. Despite differences in efficacy, vaccination was exceptionally beneficial and should be encouraged for all patients with a diagnosis of cancer.
Conflicts of Interest
None
Acknowledgments
Author contributions
None.
Data sharing
All data analyzed is available to all investigators with VA affiliations. The authors will gladly make all data available as allowed by the Department of Veterans Affairs.
This material results from work supported by resources and facilities at the James J. Peters Bronx Veterans Affairs Medical Center in Bronx, NY.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
The authors acknowledge the generous support of the Prostate Cancer Foundation and the Blavatnik Family Foundation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.seminoncol.2022.07.005.
Appendix. Supplementary materials
References
- 1.WHO. Weekly epidemiological update on COVID-19 - 27 April 2022. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—27-april-2022.
- 2.Gao YD, Ding M, Dong X, et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 3.Lowe KE, Zein J, Hatipoglu U, Attaway A. Association of smoking and cumulative pack-year exposure with COVID-19 outcomes in the Cleveland Clinic COVID-19 Registry. JAMA Intern Med. 2021;181(5):709–711. doi: 10.1001/jamainternmed.2020.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas SJ, Moreira ED, Jr., Absalon J, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774–1785. doi: 10.1056/NEJMoa2113017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadoff J, Gray G, Vandebosch A, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldberg Y, Mandel M, Bar-On YM, et al. Waning immunity after the BNT162b2 vaccine in Israel. N Engl J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajema KL, Dahl RM, Evener SL, et al. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans - five veterans affairs medical centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700–1705. doi: 10.15585/mmwr.mm7049a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944. doi: 10.1016/S0140-6736(22)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance - VISION Network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. doi: 10.15585/mmwr.mm7107e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N Engl J Med. 2022 doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Han H, He T, Labbe KE, Hernandez AV, et al. Clinical characteristics and outcomes of COVID-19-infected cancer patients: a systematic review and meta-analysis. J Natl Cancer Inst. 2021;113(4):371–380. doi: 10.1093/jnci/djaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desai A, Gupta R, Advani S, et al. Mortality in hospitalized patients with cancer and coronavirus disease 2019: a systematic review and meta-analysis of cohort studies. Cancer. 2021;127(9):1459–1468. doi: 10.1002/cncr.33386. [DOI] [PubMed] [Google Scholar]
- 15.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet (London, England) 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari BL, Ferreira CG, Menezes M, et al. Determinants of COVID-19 mortality in patients with cancer from a community oncology practice in Brazil. JCO Glob Oncol. 2021;7:46–55. doi: 10.1200/GO.20.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharafeldin N, Bates B, Song Q, et al. Outcomes of COVID-19 in patients with cancer: Report From the National COVID Cohort Collaborative (N3C) J Clin Oncol. 2021;39(20):2232–2246. doi: 10.1200/JCO.21.01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol. 2022 doi: 10.1001/jamaoncol.2022.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Zeng G, Tao H, et al. Low prevalence of IgG antibodies to SARS-CoV-2 in cancer patients with COVID-19. Int J Cancer. 2020;147(11):3267–3269. doi: 10.1002/ijc.33148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monin L, Laing AG, Muñoz-Ruiz M, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becerril-Gaitan A, Vaca-Cartagena BF, Ferrigno AS, et al. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–260. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.US Department of Veterans Affairs: COVID-19 vaccination plan for the Veterans Health Administration Version 2.0. https://www.publichealth.va.gov/docs/n-coronavirus/VHA-COVID-Vaccine-Plan-14Dec2020.pdf.
- 23.Lambrou AS, Shirk P, Steele MK, et al. Genomic surveillance for SARS-CoV-2 variants: predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants - United States, June 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(6):206–211. doi: 10.15585/mmwr.mm7106a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020;12(10):9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santesmasses D, Castro JP, Zenin AA, et al. COVID-19 is an emergent disease of aging. Aging Cell. 2020;19(10):e13230. doi: 10.1111/acel.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talic S, Shah S, Wild H, et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis. Bmj. 2021;375 doi: 10.1136/bmj-2021-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Q, Bates B, Shao YR, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the National COVID Cohort Collaborative. J Clin Oncol. 2022;40(13):1414–1427. doi: 10.1200/JCO.21.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39(8):1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Oekelen O, Gleason CR, Agte S, Srivastava K, Beach KF, Aleman A, et al. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RNA vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry C, Luttwak E, Balaban R, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv. 2021;5(16):3053–3061. doi: 10.1182/bloodadvances.2021005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maneikis K, Šablauskas K, Ringelevičiūtė U, et al. Immunogenicity of the BNT162b2 COVID-19 mRNA vaccine and early clinical outcomes in patients with haematological malignancies in Lithuania: a national prospective cohort study. Lancet Haematol. 2021;8(8):e583–ee92. doi: 10.1016/S2352-3026(21)00169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garassino MC, Whisenant JG, Huang LC, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21(7):914–922. doi: 10.1016/S1470-2045(20)30314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JT, La J, Branch-Elliman W, et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a US Nationwide Veterans Affairs Study. JAMA Oncol. 2022;8(2):281–286. doi: 10.1001/jamaoncol.2021.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massarweh A, Eliakim-Raz N, Stemmer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghione P, Gu JJ, Attwood K, et al. Impaired humoral responses to COVID-19 vaccination in patients with lymphoma receiving B-cell-directed therapies. Blood. 2021;138(9):811–814. doi: 10.1182/blood.2021012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.