Abstract
Background
The role and the durability of the immunogenicity of the third dose of vaccine against COVID-19 variants of concern in cancer patients have to be elucidated.
Patients and methods
We have prospectively evaluated the immunogenicity of the third dose of the SARS-CoV-2 BNT162b2 messenger RNA vaccine in triggering both humoral and cell-mediated immune response in patients with solid tumors undergoing active treatment 6 months after the booster. Neutralizing antibody (NT Ab) titers and total anti-spike immunoglobulin G concentrations were measured in serum. Heparinized whole blood samples were used for the SARS-CoV-2 interferon-γ release assay (IGRA).
Results
Six months after the third dose only two patients (2.4%) showed negative spike-specific immunoglobulin G antibody levels (<33.8 BAU/ml). The median level of SARS-CoV-2 NT Abs decreased and only 39/83 (47%) subjects showed maximum levels of NT Abs. T-cellular positive response was observed in 38/61 (62.3%) patients; the highest median level of response was observed 21 days after the third dose (354 mIU/ml, interquartile range 83.3-846.3 mIU/ml). The lowest median level of NT Ab response was observed against the Omicron variant (1 : 10, interquartile range 1 : 10-1 : 40) with a significant reduced rate of responder subjects with respect to the wild-type strain (77.5% versus 95%; P = 0.0022) and Delta variant (77.5% versus 93.7%; P = 0.0053). During the follow-up period, seven patients (8%) had a confirmed post-vaccination infection, but none of them required hospitalization or oxygen therapy.
Conclusions
Our work highlights a significant humoral and cellular immune response among patients with solid tumors 6 months after the third BNT162b2 vaccine dose, although a reduction in neutralizing activity against Omicron was observed.
Key words: BNT162b2 anti-SARS-CoV-2 vaccine, cancer, neutralizing antibody, third dose, VOCs
Highlights
-
•
Only two patients (2.4%) showed negative spike-specific IgG antibody levels (<33.8 BAU/ml)
-
•
Only 39/83 (47%) subjects showed maximum level of neutralizing antibodies (NT Abs).
-
•
T-cellular positive response was observed in 38/61 (62.3%) analyzed patients.
-
•
The lowest median level of NT Ab response was observed against the Omicron variant.
-
•
Seven patients (8%) had a post-vaccination infection; none of them required hospitalization or oxygen therapy.
Introduction
More than 12 billion doses have been administered and 66.7% of the world population has received at least one dose of a COVID-19 vaccine.1 According to the Centers for Disease Control and Prevention (CDC) a variant of concern (VOC) is defined as ‘a variant for which there is evidence of increased transmissibility, a more severe disease, a significant reduction in neutralization by antibodies, and with a reduced effectiveness of available diagnostic, therapeutic and vaccination strategies’.2 The emergence of SARS-CoV-2 VOCs such as Delta (B.1.617.2) and Omicron (B.1.1.529) has caused large outbreaks also among vaccinated subjects.3, 4, 5 Moreover, the risk of reinfection with Omicron is significantly higher compared with prior variants: individuals who were infected with the Delta variant have a 40% relative risk of reinfection with Omicron and those infected with the original viral strain have a 73% risk of reinfection.6
The Advisory Committee on Immunization Practices of the CDC has recommended the third dose (‘booster’) of COVID-19 vaccine to high-risk groups, including immunocompromised individuals, to deal with the potential decline in immunity against SARS-CoV-2 variants.7 Edara et al.8 evaluated the neutralizing activity of the SARS-CoV-2 Omicron variant in double-vaccinated and booster dose-vaccinated subjects. They demonstrated no neutralizing activity against Omicron in the sera from naive vaccinated subjects 6 months after the initial two-vaccine doses, whereas following a booster shot (third dose) they demonstrated that >90% of subjects show a neutralizing activity against Omicron.8 Accorsi and colleagues9 found that individuals who received three doses of COVID-19 messenger RNA (mRNA) vaccine (compared with the unvaccinated and those who received two doses) are less likely to develop a symptomatic SARS-CoV-2 infection.
A recent meta-analysis described the efficacy of COVID-19 vaccines in immunocompromised patients compared with immunocompetent people, with evidence of a lower seroconversion rate and antibody titer among immunocompromised groups studied.10 A narrative review included 54 studies about COVID-19 vaccines with evidence of a lower immunogenicity of the vaccines in terms of both humoral and cell-mediated immune responses in patients with cancer versus healthy individuals.11 A systematic review of 36 prospective studies that evaluated the safety and efficacy of COVID vaccines in patients with cancer has documented a lower seroconversion rate during cytotoxic chemotherapy, whereas the seroconversion rates during endocrine treatment or therapy with cyclin-dependent kinase 4/6 inhibitors are similar to those of immunocompetent subjects.12 Becerril-Gaitan and colleagues13 conducted a systematic review and meta-analysis of 35 original studies with the evaluation of anti-SARS-CoV-2 spike protein (S) immunoglobulin G (IgG) seroconversion rates, cell-mediated response, and documented SARS-CoV-2 infection after COVID-19 vaccination as primary endpoints. The authors highlighted that oncological patients are less likely to obtain seroconversion after incomplete [risk ratio 0.45 (95% confidence interval 0.35-0.58)] COVID-19 vaccination schemes.13 These findings suggest that the additional dose of COVID-19 vaccine may be efficacious in immunocompromised patients.
In our previous paper,14 we investigated the immunogenicity of the third dose of vaccine in triggering both humoral and cell-mediated immune response in cancer patients on active treatment.
This study prospectively evaluated these outcomes 6 months after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine with a focus also on the neutralizing activity against VOCs.
Materials and Methods
Study design and participants
Patients with solid tumors undergoing active anticancer treatment (chemotherapy/immunotherapy/or a combination of these types of therapies) were enrolled. A previous SARS-CoV-2 infection was not an exclusion criterion. The patients were referred to the Oncology Units of Fondazione IRCCS Policlinico San Matteo, Pavia and AUSL Ospedale Guglielmo Da Saliceto, Piacenza. The study (Co-Var) was conducted according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement for reporting observational studies15 and was approved by the local ethics committee (Comitato Etico Area Pavia) and institutional review board (P-0103665/21). All subjects had signed an informed written consent before enrollment.
This is a prospective follow-up report of the primary study. We considered only the patients who remained on active treatment 23-24 weeks after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine (Pfizer, New York, NY and BioNTech, Mainz, Germany).
Assessments
Patients were monitored 23-24 weeks after the third dose with blood samples for humoral and cell-mediated immune response evaluation. Throughout the study, all patients underwent a nasopharyngeal swab before each cycle of therapy.
Study endpoints
In the first publication of this study,14 the primary endpoint was assessment of the increase of IgG antibody level between the baseline (T0) and 3 weeks after the third dose of BNT162b2 SARS-CoV-2 vaccine (T1). In the present study we have provided an update about the duration of the immune response after BNT162b2 mRNA vaccination after 23-24 weeks (6 months, T2), analyzing both spike-specific interferon-γ (IFN-γ)-producing T cells and humoral response [total IgG concentration and SARS-CoV-2 neutralizing antibody (NT Ab) titer]. Subjects are defined as ‘full responders’ if there are positive anti-S IgG concentration, SARS-CoV-2 NT Ab titer and spike-specific IFN-γ-producing T cells. SARS-CoV-2 NT Ab titer against Delta (B.1.617.2) and Omicron 1 (BA.1) variants was assessed at T2. Additionally, we evaluated the incidence of virologically confirmed COVID-19 cases during the entire period of the study.
Serological assays
According to our protocol,14 the quantitative characterization of spike-specific IgG antibodies was carried out by Trimeric assay (Liaison, Diasorin, Saluggia, Italy) and results were given as BAU/ml (positive results >33.8 BAU/ml). Additionally, NT Ab titers against the B.1.1, B.1.617.2 and BA.1 strains were measured as previously reported16, 17, 18 and results were given as positive when NT Ab was ≥1 : 10.
SARS-CoV-2 IFN-γ release assay
Heparinized whole blood samples were used for SARS-CoV-2 IFN-γ release assay (IGRA), according to the manufacturer’s instructions (Euroimmun, Lübeck, Germany). Briefly, 500 μl of sample was added to the stimulator tube coated with spike antigen and to negative and positive control tubes. All the tubes were incubated overnight at 37°C, 5% CO2 and then the samples were centrifuged and the plasma supernatant was collected and stored at −80°C until testing. IFN-γ was detected automatically in the supernatants by an enzyme-linked immunosorbent assay (ELISA, Euroimmun) using the Euroimmun Analyzer I according to the manufacturer’s instructions. IFN-γ response was defined as spike-stimulated minus unstimulated. Results >200 mIU/ml were given as positive, whereas results ranging from 100 to 200 mIU/ml were defined as borderline. In case of inadequate response to the positive control, the result was given as ‘indeterminate’.
Statistical analysis
GraphPad Prism 8.3.0 (GraphPad Software, La Jolla, CA) was used for statistical analyses. We considered a two-sided P value <0.05 as statistically significant. Data were described with the median and interquartile range (IQR) if continuous and as counts and percentage if categorical. Comparison between two groups was carried out using the Mann–Whitney (unpaired samples) or Wilcoxon (paired samples) test whereas Spearman’s test was used for the correlation analysis. Fisher’s exact test was used for the comparison of categorical variables.
Results
Patients’ characteristics
The original study cohort14 consisted of 142 patients with solid tumor vaccinated with the third dose during active treatment (56 females and 86 males; median age 66 years; range 26-88 years). The current study included 83 patients with solid tumors (36 females and 47 males; median age 63 years, range 26-87 years) who were still on active treatment at the time of T2 (6 months after the third dose). A total of 43 patients (52%) had lung cancer, 15 (18%) had breast cancer, 8 (10%) had melanoma, 7 (8%) had gastrointestinal cancer, 6 (7%) had kidney cancer, and the remaining 4 patients (5%) had head and neck cancer.
In this follow-up paper, we were able to collect the samples, 6 months after the third dose, in only 83 patients out of 142. In particular, we excluded 59 patients: 6 patients refused the blood sample, 16 patients died, and 37 patients were no longer on active therapy. A total of 46 patients (55%) were on immunotherapy alone, whereas 10 patients (12%) were on chemo-immunotherapy and 27 patients (33%) were on chemotherapy (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100574).
SARS-CoV-2 humoral response
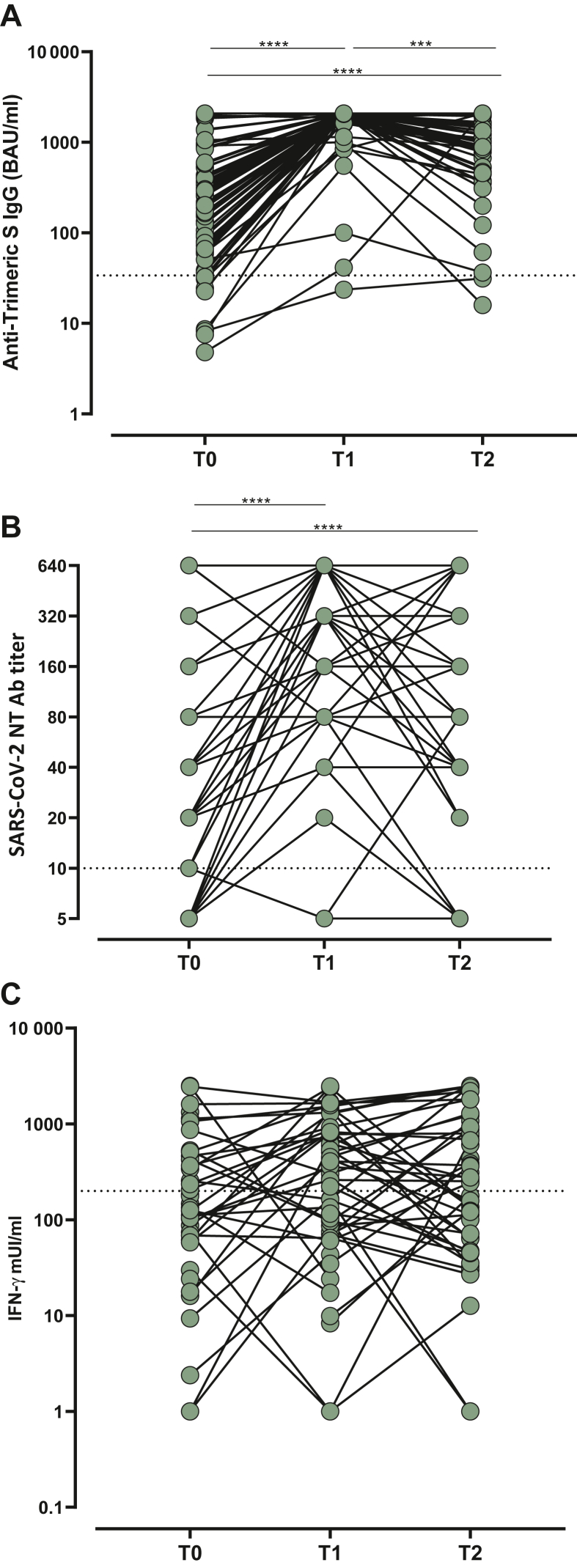
The levels of humoral immune response measured by anti-Trimeric S IgG were compared at the specified time points in the 83 patients. At baseline (T0, median 176 days, IQR 162-196 days after the first dose), 14/83 (16.9%) subjects tested negative for anti-Trimeric S IgG whereas all patients (except 1) showed a positive serological response after the third dose administration. Of note, 6 months after the third dose (T2) only two patients (2.4%) showed negative spike-specific IgG antibody levels (<33.8 BAU/ml). The median levels at T1 were significantly higher than those observed at T0 and T2; importantly the median level of response was significantly higher at T2 than at T0. Looking at SARS-CoV-2 NT Ab titers, 16/83 (19.3%) patients were negative at T0, whereas only 2/83 (2.4%) and 4/83 (4.8%) were negative at T1 and T2, respectively. In terms of SARS-CoV-2 NT Ab titers, the highest levels of response were observed at T1, with 48/83 (57.8%) patients showing NT Ab titers at the upper limit of detection of our assay (1 : 640). At T2, the median level of SARS-CoV-2 NT Abs decreased and only 39/83 (47%) subjects showed the maximum level of NT Abs. Overall, a good correlation between SARS-CoV-2 NT Ab titers and anti-Trimeric S IgG levels was observed at each time point (Figure 1).
Figure 1.
Immune response elicited by the third dose of BNT162b2 vaccine measured at T0 (before the third dose administration), T1 (3 weeks after the third dose), and T2 (6 months after the third dose). Response was measured by (A) Trimeric S IgG level and (B) SARS-CoV-2 NT Ab titer. Correlation between the two assays was measured at (C) T0, (D) T1, and (E) T2. r = correlation coefficient measured by Spearman test.
IgG, immunoglobulin G; NT Ab, neutralizing antibody.
∗∗∗P value <0.001.
∗∗∗∗P value <0.0001.
Spike-specific T-cell response
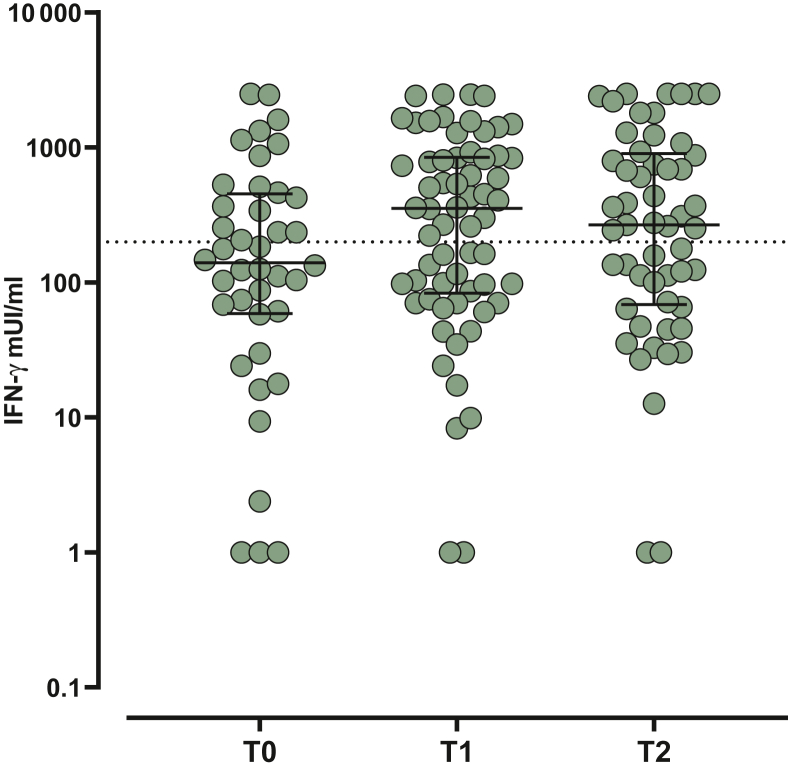
T-cell response elicited by vaccination was analyzed according to the scheduled follow-up. Valid results were obtained in 29, 47, and 61 patients at T0, T1, and T2, respectively. Overall, positive response was observed in 17/29 (58.6%), 36/47 (76.6%), and 38/61 (62.3%) analyzed patients, with the highest median level of response observed at T1 (354 mIU/ml, IQR 83.3-846.3 mIU/ml). Moreover, even if the median level of response was higher at T2 than at T0, the difference was not statistically significant (median 267.4 mIU/ml, IQR 68.6-902 mIU/ml versus 140.1 mIU/ml, IQR 59-453 mIU/ml; P = 0.2611) (Figure 2). The intra-individual kinetics of total IgG, SARS-CoV-2 NT Abs, and spike-specific T-cell response over follow-up time points are represented in Figure 3.
Figure 2.
Cell-mediated immune response elicited by the third dose of BNT162b2 vaccine measured at T0 (before the third dose administration), T1 (3 weeks after the third dose) and T2 (6 months after the third dose). Response was given as IFN-γ concentration (mUI/ml).
IFN-γ, interferon-γ.
Figure 3.
Intra-individual kinetics of (A) total IgG, (B) SARS-CoV-2 NT Abs, and (C) spike-specific T-cell response over follow-up time points. IFN-γ, interferon-γ; IgG, immunoglobulin G; NT Ab, neutralizing antibody.
∗∗∗P value <0.001.
∗∗∗∗P value <0.0001.
SARS-CoV-2 neutralizing response to VOCs
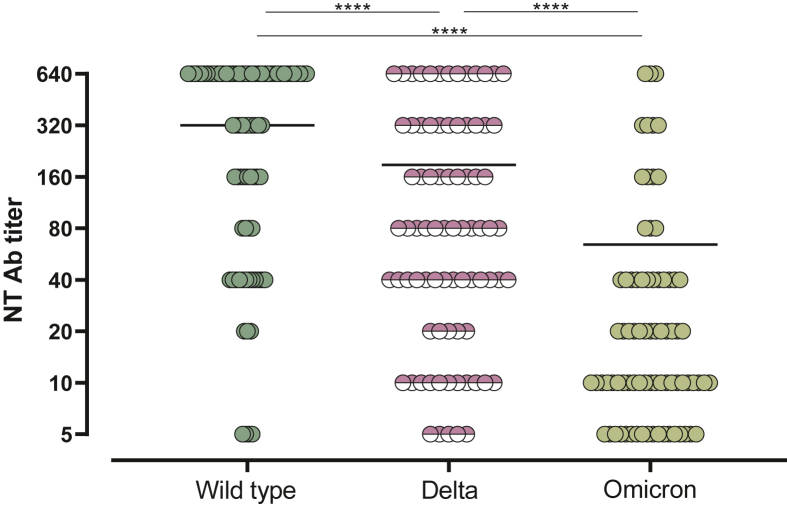
In 80 patients, SARS-CoV-2 NT Ab titer at T2 was also tested against Delta and Omicron variants. As expected, the lowest median level of NT Ab response was observed against the Omicron variant (1 : 10, IQR 1 : 10-1 : 40) with a significant reduced rate of responders in comparison to that observed against the wild-type strain (77.5% versus 95%; P = 0.0022) and Delta variant (77.5% versus 93.7%; P = 0.0053) (Figure 4).
Figure 4.
SARS-CoV-2 NT Ab titer measured at T2 against wild-type strain, Delta, and Omicron variants.
NT Ab, neutralizing antibody.
∗∗∗∗P value <0.0001.
Analysis of clinical parameters
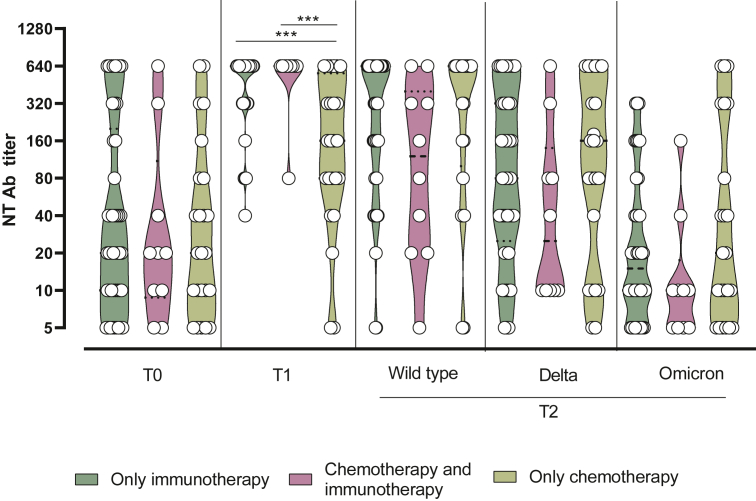
Age and sex did not affect the immune response elicited against vaccination, as well as the number of therapies administered. We observed, however, a significantly higher SARS-CoV-2 NT Ab response elicited at T1 in patients treated with only immunotherapy (median 1 : 640, IQR 1 : 320-1 : 640) and in combination with chemotherapy and immunotherapy (median 1 : 640, IQR 1 : 640-1 : 640) than the patients treated with only chemotherapy (median 1 : 160, IQR 1 : 80-1 : 320; P = 0.0004 and P = 0.0012, respectively) (Figure 5).
Figure 5.
SARS-CoV-2 NT Abs elicited by the third dose of BNT162b2 vaccine measured at T0 (before the third dose administration), T1 (3 weeks after the third dose) and T2 (6 months after the third dose) in patients treated with only immunotherapy, combination of chemotherapy and immunotherapy, and only chemotherapy.
NT Ab, neutralizing antibody.
∗∗∗P value <0.001.
Efficacy
During the follow-up period, seven patients (8%) had a confirmed post-vaccination (commonly known as ‘breakthrough’) infection [median time between the third vaccine dose and infection: 183 days (range: 105-281 days)]. The symptoms were either mild [n = 4 patients; World Health Organization (WHO) severity score 2; fever (n = 3), coryza (n = 4), anosmia (n = 1), and cough (n = 3)] or absent (n = 3 patients). Three of them received antiviral treatment (one patient) or specific monoclonal antibodies against COVID-19 (two patients). None of these patients required hospitalization or oxygen therapy (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100574).
Discussion
Our current study is a longitudinal follow-up of patients with cancer on active treatment who received the third dose of BNT162b2 COVID-19 vaccine. In our previous paper,14 we reported that the third dose of mRNA COVID-19 vaccine significantly increased IgG levels and NT Ab titers, reaching similar levels in SARS-CoV-2-naive patients to those observed in SARS-CoV-2-experienced patients after two doses of COVID-19 vaccine.
Since the onset of the COVID-19 pandemic in Wuhan (China), >12 000 mutations have been found in the SARS-CoV-2 reference sequence (hCoV-19/Wuhan/WIV04/2019).19 Specific mutations in SARS-CoV-2 S-protein have been identified in Delta (B.1.617.1 and B.1.617.2) and Omicron (BA.1/B.1.1.529) variants with higher virus infectivity and disease severity.20,21 Omicron is four times more infectious than the wild type and twice more infectious than the Delta variant,22 but the booster dose has demonstrated an increased immune response against the VOCs.6 In particular, Fendler and colleagues23 have demonstrated that SARS-CoV-2 NT Abs titers against Omicron were detectable in 37 patients with solid cancer (37%) among those who had only two doses of COVID vaccine dose, and in 104 patients (90%) among those who had a third vaccine dose.23 Overall, in patients with cancer, the third dose is able to induce a 20-fold increase in titers compared with the primary vaccine course, with a brisk B-cell anamnestic response.24
Data about the duration of the immunogenicity of the booster in patients with cancer are crucial to define the optimal boosting frequency and schedule, but they are still scare and scattered. Chevallier et al.25 have reported the waning of IgG levels concerns around 30% of 141 allo-hematopoietic stem cell transplant (allo-HSCT) recipients 6 months after the third dose of COVID-19 vaccine, although only a small proportion (19%) of these patients had IgG levels <250 BAU/ml. The benefit of the third dose of COVID vaccines in terms of seropositive rate seems more pronounced in patients with solid cancers. Ehmsen et al.26 evaluated 223 patients with solid cancers and they demonstrated that one-fifth of the patients were seronegative 3 months after the second dose but only 1 patient (<1%) was seronegative 3 months after the third dose. These results may suggest the importance of a fourth injection to enhance protection in this frail population.
Our data have highlighted the sustained benefit of the third dose: indeed, 6 months after the third dose 81 patients (98%) maintained positive spike IgG values with a higher median level than that observed 6 months after the second one, suggesting a stronger immunogenicity elicited by the third dose, also in terms of durability.
The kinetics of the humoral and cellular response showed a slight decrease during the follow up suggesting the need of a periodical boosting, but the available data do not allow us to define the most appropriate schedule. Looking at SARS-CoV-2 NT Ab titers, the median titers decreased at 6 months after the third dose and only 39/83 (47%) patients showed SARS-CoV-2 NT Abs above the upper detection limit of our assay, meaning a partial decay of neutralizing activity. Whereas only 20% of patients showed SARS-CoV-2 NT Ab titers higher than 1 : 160 6 months after the second dose, however, the percentage reached 70% 6 months after the third dose administration. As regards the cellular immunity profile, 62.3% of patients still have positive T-cell-mediated responses 6 months after the third dose.
We also observed a significantly higher SARS-CoV-2 NT Ab response elicited in patients treated with only immunotherapy. Previous papers highlighted that immune checkpoint inhibitors (ICIs) might be beneficial in amplifying antiviral T-cell immunity in the context of COVID-19 infection, with enhancement of T-cell immunity.27 In the systematic review conducted by Corti et al.,12 only a minority of patients during immunotherapy did not develop an adequate humoral response, whereas the impact of ICIs on cell-mediated immune response has still to be clarified by future studies.
Our data confirmed that the third vaccine dose of BNT162b2 is able to induce a strong immune response against the ancestral D614G variant, but also against VOCs such as the Delta and Omicron BA.1 variants, even if a significantly reduced rate of responders was observed against the BA.1 variant as also reported in other cohorts. In particular, in healthy subjects, the neutralization titers against the Omicron variant at 6 months after the booster were 6.3 times lower than at 1 month after the injection of the third dose.28 Overall, we reported only seven cases of SARS-CoV-2 infection after booster dose administration and no hospitalization was observed.
Strengths and limitations
To our knowledge, our paper is one of the first that has evaluated the immunogenicity of the third dose of the BNT162b2 vaccine by extending the follow-up to 6 months. Moreover, the strength of our paper is the simultaneous detection of both humoral and cellular immune response and we have also evaluated the activity of neutralization against the main VOCs.
The limitations of our paper consist of the lack of a control group and the small sample size that enables us to extend our conclusions in a definitive way. Valid data on T-cell responses were not obtained in all patients enrolled, so a selection bias might have occurred. Moreover, the precise correlation between the immunogenicity of COVID-19 vaccination and the vaccine efficacy has not been clarified yet. Thus, our data about the humor and cell-mediated responses cannot be directly translated to the levels of protection against COVID-19 disease.
Conclusion
Our work highlights a significant humoral and cellular immune response among patients with solid tumors 6 months after a third BNT162b2 vaccine dose, although a reduction in neutralizing activity against Omicron is observed. Given our limited sample size, future larger studies are warranted to confirm our results and to evaluate the optimal boosting schedule in patients with cancer.
Acknowledgments
Funding
This work was supported by Ricerca Corrente [grant number 08067620], Fondazione IRCCS Policlinico San Matteo, Italy, Ricerca Finalizzata [grant BIAS number 2020-12371760], and from European Commission—Horizon 2020 [EU project 101003650—ATAC].
Disclosure
The authors have declared no conflicts of interest.
Authorship
All authors contributed to the article and approved the submitted version.
Supplementary data
References
- 1.Coronavirus (COVID-19) Vaccinations Our World in Data. https://ourworldindata.org/covid-vaccinations Available at.
- 2.CDC SARS-CoV-2 Variant Classifications and Definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html Available at.
- 3.Shitrit P., Zuckerman N.S., Mor O., Gottesman B.S., Chowers M. Nosocomial outbreak caused by the SARS-CoV-2 delta variant in a highly vaccinated population, Israel, July 2021. Euro Surveill. 2021;26(39) doi: 10.2807/1560-7917.ES.2021.26.39.2100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karim S.S.A., Karim Q.A. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–2128. doi: 10.1016/S0140-6736(21)02758-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mair M.J., Mitterer M., Gattinger P., et al. Enhanced SARS-CoV-2 breakthrough infections in patients with hematologic and solid cancers due to Omicron. Cancer Cell. 2022;40(5):444–446. doi: 10.1016/j.ccell.2022.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chenchula S., Karunakaran P., Sharma S., Chavan M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: a systematic review. J Med Virol. 2022;94:2969–2976. doi: 10.1002/jmv.27697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mbaeyi S., Oliver S.E., Collins J.P. Morbidity and Mortality Weekly Report The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines - United States, 2021. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html Available at. [DOI] [PMC free article] [PubMed]
- 8.Edara V.V., Manning K.E., Ellis M., et al. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep Med. 2022;3(2) doi: 10.1016/j.xcrm.2022.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Accorsi E.K., Britton A., Fleming-Dutra K.E., et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 Omicron and Delta variants. JAMA. 2022;327(7):639–651. doi: 10.1001/jama.2022.0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee A.R.Y.B., Wong S.Y., Chai L.Y.A., et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seneviratne S.L., Yasawardene P., Wijerathne W., Somawardana B. COVID-19 vaccination in cancer patients: a narrative review. J Int Med Res. 2022;50(3) doi: 10.1177/03000605221086155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti C., Antonarelli G., Scotté F., et al. Seroconversion rate after vaccination against COVID-19 in patients with cancer-a systematic review. Ann Oncol. 2022;33(2):158–168. doi: 10.1016/j.annonc.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becerril-Gaitan A., Vaca-Cartagena B.F., Ferrigno A.S., et al. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2022;160:243–260. doi: 10.1016/j.ejca.2021.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lasagna A., Bergami F., Lilleri D., et al. Immunogenicity and safety after the third dose of BNT162b2 anti-SARS-CoV-2 vaccine in patients with cancer on active treatment: a prospective cohort study. ESMO Open. 2022;7(2) doi: 10.1016/j.esmoop.2022.100458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(suppl 1):S31–S34. doi: 10.4103/sja.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percivalle E., Cambiè G., Cassaniti I., et al. Prevalence of SARS-CoV-2 specific neutralising antibodies in blood donors from the Lodi Red Zone in Lombardy, Italy, as at 06 April 2020. Euro Surveill. 2020;25(24) doi: 10.2807/1560-7917.ES.2020.25.24.2001031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasagna A., Lilleri D., Agustoni F., et al. Analysis of the humoral and cellular immune response after of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors with or without chemotherapy: an update after six months of follow up. ESMO Open. 2021;7(1) doi: 10.1016/j.esmoop.2021.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lasagna A., Agustoni F., Percivalle E., et al. A snapshot of the immunogenicity, efficacy and safety of a full course of BNT162b2 anti-SARS-CoV-2 vaccine in cancer patients treated with PD-1/PD-L1 inhibitors: a longitudinal cohort study. ESMO Open. 2021;6(5) doi: 10.1016/j.esmoop.2021.100272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dejnirattisai W., Zhou D., Supasa P., et al. Antibody evasion by the P.1 strain of SARS-CoV-2. Cell. 2021;184:2939–2954. doi: 10.1016/j.cell.2021.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eyre D.W., Taylor D., Purver M., et al. Effect of covid-19 vaccination on transmission of alpha and delta variants. N Engl J Med. 2022;386(8):744–756. doi: 10.1056/NEJMoa2116597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386(5):494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beeraka N.M., Tulimilli S.V., Greeshma M.V., et al. COVID-19 effects on geriatric population and failures of aminoquinoline therapy: compilation of studies from EU, USA, and China; safety and efficacy of vaccines in the prevention & treatment of COVID-19. Curr Med Chem. 2022;29:3601–3621. doi: 10.2174/0929867329666220301113146. [DOI] [PubMed] [Google Scholar]
- 23.Fendler A., Shepherd S.T.C., Au L., et al. Omicron neutralising antibodies after third COVID-19 vaccine dose in patients with cancer. Lancet. 2022;399(10328):905–907. doi: 10.1016/S0140-6736(22)00147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan Q.J., Bivona C.R., Martin G.A., et al. Evaluation of the durability of the immune humoral response to COVID-19 vaccines in patients with cancer undergoing treatment or who received a stem cell transplant. JAMA Oncol. 2022;8:1053–1058. doi: 10.1001/jamaoncol.2022.0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chevallier P., Jullien M., Peterlin P., et al. Effectiveness of a third dose of BNT162b2 anti-SARS-CoV-2 mRNA vaccine over a 6-month follow-up period in allogenic hematopoietic stem cells recipients. Hematol Oncol. 2022 doi: 10.1002/hon.3006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehmsen S., Asmussen A., Jeppesen S.S., et al. Antibody responses following third mRNA COVID-19 vaccination in patients with cancer and potential timing of a fourth vaccination. Cancer Cell. 2022;40(4):338–339. doi: 10.1016/j.ccell.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yatim N., Boussier J., Tetu P., et al. Immune checkpoint inhibitors increase T cell immunity during SARS-CoV-2 infection. Sci Adv. 2021;7(34) doi: 10.1126/sciadv.abg4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pajon R., Doria-Rose N.A., Shen X., et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N Engl J Med. 2022;386(11):1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.