Abstract
Our understanding of the genomics and epigenomics of medullary thyroid carcinoma (MTC) has advanced since the initial recognition of RET as a driver of MTC tumorigenesis in familial MTC. We now have insight into the frequency and prognostic significance of specific RET mutations in sporadic MTC. For example, the most common RET mutation in sporadic MTC is the RET Met918Thr mutation, the same mutation that underlies MEN2B and a poor prognosticator. This mutation is relatively infrequent in medullary thyroid microcarcinomas but is over-represented in advanced-stage disease. RAS mutations are detected in 70% of sporadic, RET wild-type MTC. Although next-generation and whole-exome sequencing studies have shown that tumors that are wild-type for RET and RAS mutations essentially lack other recurrent mutations, additional pathways and epigenetic alterations have been implicated in MTC tumorigenesis. Increased insight into the clinical course of patients with familial MTC with specific RET mutations has guided treatment recommendations for these patients. Finally, an understanding of the genomics has informed treatment for patients with advanced MTC. In this review, we will examine the genomics and epigenomics of sporadic and familial MTC, along with the prognostic significance of molecular alterations, management of patients with germline RET mutations, and treatment strategies for MTC patients.
Keywords: Medullary thyroid carcinoma, RET, Sporadic medullary thyroid carcinoma, Familial medullary thyroid carcinoma, Medullary thyroid cancer
Introduction
Medullary thyroid carcinoma (MTC) accounts for approximately 2% of thyroid malignancies and 8% of thyroid cancer-related deaths in the USA [1, 2]. The first reported series of MTC was by Hazard in 1959 who described it as a tumor with a “solid and non-follicular histologic pattern, the presence of amyloid in the stroma, and a high incidence of lymph node metastases” [3]. Seven years later, Williams reported that MTC was derived from parafollicular cells and postulated that these cells may be responsible for the production of calcitonin [4]. In 1985, Takahashi and colleagues discovered the RET (RE arranged during transfection) oncogene [5], and by 1993–1996, it was known that RET mutations are responsible for virtually all cases of MEN2A and MEN2B and that somatic RET mutations are present in a significant proportion of sporadic MTC [6-11]. In this review, we will examine the genomics and epigenomics of sporadic and familial MTC, along with the prognostic significance of molecular alterations, management of patients with germline RET mutations, and treatment strategies for MTC patients.
Sporadic Medullary Thyroid Carcinoma
Sporadic MTC accounts for up to 75% of cases. The average age at diagnosis is 45–55 years, and there is a slight female predominance [12, 13]. Approximately half of patients with sporadic MTC have lymph node metastases at diagnosis and 15% have distant metastases [13]. Stage is the strongest independent predictor of survival on multivariate analysis [13-15], with 10-year survival rates of approximately 95%, 75%, and 40% for patients with tumors confined to the thyroid, those with regional stage disease, and those with distant metastases, respectively [15].
The most frequent molecular alterations detected in sporadic MTC are activating RET mutations. RET is a 21-exon gene located on the long arm of chromosome 10 (10q11.2) [16]. It encodes a tyrosine kinase transmembrane receptor and is involved in the normal development of the central and peripheral nervous systems as well as the genitourinary system and, in adults, is predominantly expressed in neural-crest-derived tissues [17-19]. RET activates multiple pathways including the mitogen-activated protein kinase (MAPK) and the phosphoinositide 3-kinase (PI3K) pathways (among others), which, in turn, promote cell growth, proliferation, survival, and differentiation [20]. The RET receptor is composed of an N-terminal extracellular domain (with four cadherin-like regions and a cysteine-rich region), a transmembrane domain, and a cytoplasmic domain with two tyrosine kinase domains [21, 22]. RET mutations in MTC all result in ligand-independent constitutive activation; however, the mechanism of action differs depending on the mutation. For example, the RET Cys634Arg mutation in exon 11 (a mutation in the cysteine-rich region of the extracellular domain) leads to formation of disulfide-bonded RET homodimers and subsequent constitutive activation [23], whereas the RET Met918Thr mutation in exon 16 (a mutation in the intracellular tyrosine kinase domain) results in autophosphorylation of the tyrosine kinase domain [24, 25]. Additionally, different RET mutations have different transforming activity. For example, the RET Met918Thr mutation has been shown to have higher transforming activity than other RET mutations [25].
RET mutations have been reported in approximately half of sporadic MTC [10, 26-31]. For example, in a recent large cohort of sporadic MTC analyzed by next-generation sequencing, 101 of 181 (56%) cases analyzed were found to have a RET mutation [27]. The most common RET mutation in sporadic MTC is the RET Met918Thr mutation (accounting for up to 80% of RET mutations in sporadic MTC) [10, 26-29, 31, 32]. The second-most affected codon is codon 634, with mutations at codon 634 accounting for approximately 15% of RET mutations [28]. Other RET mutations that have been reported include additional mutations in the cysteine-rich region of the extracellular domain such as mutations at codons 611 and 618 (both in exon 10) and 620 and 630 (both in exon 11) and additional mutations affecting the intracellular tyrosine kinase domains such as mutations at codons 768 and 791 (both in exon 13) and codon 883 (in exon 15) [27, 28]. Deletions and complex RET alterations occur in a small percentage of sporadic MTC [27, 33, 34]. The prevalence of RET mutations depends on the size of the tumor [28, 31]. In a study interrogating for the RET Met918Thr mutation, it was found that the rate of this mutation was significantly less in medullary thyroid microcarcinomas, with this mutation detected in 11% of tumors under 1 cm in size compared with 59% of tumors over 3 cm [31]. Moreover, the RET Met918Thr mutation has a high frequency (present in over 70% of cases) in advanced disease (defined in the referenced study as patients with histologically unresectable locally advanced or metastatic MTC that is clinically symptomatic or shows radiographic disease progression) [33]. In the vast majority of sporadic MTC, a single RET mutation is detected; however, about 7% of advanced MTC demonstrate double RET mutations [33]. Interestingly, it has also been shown that RET mutations are not uniform throughout individual tumors or consistent across metastases in sporadic MTC [34, 35]. Romei and colleagues found that in 20% of sporadic MTC, there was a different RET mutation profile when comparing the primary tumor and its corresponding metastases, and in 8% of tumors, RET intratumor heterogeneity was observed [34]. Based on these findings, it seems that RET Met918Thr mutation may not be an early event in MTC tumorigenesis, but instead a mutation that arises during clonal evolution of the tumor, occurring within an established primary tumor or within a metastatic clone. Finally, about a quarter of sporadic MTC have chromosome 10 aneuploidy (trisomy and tetrasomy) in a variable percentage of cells that results in RET copy number alterations, with a higher prevalence of RET copy number alterations detected in RET-mutant MTC [36]. Ciampi and colleagues hypothesized that chromosome 10 aneuploidy might confer a higher rate of genomic instability thus facilitating the onset of the somatic RET mutations [36].
Tumors that are wild type for RET have a significant rate of RAS mutations [26, 27, 32, 37, 38]. RAS mutations are detected in approximately 70% of RET wild-type tumors, with HRAS mutations most common, KRAS mutations occurring at a lower frequency, and rare NRAS mutations [26, 27, 32]. For example, Ciampi and colleagues evaluated 181 sporadic MTC and found that RAS alterations were present in 24%, with 17% harboring HRAS mutations, 7% with KRAS mutations, and < 1% with an NRAS mutation [27]. Although RET and RAS mutations are nearly always mutually exclusive, rare tumors have been reported to have both a RET and a RAS mutation [27, 32]. Mutations in RAS genes are generally in hotspots, that is, codon 12 and 13 (exon 2) mutations and codon 63 (exon 3) mutations; however, exon 4 (non-hotspot) mutations also occur [26]. In studies utilizing next-generation sequencing, under 20% of sporadic MTC are wild-type for both RET and RAS [27, 37].
RET status has been shown to be prognostically significant in sporadic MTC. The presence of a RET mutation has been shown to correlate with increased stage at diagnosis, residual/recurrent disease, and survival [28, 33, 39, 40]. In a study by Elisei and colleagues evaluating 100 sporadic MTC patients with a 10.2-year mean follow-up, somatic RET mutations were found in 43%, with the RET Met918Thr mutation accounting for 79% of mutations [28]. The authors found that RET mutations occurred more frequently in larger tumors and in MTC with lymph node and distant metastases; thus, there was a significant correlation between the presence of a RET mutation and more advanced stage at diagnosis. Moreover, the presence of a RET mutation independently correlated with decreased survival on multivariate analysis. In a recent meta-analysis of 23 studies with 964 sporadic MTC, the presence of a RET mutation was associated with an elevated risk of lymph node metastases (OR = 3.61), distant metastases (OR = 2.85), advanced tumor stage (OR = 3.25), tumor recurrence (OR = 3.01), and patient mortality (OR = 2.43) [40]. However, the prognosis depends on the specific RET mutation identified. Moura and colleagues showed that patients with tumors with RET mutations in exons 15 and 16 (which includes the RET Met918Thr mutation) were associated with the worst outcome, whereas cases with other RET mutations had the most indolent course, and those with no RET mutations were clinically intermediate. Thus, the poor prognosis reported for RET-mutant sporadic MTC is largely driven by the high rate of the RET Met918Thr mutation. Additionally, it has been reported that the presence of double RET mutations in patients with advanced sporadic MTC correlates with worse outcome [33]. RET copy number alterations may also be a poor prognostic factor potentiating the effect of a RET mutation [36]. The clinical significance of a RAS mutation is not established. However, Moura and colleagues reported that tumors with RAS mutations behaved less aggressively than those with RET mutations in exons 15 and 16, but more aggressively than those with other RET mutations.
Next-generation and whole-exome sequencing studies have shown that tumors that are wild type for RET and RAS mutations essentially lack other recurrent mutations [27, 37, 38, 41, 42]. However, there have been studies implicating alterations in additional pathways as well as studies demonstrating epigenetic alterations in sporadic MTC. Grubbs and colleagues demonstrated somatic copy number loss of the CDKN2C gene in approximately one fifth of tumors (half of which harbored the RET Met918Thr mutation) and found that the presence of CDKN2C loss was associated with distant metastases, overall AJCC stage, and decreased overall survival in their cohort of 62 sporadic MTC [43]. In fact, they reported that the median overall survival of patients with tumors with and without a somatic CDKN2C copy number loss was 4.1 and 18.3 years, respectively. The mTOR pathway has also been shown to be activated in sporadic MTC [44, 45]. Tamburrino and colleagues reported that mTOR pathway activation was especially prevalent in lymph node metastases in patients with sporadic MTC [45]. Lyra and colleagues showed that mTOR pathway activation was associated with lymph node metastases, but also reported increased activation in RAS-mutant MTC [44]. Overexpression of hepatocyte growth factor (HGF) and its receptor (MET) has also been shown in MTC and may be associated with tumor multifocality [46].
There are also studies that have evaluated epigenetics of MTC. For example, Sponziello and colleagues investigated the expression of epigenetic regulators in a cohort that included 41 sporadic MTC [47]. They found that more aggressive tumors (i.e., those with associated metastases, persistent disease, or disease-related death) had a significant increase in expression of the histone methyltransferases EZH2 and SMYD3. The levels of gene expression did not correlate with RET or RAS mutational status, potentially suggesting that MTC progression may depend on epigenetic factors that are independent of RET and RAS pathways. TERT promoter mutations are not present in MTC; however, TERT copy number gain and TERT promoter methylation have been reported in MTC, with TERT promoter methylation (which results in TERT expression and telomerase activation) reported to correlate with decreased disease-free and overall survival in MTC [48]. Overexpression of microRNA (miRNA) has been implicated in tumorigenesis via the down regulation of tumor suppressor genes [49]. Abraham and colleagues evaluated the miRNA profile of a cohort or sporadic and hereditary MTC and found that MiRs-183 and 375 were overexpressed in sporadic versus hereditary MTC. Moreover, they found that overexpression of miRs-183 and 375 in MTC predicted lateral lymph node metastases and was associated with residual disease, distant metastases, and mortality [50]. Additionally, Aubert and colleagues reported that tumor expression levels of miR-21 and miR-183 were significantly higher in patients with sporadic MTC associated with lymph node metastases. Finally, Coelin and colleagues evaluated global DNA methylation levels in peripheral blood leukocytes in patients with sporadic and hereditary MTC and found that the global methylation level was higher in patients with sporadic versus hereditary disease (no association was identified between methylation and mutational profile, age at diagnosis, tumor size, or metastatic disease) [51]. Although the explanation for this finding is not known, the authors speculated that a potential explanation might be that while hereditary MTC is triggered by a germline driver mutation, the development of sporadic MTC may depend more on environmental factors which affect the methylation status.
Familial (Inherited) Medullary Thyroid Carcinoma
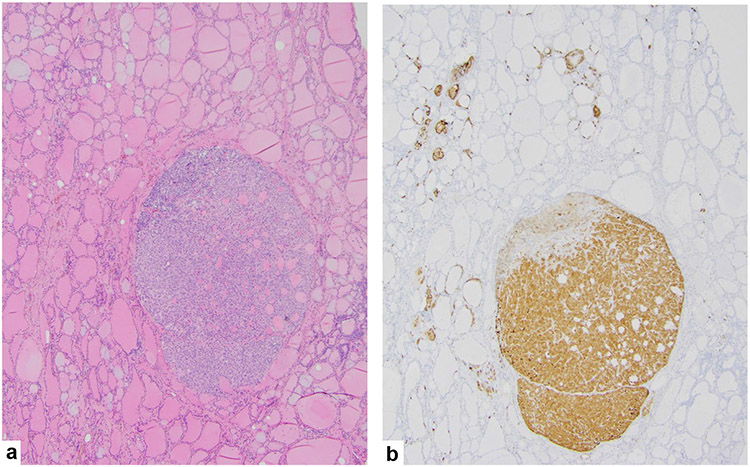
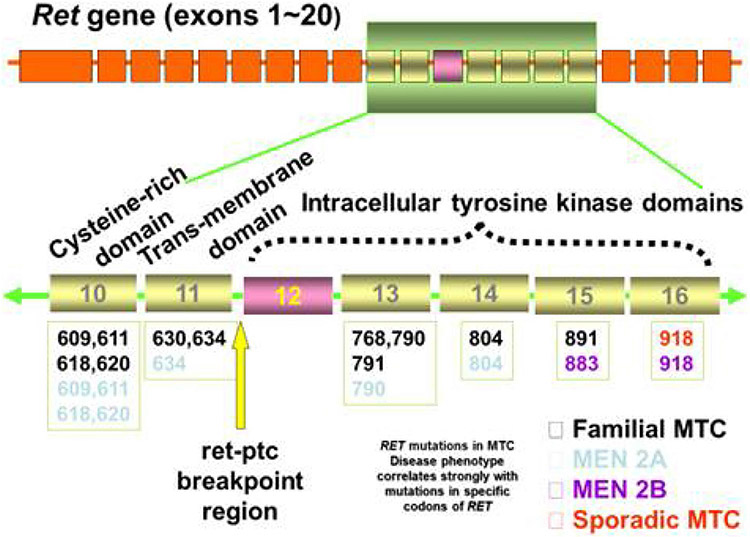
As noted, up to 75% of MTC arise spontaneously, but notoriously, this tumor type is linked with familial disease that accounts for the remaining incidence. Unlike many cancers that have loss of function mutations, these tumors harbor gain of function mutations in the RET proto-oncogene [52]. Thus, both sporadic (de novo) and inherited forms of MTC share the same driver, although there is a shifted timeframe to earlier occurrence with familial MTC. Additionally, familial MTC is often multifocal and arises in a background of C cell hyperplasia that can be highlighted with immunohistochemistry for calcitonin (Fig. 1). The inherited forms of MTC, which follow an autosomal dominant pattern of inheritance, are multiple endocrine neoplasia (MEN) types 2A and 2B and familial medullary thyroid carcinoma (FMTC), which is considered a subtype of MEN2A [53]. The more recently accepted classifications of the clinically distinct types of MEN2 syndrome are MEN2A and MEN2B. Within MEN2A, there are four variants: (1) MEN2A, classical type, represented by the presence of MTC and pheochromocytoma and/or hyperparathyroidism, or both; (2) MEN2A with cutaneous lichen amyloidosis; (3) MEN2A with Hirschsprung disease; and (4) familial medullary thyroid carcinoma-only where families or individuals present MTC-only. MEN2B accounts for 5–10% of cases of MEN2. Approximately 95% of patients with MEN2B harbor the aggressive RET M918T mutation and under 5% have an A883F mutation [54]. For patients with MEN2B, approximately 75% have de novo RET mutations, while 25% of cases occur in families with previous or current manifestations of MEN2B [54]. MEN2A comprises the remainder of MEN2 cases, with classical MEN2A the most common MEN2A variant. Of patients with classical MEN2A, 95% have RET germline mutations that occur in codons 609, 611, 618, or 620 of exon 10 or codon 634 of exon 11 [54]. See Fig. 2 for the main mutations associated with MEN2A, MEN2B, and FMTC.
Fig. 1.
A familial medullary thyroid carcinoma a arising in the setting of C cell hyperplasia highlighted with a calcitonin immunohistochemical stain b
Fig. 2.
The most common RET mutations associated with familial medullary thyroid carcinoma
MTC is the hallmark of MEN types 2A, 2B, and FMTC, with essentially all patients developing MTC if untreated [55]. In addition to MTC, patients with MEN2A may develop pheochromocytomas, hyperparathyroidism, cutaneous lichen amyloidosis, Hirschsprung disease, and/or thickened corneal nerves [55, 56]. Those with MEN2B additionally develop pheochromocytoma, mucosal neuromas, ocular features (subluxation of lens, keratoconus), Marfanoid body habitus, constipation, and ganglioneuromatosis of the gastrointestinal tract [56]. MTC is the only manifestation of FMTC. See Table 1 for a summary of clinical characteristics of syndromes. Although there are about 90 known pathogenic variants associated with MEN2, they tend to cluster in hotspots in the extracellular domain and intracellular kinase domains, the location of which determines disease manifestation and aggression of clinical course. The American Thyroid Association (ATA) risk stratifies germline mutations [54]. Mutations are categorized as highest, high, and moderate (see Table 2), with aggressiveness based on the development of MTC at an early age, frequently in association with metastatic disease.
Table 1.
Clinical characteristics of familial syndromes associated with medullary thyroid carcinoma (MTC)
| MEN-2A | MEN-2B | FMTC | |
|---|---|---|---|
| % of inherited MTC | ~ 65% | ~ 5-10% | ~ 25% |
| Presenting age (years) | 25–35 | 10–20 | Variable |
| Endocrine-related manifestations | MTC (95–100%) | MTC (100%) | MTC (100%) |
| Pheo (50%) | Pheo (50%) | ||
| HPT (20–30%) | |||
| Other manifestations | CLA (~ 30% [codon 634-mutated]); Hirschsprung disease (< 1%) | Mucosal/intestinal neuroma (100%); Marfanoid habitus (100%) | None |
| % of MTC with metastasis | 14% LN | 38% LN | Variable |
| 3% D | 20% D |
Pheo pheochromocytoma, HPT hyperparathyroidism, CLA cutaneous lichen amyloidosis, LN lymph node metastasis, D distant metastasis
Table 2.
American Thyroid Association (ATA) risk stratification for common RET mutations
| 2009 ATA risk level | 2015 revised ATA risk level | Pathogenic variants |
|---|---|---|
| D | Highest | p.M918T |
| C | High | p.A883F |
| p.C634F/G/R/S/W/Y | ||
| B | Moderate | Other pathogenic variants |
| A |
In inherited forms of MTC, the location of the mutation will determine not only the timeline of disease manifestation, but also suggest the appropriate intervention; earliest in those with MEN2B, who may manifest symptoms by 5 years of age but with evidence of C cell hyperplasia (nodular or diffuse) or medullary thyroid microcarcinoma even earlier [57]. As the vast majority of patients with MEN2B harbor the aggressive RET M918T mutation, if unrecognized, they typically die by the age of 21. They may be known to be at risk from birth, allowing for early surgical intervention within the first year or first months of life with total thyroidectomy and central lymph node dissection [54]. Most of these patients will have a minimum of C cell hyperplasia even before the age of 5. MEN2A patients present with MTC between 25 and 35 years of age, with lymph node metastases present in over 70%. If MEN2A patients are known, they will often start having serum calcitonin screens at birth, with total thyroidectomy before the age of 5 based on serum calcitonin levels or once serum calcitonin levels become elevated. Intervention may be done earlier by parental request, as these patients will inevitably develop MTC [54]. The age of onset in MEN2A is determined by the mutation present, with those with the exon 11 RET C634 mutation demonstrating an earlier onset than all other mutations (largely in exon 10). About 50% of these patients develop pheochromocytoma by the 5th decade with increasing incidence from there [58]. Hyperparathyroidism develops in approximately 30% of patients with variants involving C634. Given the clinical variability that depends on specific mutation present, it is recommended that all patients with a diagnosis of MTC have genetic testing performed. This is particularly important in the case of FMTC in which the only manifestation is MTC that presents at a mean of 45–55 years of age, that is, at an age similar to that seen with spontaneously occurring disease [57]. Regarding MEN2, a recent review proposed a “5P” strategy for MEN2 management involving prevention, prediction, personalization, psychological support, and participation in order to improve clinical outcomes [56]. This is particularly helpful as this constellation of diseases of which MTC is at the forefront have variable onset and manifestations, particularly from a young age, which will require adaptation by patients and their families. Moreover, early detection in familial cases of MTC presents a host of early interventional opportunities with a keen awareness of concomitant or eventually to manifest disease processes that will not be eliminated by early thyroidectomy. Moreover, given the phenotypic spectrum with different germline RET mutations, the personalization aspect is of particular importance. Treatment strategies for MTC (described below) are similar whether the tumor is sporadic or familial. Obviously, the greatest chance for cure for MTC is the earliest possible intervention, which will be mutation-based. If total thyroidectomy with central neck dissection demonstrates C cell hyperplasia only or even medullary thyroid microcarcinoma with no evidence of nodal disease, a poor outcome has been avoided. Finally, although RET mutations underlie virtually all inherited forms of MTC, the identification of pathogenic variants in MET (p.Arg417Gln; 7q31.2) involving the extracellular Serna domain of the MET gene in two siblings with inherited MTC and wild-type RET has expanded the germline correlates of this disease [59].
Treatment of Medullary Thyroid Carcinoma
An understanding of the genomics has informed treatment strategies for MTC patients, with treatment modalities for advanced MTC becoming remarkably refined. However, first, it is important to recognize that not all patients with metastatic disease receive systemic therapy. When possible, metastases are surgically resected. Moreover, surveillance is appropriate for patients with elevated tumor markers only or a small metastatic burden. As indicated in the revised American Thyroid Association (ATA) guidelines on the management of MTC, “considering that metastatic MTC is incurable, the management goals are to provide loco-regional disease control, palliate symptoms of hormonal excess (such as diarrhea or Cushing’s syndrome), palliate symptomatic metastases (such as pain or bone fracture), and control metastases that threaten life (such as bronchial obstruction or spinal cord compression)” [54]. Thus, at this time, systemic treatment is typically reserved for patients with significant tumor burden and symptomatic or progressive metastatic disease according to RECIST (growth rate of MTC can be estimated from sequential imaging studies using response evaluation criteria in solid tumors [RECIST] that document incremental increases in tumor size over time) [54, 60].
Because cytotoxic chemotherapeutic regimens had low response rates [54], there was a need for targeted therapies for MTC. In 2011 and 2012 (on the basis of the phase III clinical trials that are described below), the Food and Drug Administration (FDA) approved two multi-kinase inhibitors (MKI) vandetanib and cabozantinib for the treatment of patients with advanced progressive MTC. Vandetanib is an oral inhibitor of RET kinase, vascular endothelial growth factor receptor, and epidermal growth factor receptor signaling. The prospective, randomized, double-blind, phase III trial conducted to compare the efficacy of vandetanib to placebo included 331 MTC patients and demonstrated a significantly improved progression-free survival (PFS) for the vandetanib arm (30.5 months, predicted median PFS) versus the placebo arm (19.3-month median PFS) [61]. Moreover, objective responses were observed in 45% of vandetanib-treated patients, with a predicted median duration of response of 22 months. RET mutation status was also evaluated in this trial. A RET mutation was present in 52%, no RET mutation was present in 3%, and the RET mutation status was unknown in 45% (there was a high number of patients with unknown RET mutation status because of inadequate DNA for analysis). The small number of RET-negative patients meant that subgroup analyses of PFS and objective response rate by RET mutation status were inconclusive. However, in patients with sporadic MTC, a subgroup analysis of PFS by M918T mutation suggested that M918T mutation-positive patients had a higher response rate to vandetanib compared with M918T mutation-negative patients [61]. Cabozantinib is a tyrosine kinase inhibitor of hepatocyte growth factor receptor (MET), vascular endothelial growth factor receptor 2, and RET. A double-blind, phase III trial was conducted to compare cabozantinib with placebo in 330 patients with documented radiographic progression of metastatic MTC [62]. The study demonstrated an estimated median PFS of 11.2 months for cabozantinib versus 4.0 months for placebo. The response rate was 28% for cabozantinib, with a median estimated duration of response of 14.6 months. Prolonged PFS with cabozantinib was observed regardless of RET mutation status [62]; however, the results of a subsequent exploratory analysis of the phase 3 trial data that evaluated the influence of RET and RAS mutations on cabozantinib clinical activity suggested that cabozantinib provides the greatest clinical benefit in patients with MTC who have RET M918T or RAS mutations [63]. Despite these findings, there are currently no recommendations for treatment with vandetanib or cabozantinib based on RET/RAS status.
Although MKI therapy was a major advance, these drugs have overall modest response rates and significant toxicities. Moreover, with time, virtually all patients cease to respond to these drugs. As a result, there is interest in identifying other drugs to treat advanced MTC. Selpercatinib (formerly known as LOXO-292) is a selective RET inhibitor that inhibits activating RET mutations, RET fusions, and RET-acquired resistance mutations. Selpercatinib has been shown to demonstrate potent and selective anti-RET activity preclinically and was shown to be efficacious in a patient with RET M918T-mutant MTC metastatic to the liver and an acquired RET V804M gatekeeper resistance mutation [64]. A recent phase 1–2 trial evaluated selpercatinib in a cohort of 531 patients, including patients with RET-mutant MTC with or without a history of previous MKI therapy [65]. In 88 patients with RET-mutant MTC who had not previously received these therapies, the response rate was 73%, and 1-year progression-free survival was 92% (95% CI, 82 to 97). For the 55 patients with RET-mutant MTC who had previously received a MKI, 69% demonstrated a complete or partial response and the 1-year progression-free survival was 82%. Only 2% of patients discontinued treatment due to drug-related adverse events. The most common adverse event (not necessarily resulting in discontinuation) was hypertension. BLU-667 is a second selective RET inhibitor that in vitro has demonstrated at least tenfold increased potency over approved MKI against oncogenic RET variants and resistance mutants [66]. Clinical trials for selpercatinib and BLU-667 are accruing patients (ClinicalTrials.gov, identifiers NCT03157128, and NCT03037385, respectively); thus, more data will be available in the future. However, the results thus far are encouraging, suggesting that tumors that have ceased to respond to vandetanib and/or cabozantinib may show durable responses to targeted RET inhibitors even in the context of acquired resistance mutations. Beyond RET inhibition, based on evidence of mTOR pathway activation in MTC, there has been interest in targeting the mTOR pathway. Small phase 2 trials evaluating the efficacy of everolimus, an mTOR inhibitor, in patients with progressive metastatic or inoperable MTC reported limited results [67, 68]. However, it may be that a dual targeting strategy utilizing a RET kinase inhibitor and an mTOR inhibitor might be more effective. For example, one MTC patient with progressive disease who received everolimus in combination with vandetanib showed a 25% tumor reduction [42]. Further clinical trials are needed to evaluate this treatment strategy. Finally, immune checkpoint inhibitors have not played a large role in MTC treatment thus far, although combination trials are underway to investigate stimulating this response [69].
Conclusion
It has been almost two decades since RET mutations were described in patients with MEN2A and MEN2B. Since that time, our understanding of the genomics and epigenomics of familial and sporadic MTC has deepened. An evolution in our ability to subtype disease and predict behavior and response to treatment has paralleled advances in molecular diagnostics. Further, a revolution in the development of small molecule pharmaceuticals that target specific gene products and gene-driven pathways without disruptions of broader, less specific physiologic functions has provided an opportunity for a more effective, more durable, and less morbid approach to treatment of patients with advanced MTC.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
References
- 1.Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA 317: 1338–1348, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P Thyroid Cancer Incidence Trends in the United States: Association with Changes in Professional Guideline Recommendations. Thyroid 30: 1132–1140, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Hazard JB, Hawk WA, Crile G Jr. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J Clin Endocrinol Metab 19: 152–161, 1959. [DOI] [PubMed] [Google Scholar]
- 4.Williams ED Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol 19: 114–118, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi M, Ritz J, Cooper GM Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 42: 581–588, 1985. [DOI] [PubMed] [Google Scholar]
- 6.Carlson KM, Dou S, Chi D et al. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci U S A 91: 1579–1583, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donis-Keller H, Dou S, Chi D et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet 2: 851–856, 1993. [DOI] [PubMed] [Google Scholar]
- 8.Eng C, Smith DP, Mulligan LM et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet 3: 237–241, 1994. [DOI] [PubMed] [Google Scholar]
- 9.Hofstra RM, Landsvater RM, Ceccherini I et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature 367: 375–376, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Marsh DJ, Learoyd DL, Andrew SD et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinoma. Clin Endocrinol (Oxf) 44: 249–257, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Mulligan LM, Kwok JB, Healey CS et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature 363: 458–460, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 88: 1139–1148, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Modigliani E, Cohen R, Campos JM et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d’etude des tumeurs a calcitonine. Clin Endocrinol (Oxf) 48: 265–273, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Kuo EJ, Sho S, Li N, Zanocco KA, Yeh MW, Livhits MJ Risk Factors Associated With Reoperation and Disease-Specific Mortality in Patients With Medullary Thyroid Carcinoma. JAMA Surg 153: 52–59, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman S, Lin R, Sosa JA Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107: 2134–2142, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Ishizaka Y, Itoh F, Tahira T et al. Human ret proto-oncogene mapped to chromosome 10q11.2. Oncogene 4: 1519–1521, 1989. [PubMed] [Google Scholar]
- 17.Avantaggiato V, Dathan NA, Grieco M et al. Developmental expression of the RET protooncogene. Cell Growth Differ 5: 305–311, 1994. [PubMed] [Google Scholar]
- 18.Pasini B, Hofstra RM, Yin L et al. The physical map of the human RET proto-oncogene. Oncogene 11: 1737–1743, 1995. [PubMed] [Google Scholar]
- 19.Takahashi M, Buma Y, Iwamoto T, Inaguma Y, Ikeda H, Hiai H Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene 3: 571–578, 1988. [PubMed] [Google Scholar]
- 20.Romei C, Ciampi R, Elisei R A comprehensive overview of the role of the RET proto-oncogene in thyroid carcinoma. Nat Rev Endocrinol 12: 192–202, 2016. [DOI] [PubMed] [Google Scholar]
- 21.Anders J, Kjar S, Ibanez CF Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem 276: 35808–35817, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Ibanez CF Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol 5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asai N, Iwashita T, Matsuyama M, Takahashi M Mechanism of activation of the ret proto-oncogene by multiple endocrine neoplasia 2A mutations. Mol Cell Biol 15: 1613–1619, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gujral TS, Singh VK, Jia Z, Mulligan LM Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2B. Cancer Res 66: 10741–10749, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Salvatore D, Melillo RM, Monaco C et al. Increased in vivo phosphorylation of ret tyrosine 1062 is a potential pathogenetic mechanism of multiple endocrine neoplasia type 2B. Cancer Res 61: 1426–1431, 2001. [PubMed] [Google Scholar]
- 26.Boichard A, Croux L, Al Ghuzlan A et al. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J Clin Endocrinol Metab 97: E2031–2035, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciampi R, Romei C, Ramone T et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience 20: 324–336, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elisei R, Cosci B, Romei C et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab 93: 682–687, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Mian C, Pennelli G, Barollo S et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratification. Eur J Endocrinol 164: 971–976, 2011. [DOI] [PubMed] [Google Scholar]
- 30.Moura MM, Cavaco BM, Pinto AE et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer 100: 1777–1783, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romei C, Ugolini C, Cosci B et al. Low prevalence of the somatic M918T RET mutation in micro-medullary thyroid cancer. Thyroid 22: 476–481, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Moura MM, Cavaco BM, Pinto AE, Leite V High prevalence of RAS mutations in RET-negative sporadic medullary thyroid carcinomas. J Clin Endocrinol Metab 96: E863–868, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Romei C, Casella F, Tacito A et al. New insights in the molecular signature of advanced medullary thyroid cancer: evidence of a bad outcome of cases with double RET mutations. J Med Genet 53: 729–734, 2016. [DOI] [PubMed] [Google Scholar]
- 34.Romei C, Ciampi R, Casella F et al. RET mutation heterogeneity in primary advanced medullary thyroid cancers and their metastases. Oncotarget 9: 9875–9884, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eng C, Mulligan LM, Healey CS et al. Heterogeneous mutation of the RET proto-oncogene in subpopulations of medullary thyroid carcinoma. Cancer Res 56: 2167–2170, 1996. [PubMed] [Google Scholar]
- 36.Ciampi R, Romei C, Cosci B et al. Chromosome 10 and RET gene copy number alterations in hereditary and sporadic Medullary Thyroid Carcinoma. Mol Cell Endocrinol 348: 176–182, 2012. [DOI] [PubMed] [Google Scholar]
- 37.Agrawal N, Jiao Y, Sausen M et al. Exomic sequencing of medullary thyroid cancer reveals dominant and mutually exclusive oncogenic mutations in RET and RAS. J Clin Endocrinol Metab 98: E364–369, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simbolo M, Mian C, Barollo S et al. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch 465: 73–78, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Schilling T, Burck J, Sinn HP et al. Prognostic value of codon 918 (ATG-->ACG) RET proto-oncogene mutations in sporadic medullary thyroid carcinoma. Int J Cancer 95: 62–66, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Vuong HG, Odate T, Ngo HTT et al. Clinical significance of RET and RAS mutations in sporadic medullary thyroid carcinoma: a meta-analysis. Endocr Relat Cancer 25: 633–641, 2018. [DOI] [PubMed] [Google Scholar]
- 41.Chang YS, Chang CC, Huang HY, Lin CY, Yeh KT, Chang JG Detection of Molecular Alterations in Taiwanese Patients with Medullary Thyroid Cancer Using Whole-Exome Sequencing. Endocr Pathol 29: 324–331, 2018. [DOI] [PubMed] [Google Scholar]
- 42.Heilmann AM, Subbiah V, Wang K et al. Comprehensive Genomic Profiling of Clinically Advanced Medullary Thyroid Carcinoma. Oncology 90: 339–346, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grubbs EG, Williams MD, Scheet P et al. Role of CDKN2C Copy Number in Sporadic Medullary Thyroid Carcinoma. Thyroid 26: 1553–1562, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyra J, Vinagre J, Batista R et al. mTOR activation in medullary thyroid carcinoma with RAS mutation. Eur J Endocrinol 171: 633–640, 2014. [DOI] [PubMed] [Google Scholar]
- 45.Tamburrino A, Molinolo AA, Salerno P et al. Activation of the mTOR pathway in primary medullary thyroid carcinoma and lymph node metastases. Clin Cancer Res 18: 3532–3540, 2012. [DOI] [PubMed] [Google Scholar]
- 46.Papotti M, Olivero M, Volante M et al. Expression of Hepatocyte Growth Factor (HGF) and its Receptor (MET) in Medullary Carcinoma of the Thyroid. Endocr Pathol 11: 19–30, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Sponziello M, Durante C, Boichard A et al. Epigenetic-related gene expression profile in medullary thyroid cancer revealed the overexpression of the histone methyltransferases EZH2 and SMYD3 in aggressive tumours. Mol Cell Endocrinol 392: 8–13, 2014. [DOI] [PubMed] [Google Scholar]
- 48.Wang N, Kjellin H, Sofiadis A et al. Genetic and epigenetic background and protein expression profiles in relation to telomerase activation in medullary thyroid carcinoma. Oncotarget 7: 21332–21346, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volinia S, Calin GA, Liu CG et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103: 2257–2261, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abraham D, Jackson N, Gundara JS et al. MicroRNA profiling of sporadic and hereditary medullary thyroid cancer identifies predictors of nodal metastasis, prognosis, and potential therapeutic targets. Clin Cancer Res 17: 4772–4781, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Ceolin L, Goularte APP, Ferreira CV, Romitti M, Maia AL Global DNA methylation profile in medullary thyroid cancer patients. Exp Mol Pathol 105: 110–114, 2018. [DOI] [PubMed] [Google Scholar]
- 52.Santoro M, Carlomagno F, Romano A et al. Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science 267: 381–383, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Larouche V, Akirov A, Thomas CM, Krzyzanowska MK, Ezzat S A primer on the genetics of medullary thyroid cancer. Curr Oncol 26: 389–394, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells SA Jr., Asa SL, Dralle H et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25: 567–610, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guilmette J, Nose V Hereditary and familial thyroid tumours. Histopathology 72: 70–81, 2018. [DOI] [PubMed] [Google Scholar]
- 56.Li SY, Ding YQ, Si YL, Ye MJ, Xu CM, Qi XP 5P Strategies for Management of Multiple Endocrine Neoplasia Type 2: A Paradigm of Precision Medicine. Front Endocrinol (Lausanne) 11: 543246, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nose V Diagnostic Familial Cancer Syndromes: 170–187, 2020. [Google Scholar]
- 58.Wells SA Jr. Advances in the management of MEN2: from improved surgical and medical treatment to novel kinase inhibitors. Endocr Relat Cancer 25: T1–T13, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sponziello M, Benvenuti S, Gentile A et al. Whole exome sequencing identifies a germline MET mutation in two siblings with hereditary wild-type RET medullary thyroid cancer. Hum Mutat 39: 371–377, 2018. [DOI] [PubMed] [Google Scholar]
- 60.Therasse P, Arbuck SG, Eisenhauer EA et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Wells SA Jr., Robinson BG, Gagel RF et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30: 134–141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elisei R, Schlumberger MJ, Muller SP et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 31: 3639–3646, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sherman SI, Clary DO, Elisei R et al. Correlative analyses of RET and RAS mutations in a phase 3 trial of cabozantinib in patients with progressive, metastatic medullary thyroid cancer. Cancer 122: 3856–3864, 2016. [DOI] [PubMed] [Google Scholar]
- 64.Subbiah V, Velcheti V, Tuch BB et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol 29: 1869–1876, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wirth LJ, Sherman E, Robinson B et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med 383: 825–835, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Subbiah V, Gainor JF, Rahal R et al. Precision Targeted Therapy with BLU-667 for RET-Driven Cancers. Cancer Discov 8: 836–849, 2018. [DOI] [PubMed] [Google Scholar]
- 67.Lim SM, Chang H, Yoon MJ et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann Oncol 24: 3089–3094, 2013. [DOI] [PubMed] [Google Scholar]
- 68.Schneider TC, de Wit D, Links TP et al. Beneficial Effects of the mTOR Inhibitor Everolimus in Patients with Advanced Medullary Thyroid Carcinoma: Subgroup Results of a Phase II Trial. Int J Endocrinol 2015: 348124, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castellone MD, Melillo RM RET-mediated modulation of tumor microenvironment and immune response in multiple endocrine neoplasia type 2 (MEN2). Endocr Relat Cancer 25: T105–T119, 2018. [DOI] [PubMed] [Google Scholar]