Abstract
Tumour budding (TB) has been associated with adverse clinicopathological factors and poor survival in a plethora of therapy‐naïve carcinoma entities including gastric adenocarcinoma (GC). As conventional histopathological grading is usually omitted in the post‐neoadjuvant setting of GC, our study aimed to investigate the prognostic impact of TB in GCs resected after neoadjuvant therapy. We evaluated TB according to the criteria from the International Tumour Budding Consensus Conference (ITBCC) in 167 post‐neoadjuvant resections of intestinal‐type GC and correlated the results with overall survival (OS) and clinicopathological parameters. GCs were categorised into Bd1 (0–4 buds, low TB), Bd2 (5–9 buds, intermediate TB), and Bd3 (≥10 buds, high TB). Carcinomas with intermediate and high TB were significantly enriched in higher ypTNM stages and strongly associated with reduced 5‐year OS in univariable analyses (p < 0.001). In multivariable analyses including sex, age, resection status, UICC stage, and tumour regression grading, TB remained a stage‐independent predictor of survival (p < 0.001, hazard ratio Bd2: 2.60, Bd3: 4.74). The assessment of TB according to the ITBCC criteria provides valuable prognostic information in the post‐neoadjuvant setting of intestinal‐type GC and may be a considerable substitute for the conventional grading system in GCs after neoadjuvant therapy.
Keywords: gastric adenocarcinoma, tumour budding, neoadjuvant therapy, prognosis
Introduction
Gastric adenocarcinoma (GC) is the fourth most common cause of cancer‐related deaths worldwide [1]. Even surgically resectable cases are often diagnosed in clinically advanced stages that require neoadjuvant treatment, which usually means administration of platinum‐based neoadjuvant chemotherapy [2, 3, 4, 5].
Tumour budding (TB) is defined as the presence of invasive single cells or tumour cell complexes <5 cells surrounded by desmoplastic stroma and has been discussed as a morphological correlate of epithelial–mesenchymal transition [6, 7, 8]. Numerous studies across various carcinoma entities throughout the body have identified TB as an independent predictor of poor survival in therapy‐naïve tumours [7, 9, 10, 11, 12, 13, 14, 15, 16, 17]. Results from recent post‐neoadjuvant studies investigating TB in oesophageal squamous cell carcinoma and rectal adenocarcinoma suggest that even after neoadjuvant treatment, where conventional histopathological grading is usually omitted, the prognostic power of TB is retained [18, 19, 20, 21].
For GC, several studies were able to reproduce the strong prognostic power of TB in primary resected cancers [16, 22, 23]. However, there are no available studies that investigated TB in GCs after neoadjuvant treatment, where additional reliable prognostic factors are urgently needed.
To address this question, we analysed TB using the standardised criteria recommended by the International Tumour Budding Consensus Conference (ITBCC) [6] in a cohort of 167 intestinal‐type GCs resected after neoadjuvant chemotherapy and correlated the results with ypTNM‐stage, tumour regression grade, and 5‐year overall survival (OS).
Materials and methods
Cohort
We investigated a cohort of 167 patients with resected GC including tumours of the gastro‐oesophageal junction (AEG II and AEG III according to Siewert and Stein [24]), who received neoadjuvant chemotherapy prior to their resection. The patients underwent resection at the University Hospital Rechts der Isar of the Technical University of Munich or the University Hospital Heidelberg and were part of previously described GC cohorts [25, 26]. This study was approved by the local ethical committees of the University Hospital Heidelberg (reference: 301/2001) and of the University Hospital Rechts der Isar of the Technical University of Munich (reference number: 503/16 s).
Only patients with intestinal‐ (and indeterminate) type adenocarcinomas according to the Lauren classification (papillary, tubular, and solid according to the WHO classification) were considered in this study [27, 28]. Carcinomas with a diffuse or mixed histology according to the Lauren classification that showed parts of a diffuse/signet‐ring cell carcinoma were excluded from this study as they per se show diffuse and infiltrative growth suggesting a high TB activity.
Survival data as well as clinicopathological characteristics from all patients were extracted from local cancer registries or from hospital records; events were recorded as described previously [29]. OS was defined as the time between the date of surgery and death of any cause. Follow‐up of all patients with no events was censored after 60 months. Median age was 63.9 years (range: 30–86 years). One hundred and forty‐seven patients were male (88%) and 20 were female (12%). One hundred and twenty‐five tumours involved the proximal stomach (AEG II/II, cardia region, fundus; 75%) and 42 tumours were located distally within the stomach (corpus/antrum). All patients received neoadjuvant treatment with platinum/5 FU based chemotherapeutic regimens with or without taxane or anthracycline (for details, see supplementary material, Table S1). Incomplete therapy cycles were not documented. ypTNM staging was performed according to the eighth edition of the TNM classification of malignant tumours [30]. Following the resection, 22 cases were UICC stage I (13.2%), 59 were stage II (35.3%), 57 were stage III (34.1%), and 29 were stage IV (17.4%). The microsatellite status was available for 93% of the patients. After neoadjuvant treatment, tumour regression was determined according to Becker et al [31] (TRG1a: complete response, n = 0/167; TRG1b: less than 10% viable tumour, n = 19/167, 11.4%; TRG2: 10–50% viable tumour, n = 67/167, 40.1%; TRG3: more than 50% viable tumour, n = 81/167, 48.5%). Adjuvant therapy following the resection was documented for 25 patients (chemotherapy: n = 23, radiochemotherapy: n = 2). Microsatellite instability analysis was performed as described previously [26]. The detailed clinicopathological characteristics are given in Table 1.
Table 1.
Clinicopathological cohort characteristics in association with 5‐year OS
| Overall | Mean OS (95% CI) | Median OS (95% CI) | P value (log‐rank) | |
|---|---|---|---|---|
| 167 (100%) | ||||
| Age (median: 63.9 years; range: 30–86 years) | 0.406* | |||
| Gender | 0.055 | |||
| Male | 147 (88%) | 36.1 (32.2–40.1) | 32.2 (19.7–44.79) | |
| Female | 20 (12%) | 46.4 (37.9–54.9) | Not reached | |
| Location | 0.559 | |||
| Cardia/fundus | 125 (74.9%) | 36.7 (32.5–40.9) | 35.9 (21.0–50.7) | |
| Corpus | 24 (14.4%) | 41.7 (32.9–50.4) | Not reached | |
| Antrum | 18 (10.7%) | 37.6 (25.2–50.0) | Not reached | |
| ypT | <0.001 | |||
| 1 | 13 (7.8%) | 55.1 (46.0–60.0) | Not reached | |
| 2 | 25 (15%) | 48.6 (41.1–56.1) | Not reached | |
| 3 | 102 (61.1%) | 36.7 (32.2–41.2) | 35.1 (21.5–48.6) | |
| 4 | 27 (16.2%) | 22.1 (12.9–31.2) | 13.3 (3.9–22.6) | |
| ypN | <0.001 | |||
| 0 | 53 (31.7%) | 50.3 (44.9–55.6) | Not reached | |
| 1 | 41 (24.6%) | 37.6 (30.8–44.3) | 38.7 (24.5–52.8) | |
| 2 | 34 (20.4%) | 31.9 (24.0–39.7) | 24.9 (17.6–32.1) | |
| 3 | 39 (23.4%) | 25.8 (18.6–32.8) | 17.1 (10.8–23.3) | |
| ypM | <0.001 | |||
| 0 | 138 (82.6%) | 40.6 (36.6–44.5) | Not reached | |
| 1 | 29 (17.4%) | 24.4 (17.1–31.5) | 18.1 (16.4–19.7) | |
| UICC stage | <0.001 | |||
| 1 | 22 (13.2%) | 53.1 (45.6–60.5) | Not reached | |
| 2 | 59 (35.3%) | 44.9 (39.6–50.2) | Not reached | |
| 3 | 57 (34.1%) | 31.1 (24.7–37.6) | 25.3 (13.3–37.2) | |
| 4 | 29 (17.4%) | 24.4 (17.1–31.5) | 18.1 (16.4–19.7) | |
| Resection status | 0.002 | |||
| R0 | 132 (79%) | 40.3 (36.3–44.4) | Not reached | |
| R1 | 35 (21%) | 26.2 (19.2–33.1) | 17.1 (8.8–25.3) | |
| Microsatellite status | 0.200 | |||
| MSS | 143 (85.6%) | 35.9 (32.0–39.9) | 32.2 (21.7–42.7) | |
| MSI‐H | 12 (7.2%) | 45.2 (32.8–57.6) | Not reached | |
| Not available | 12 (7.2%) | |||
| Tumour regression grade | 0.405 | |||
| 1b | 19 (11.4%) | 43.9 (34.8–52.8) | Not reached | |
| 2 | 67 (40.1%) | 36.9 (31.4–42.5) | 31.1 (12.3–49.8) | |
| 3 | 81 (48.5%) | 36.4 (30.8–42.0) | 38.7 (21.8–55.6) |
Age is a continuous variable, P value for survival therefore calculated with a univariable Cox regression.
Histopathological evaluation
Haematoxylin and eosin‐stained slides of the GCs resected after neoadjuvant therapy were evaluated by an experienced gastrointestinal pathologist (MJ), who was blinded to all clinicopathological data. An Olympus BX46 microscope (Olympus, Shinjuku, Tokyo, Japan) with a field diameter of 0.55 mm (0.24 mm2, ×40) was used for all histopathological analyses. Appropriate normalisations (normalisation factor 1.21) as recommend by the ITBCC consensus paper [6] were performed. A defined set of 30 randomly selected cases was also investigated by a second pathologist (AG), who was blinded to the results of the initial observation and to all clinicopathological parameters.
TB was defined as the presence of small, stroma invasive tumour complexes of less than 5 tumour cells (1–4 cells) surrounded by a desmoplastic stromal reaction. The extent of TB was scored in analogy to the scoring algorithm proposed for colorectal cancer by the ITBCC from 2016, which has already been used for the assessment of TB in GCs without prior neoadjuvant treatment [6, 16].
All the available tumour‐carrying slides of each case were initially investigated at scanning magnification in order to identify the tumour area suspicious of the highest budding activity. TB activity was then assessed at ×20 objective magnification and the number of tumour buds was manually counted. Tumours that showed a range of 0–4 tumour buds were assigned to the Bd1 category (low TB), tumours that showed a maximal budding activity of 5–9 buds were classified as Bd2 (intermediate TB), and highly dissociative cancers that showed 10 or more tumour buds at ×20 magnification were assigned to the Bd3 category (high TB). Because a clear invasive front was not always identifiable in all cases because of the regressive effects caused by neoadjuvant chemotherapy (mainly in TRG1b/TRG2 cancers), all tumour regions were considered for the assessment of TB in this post‐neoadjuvant setting. Examples of the three ITBCC TB subgroups are shown in Figure 1.
Figure 1.
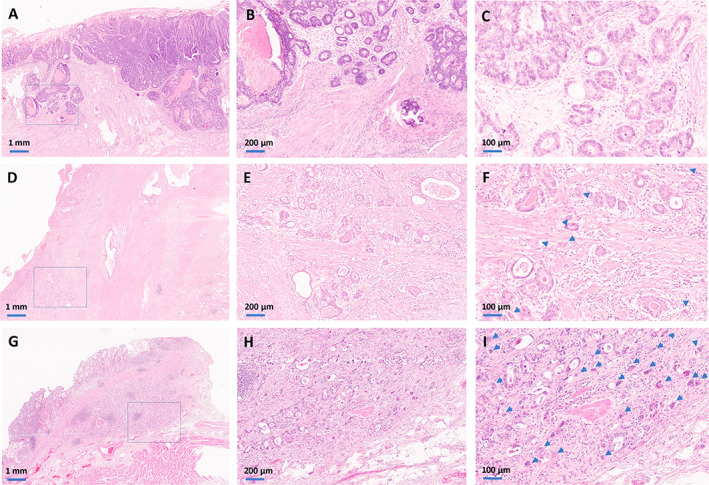
Histopathology of the ITBCC TB subgroups in resected intestinal‐type GC after neoadjuvant therapy. (A–C) The upper panels show an adenocarcinoma from the Bd1 subgroup. Scanning magnification (×2, A) of an adenocarcinoma with minimal regressive changes and a pushing pattern of invasion. The box shows the tumour area that was suspected to show the highest degree of dissociative growth, which is seen in ×10 magnification in the middle panel (B). In the ×20 magnification (C), we see that there are no cell clusters consisting of less than five cells. (D–F) The central panels show an adenocarcinoma from the Bd2 subgroup. The scanning magnification (×2, D) shows a tubular adenocarcinoma with moderate regressive changes. The box shows the tumour area that was suspicious of showing the highest budding activity, which is seen in ×10 magnification in the centre (E). In the ×20 magnification (F), an intermediate TB activity is observed with more than five, but less than 10 tumour buds (arrows). (G–I) The lower panels show an adenocarcinoma from the Bd3 subgroup. The scanning magnification (×2, G) shows a pT1 tumour, where the partially dissociative growth can already be appreciated. The box shows the tumour area that was suspicious of showing the highest budding activity, which is seen in ×10 magnification in the centre (H). In the ×20 magnification (I), a high TB activity is seen with far more than 10 tumour buds (arrows).
Statistics
The distribution of qualitative data is presented by absolute and relative frequencies. Hypothesis tests of associations of morphological characteristics with clinicopathological parameters were performed by chi‐square test and Fisher's exact tests (two‐sided). Univariable survival probabilities were estimated with the Kaplan–Meier method and log‐rank tests were used to probe the statistical significance of differences. Mean and median survival is presented with 95% confidence intervals (CIs). Hazard ratio (HR) for univariable survival analyses was determined using the univariate Cox proportional hazards regression model. Multivariable survival analysis was performed with the Cox proportional hazards model and respective effect estimates of the HR are presented with 95% CIs. Cohen's kappa was used to estimate interobserver agreement. Exploratory P values of ≤0.05 were considered statistically significant. Analyses were performed with IBM SPSS Statistics Version 27.0. (IBM Corp, Armonk, NY, USA).
Results
Impact of classical clinicopathological parameters on patient survival
As expected, neoadjuvant ypT, ypN, and ypM and also the resulting UICC stage (p < 0.001, respectively) as well as resection status (p = 0.002) were strongly related to patient survival. Females trended towards a better survival outcome (p = 0.055). Tumour regression grade was not associated with patient survival; however, only 11% of the patients were in the TRG1b subgroup and (naturally) no complete responders were included. No survival advantages were observed for those patients for whom an adjuvant therapy following the resection was documented (p = n.s.). Detailed survival information regarding the common clinicopathological variables is provided in Table 1.
Frequency of ITBCC TB subgroups and association with clinicopathological parameters
Out of the 167 resected intestinal‐type adenocarcinomas after neoadjuvant treatment, 49 tumours were assigned to the Bd1 (low TB, 0–4 buds, 29%) category, 41 cancers fell into the Bd2 (intermediate TB, 5–9 buds, 25%) category, and 77 neoplasms were allocated to the Bd3 (high TB, ≥10 buds, 46%) category. Tumours with high TB activity were more likely to show advanced ypT (p < 0.001), ypN (p = 0.045), and ypM stages (p = 0.050), and were therefore naturally associated with higher UICC stages (p < 0.001). An R1 resection was far more frequent in Bd3 carcinomas (p = 0.003). No significant association of the TB subgroups with age, sex, tumour localisation, microsatellite status, predominant histological growth pattern (tubular, papillary, solid), or tumour regression grade was noted.
Association of ITBCC TB subgroups with 5‐year survival in the overall cohort and in specific subgroups
As depicted in Figure 2 and supplementary material, Table S2, we observed striking survival differences between the three ITBCC TB subgroups (p < 0.001) in univariable statistical analyses of the 5‐year OS within the whole cohort. Patients whose tumours were assigned to the Bd1 subgroup had a mean OS of 51.7 months (95% CI: 46.5–56.8 months) compared to 37.4 months for Bd2 (95% CI: 30.3–44.5 months; HR: 3.48, 95% CI: 1.57–7.73) and 28.1 months for Bd3 carcinomas (95% CI: 23.2–33.1 months; HR: 6.26, 95% CI: 3.06–12.81).
Figure 2.
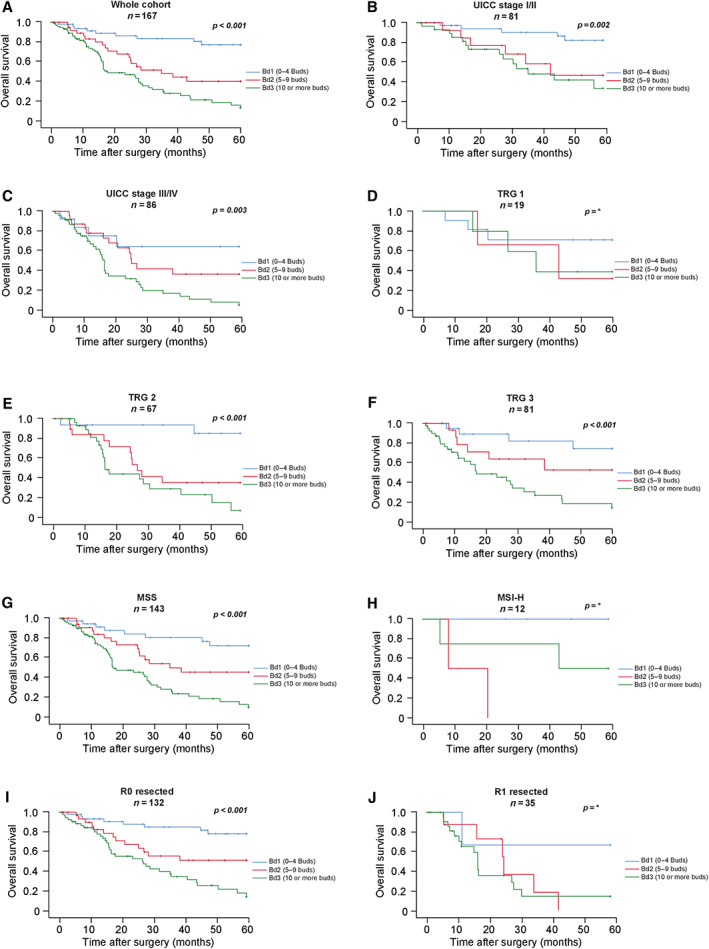
Impact of the ITBCC TB subgroups on survival parameters in (A) the overall cohort, (B) UICC stage I/II, and (C) UICC stage III/IV tumours; in (D) TRG1b, (E) TRG2, and (F) TRG3 tumours; in (G) microsatellite‐stable (MSS) and (H) microsatellite‐unstable (MSI‐H) gastric cancers as well as in (I) R0 resected and (J) R1 resected tumours. *P values for D, H, and J are not given because too few events were present for a log‐rank test in these subgroups.
The TB subgroups retained their statistical significance in subgroup analyses of UICC stage I/II (p = 0.002; HR for Bd2: 2.42, 95% CI: 0.68–8.63; HR for Bd3: 3.68, 95% CI: 1.34–10.06) and UICC stage III/IV carcinomas (p < 0.001; HR for Bd2: 2.92, 95% CI: 0.84–10.12; HR for Bd3: 5.86, 95% CI: 1.80–19.13) as well as in microsatellite‐stable (p < 0.001, MSS, HR for Bd2: 2.58, 95% CI: 1.09–6.10; HR for Bd3: 5.60, 95% CI: 2.62–11.96) and R0 resected cancers (p < 0.001, HR for Bd2: 2.98, 95% CI: 1.23–7.21; HR for Bd3: 6.07, 95% CI: 2.79–13.18). Within the different regression subgroups, the ITBCC subgroups retained their statistical significance in TRG2 (p < 0.001, HR for Bd2: 6.85, 95% CI: 1.51–31.05; HR for Bd3: 11.08, 95% CI: 2.56–48.01) and TRG3 carcinomas (p < 0.001, HR for Bd2: 2.45, 95% CI: 0.69–8.71; HR for Bd3: 6.07, 95% CI: 2.10–17.44). An exploratory survival analysis between gastrooesophageal (GE) junction (AEG II/III, p < 0.001; mean OS: Bd1: 51.3 months, Bd2: 36.5 months [HR: 3.55, 95% CI: 1.44–8.75], Bd3: 27.9 months [HR: 6.02, 95% CI: 2.67–13.58], data not shown) and pure gastric cancers (p = 0.016; mean OS: Bd1: 52.6 months, Bd2: 40.5 months [HR: 3.26, 95% CI: 0.59–17.87], Bd3: 28.5 months [HR: 6.76, 95% CI: 1.48–30.88], data not shown) showed a prognostic impact of the ITBCC subgroups in both localisations.
In the very small microsatellite‐unstable (MSI‐H) subgroup (n = 12), higher TB activity trended towards a worse outcome; however, the low number of patients and events in this subgroup prevented a reliable survival analysis. This was also the case for R1 resected (n = 35) and TRG1b carcinomas (n = 19), where exploratory statistical analyses did not point towards statistical significance.
Multivariable survival analysis
In a multivariable survival analysis (Cox regression model) including age (continuous variable), sex, resection status (R0 versus R1), UICC stage (I–IV), and tumour regression grading (TRG1b, TRG2, TRG3), the ITBCC TB subgroups (Bd1 versus Bd2 versus Bd3 [p < 0.001]) and UICC stage (p = 0.005) proved to be independent, highly significant survival parameters. Taking the Bd1 subgroups as reference, the HR was 2.60 for Bd2 and 4.74 for Bd3 carcinomas (for details, see Table 2).
Table 2.
Multivariable survival analysis (Cox proportional hazards regression model) for OS including age, sex, resection status (R0 versus R1), UICC stage (I–IV), tumour regression grading (TRG1b, TRG2, TRG3), and the ITBCC TB subgroups (Bd1 versus Bd2 versus Bd3)
| HR (OS) | Lower CI (95%) | Upper CI (95%) | P value | |
|---|---|---|---|---|
| TB groups (ITBCC) | <0.001 | |||
| Bd1 (low budding) | 1 | |||
| Bd2 (intermediate budding) | 2.60 | 1.14 | 5.95 | |
| Bd3 (high budding) | 4.74 | 2.25 | 10.03 | |
| UICC stage | 0.005 | |||
| I | 1 | |||
| II | 2.29 | 0.62 | 8.45 | |
| III | 4.72 | 1.22 | 18.26 | |
| IV | 5.90 | 1.51 | 22.94 | |
| Gender | 0.070 | |||
| Male | 1 | |||
| Female | 0.45 | 0.19 | 1.06 | |
| Age | 1.27 | 0.99 | 1.03 | 0.262 |
| Resection status | 0.600 | |||
| R0 | 1 | |||
| R1 | 1.15 | 0.67 | 1.98 | |
| Tumour regression grade (Becker et al) | 0.726 | |||
| 1b (<10% viable tumour) | 1 | |||
| 2 (10–50% viable tumour) | 0.79 | 0.33 | 1.88 | |
| 3 (>50% viable tumour) | 0.85 | 0.35 | 2.04 |
Interobserver variability of TB assessment
To investigate the interobserver variability of the assessment of the ITBCC TB groups in the neoadjuvant setting, 30 randomly selected cases were independently evaluated by a second pathologist who was blinded to clinicopathological data and to the initial assessment made by the main observer. Interobserver analysis revealed a high concordance between the two observers (p < 0.001, Cohen's kappa value: 0.77). Discrepancies of more than one grade were not observed (supplementary material, Table S3).
Discussion
Even resectable GC is often diagnosed in advanced stages, which require neoadjuvant chemotherapy prior to resection [5]. Through ypTNM staging [30] and determination of histopathological response [31], pathological examination of the resection specimen delivers crucial information about tumour vitality and disease burden in the post‐neoadjuvant setting. However, especially in cases with high post‐neoadjuvant tumour stage and no or incomplete histopathological response, clinical guidelines regarding the further clinical management of these patients are ill defined [32, 33, 34, 35]. Therefore, additional biomarkers that could help to stratify those patients into different prognostic, and potentially treatment, groups would be highly beneficial in this specific situation. The most prominent non‐staging related histopathological parameter given in pathology reports of therapy‐naïve GCs – the conventional WHO grading based on the extent of gland formation – is usually omitted in reports of neoadjuvant resections, as the applied cytotoxic agents are known to influence the histopathological appearance of malignant tumours [28, 36, 37].
TB has been identified as a highly valuable histopathological parameter in a plethora of carcinoma entities throughout the body [8, 12, 13, 14, 15, 38, 39, 40, 41] and recent studies on other gastrointestinal carcinomas were able to demonstrate that, as opposed to conventional grading systems, TB remains a reliable prognosticator even after neoadjuvant therapy [18, 19, 20, 21]. For GC, several previous studies have already demonstrated the high prognostic relevance of TB in therapy‐naïve tumours [16, 22, 23, 42]. However, in routine daily clinicopathological practice, TB is still not a standardly assessed morphological parameter in histopathological reports of GC, which is for instance reflected by the fact that it is barely mentioned in its recent WHO classification [28].
In contrast to therapy‐naïve tumours, there are no consistent data regarding the prognostic relevance of TB in GCs resected after neoadjuvant therapy. Therefore, this study investigated TB according to the ITBCC criteria [6] in 167 intestinal‐type GCs including adenocarcinomas of the gastro‐oesophageal junction (AEG II/III) and identified TB as a very strong and reproducible prognostic factor in the post‐neoadjuvant setting, which is independent of stage and regression grade. While tumours with no or low TB activity showed a comparatively long 5‐year survival, those patients whose tumours fells into the high‐grade budding category showed a highly dismal clinical course compared to the low budding subgroup. Although slightly less accentuated, we still observed significant survival differences between tumours with intermediate (Bd2) and high (Bd3) TB activity. These findings are very important, as they prove that post‐neoadjuvant assessment of TB categories according to the ITBCC criteria is feasible and that the prognostic power of TB is not disrupted by chemotherapy effects. This assumption is also supported by the observation that the distribution of budding groups in our neoadjuvant cohort is generally in line with those described for primary resected GCs [16]. Our data almost perfectly recapitulate post‐neoadjuvant studies from rectal or oesophageal carcinomas, where TB remains a stage‐independent prognostic parameter that even outperformed conventional ypTNM staging [18, 19, 20, 21]. Therefore, we believe that the assessment of TB can serve as a very strong additional histopathological parameter that should be included in the pathology reports of GCs after neoadjuvant treatment.
For this particular neoadjuvant setting, we considered all tumour areas for the assessment of TB, as a clear invasive front is often hard to determine in tumours with a marked response to neoadjuvant treatment, rendering the evaluation of peritumoural budding very difficult to impossible in those cases. However, previous studies demonstrated a very high correlation between intra‐ and peri‐tumoural budding activity and even suggested intratumoural budding as a surrogate marker for the determination of the general TB activity for those cases, in which the assessment of peritumoural budding is hard to assess [43, 44].
Our study only included intestinal‐type adenocarcinomas [27] according to the Lauren classification. As opposed to intestinal‐type adenocarcinomas, diffuse gastric carcinomas per definition show a highly dissociative growth pattern that would assign them into the high‐grade budding category, which is probably one of the reasons for their generally dismal clinical survival. In stark contrast, a large fraction of conventional intestinal‐type adenocarcinomas shows no or low TB activity, as nicely demonstrated in a recent study of untreated GC by Ulase et al [16] and by our study. Furthermore, it is already known that GCs with a post‐neoadjuvant diffuse/signet‐ring morphology are associated with a highly adverse outcome, which is comparable to the dismal survival of the intestinal‐type adenocarcinomas in our study that fall into the high TB category [45, 46]. Therefore, post‐neoadjuvant TB assessment appears to be a valid method to identify those tumours that, although they show an intestinal‐type architecture, have considerable cellular dissociation capacity, which corresponds to an aggressive biological behaviour comparable to adenocarcinomas classified as diffuse by the Lauren classification.
This study has several limitations. First, our analyses are retrospective in nature and further prospective (and multicentric) studies are warranted to further investigate potential therapeutic implications of TB in larger neoadjuvant cohorts. Second, our cohort only includes a small number of patients whose tumours showed a marked response to the neoadjuvant treatment (TRG1b, <10% viable tumour cells) and also very few microsatellite‐unstable tumours, making statistically reliable analyses impossible for these subgroups. For this reason, our study cannot conclusively answer the question of whether the assessment of TB also retains its prognostic relevance in these specific and clinically very important subgroups.
In conclusion, our study of 167 intestinal‐type GCs demonstrates that the assessment of TB according to the ITBCC criteria is feasible and provides stage‐ and regression‐grade independent prognostic information in the post‐neoadjuvant setting. Considering our data for the neoadjuvant setting, and the impressive findings from previous studies on therapy‐naïve GCs, we believe that the implementation of TB according to the ITBCC criteria should be strongly considered for routine pathology reports of GC.
Author contributions statement
MJ and GK designed and supervised this study and wrote the manuscript with assistance from WW, SL, A‐LH and CD. MJ, MS, AH and GK performed statistical analyses. A‐LH, MK, KO, AN and TS collected clinical data. MJ, AG, MG and WW performed histopathological analyses.
Supporting information
Table S1. Detailed listing of the applied chemotherapy regimens
Table S2. Detailed survival of the ITBCC TB subgroups within the overall cohort and in specific subgroups
Table S3. Detailed interobserver analysis regarding the ITBCC TB subgroups between the two observers
Acknowledgements
We acknowledge the iBio biomaterial bank of the Technical University Munich and the Biomaterialbank (BMBH) Heidelberg for technical support. Open Access funding enabled and organized by Projekt DEAL.
No conflicts of interest were declared.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network . Gastric Cancer. National Comprehensive Cancer Network (NCCN) Guidelines. Version 1.2020. 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf
- 3. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021; 71: 264–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sexton RE, Al Hallak MN, Diab M, et al. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev 2020; 39: 1179–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11–20. [DOI] [PubMed] [Google Scholar]
- 6. Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 2017; 30: 1299–1311. [DOI] [PubMed] [Google Scholar]
- 7. Lugli A, Zlobec I, Berger MD, et al. Tumour budding in solid cancers. Nat Rev Clin Oncol 2021; 18: 101–115. [DOI] [PubMed] [Google Scholar]
- 8. Bronsert P, Enderle‐Ammour K, Bader M, et al. Cancer cell invasion and EMT marker expression: a three‐dimensional study of the human cancer‐host interface. J Pathol 2014; 234: 410–422. [DOI] [PubMed] [Google Scholar]
- 9. Zlobec I, Dawson HE, Blank A, et al. Are tumour grade and tumour budding equivalent in colorectal cancer? A retrospective analysis of 771 patients. Eur J Cancer 2020; 130: 139–145. [DOI] [PubMed] [Google Scholar]
- 10. Zlobec I, Lugli A. Tumour budding in colorectal cancer: molecular rationale for clinical translation. Nat Rev Cancer 2018; 18: 203–204. [DOI] [PubMed] [Google Scholar]
- 11. Karamitopoulou E, Wartenberg M, Zlobec I, et al. Tumour budding in pancreatic cancer revisited: validation of the ITBCC scoring system. Histopathology 2018; 73: 137–146. [DOI] [PubMed] [Google Scholar]
- 12. Boxberg M, Jesinghaus M, Dorfner C, et al. Tumour budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: proposal for an adjusted grading system. Histopathology 2017; 70: 1125–1137. [DOI] [PubMed] [Google Scholar]
- 13. Boxberg M, Kuhn PH, Reiser M, et al. Tumor budding and cell nest size are highly prognostic in laryngeal and hypopharyngeal squamous cell carcinoma: further evidence for a unified histopathologic grading system for squamous cell carcinomas of the upper aerodigestive tract. Am J Surg Pathol 2019; 43: 303–313. [DOI] [PubMed] [Google Scholar]
- 14. Jesinghaus M, Brühl F, Steiger K, et al. Cellular dissociation grading based on the parameters tumor budding and cell nest size in pretherapeutic biopsy specimens allows for prognostic patient stratification in esophageal squamous cell carcinoma independent from clinical staging. Am J Surg Pathol 2019; 43: 618–627. [DOI] [PubMed] [Google Scholar]
- 15. Kadota K, Nitadori J, Woo KM, et al. Comprehensive pathological analyses in lung squamous cell carcinoma: single cell invasion, nuclear diameter, and tumor budding are independent prognostic factors for worse outcomes. J Thorac Oncol 2014; 9: 1126–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ulase D, Heckl S, Behrens HM, et al. Prognostic significance of tumour budding assessed in gastric carcinoma according to the criteria of the International Tumour Budding Consensus Conference. Histopathology 2020; 76: 433–446. [DOI] [PubMed] [Google Scholar]
- 17. Zare SY, Aisagbonhi O, Hasteh F, et al. Independent validation of tumor budding activity and cell nest size as determinants of patient outcome in squamous cell carcinoma of the uterine cervix. Am J Surg Pathol 2020; 44: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 18. Jesinghaus M, Boxberg M, Wilhelm D, et al. Post‐neoadjuvant cellular dissociation grading based on tumour budding and cell nest size is associated with therapy response and survival in oesophageal squamous cell carcinoma. Br J Cancer 2019; 121: 1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trotsyuk I, Sparschuh H, Müller AJ, et al. Tumor budding outperforms ypT and ypN classification in predicting outcome of rectal cancer after neoadjuvant chemoradiotherapy. BMC Cancer 2019; 19: 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miyata H, Yoshioka A, Yamasaki M, et al. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer 2009; 115: 3324–3334. [DOI] [PubMed] [Google Scholar]
- 21. Du C, Xue W, Ikäläinen J, et al. Morphology and prognostic value of tumor budding in rectal cancer after neoadjuvant radiotherapy. Hum Pathol 2012; 43: 1061–1067. [DOI] [PubMed] [Google Scholar]
- 22. Kemi N, Eskuri M, Ikäläinen J, et al. Tumor budding and prognosis in gastric adenocarcinoma. Am J Surg Pathol 2019; 43: 229–234. [DOI] [PubMed] [Google Scholar]
- 23. Guo YX, Zhang ZZ, Zhao G, et al. Prognostic and pathological impact of tumor budding in gastric cancer: a systematic review and meta‐analysis. World J Gastrointest Oncol 2019; 11: 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85: 1457–1459. [DOI] [PubMed] [Google Scholar]
- 25. Bauer L, Hapfelmeier A, Blank S, et al. A novel pretherapeutic gene expression‐based risk score for treatment guidance in gastric cancer. Ann Oncol 2018; 29: 127–132. [DOI] [PubMed] [Google Scholar]
- 26. Kohlruss M, Grosser B, Krenauer M, et al. Prognostic implication of molecular subtypes and response to neoadjuvant chemotherapy in 760 gastric carcinomas: role of Epstein‐Barr virus infection and high‐ and low‐microsatellite instability. J Pathol Clin Res 2019; 5: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lauren P. The two histological main types of gastric carcinoma: diffuse and so‐called intestinal‐type carcinoma. An attempt at a histo‐clinical classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 28. Fukayama M, Rugge M, Washington MK. Tumours of the stomach. In: WHO Classification of Tumours, Digestive System Tumours (5th edn). IARC: Lyon, 2019. [Google Scholar]
- 29. Konukiewitz B, Kasajima A, Schmitt M, et al. Neuroendocrine differentiation in conventional colorectal adenocarcinomas: incidental finding or prognostic biomarker? Cancers (Basel) 2021; 13: 5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gospodarowicz MK, Brierley JD, Wittekind C. TNM Classification of Malignant Tumours. Hoboken, New Jersey, USA: John Wiley & Sons, 2017. [Google Scholar]
- 31. Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011; 253: 934–939. [DOI] [PubMed] [Google Scholar]
- 32. Moehler M, Al‐Batran SE, Andus T, et al. S3‐Leitlinie Magenkarzinom – Diagnostik und Therapie der Adenokarzinome des Magens und des ösophagogastralen Übergangs – Langversion 2.0 – August 2019. AWMF‐Registernummer: 032/009OL. Z Gastroenterol 2019; 57: 1517–1632. [DOI] [PubMed] [Google Scholar]
- 33. Leitlinienprogramm Onkologie . S3 Leitlinie Magenkarzinom. Version 2.0. August 2019. S3‐Leitlinie Magenkarzinom Diagnostik und Therapie der Adenokarzinome des Magens und ösophagogastralen Übergangs. [Accessed 10 June 2022]. Available from: https://www.awmf.org/uploads/tx_szleitlinien/032‐009l_S3_Magenkarzinom_Diagnostik_Therapie_Adenokarzinome_oesophagogastraler_Uebergang_2019‐12.pdf
- 34. Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014; 32: 2983–2990. [DOI] [PubMed] [Google Scholar]
- 35. Schmidt T, Sicic L, Blank S, et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer 2014; 110: 1712–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McCluggage WG, Lyness RW, Atkinson RJ, et al. Morphological effects of chemotherapy on ovarian carcinoma. J Clin Pathol 2002; 55: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCarthy AJ, Rouzbahman M, Thiryayi SA, et al. Neoadjuvant therapy in gynaecological malignancies: what pathologists need to know. J Clin Pathol 2019; 72: 102–111. [DOI] [PubMed] [Google Scholar]
- 38. Boxberg M, Bollwein C, Jöhrens K, et al. Novel prognostic histopathological grading system in oral squamous cell carcinoma based on tumour budding and cell nest size shows high interobserver and intraobserver concordance. J Clin Pathol 2019; 72: 285–294. [DOI] [PubMed] [Google Scholar]
- 39. Chouat E, Zehani A, Chelly I, et al. Tumor budding is a prognostic factor linked to epithelial mesenchymal transition in pancreatic ductal adenocarcinoma. Study report and literature review. Pancreatology 2018; 18: 79–84. [DOI] [PubMed] [Google Scholar]
- 40. Hong KO, Oh KY, Shin WJ, et al. Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial‐mesenchymal transition process. Hum Pathol 2018; 80: 123–129. [DOI] [PubMed] [Google Scholar]
- 41. Jesinghaus M, Boxberg M, Konukiewitz B, et al. A novel grading system based on tumor budding and cell nest size is a strong predictor of patient outcome in esophageal squamous cell carcinoma. Am J Surg Pathol 2017; 41: 1112–1120. [DOI] [PubMed] [Google Scholar]
- 42. Shi H, Ye L, Lu W, et al. Grading of endocervical adenocarcinoma: a novel prognostic system based on tumor budding and cell cluster size. Mod Pathol 2022; 35: 524–532. [DOI] [PubMed] [Google Scholar]
- 43. Thies S, Guldener L, Slotta‐Huspenina J, et al. Impact of peritumoral and intratumoral budding in esophageal adenocarcinomas. Hum Pathol 2016; 52: 1–8. [DOI] [PubMed] [Google Scholar]
- 44. Lugli A, Vlajnic T, Giger O, et al. Intratumoral budding as a potential parameter of tumor progression in mismatch repair‐proficient and mismatch repair‐deficient colorectal cancer patients. Hum Pathol 2011; 42: 1833–1840. [DOI] [PubMed] [Google Scholar]
- 45. Koh YW, Park YS, Ryu MH, et al. Postoperative nodal status and diffuse‐type histology are independent prognostic factors in resectable advanced gastric carcinomas after preoperative chemotherapy. Am J Surg Pathol 2013; 37: 1022–1029. [DOI] [PubMed] [Google Scholar]
- 46. Schirren R, Novotny A, Oesterlin C, et al. Significance of Lauren classification in patients undergoing neoadjuvant/perioperative chemotherapy for locally advanced gastric or gastroesophageal junction cancers – analysis from a large single center cohort in Germany. Cancers (Basel) 2021; 13: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detailed listing of the applied chemotherapy regimens
Table S2. Detailed survival of the ITBCC TB subgroups within the overall cohort and in specific subgroups
Table S3. Detailed interobserver analysis regarding the ITBCC TB subgroups between the two observers


