Key Points
Question
How useful are tumor bed intraoperative frozen section histopathology (IFSH) samples for assessing final margin status in patients undergoing surgery for oral cavity squamous cell carcinoma (SCC)?
Findings
In this cross-sectional study, 4821 tumor bed IFSH samples were examined from 1104 patients undergoing surgery for oral cavity SCC between 2000 and 2015. Compared with final tumor margin samples, the sensitivity of IFSH samples was 11%, and the specificity was 99%.
Meaning
This study suggests that IFSH samples from the tumor bed have low sensitivity for assessing final margin tumor status.
Abstract
Importance
Methods of assessing final margin status in patients undergoing surgery for oral cavity squamous cell carcinoma, such as intraoperative frozen section histopathology (IFSH) taken from the tumor bed, may have limitations in accuracy.
Objective
To evaluate the accuracy and implications of using IFSH samples to assess tumor bed margins in patients undergoing surgery for oral cavity squamous cell carcinoma (SCC).
Design, Setting, and Participants
This cross-sectional study included 1257 patients who underwent surgery for oral cavity SCC between January 1, 2000, and December 31, 2015, at an academic cancer center. A total of 4821 IFSH samples were examined from 1104 patients (87.8%) who had at least 1 IFSH sample. Institutional practice is to harvest margins for IFSH from the tumor bed. Statistical analysis was performed from August 1, 2021, to April 4, 2022.
Main Outcomes and Measures
Sensitivity and specificity were calculated for IFSH samples of margins compared with the permanent pathology samples of the same tissue and for IFSH compared with the final tumor specimen histopathology (FTSH). Results were classified using a binary method, with histopathologic reports interpreted as either negative (including negative or atypia or dysplasia) or positive (including carcinoma in situ, suspicious, or positive).
Results
A total of 1257 patients met the inclusion criteria, including 709 men (56.4%), with a median age of 62 years (IQR, 52-73 years); 1104 patients (627 men [56.8%]; median age, 62 years [IQR, 52-72 years]) had IFHS samples. For IFSH relative to permanent sections of the IFSH tissue, sensitivity and specificity of IFSH were high (sensitivity, 76.5% [95% CI, 67.5%-85.5%]; specificity, 99.9% [95% CI, 99.8%-100%]), with discordant results in 24 of 4821 total specimens (0.5%). Final specimen margins were positive in 11.7% of patients (147 of 1257). Compared with FTSH, the sensitivity of IFSH for defining margin status was 10.8% (95% CI, 5.8%-15.8%), and the specificity was 99.1% (95% CI, 98.8%-99.4%). The rate of discordance was 4.0% (171 of 4284 specimens) between IFSH and FTSH.
Conclusions and Relevance
The findings of this cross-sectional study suggest that IFSH is accurate compared with permanent pathologic characteristics of the same tissue, but less reliable at assessing final margin status on the tumor specimen. Despite a high specificity, the sensitivity of IFSH compared with FTSH is low, which may be associated with the inherent inability of tumor bed IFSH margin analysis to accurately account for the 3-dimensional association of tumor margins with the periphery of the specimen and the overall low rate of positive final tumor margins. Although tumor bed IFSH is widely used in the management of oral cavity cancer, this study suggests that there are limitations of this modality in assessing the final surgical margin status.
This cross-sectional study evaluates the accuracy and implications of using intraoperative frozen section histopathology samples to assess tumor bed margins in patients undergoing surgery for oral cavity squamous cell carcinoma.
Introduction
Debate regarding the management of surgical margins in oral squamous cell carcinoma (SCC) continues despite extensive research.1,2,3,4 The oral cavity is the most likely site for a positive surgical margin in solid tumors.5 It is generally held that involved surgical margins seen on the final tumor specimen histopathology (FTSH) are associated with prognosis, but the extent to which a margin needs to be cleared has yet to be fully defined.4 Extirpation of oral cavity SCC relies extensively on visual inspection and palpation, which has obvious limitations; therefore, intraoperative frozen section histopathology (IFSH) analysis of margins is commonly used to guide margin assessment and improve tumor ablation.
Although IFSH analysis of margins is used as an adjunct by more than 90% of surgeons, there is still discussion in the literature regarding their utility in oral cavity SCC.2,6,7,8 The optimal technique for obtaining margins is not standardized, with some clinicians favoring obtaining margins from the tumor bed and others favoring obtaining margins from the specimen.6,8 Although frozen section analysis is in itself accurate, IFSH does not always correlate well with final margin status on the tumor specimen.6,9,10
There is conflicting evidence regarding the association of IFSH with survival or recurrence outcomes, with some investigators finding no association,9,11 while others found improved oncologic outcomes.2 Whether IFSH or FTSH has a greater association with oncologic outcomes has not been determined, although most studies use FTSH to define margin status. Refinements in the frozen section technique may improve the utility of IFSH in oral cavity SCC, but there is still significant variance in practice across surgeons and institutions.2,8,12 Conflicting data also exist regarding the association with outcomes of additional reresection to clear a positive frozen section margin.2,10,13,14
Most head and neck surgeons continue to perform IFSH assessments to guide surgical resection despite the lack of consistently demonstrated benefit, the significant cost,15 the requirement for specialist personnel, and the heterogeneity in technique.8 Large-scale studies are needed to evaluate whether IFSH is reliably associated with tumor margin status or whether better intraoperative modalities are needed for assessment of tumor margins. This study had 3 aims: (1) to evaluate the accuracy of IFSH compared with the permanent histopathology of the same tissue used for IFSH analysis, (2) to evaluate how the IFSH result compared with the FTSH result, and (3) to evaluate whether any differences in sensitivity and specificity exist between radial vs deep frozen section margins in oral cavity SCC.
Methods
The study received approval from the institutional review board of Memorial Sloan Kettering Cancer Center, and patient informed consent was waived because the data were deidentified. The Head and Neck Surgery Service at Memorial Sloan Kettering Cancer Center maintains a prospective database of patients with oral cavity SCC. This database was queried from January 1, 2000, to December 31, 2015, yielding a total of 1257 operative cases during the 16-year period. Patients met inclusion criteria if they underwent surgery for biopsy-proven oral cavity SCC (all subsites) during the specified period. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
All patients were treated with surgical removal of the primary tumor with or without neck dissection. Patients were excluded if there was inadequate clinicopathologic information for analysis. Close margins were excluded from the sensitivity and specificity calculations because this information is available for FTSH but not IFSH. Margins were considered close if they were 1.0 to 4.9 mm from the tumor or if a “close margin” was identified by the reporting pathologist. Data were extracted from the database and supplemented by information from the medical record when necessary. Collated data included demographic characteristics, clinicopathologic features, and treatment information. All tumors were staged using the eighth edition of the American Joint Committee on Cancer.16 There was detailed data collection regarding intraoperative and postoperative pathologic margins.
At the discretion of the operating surgeon, margins were assessed intraoperatively using IFSH with a subsequent postoperative final histopathologic assessment of that same specimen. The general practice at our institution has been to take IFSH margins from the tumor bed rather than from the specimen. Intraoperative frozen section histopathology specimens were excluded if they were taken for diagnostic assessment rather than for margin assessment. A comprehensive postoperative assessment of permanent margins on the FTSH was performed by oncologic pathologists for all cases.
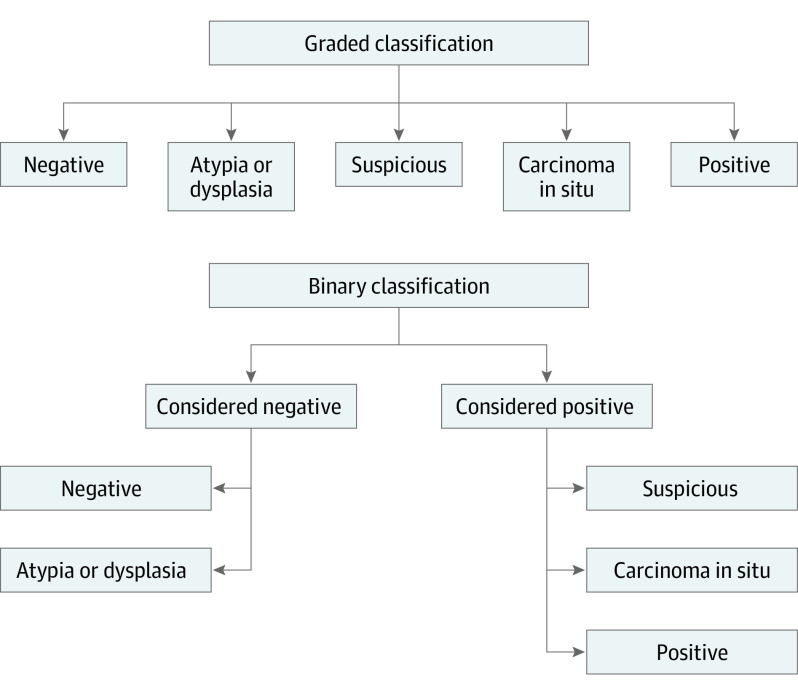
The IFSH report, the final postoperative report of the same intraoperative specimen used for IFSH, and the FTSH report were reviewed. Margin results were classified in 2 ways (Figure). First, a graded classification system was developed with histopathologic reports interpreted as negative, atypia or dysplasia, carcinoma in situ, suspicious, or positive. Second, a binary classification system was developed, with histopathologic reports interpreted as either negative (including negative or atypia or dysplasia) or positive (including carcinoma in situ, suspicious, or positive). Although the graded classification is a strict reflection of the histopathologic report, the binary classification is designed to better reflect the intraoperative clinical utility of the histopathologic information based on IFSH.
Figure. Classification Systems for Frozen Section Margin Status.
The graded classification system included negative, atypia or dysplasia, suspicious, carcinoma in situ, and positive results. The binary classification system grouped negative and atypia or dysplasia into a single negative category and suspicious, carcinoma in situ, and positive into a single positive category.
To assess whether the IFSH margins were reliable compared with formal histopathologic techniques, each IFSH margin was compared with the final postoperative histopathologic characteristics of the same specimen used for IFSH. This comparison was performed using both classification systems. Specimens were denoted as true negative, true positive, false negative, or false positive against the final postoperative pathology report.
To assess whether IFSH margins were representative of the FTSH margins, the postoperative histopathologic characteristics of the same specimens used for the IFSH margins were correlated with the margins on the FTSH using both classification systems. This correlation was performed only for cases that had at least 1 IFSH margin and a final tumor specimen margin recorded.
The data set was then divided into 2 groups according to whether or not cases used IFSH. Statistical analysis was performed from August 1, 2021, to April 4, 2022. Baseline clinicopathologic and treatment data were compared between the 2 groups using Pearson χ2 and Fisher exact tests. Effect sizes and 95% CIs were calculated for each variable. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. Data were collated using Excel, version 16.6.11 (Microsoft Corp) for Mac. Statistical analyses were performed in Stata, version 16 (StataCorp LLC).
Results
Baseline Characteristics
A total of 1257 patients met the inclusion criteria, including 709 male patients (56.4%) and 548 female patients (43.6%), with a median age of 62 years (IQR, 52-73 years). For the overall cohort, the tongue was the most common primary site of SCC (680 [54.1%]), followed by the gingiva (253 [20.1%]). For the 1196 patients with available pathologic T staging information, most tumors were classified as pT1 (414 [34.6%]) or pT2 (323 [27.0%]). Of the 1193 patients with available overall pathologic stage, 606 (50.8%) had stage I to II disease and 587 (49.2%) had stage III to IV disease. Patient, tumor, and treatment characteristics for the total cohort as well as by frozen section status are shown in Table 1.
Table 1. Patient, Tumor, and Treatment Characteristics Among Patients With IFSH Results.
| Characteristic | Patients with IFSH results (n = 1104) |
|---|---|
| Age, median (IQR), y | 62 (52-72) |
| Sex, No. (%) | |
| Female | 477 (43.2) |
| Male | 627 (56.8) |
| Tobacco use, No. (%) | |
| Never | 420 (38.0) |
| Yes | 684 (62.0) |
| Alcohol use, No. (%) | |
| Never | 325 (29.4) |
| Yes | 779 (70.6) |
| Washington University Head and Neck Comorbidity Index | |
| 0 | 781 (70.7) |
| >1 | 323 (29.3) |
| Primary site | |
| Tongue | 639 (57.9) |
| Floor of mouth | 123 (11.1) |
| Hard palate | 17 (1.5) |
| Lower or upper gingiva | 192 (17.4) |
| Retromolar trigone or buccal mucosa | 133 (12.0) |
| pT stage (n = 1059) | |
| T1-T2 | 661 (62.4) |
| T3-T4 | 398 (37.6) |
| pN stage (n = 1087) | |
| Nx | 270 (24.8) |
| N0 | 472 (43.4) |
| N1 | 104 (9.6) |
| N2 | 109 (10.0) |
| N3 | 132 (12.1) |
| Overall stage (n = 1054) | |
| I-II | 537 (50.9) |
| III-IV | 517 (49.1) |
| Lymphovascular invasion (n = 1040) | |
| No | 879 (84.5) |
| Yes | 161 (15.5) |
| Perineural invasion (n = 1040) | |
| No | 693 (66.6) |
| Yes | 347 (33.4) |
| Adjuvant treatment | |
| None | 693 (62.8) |
| Radiotherapy | 300 (27.2) |
| Chemoradiotherapy | 111 (10.1) |
Abbreviation: IFSH, intraoperative frozen section histopathology.
Of 1257 total patients, 1104 patients (87.8%) had at least 1 IFSH assessment performed, and 153 patients (12.2%) did not. A comparison of these groups was not one of the primary aims of this study because the decision on whether to perform an IFSH assessment was dictated by surgeon preference and the number of patients who did not undergo IFSH was low. The analysis between these groups is therefore included as the eTable in the Supplement. In brief, significant differences were found in the primary site distribution between groups, with a high proportion of patients in the IFSH group with an oral tongue primary site (639 of 1104 [57.9%]) and a high proportion of patients in the no-IFSH group with an upper or lower gingival primary site (61 of 153 [39.9%]). A greater proportion of Nx or N0 tumors were in the no-IFSH group (120 of 153 [78.4%] vs 742 of 1087 [68.3%]; difference, 10.1% [95% CI, 3.0%-17.2%]); however, there was no meaningful difference between these groups based on overall stage.
Of the 1104 patients with available frozen section margins, there were a total of 5093 individual frozen section margins, of which 4821 were included in analysis. The remaining 272 individual frozen section margins did not have final histopathologic results and therefore could not be correlated. Of the 4821 individual frozen section margins, there were 3939 radial margins (81.7%) and 882 deep margins (18.3%). The median number of frozen section margins per patient was 5 (IQR, 4-5).
Analysis of IFSH for Margins in Oral SCC
Table 2 depicts the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the IFSH result compared with the final histopathologic result of the same tissue using both the graded and binary classifications systems. With the graded classification system, the sensitivity was 75.9% (95% CI, 70.9%-80.8%), the specificity was 97.7% (95% CI, 97.2%-98.1%), the PPV was 67.7% (95% CI, 62.6%-72.8%), and the NPV was 98.4% (95% CI, 98.1%-98.8%). With the use of the graded classification system, 175 of 4821 specimens (3.6%) had discordant results. Among these specimens with discordant results, the IFSH “overcalled” (the IFSH was worse than the specimen it is being compared with) the diagnosis for 105 margin samples (2.2%) and “undercalled” (the IFSH was better than the specimen it is being compared with) the diagnosis for 70 margin samples (1.5%) (per the graded classification in the Figure). When the binary classification system was used, the sensitivity was 76.5% (95% CI, 67.5%-85.5%), the specificity was 99.9% (95% CI, 99.8%-100%), the PPV was 94.2% (95% CI, 88.7%-99.7%), and the NPV was 99.6% (95% CI, 99.4%-99.8%). With the use of the binary system, 24 of 4821 specimens (0.5%) had discordant results.
Table 2. IFSH Margin Compared With Final Result of Same Specimen.
| IFSH vs final frozen section margin | Final positive, No. | Final negative, No. | Total, No. |
|---|---|---|---|
| Graded classification | |||
| IFSH margin | |||
| Positive | 220 (TP) | 105 (FP) | 325 |
| Negative | 70 (FN) | 4426 (TN) | 4496 |
| Total | 290 | 4531 | 4821 |
| Binary classificationa | |||
| IFSH margin | |||
| Positive | 65 (TP) | 4 (FP) | 69 |
| Negative | 20 (FN) | 4732 (TN) | 4752 |
| Total | 85 | 4736 | 4821 |
Abbreviations: FN, false negative; FP, false positive; IFSH, intraoperative frozen section histopathology; TN, true negative; TP, true positive.
Negative or dysplasia vs carcinoma in situ, suspicious, or positive.
Association of IFSH With FTSH for Margins in Oral SCC
Of the total cohort, 11.7% (147 of 1257) of patients had at least 1 final positive margin. Table 3 shows the sensitivity, specificity, PPV, and NPV of the IFSH compared with the FTSH margin status. With the graded classification, the sensitivity was 15.2% (95% CI, 9.9%-20.4%), the specificity was 93.9% (95% CI, 93.2%-94.6%), the PPV was 9.7% (95% CI, 6.2%-13.2%), and the NPV was 96.2% (95% CI, 95.6%-96.8%). There were 402 of 4284 specimens with discordant results (sampling error rate, 9.4%). With the binary classification, the sensitivity was 10.8% (95% CI, 5.8%-15.8%), the specificity was 99.1% (95% CI, 98.8%-99.4%), the PPV was 29.1% (95% CI, 17.1%-41.1%), and the NPV was 96.9% (95% CI, 96.4%-97.4%). With the binary classification system, there were 171 of 4282 total specimens with discordant results (sampling error rate, 4.0%).
Table 3. IFSH Margin Compared With FTSH Margin.
| IFSH vs final frozen section margin | FTSH margin | Total, No. | |
|---|---|---|---|
| Positive, No. | Negative, No. | ||
| Graded classification | |||
| IFSH margin | |||
| Positive | 27 (TP) | 251 (FP) | 278 |
| Negative | 151 (FN) | 3855 (TN) | 4006 |
| Total | 178 | 4106 | 4284 |
| Binary classificationa | |||
| IFSH margin | |||
| Positive | 16 (TP) | 39 (FP) | 55 |
| Negative | 132 (FN) | 4097 (TN) | 4229 |
| Total | 148 | 4136 | 4284 |
Abbreviations: FN, false negative; FP, false positive; FTSH, final tumor specimen histopathology; IFSH, intraoperative frozen section histopathology; TN, true negative; TP, true positive.
Negative or dysplasia vs carcinoma in situ, suspicious, or positive.
Radial vs Deep Margins
Sensitivity and Specificity of IFSH Compared With the Same Tissue
Radial and deep IFSH margins were separately considered using the same approach with both classification systems. For the deep margins, the sensitivity of IFSH result compared with the final histopathologic result of the same tissue was 66.7% (95% CI, 40.0%-93.3%) using the graded classification vs 75% (95% CI, 50.5%-99.5%) using the binary classification, and the specificity was 99.9% (95% CI, 99.7%-100%) using the graded classification vs 100% (95% CI, 100%-100%) using the binary classification. For the radial margins, the sensitivity of IFSH was 76.3% (95% CI, 71.3%-81.3%) using the graded classification vs 76.7% (95% CI, 67.0%-86.4%) using the binary classification, and the specificity was 97.2% (95% CI, 96.6%-97.7%) using the graded classification vs 99.9% (95% CI, 99.8%-100%) using the binary classification. Although the sensitivity of IFSH was slightly lower for deep IFSH when the graded classification system was used, the sensitivity was similar to that for radial IFSH using the binary classification system.
Sensitivity and Specificity of IFSH Compared With FTSH
Comparing the IFSH of the deep margins with FTSH margins using the graded classification system, the sensitivity was 12.5% (95% CI, 1.0%-24%), the specificity was 99.3% (95% CI, 98.7%-99.9%), the PPV was 44.4% (95% CI, 12%-76.9%), and the NPV was 96.2% (95% CI, 94.8%-97.6%) (Table 4). With the use of the binary classification system, the sensitivity was 15.6% (95% CI, 3.0%-28.2%), the specificity was 99.4% (95% CI, 98.9%-100%), the PPV was 55.6% (95% CI, 23.1%-88%), and the NPV was 96.3% (95% CI, 94.9%-97.7%).
Table 4. IFSH Margin Compared With FTSH Margin, Deep vs Radial.
| Comparison | FTSH margin | Total, No. | |
|---|---|---|---|
| Positive, No. | Negative, No. | ||
| Deep IFSH margin vs FTSH margin, graded classification | |||
| Deep IFSH margin | |||
| Positive | 4 (TP) | 5 (FP) | 9 |
| Negative | 28 (FN) | 704 (TN) | 732 |
| Total | 32 | 709 | 741 |
| Deep IFSH margin vs FTSH margin, binary classificationa | |||
| Deep IFSH margin | |||
| Positive | 5 (TP) | 4 (FP) | 9 |
| Negative | 27 (FN) | 705 (TN) | 732 |
| Total | 32 | 709 | 741 |
| Radial IFSH margin vs FTSH margin, graded classification | |||
| Radial IFSH margin | |||
| Positive | 23 (TP) | 246 (FP) | 269 |
| Negative | 123 (FN) | 3152 (TN) | 3275 |
| Total | 146 | 3398 | 3544 |
| Radial IFSH margin vs FTSH margin, binary classificationa | |||
| Radial IFSH margin | |||
| Positive | 11 (TP) | 35 (FP) | 46 |
| Negative | 105 (FN) | 3393 (TN) | 3498 |
| Total | 116 | 3428 | 3544 |
Abbreviations: FN, false negative; FP, false positive; FTSH, final tumor specimen histopathology; IFSH, intraoperative frozen section histopathology; TN, true negative; TP, true positive.
Negative or dysplasia vs carcinoma in situ, suspicious, or positive.
Comparing the IFSH of the radial margins with FTSH margins using the graded classification system, the sensitivity was 15.8% (95% CI, 9.8%-21.7%), the specificity was 92.8% (95% CI, 91.9%-93.6%), the PPV was 8.6% (95% CI, 5.2%-11.9%), and the NPV was 96.2% (95% CI, 95.6%-96.9%) (Table 4). With the use of the binary classification system, the sensitivity was 9.5% (95% CI, 4.2%-14.8%), the specificity was 99.0% (95% CI, 98.6%-99.3%), the PPV was 23.9% (95% CI, 11.6%-36.2%), and the NPV was 97.0% (95% CI, 96.%-97.6%).
Overall, across both classification systems, the PPV was approximately 44% to 55% for deep margins and 8% to 24% for radial margins. Across both classification systems, the NPV was approximately 96% for deep margins and 96% to 97% for radial margins.
Discussion
This study delineates the limitations of using IFSH to assess oral cavity SCC and furthers our understanding of the clinical utility of this technique. The weaknesses of IFSH have been suggested previously in smaller studies often containing all head and neck subsites.15,17,18 In our study, even in the fewer than 4% of cases when the result communicated by the pathologist intraoperatively differed from the permanent result, it only rarely differed to an extent that could potentially alter a surgeon’s management intraoperatively. Despite this finding, the low sensitivity of IFSH compared with FTSH demonstrates that IFSH does not have the same reliability in indicating margin status on FTSH.
Approximately 11% of patients had at least 1 positive margin on the final permanent tumor specimen, which is in line with other literature.5 Our study is novel in its consideration of deep and radial IFSH separately, as well as its use of a dual classification system for determining the sensitivity and specificity of IFSH compared with both the final pathology of the same specimen as well as the final tumor specimen margin status. By determining the sensitivity and specificity in this way, we better demonstrate the clinical relevance of any discordance associated with IFSH. Because most head and neck surgeons consider carcinoma in situ to be a positive finding, the binary classification system used in our study provides the most clinically relevant information for intraoperative decision-making.7,8 Despite the improved results with the binary system compared with the graded system, IFSH is still shown to correlate well with the permanent pathology of the same specimen but not with FTSH.
Interpretive and sampling errors can both occur in IFSH. Interpretive errors are less common because IFSH has been shown to be 96% to 99% accurate when the same tissue undergoes formal histopathology.10,15,18,19 Sampling errors are more likely because assessing a close or positive final tumor specimen margin relies on a representative IFSH.15,19,20 Ord and Aisner17 reported 307 frozen sections from 49 patients with oral cavity SCC to assess the accuracy of the frozen section technique. Only 2 false negatives and 1 false positive were found when comparing the frozen section result with the permanent section of the same tissue, yielding an accuracy rate of 99%. In a more recent study of 3308 IFSH margins, Buchakjian et al10 similarly found IFSH to have 99% accuracy compared with the permanent pathology of the same tissue. The high accuracy in our study using both classification systems corroborates that from these smaller prior studies.10,15,17,18,19
When comparing IFSH against FTSH in our study, IFSH had a high NPV (approximately 96%) but a low PPV (<30%), demonstrating that a positive IFSH does not correlate well to FTSH margin status. These patterns remained true for both deep and radial IFSH, but deep IFSH yielded higher PPV compared with radial IFSH (deep, 44%-56%; radial, 9%-24%).
Buchakjian et al10 also found that IFSH did not strongly correlate with FTSH margin status, with an accuracy of just 66%. Even when they considered only invasive carcinoma as positive, the PPV was just 45% (95% CI, 33%-57%). Our study supports the poor association between IFSH and FTSH margin status, demonstrating that reliance on IFSH results to guide tumor excision needs careful consideration.
Limitations
This study has some limitations. It represents a historical data set, and the pathology records were retrospectively reviewed. The findings of our study relate to a summation of all oral cavity subsites and predominantly reflects a tumor bed margin technique. In a retrospective study of 108 patients with mostly oral cavity cancer, Varvares et al2 found that local recurrence rates were higher when margins were taken from the tumor bed compared with specimen-based margins. Others have also proposed the specimen-based method for margin assessment on the grounds that it affords better local control.12 A complete discussion on the differences between these 2 methods and their corresponding advantages is outside the scope of this article, but it is an important topic and one of ongoing debate. In addition, the technique for obtaining IFSH specimens differs from that performed by the pathologists for the final margin sampling: intraoperatively, IFSH specimens are obtained from the tumor bed by a cut shaving, whereas the sampled sections of final margins are taken perpendicularly from the area closest to the tumor. This difference and the subsequent potential for sampling error demonstrate the limitations of comparing IFSH and FTSH and are likely associated with the low sensitivity rate.
In addition, because the PPV and the NPV are associated with prevalence, the low prevalence of positive margins (approximately 11%) in our study could have been associated with these results. Most patients in our study had at least 1 IFSH sample for analysis, with only 12% of patients without any IFSH sample. Additional studies with a larger cohort of patients without IFSH samples may be needed to definitively compare groups.
Conclusions
This cross-sectional study found that the sensitivity of tumor bed IFSH compared with the final specimen margin status is low (approximately 10%-15%) for all margins, as well as for radial and deep margins when considered separately. This finding suggests that the IFSH result communicated intraoperatively should not be used to assess the final specimen margin status and, thus, questions the clinical utility of tumor bed frozen margin analysis.
eTable. Patient, Tumor, and Treatment Characteristics Between Patients With IFSH and Those With No IFSH
References
- 1.Ch’ng S, Corbett-Burns S, Stanton N, et al. Close margin alone does not warrant postoperative adjuvant radiotherapy in oral squamous cell carcinoma. Cancer. 2013;119(13):2427-2437. doi: 10.1002/cncr.28081 [DOI] [PubMed] [Google Scholar]
- 2.Varvares MA, Poti S, Kenyon B, Christopher K, Walker RJ. Surgical margins and primary site resection in achieving local control in oral cancer resections. Laryngoscope. 2015;125(10):2298-2307. doi: 10.1002/lary.25397 [DOI] [PubMed] [Google Scholar]
- 3.Barry CP, Ahmed F, Rogers SN, et al. Influence of surgical margins on local recurrence in T1/T2 oral squamous cell carcinoma. Head Neck. 2015;37(8):1176-1180. doi: 10.1002/hed.23729 [DOI] [PubMed] [Google Scholar]
- 4.Zanoni DK, Migliacci JC, Xu B, et al. A proposal to redefine close surgical margins in squamous cell carcinoma of the oral tongue. JAMA Otolaryngol Head Neck Surg. 2017;143(6):555-560. doi: 10.1001/jamaoto.2016.4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orosco RK, Tapia VJ, Califano JA, et al. Positive surgical margins in the 10 most common solid cancers. Sci Rep. 2018;8(1):5686. doi: 10.1038/s41598-018-23403-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kain JJ, Birkeland AC, Udayakumar N, et al. Surgical margins in oral cavity squamous cell carcinoma: current practices and future directions. Laryngoscope. 2020;130(1):128-138. doi: 10.1002/lary.27943 [DOI] [PubMed] [Google Scholar]
- 7.Meier JD, Oliver DA, Varvares MA. Surgical margin determination in head and neck oncology: current clinical practice: the results of an International American Head and Neck Society member survey. Head Neck. 2005;27(11):952-958. doi: 10.1002/hed.20269 [DOI] [PubMed] [Google Scholar]
- 8.Bulbul MG, Zenga J, Tarabichi O, et al. Margin practices in oral cavity cancer resections: survey of American Head and Neck Society members. Laryngoscope. 2021;131(4):782-787. doi: 10.1002/lary.28976 [DOI] [PubMed] [Google Scholar]
- 9.Mair M, Nair D, Nair S, et al. Intraoperative gross examination vs frozen section for achievement of adequate margin in oral cancer surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;123(5):544-549. doi: 10.1016/j.oooo.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 10.Buchakjian MR, Tasche KK, Robinson RA, Pagedar NA, Sperry SM. Association of main specimen and tumor bed margin status with local recurrence and survival in oral cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142(12):1191-1198. doi: 10.1001/jamaoto.2016.2329 [DOI] [PubMed] [Google Scholar]
- 11.Pathak KA, Nason RW, Penner C, Viallet NR, Sutherland D, Kerr PD. Impact of use of frozen section assessment of operative margins on survival in oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(2):235-239. doi: 10.1016/j.tripleo.2008.09.028 [DOI] [PubMed] [Google Scholar]
- 12.Maxwell JH, Thompson LDR, Brandwein-Gensler MS, et al. Early oral tongue squamous cell carcinoma: sampling of margins from tumor bed and worse local control. JAMA Otolaryngol Head Neck Surg. 2015;141(12):1104-1110. doi: 10.1001/jamaoto.2015.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulbul MG, Tarabichi O, Sethi RK, Parikh AS, Varvares MA. Does clearance of positive margins improve local control in oral cavity cancer? a meta-analysis. Otolaryngol Head Neck Surg. 2019;161(2):235-244. doi: 10.1177/0194599819839006 [DOI] [PubMed] [Google Scholar]
- 14.Scholl P, Byers RM, Batsakis JG, Wolf P, Santini H. Microscopic cut-through of cancer in the surgical treatment of squamous carcinoma of the tongue: prognostic and therapeutic implications. Am J Surg. 1986;152(4):354-360. doi: 10.1016/0002-9610(86)90304-1 [DOI] [PubMed] [Google Scholar]
- 15.DiNardo LJ, Lin J, Karageorge LS, Powers CN. Accuracy, utility, and cost of frozen section margins in head and neck cancer surgery. Laryngoscope. 2000;110(10, pt 1):1773-1776. doi: 10.1097/00005537-200010000-00039 [DOI] [PubMed] [Google Scholar]
- 16.Amin M, Edge S, Greene F, et al. , eds. American Joint Committee on Cancer (AJCC) Staging Manual. 8th ed. Springer International Publishing; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 17.Ord RA, Aisner S. Accuracy of frozen sections in assessing margins in oral cancer resection. J Oral Maxillofac Surg. 1997;55(7):663-669. doi: 10.1016/S0278-2391(97)90570-X [DOI] [PubMed] [Google Scholar]
- 18.Du E, Ow TJ, Lo YT, et al. Refining the utility and role of frozen section in head and neck squamous cell carcinoma resection. Laryngoscope. 2016;126(8):1768-1775. doi: 10.1002/lary.25899 [DOI] [PubMed] [Google Scholar]
- 19.Gandour-Edwards RF, Donald PJ, Wiese DA. Accuracy of intraoperative frozen section diagnosis in head and neck surgery: experience at a university medical center. Head Neck. 1993;15(1):33-38. doi: 10.1002/hed.2880150108 [DOI] [PubMed] [Google Scholar]
- 20.Layfield EM, Schmidt RL, Esebua M, Layfield LJ. Frozen section evaluation of margin status in primary squamous cell carcinomas of the head and neck: a correlation study of frozen section and final diagnoses. Head Neck Pathol. 2018;12(2):175-180. doi: 10.1007/s12105-017-0846-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Patient, Tumor, and Treatment Characteristics Between Patients With IFSH and Those With No IFSH