Abstract
Circular RNAs (circRNAs) are a special type of endogenous RNAs with extensive roles in multiple human diseases. They are formed by back-splicing of partial sequences of the parental precursor mRNAs. Unlike linear RNAs, their covalently closed loop structure without a 5′ cap and a 3′ polyadenylated tail confers on them high stability and they are difficult to be digested by RNase R. Increasing evidence has proved that aberrant expressions of many circRNAs are detected and that circRNAs exert essential biological functions in disease development and progression via acting as a molecular sponge of microRNA, interacting with proteins as decoys or scaffolds, or self-encoding small peptides. Circular RNA zinc finger protein 609 (circ-ZNF609) originates from exon2 of ZNF609, which is located at chromosome 15q22.31, and it has recently been proved that it can translate into a protein. Being aberrantly upregulated in various diseases, it could promote malignant progression of human tumors, as well as tumor cell proliferation, migration, and invasion. Here in this review, we concluded the biological functions and potential mechanisms of circ-ZNF609 in multiple diseases, which could be further explored as a targetable molecule in future accurate diagnosis and prognosis.
Keywords: circular RNA, ZNF609, human diseases, tumor malignant progression, mechanism
Introduction
In 1976, circular RNAs (circRNAs) were found in viroids and eukaryotic cells (Kolakofsky, 1976; Sanger et al., 1976). In the process of transcription from parental gene to RNA, circular RNAs are back-spliced to form a loop structure without a 5′ cap and a 3′ polyadenylated tail (Jeck et al., 2012; Chen and Yang, 2015). The special circular structure endows them with a characteristic so that they can resist the digestion of exonuclease RNase R and become a class of relatively stable RNAs (Suzuki et al., 2006; Xiao and Wilusz, 2019).
With the development of high-throughput RNA sequencing and bioinformatics, the mechanisms and functions of circRNAs have been gradually elucidated (Memczak et al., 2013; Chen and Yang, 2015). According to the current research, circRNAs are generally divided into three subtypes: exonic circRNAs (ecircRNAs) (Jeck et al., 2012), exon–intron circRNAs (eicircRNAs) (Li et al., 2015), and intronic circRNAs (ciRNAs) (Zhang et al., 2013). The majority is ecircRNAs located in the cytoplasm mostly, and the other two exist in the nucleus mostly (Jeck et al., 2012; Zhang et al., 2013; Li et al., 2015). CircRNAs are ubiquitous in tissue cells, blood cells, serum, and exosomes (Salzman et al., 2012; Rong et al., 2019; Shi et al., 2020a). The primary functions of circRNAs are as follows: the ability to regulate the transcription of parental mRNAs (Li et al., 2015); acting as molecular sponges to regulate the expressions of target genes (Thomson and Dinger, 2016); binding to and sequestering proteins to regulate the expressions of the associated proteins (Kristensen et al., 2019; Chen, 2020); and translating into proteins to perform their functions (Legnini et al., 2017; Shi et al., 2020b). CircRNAs play important roles in the occurrence and development of human diseases, such as promoting cell proliferation, migration, and invasion in tumors, regulating drug resistance, and the progression of cardiovascular disease, as well as regulating neovascularization (Liu et al., 2017; Aufiero et al., 2019; Rossi et al., 2019; Li et al., 2020a; Xiao et al., 2020; Ding et al., 2021; Kim et al., 2021; Verduci et al., 2021).
Circular RNA zinc finger protein 609 (circ-ZNF609) is highly expressed in normal human neurons and maintains physiological functions (Rybak-Wolf et al., 2015; Legnini et al., 2017). It is differentially expressed in a variety of human diseases, as a competitive endogenous RNA to regulate target genes and affect disease progression. In the present review, we aim to gain insights into the relationship between circ-ZNF609 and human diseases and provide a theoretical basis for clinical diagnosis and targeted therapy.
Structure and Biological Function of Circ-ZNF609
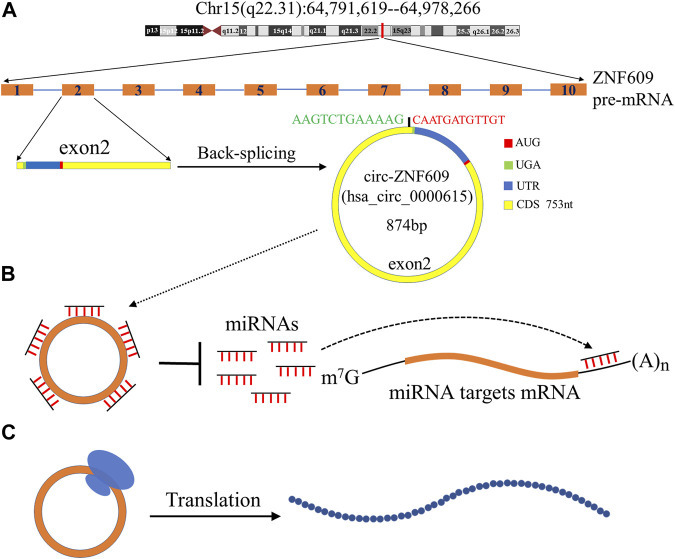
Circ-ZNF609 is a covalently closed circular RNA, which originates from the primary transcript of exon2 of ZNF609 located on chromosome 15q22.31. It is usually formed by back-splicing, a downstream splice-donor site is joined to an upstream splice-acceptor site, resulting in the loop structure of circ-ZNF609 and containing a specific junction site. It contains 874 bp nucleotides (Legnini et al., 2017); as a kind of circRNA that could be translated, its coding sequence contains 753 bp nucleotides, the start codon is located at position 128, and the stop codon is located at position 6 (Figure 1). There is one of the RNA binding proteins that can regulate the biogenesis of circ-ZNF609. Liu et al. (Liu et al., 2021a) reported that the expression of circ-ZNF609 could be upregulated by RNA binding protein fused in sarcoma (FUS), which could modulate the back-splicing reaction (Liu et al., 2021a). The FUS protein could induce the splicing and circularization of circ-ZNF609 by binding to the upstream exon2 of ZNF609 pre-mRNA (Liu et al., 2021a).
FIGURE 1.
Biogenesis and biological functions of circ-ZNF609. (A) ZNF609 is located in chromosome 15q22.31 in humans; the red line indicates its approximate location. ZNF609 contains 10 exons, and the exon2 is spliced in reverse to form circ-ZNF609. The total length of circ-ZNF609 is 874nt and contains a 753nt coding region, the promoter of circ-ZNF609 is at position 128, and the terminator is at position 6. (B) Circ-ZNF609 as a molecular sponge to adsorb microRNAs, reducing its binding to the 3′ UTR of the target mRNA, and regulate gene expression. (C) Circ-ZNF609 can be translated into a protein.
Considering the loop structure of circ-ZNF609 without a 5′ cap, its translation relies on a splicing-dependent/cap-independent manner (Legnini et al., 2017). However, Hung et al. (Ho-Xuan et al., 2020a) found that its translation may be originated from trans-splicing the byproducts of the overexpression of artificial circRNAs. They thought while performing functional studies of overexpression constructs of circRNA, it should be evaluated carefully (Ho-Xuan et al., 2020a). The primary biological function of circ-ZNF609 was to act as a molecular sponge of endogenous microRNAs to sequester and inhibit the microRNA activity, which led to regulating the target gene expression (Chen, 2020; Xiao et al., 2020). As in hepatocellular carcinoma, circ-ZNF609 inhibits miR-15a-5p/15b-5p expression and then elevates GLI2 (a key protein molecule concerning the Hedgehog pathway) expression, activating the Hedgehog pathway to promote hepatocellular carcinoma (HCC) proliferation and metastasis (He et al., 2020).
Circ-ZNF609 in Multiple Human Cancers
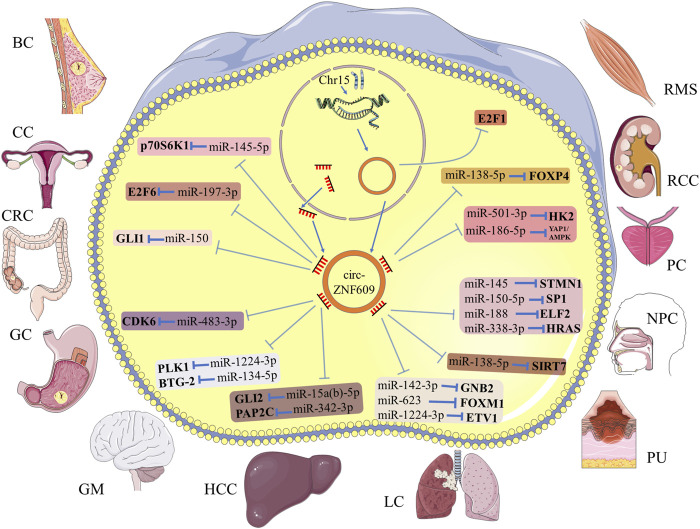
The present studies suggested that the main function of circ-ZNF609 is the posttranscriptional regulation, by acting as a molecular sponge of target microRNA (Figure 2; Table 1). As a carcinogen, circ-ZNF609 was abnormally upregulated in tumor tissues and cell lines. It also promoted tumor proliferation, migration, invasion, and other malignant phenotypes. The following content describes the molecular mechanisms of circ-ZNF609 in human cancers, in order by cancer names.
FIGURE 2.
Schematic diagram for the molecular mechanism of circ-ZNF609 in human cancers. Circ-ZNF609 acts as a molecular sponge of specific miRNA to regulate the expression of targeted genes in multiple cancers. T-shaped bars represent inhibition.
TABLE 1.
Mechanism of circ-ZNF609 in human cancers.
| Cancers | Expression Change | Targeted miRNAs | Targeted Genes | Tumor’s progression | Clinicopathological Features | References |
|---|---|---|---|---|---|---|
| Breast cancer (BC) | Upregulated | miR-145-5p | p70S6K1 | Promoting | Lymph node metastasis, advanced TNM stage, poor overall survival | Wang et al. (Wang et al., 2018a) |
| Cervical cancer (CC) | Upregulated | miR-197-3p | E2F6 | Promoting | — | Gu et al. (Gu et al., 2021) |
| Colorectal cancer (CRC) | Upregulated | miR-150 | GLI1 | Promoting | Lymph node metastasis, Dukes stage | Wu et al. (Wu et al., 2018) |
| Gastric cancer (GC) | Upregulated | miR-483-3p | CDK6 | Promoting | Advanced TNM stage, poor overall survival | Wu et al. (Wu et al., 2019) |
| Glioma (GM) | Upregulated | miR-1224-3p | PLK1 | Promoting | Advanced clinical grade | Du et al. (Du et al., 2021) |
| miR-134-5p | BTG-2 | Promoting | — | Tong et al. (Tong et al., 2019) | ||
| Hepatocellular carcinoma (HCC) | Upregulated | miR-15a(b)-5p | GLI2 | Promoting | — | He et al. (He et al., 2020) |
| miR-342-3p | PAP2C | Promoting | Lymph node metastasis, advanced TNM stage, poor overall survival | Liao et al. (Liao et al., 2020) | ||
| Lung cancer (LC) | Upregulated | miR-142-3p | GNB2 | Promoting | — | Liu et al. (Liu et al., 2021a) |
| miR-623 | FOXM1 | Promoting | Lymph node metastasis, advanced TNM stage, poor overall survival | Wang et al. (Wang et al., 2021a) | ||
| miR-1224-3p | ETV1 | Promoting | — | Zuo et al. (Zuo et al., 2020) | ||
| Melanoma (MM) | Upregulated | miR-138-5p | SIRT7 | Promoting | — | Liu et al. (Liu et al., 2021b) |
| Nasopharyngeal carcinoma (NPC) | Upregulated | miR-145 | STMN1 | Promoting | Lymph node metastasis, advanced clinical stage | Wang et al. (Wang et al., 2021b) |
| miR-150-5p | SP1 | Promoting | — | Zhu et al. (Zhu et al., 2019) | ||
| miR-188 | ELF2 | Promoting | — | Li et al. (Li et al., 2020b) | ||
| miR-338-3p | HRAS | Promoting | Poor overall survival | Liu et al. (Liu et al., 2021c) | ||
| Prostate cancer (PC) | Upregulated | miR-186-5p | YAP1/AMPK | Promoting | — | Jin et al. (Jin et al., 2019) |
| miR-501-3p | HK2 | Promoting | Advanced TNM stage, metastasis | Du et al. (Du et al., 2020) | ||
| Renal cell carcinoma (RCC) | Upregulated | miR-138-5p | FOXP4 | Promoting | — | Xiong et al. (Xiong et al., 2019) |
| Rhabdomyosarcoma (RMS) | Upregulated | — | E2F1 | Promoting | — | Rossi et al. (Rossi et al., 2019) |
Breast Cancer
Breast cancer is the most common cancer and the second leading cause of cancer lethality among women (DeSantis et al., 2017). In comparison with conventional surgery, neoadjuvant therapy has become a more widely used option, and novel targeted therapies play an important role in long-term disease control of metastatic breast cancer (Harbeck and Gnant, 2017). Being highly expressed in breast tumor tissues; circ-ZNF609 leads to a poor outcome in the overall survival and is closely associated with lymph node metastasis and advanced TNM stage (Wang et al., 2018a). Wang et al. proved that circ-ZNF609 knockdown inhibits the formation of malignant phenotypes of breast cancer cells and delays the tumor growth rate in vivo. It exerted biological function by sponging miR145-5p, which targeted oncogenic ribosomal protein S6 kinase, polypeptide 1 (p70S6K1), and promoted breast cancer progression (Wang et al., 2018a). They demonstrated that circ-ZNF609 regulated the miR-145-5p/p70S6K1 axis and could become a potential posttreatment prognostic biomarker in breast cancer.
Cervical Cancer
Each year, more than 500,000 women are diagnosed with cervical cancer. Advances in radiotherapy technology have significantly reduced treatment-related toxicity (Cohen et al., 2019); however, the overall survival of metastatic cervical tumors is still poor. Gu et al. (Gu et al., 2021) found that circ-ZNF609 was overexpressed in cervical cancer tissues and cell lines, and knockdown of circ-ZNF609 suppresses the malignant phenotype of the tumor. Circ-ZNF609 acted as the sponge of miR-197-3p, directly upregulating the expression of E2F transcription factor 6 (E2F6). The overexpression of E2F6 can partially reverse the inhibition of cell proliferation, migration, and invasion caused by circ-ZNF609 depletion (Gu et al., 2021). Their research suggested that the circ-ZNF609/miR-197-3p/E2F6 regulatory axis proposed a new insight into the progression of cervical cancer.
Colorectal Cancer
Colorectal cancer is the third most commonly diagnosed cancer in males and the second in females (Torre et al., 2015). Exploring the potential diagnostic biomarkers of colorectal cancer is of great significance to contemporary medicine. Emerging evidence has shown the vital role of circ-ZNF609 in colorectal cancer development and progression. The expression level of circ-ZNF609 was reported to be positively correlated with GLI family zinc finger 1 (GLI1) and negatively correlated with miR-150. In particular, it acted as a molecular sponge of miR-150 to downregulate the expression of GLI1 and then promoted colorectal cancer cell proliferation and migration (Wu et al., 2018). Later, Hung et al. (Ho-Xuan et al., 2020b) found that circ-ZNF609 acted as an oncogene during colorectal cancer progression and metastasis. The overexpression of circ-ZNF609 leads to increased tumor growth, while knockdown led to contrasting effects in mouse xenograft models. However, Zhang et al. (Zhang et al., 2019a) reported discrepant results that circ-ZNF609 is downregulated in colorectal cancer tissues and patient serum samples, and it also induced cell apoptosis via upregulating p53. To summarize, the specific role and regulating mechanism of circ-ZNF609 in colorectal cancer are still unclear, requiring further elucidation.
Gastric Cancer
Gastric cancer is the second leading cause of cancer-related deaths and the incidence ranks fourth worldwide, which mainly relies on pathological examination (Torre et al., 2015; Sitarz et al., 2018). Surgical resection and chemotherapy are the principal treatment approaches; however, lymph node and distant metastases during advanced stages limit the therapeutic effect (Sitarz et al., 2018). Therefore, seeking early biomarkers of gastric cancer makes all the difference between accurate diagnosis and treatment (Zhang and Zhang, 2017). Wu et al. (Wu et al., 2019) proved that circ-ZNF609 is overexpressed in cancer tissues and cell lines of gastric cancer patients, and it was positively correlated with a higher TNM stage and a lower 5-year survival rate. It acted as a sponge of miR-483-3p, upregulated the expression of cell-promoting factor cyclin-dependent kinase 6 (CDK6), and promoted the proliferation and migration of gastric cancer cells through the circ-ZNF609/miR-483-3p/CDK6 axis (Wu et al., 2019). In interest, Liu et al. (Liu et al., 2019) had discovered different mechanisms of circ-ZNF609 in gastric cancer, through binding to miR-145-5p and negatively regulating its expression. Knockdown of circ-ZNF609 inhibited cell proliferation and induced apoptosis, which could be partially reversed by miR-145-5p overexpression (Liu et al., 2019).
Glioma
Glioma is a primary brain tumor that is highly metastatic and aggressive (Weller et al., 2015; Chen et al., 2017). It is of great significance to determine the potential molecular mechanism of circ-ZNF609 in glioma. Du et al. (Du et al., 2021) proved that circ-ZNF609 was overexpressed in glioma tissues and cell lines and was significantly overexpressed in high-grade glioma than in low-grade glioma. Silencing circ-ZNF609 could inhibit the proliferation and migration of glioma cells. In routine, it promoted the expression of polo-like kinase-1 (PLK1) by competitively binding to miR-1224-3p, and circ-ZNF609 also promoted tumor growth in vivo (Du et al., 2021). Meanwhile, Tong et al. (Tong et al., 2019) found that miR-134-5p inhibited the expression of BTG antiproliferation factor 2 (BTG-2) and inhibited the proliferation and migration of glioma. Circ-ZNF609 positively regulated the expression of BTG-2 through competitively binding to miR-134-5p, leading to the proliferation and migration of glioma. It proposed a novel mechanism of circ-ZNF609 in regulating the progression of glioma (Tong et al., 2019).
Hepatocellular Carcinoma
Liver cancer is the fourth leading cause of cancer deaths worldwide (Villanueva, 2019). Existing studies have demonstrated that circRNAs could promote the progress of HCC by regulating microRNAs (Zhang and Wang, 2021). Circ-ZNF609 was highly expressed in HCC tissues and cell lines. Knockdown of circ-ZNF609 could inhibit the proliferation, migration, and invasion of HCC and promote apoptosis (He et al., 2020). Adding SAG, an agonist of the Hedgehog signal pathway, could restore the phenotype caused by circ-ZNF609 knockdown (He et al., 2020). Bioinformatics analysis and experiments validated that circ-ZNF609 regulated the expressions of miR-15a-5p/15b-5p and GLI family zinc finger 2 (GLI2) and promoted the malignant phenotype of HCC through the Hedgehog pathway (He et al., 2020). Liao et al. (Liao et al., 2020) also proved that silencing circ-ZNF609 could inhibit the proliferation of HCC and upregulate the expression of RAP2C (member of the RAS oncogene family) by acting as a sponge of miR-342-3p. The experiments in vivo showed that circ-ZNF609 facilitated tumor growths, confirming the findings in vitro.49
Lung Cancer
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer (Devesa et al., 2005), and circRNAs play a significant role in its pathogenesis and progression (Di et al., 2019; Huang et al., 2019). Fork head box protein M1 (FOXM1) is overexpressed in various cancers, which is a necessary transcription factor for cell proliferation (Liao et al., 2018). In NSCLC, it was targeted by miR-623 and circ-ZNF609. Knocking down circ-ZNF609 inhibited cell viability, migration, and invasion and promoted apoptosis, and knocking down miR-623 or overexpressed FOXM1 could weaken these effects (Wang et al., 2021a). Lung adenocarcinoma (LUAD) is one of the histological subtypes of lung cancer with a poor prognosis (Devesa et al., 2005); circ-ZNF609 was overexpressed in LUAD, acting as a sponge of miR-1224-3p to promote the cell proliferation of LUAD, which negatively regulated the expression of ETS variant transcription factor 1 (ETV1). They verified that circ-ZNF609 promoted LUAD proliferation through the miR-1224-3p/ETV1 axis (Zuo et al., 2020). In lung cancer, it was also found that FUS RNA binding protein could bind to the intron1 region of pre-mRNA of ZNF609, but not to exon1 and exon2. The specific binding may regulate the back-splicing of exon2, leading to upregulation of circ-ZNF609 and promoting the malignant progression of lung cancer through the miR-142-3p/GNB2 axis (Liu et al., 2021a). These studies provided different insights for understanding the value of circ-ZNF609 in different histological subtypes of lung cancer, indicating that the pathogenic mechanism of circ-ZNF609 in lung cancer was tissue specific.
Melanoma
Melanoma is a prevalent malignant skin cancer. Its incidence and mortality rates vary greatly worldwide. Once melanoma spreads, it will quickly become life-threatening (Schadendorf et al., 2018). As stated, circ-ZNF609-mediated DNA damage plays an important role in the development of melanoma. Knocking down circ-ZNF609 could inhibit the proliferation, migration, and invasion of melanoma cell lines, reduce cell survival rate, and promote apoptosis (Liu et al., 2021b). Comet assays showed that the tail length was elevated and the expression level of γH2AX variant histone (γH2AX) was increased after circ-ZNF609 depletion, suggesting that circ-ZNF609 inhibited the DNA damage in melanoma (Liu et al., 2021b). Circ-ZNF609 repressed DNA oxidative damage by acting as a sponge of miR-138-5p, which induced DNA oxidative damage by targeting sirtuin 7 (SIRT7). Adding miR-138-5p inhibitor or overexpression of SIRT7 partially reversed the DNA damage phenotype caused by circ-ZNF609 depletion, and circ-ZNF609 depletion reduced the tumor size, tumor volume, and tumor weight through the miR-138-5p/SIRT7 axis in vivo (Liu et al., 2021b). This study provided a new mechanism for the pathogenesis of DNA damage in melanoma, suggesting that circRNA-mediated DNA oxidative damage may be a valuable direction for melanoma biogenesis.
Nasopharyngeal Carcinoma
Nasopharyngeal carcinoma (NPC) is a common malignant tumor of the head and neck, and chemotherapy is an effective treatment method (Chen et al., 2019). However, since it is prone to lymph node metastasis and the degree of malignancy is high (Chua et al., 2016), it is of great significance to study the mechanism of occurrence and development. Pathological angiogenesis is a hallmark of cancer progression (Carmeliet and Jain, 2000), which is an important cause of the metastasis of NPC (Bao et al., 2018). The expression of vascular endothelial growth factor (VEGF) after the knockdown of circ-ZNF609 in NPC cells was downregulated. The supernatant was added to treat human umbilical vein endothelial cells (HUVEC), and the proteins of VEGF receptor-1 and VEGF receptor-2 in HUVECs are reduced. It could be observed that the total tube length was shortened, and the nodules were reduced when knocking down circ-ZNF609 in HUVEC. These proved that the angiogenesis was reduced after knocking down circ-ZNF609. It negatively regulated the expression of miR-145 and upregulated stathmin 1 (STMN1) to promote the proliferation, migration, and angiogenesis of NPC, forming a new regulatory mechanism for the pathological angiogenesis of NPC (Wang et al., 2021b). Zhu et al. (Zhu et al., 2019) found that circ-ZNF609 promoted the growth and metastasis of NPC and exerted carcinogenic influence by competing with miR-150-5p which degraded Sp1 expression. Liu et al. (Liu et al., 2021c) also proposed that circ-ZNF609 was highly expressed in NPC tissues and cell lines, by binding to miR-338-3p to negatively regulate its expression, upregulating histidyl-tRNA synthetase (HARS) that promoted the proliferation, migration, invasion, and glycolysis of NPC, and xenograft experiments proved the result in vitro. The results of Li et al. (Li et al., 2020b) also showed that circ-ZNF609 was overexpressed in NPC tissues and cell lines, knocking down it inhibited NPC cell proliferation and cell cycle transition, as well as accelerated apoptosis, and the carcinogenic effect was achieved through the circ-ZNF609/miR-188/ELF2 axis. Their studies had shown that circ-ZNF609 was overexpressed in NPC, as a molecular sponge of related microRNA and upregulated the expression of the target gene, achieving carcinogenic effects, and these suggest that circ-ZNF609 may be a new therapeutic target for NPC. The molecular mechanism of circ-ZNF609 in NPC had been inconsistently reached by different research teams, which may be caused by differences in patient samples. The mechanism of circ-ZNF609 in NPC required a more rigorous study to reveal a clear conclusion.
Prostate Cancer
Prostate cancer is a common malignant tumor of the urinary system (Torre et al., 2015), and radiotherapy is the main treatment modality. However, the metastasis of advanced patients limits the application of radiotherapy (Mohler et al., 2010). It is of great significance to understand the mechanisms of radiological resistance. Even in the presence of oxygen and fully functional mitochondria, tumor cells increased glucose uptake and fermentation of glucose to lactate, and the process is called the Warburg effect. It is characterized by changes in glycolysis and metabolism, which can promote tumor metastasis (Liberti and Locasale, 2016). Circ-ZNF609 was highly expressed in prostate cancer tissues and cells, and silencing it could repress cell viability, inhibit cell migration and invasion, and induce cell apoptosis (Du et al., 2020). Circ-ZNF609 silencing decreased the glucose uptake and lactate product of tumor cells, and overexpression of circ-ZNF609 could increase the radioresistance of cells. However, the radioresistance was significantly inhibited by the addition of glycolysis inhibitor 2-deoxy-D-glucose (2-DG), suggesting that circ-ZNF609 promoted glycolysis to improve the radioresistance of cells (Du et al., 2020). Circ-ZNF609 acted as a molecular sponge of miR-501-3p, and 2-DG could significantly inhibit the promotion of glycolysis by anti-miR-501-3p. The circ-ZNF609/miR-501-3p axis was targeted to upregulate the expression of hexokinase 2 (HK2), a key enzyme of glycolysis, and then improved the radioresistance of tumor cells both in vitro and in vivo (Du et al., 2020). Jin et al. (Jin et al., 2019) proposed that silencing circ-ZNF609 could restrain Yes1-associated transcriptional regulator (YAP1) and AMP-activated protein kinase (AMPK) signaling pathways by upregulating miR-186-5p, thereby inhibiting cell proliferation, migration, and invasion, and inducing apoptosis. In conclusion, circ-ZNF609 could promote prostate cancer progression through multiple mechanisms, including regulated glycolysis and metabolism, promoting radioresistance and activating signaling pathways.
Renal Cell Carcinoma
Renal cell carcinoma (RCC) is a common tumor of the urinary system (Rini et al., 2009). Some ncRNAs have been proved to be involved in the biological process of kidney cancer, providing molecular targets for the treatment (Li et al., 2017; Wang et al., 2017; Shelar et al., 2018). Xiong et al. (Xiong et al., 2019) proved that circ-ZNF609 represented a circular structure that was resistant to the digestion of RNase R. It was also overexpressed in renal cancer cell lines than in renal epithelial cells. By targeted binding to miR-138-5p, circ-ZNF609 upregulated the expression of the transcription factor forkhead box P4 (FOXP4) and promoted the proliferation, migration, and invasion of renal cancer cells. Knocking down of circ-ZNF609 inhibited the malignant phenotype in RCC (Xiong et al., 2019).
Rhabdomyosarcoma
RMS is a pediatric skeletal muscle malignancy that accounts for roughly 5% of all pediatric tumors (Egas-Bejar and Huh, 2014). Rhabdomyosarcoma in children is usually divided into two main histological subtypes, the embryonal rhabdomyosarcoma (ERMS) and the alveolar rhabdomyosarcoma (ARMS), and the latter has a generally worse prognosis (Egas-Bejar and Huh, 2014; Sun et al., 2015). Rossi et al. (Rossi et al., 2019) reported that circ-ZNF609 was upregulated in biopsies from ERMS and ARMS. Circ-ZNF609 knockdown induced a significant decrease in the p-Akt protein level, which modulated cell proliferation-related pathways and an alteration of the p-Rb/Rb ratio in an ERMS-derived cell line. The hypophosphorylated Rb protein could bind E2F transcription factor 1 (E2F1) to reduce the activation of S-phase transcription factors such as TCF19 and MCMs, which caused a specific block of ERMS from the G1 to the S phase. Differently to ERMS, in the ARMS-derived cells, due to the lower p53 that was involved in cell cycle arrest, ARMS does not undergo G1-S arrested after the circ-ZNF609 knockdown, which means that circ-ZNF609 knockdown was not enough to significantly inhibit ARMS cell proliferation (Rossi et al., 2019).
Circ-ZNF609 in Other Human Diseases
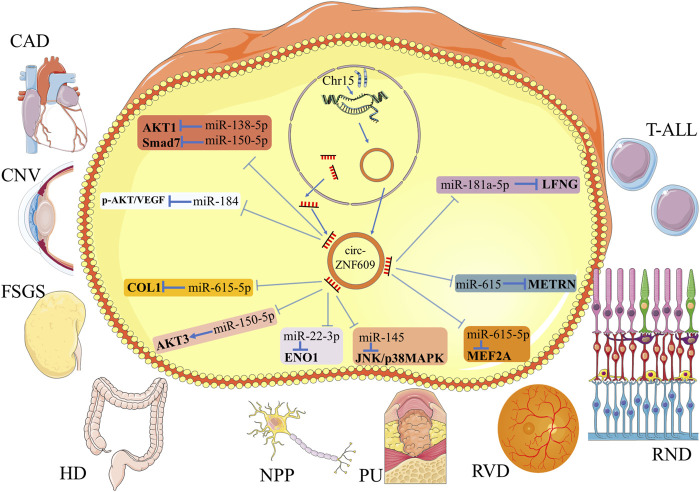
Similar to the function in human tumors, circ-ZNF609 also acted as a sponge of microRNAs in nontumor diseases (Figure 3; Table 2). In nontumor diseases, the expression level of circ-ZNF609 had not been verified in the human tissues. On accounting for cell lines and animal models, researchers found that circ-ZNF609 promoted cell proliferation and induced poor phenotypes in most nontumorous diseases. However, in coronary heart disease and Hirschsprung’s disease, it was downregulated, representing an opposite function as a protective regulator. Therefore, we summarized the role of circ-ZNF609 in nontumorous diseases but not only in human cancers here.
FIGURE 3.
Schematic diagram for the molecular mechanism of circ-ZNF609 in nontumor diseases. Circ-ZNF609 acts as a molecular sponge of specific miRNA to regulate the expression of targeted genes in nontumor diseases. T-shaped bars represent inhibition, and arrows represent promotion.
TABLE 2.
Mechanism of circ-ZNF609 in other human diseases.
| Diseases | Expression Change | Targeted miRNAs | Targeted genes | Diseases Progression | References |
|---|---|---|---|---|---|
| Coronary artery disease (CAD) | Downregulated | miR-138-5p a | AKT1 a | Inhibiting | Liang et al. (Liang et al., 2020) |
| miR-150-5p a | Smad7 a | ||||
| Corneal neovascularization (CNV) | Upregulated | miR-184 | p-AKT/VEGF | Promoting | Wu et al. (Wu et al., 2020) |
| Focal segmental glomerulosclerosis (FSGS) | Upregulated | miR-615-5p | COL1 | Promoting | Cui et al. (Cui et al., 2020) |
| Hirschsprung’s disease (HD) | Downregulated | miR-150-5p | AKT3 | Inhibiting | Peng et al. (Peng et al., 2017) |
| Neuropathic pain (NPP) | Upregulated | miR-22-3p | ENO1 | Promoting | Li et al. (Li et al., 2020c) |
| Pressure ulcer (PU) | Upregulated | miR-145 | JNK/p38MAPK | Promoting | Ge and Gao (Ge and Gao, 2020) |
| Retinal neurodegeneration (RND) | Upregulated | miR-615 | METRN | Promoting | Wang et al. (Wang et al., 2018b) |
| Retinal vascular dysfunction (RVD) | Upregulated | miR-615-5p | MEF2A | Promoting | Liu et al. (Liu et al., 2017) |
| T-cell acute lymphoblastic leukemia (T-ALL) | Upregulated | miR-181a-5p | LFNG | Promoting | Buratin et al. (Buratin et al., 2020) |
Based on bioinformatics prediction and literature reports.
Coronary Artery Disease
Coronary artery disease (CAD) is the major cause of mortality globally (Dagenais et al., 2020), and the inflammatory response theory has been widely recognized in its pathogenic mechanism (Hansson, 2005). It has been reported that circRNAs were involved in several cardiovascular pathological processes (Wang et al., 2016; Shen et al., 2019). Compared with normal populations, the expression of circ-ZNF609 was downregulated in CAD patients. Logistic analysis suggested that a low circ-ZNF609 level was an independent risk factor for CAD. Overexpression of circ-ZNF609 in cells would cause the decrease of IL-6 and TNF-α and an increase in IL-10 expressions, suggesting its antiinflammatory effects, and could alleviate the development of CAD (Liang et al., 2020). Based on the bioinformatics prediction and the literature reports, researchers speculated that circ-ZNF609 exerted a protective function in CAD by sponging microRNA and regulated the miR-138-5p/AKT1 or miR-150-5p/Smad7 axis to interrupter inflammation pathways.
Corneal Neovascularization
The cornea lacks blood vessels to ensure that light passes through the lens, and pathological corneal neovascularization derived from the corneal limbus which is filled with blood vessels can affect the function of the transparent cornea and threaten vision (Feizi et al., 2017; Mobaraki et al., 2019). The study of corneal neovascularization by Wu et al. (Wu et al., 2020) suggested that in the rat corneal suture model, the overexpression of circ-ZNF609 and a decrease of miR-184 were observed in the corneal epithelia of rats after corneal suture surgery, and circ-ZNF609 acted as a sponge of miR-184 to regulate the AKT/β-catenin/VEGF signaling pathway and then promoted cell proliferation, migration in vitro, and angiogenesis in vivo.84 Their study proved that inhibiting circ-ZNF609 may be a new therapeutic method for the treatment of pathological corneal neovascularization. The role of circ-ZNF609 in rat corneal neovascularization was different from that of human tumors; although there was no more cell line model to validate the finding, it could be validated in more animal models to ensure the conclusion.
Focal Segmental Glomerulosclerosis
Focal segmental glomerulosclerosis (FSGS) is the main cause of kidney disease worldwide, and it is a complex syndrome that arises after podocyte injury in general (Rosenberg and Kopp, 2017). In the mice model of FSGS, the expression of circ-ZNF609 was increased in biopsies compared to normal control mice. The expression of circ-ZNF609 was positively correlated with the degree of podocyte destruction and renal fibrosis, but miR-615-5p was negatively correlated with circ-ZNF609 (Cui et al., 2020). In mechanism, it acted as a molecular sponge of miR-615-5p to downregulate the expression of podocyte biomarkers WT1 and upregulate fibrotic proteins including COL1, promoting the progression of FSGS (Cui et al., 2020). The expression of circ-ZNF609 in the kidney was limited not only to RCC but also to FSGS, indicating that the scope of research could be extended to other nontumor sites. This study suggested that circ-ZNF609 might be a potential biomarker for the diagnosis of kidney disease.
Hirschsprung’s Disease
Hirschsprung’s disease (HSCR) is caused by a lack of enteric nerve cells in the variable part of the distal intestine, and infants with related genetic changes usually develop intestinal obstruction a few days after birth (Kenny et al., 2010). The expression of circ-ZNF609 was downregulated in HSCR compared with normal colon tissues and inhibited the proliferation and migration of HSCR cells. In mechanism, it downregulated the expression of AKT serine/threonine kinase 3 (AKT3) through acting as a sponge of miR-150-5p and promoted disease progression (Peng et al., 2017). However, Rossi et al. (Rossi et al., 2019) found that the expressions of miR-150-5p and AKT3 were not affected by the downregulation of circ-ZNF609; therefore, the mechanism of circ-ZNF609-regulated HSCR progression needs further study.
Neuropathic Pain
The widely accepted definition of neuropathic pain is the pain caused by a lesion or disease of the somatosensory system (Colloca et al., 2017). Due to the aging of the global population, the increasing incidence of cancer, and the consequences of chemotherapy, neuropathic pain may become more common (Colloca et al., 2017), and therefore finding its therapeutic targets has important clinical significance. The expression level of miR-22-3p was reduced in rat models with chronic constrictive injury and was involved in the progression of neuropathic pain, and miR-22-3p downregulation promoted neuropathic pain by targeting enolase 1 (ENO1) to regulate the expression of inflammatory factors (Li et al., 2020c). Li et al. (Li et al., 2020c) found that circ-ZNF609 regulated the expression of inflammatory factors TNF-α, IL-1, and IL-6 to promote neuropathic pain progression through the miR-22-3p/ENO1 axis (Li et al., 2020c). Although it was inappropriate to quantify the neuropathic pain phenotype with the level of inflammatory factors, this paper provided a new molecular mechanism for circ-ZNF609 in the regulation of inflammatory factors.
Pressure Ulcers
Pressure ulcers (PU) mostly occur in paralyzed and bedridden patients and are localized injuries to the skin and/or underlying tissues, usually over a bony prominence as a result of pressure combined with friction (Agrawal and Chauhan, 2012). PU are usually accompanied by skin oxidative damage, and drugs with antioxidant function are considered for treatment (Liu et al., 2018; Zhang et al., 2019b). In the PU model of HaCaT cells treated with H2O2, the expression of circ-ZNF609 was promoted in the model, and silencing of the expression of circ-ZNF609 alleviated oxidative stress damage including the viability loss, apoptosis, and ROS generation of HaCaT cells, through inhibiting the JNK and p38MAPK signaling pathways via acting as the sponge of miR-145 (Ge and Gao, 2020). The study in the model of PU provided new evidence for circ-ZNF609 in oxidative stress damage.
Retinal Neurodegeneration
Glaucoma is mainly manifested by visual field loss and irreversible blindness caused by progressively retinal neurodegenerative diseases, and the death of retinal ganglion cells (RGC) and high intraocular pressure are pathophysiological characteristics (Almasieh et al., 2012; Danesh-Meyer and Levin, 2015). In the rat model, circ-ZNF609 was significantly upregulated in the degeneration of the optic nerve induced by high intraocular pressure, and silencing it could inhibit the proliferation of RGC and optic nerve damage caused by high intraocular pressure (Wang et al., 2018b). Their previous studies suggested that circ-ZNF609 acted as a sponge of miR-615 to promote the proliferation of vascular endothelial cells. Circ-ZNF609 in RGC also acted as a sponge of miR-615 and then upregulated mentoring glial cell differentiation regulator (METRN) expression, promoting cell proliferation (Wang et al., 2018b). This study of circ-ZNF609 provided new insight into circRNAs in the growth of nerves, and it reflected the breadth of the role of circRNAs.
Retinal Vascular Dysfunction
Vascular dysfunction is a hallmark of pathological angiogenesis and contributes to the progression of various diseases (Puro et al., 2016), the expression of circRNAs is dysregulated in cardiovascular disease that is accompanied by vascular dysfunction, and endothelial cell regulation plays an important role in it (Eelen et al., 2015; Aufiero et al., 2019). The retinal vascular system can be observed by noninvasive means, which can be used to investigate the mechanism of vascular dysfunction (Flammer et al., 2013). Circ-ZNF609 promoted pathological angiogenesis and made endothelial cells more susceptible to oxidative stress and hypoxia (Liu et al., 2017; Wang et al., 2021b). Knocking down circ-ZNF609 in the mouse model of oxygen-induced retinopathy did not affect the development of normal retinal vascular, while it reduced avascular area and reduced pathological retinal angiogenesis (Liu et al., 2017). The study revealed the mechanism of action for the circ-ZNF609/miR-615-5p/MEF2A axis in the mediation of vascular endothelial dysfunction, and since pathological angiogenesis is a hallmark of tumors, this study also verified that circ-ZNF609 can cause tumors.
T-Cell Acute Lymphoblastic Leukemia
The expression level of circRNAs differs among normal blood cell types, but the expression in T-ALL is still unclear (Nicolet et al., 2018). RNA sequencing data from 25 T-ALL patients were analyzed and most circRNAs were found to be downregulated in expression in malignant T-ALL (Buratin et al., 2020). In particular, circ-ZNF609 was overexpressed in immature T-ALL, knocking down circ-ZNF609-inhibited cell proliferation and survival compared with normal control (Buratin et al., 2020). Bioinformatics analysis suggested that circ-ZNF609 was bound to miR-181a-5p in immature T-ALL, which by targeting O-fucosylpeptide 3-beta-N-acetylglucosaminyltransferase (LFNG) to promote leukemogenic potential through the Notch1 signaling pathway in T-ALL (Buratin et al., 2020). With only the predicted results of the bioinformatics analysis available here, researchers could verify the molecular mechanism of circ-ZNF609 in T-ALL through more cell experiments and animal experiments.
Conclusion and Future Prospects
The present review described the basic biological functions of circ-ZNF609 and systematically concluded its differential expression and its underlying molecular mechanism in human diseases. We found that the expression of circ-ZNF609 in various cancer tissues was higher than in adjacent normal tissues and dysregulation in nontumor diseases. When circ-ZNF609 is overexpressed in tumor tissues, it could result in poor overall survival, and it positively correlated with lymph node metastasis and advanced TNM or clinical stage. Relevant research on circ-ZNF609 helped us understand the pathogenesis of many diseases, and it was proved that circ-ZNF609 might be an effective and promising biomarker for diagnosis.
CircRNAs not only exist in human tissues and blood cells but are also differentially expressed in the serum and exosomes, playing an important role in disease progression (Devaux, 2017; Vea et al., 2018; Kristensen et al., 2019; Wang et al., 2019). Therefore, researchers can use the circRNAs in the patient’s body fluid as a noninvasive molecular marker. Emerging circRNAs have become potential therapeutic targets for human diseases, and circ-ZNF609 is one of them. In particular, circ-ZNF609 is an RNA that can be translated into a protein, giving it a broader role. Nowadays, the COVID-19 epidemic is serious, and the mRNA-based vaccine still has its limitations (Alameh et al., 2020; Corbett et al., 2020). The loop structure of circ-ZNF609 prevents its degradation and confers stronger stability to it compared with linear mRNA (Xiao and Wilusz, 2019). It is a promising research direction to develop a circRNA-based vaccine, by integrating an antigen-encoding sequence of COVID-19 into circ-ZNF609. The internal ribosomal entry site of circ-ZNF609 confers the translational function, making it possible to express the antigen of COVID-19. Qu et al. (Qu et al., 2022) reported a circRNA vaccine that elicited potent neutralizing antibodies and T-cell responses in an animal model (Qu et al., 2022; Szabó et al., 2022). The above studies indicated that circ-ZNF609 might become an effective and safe molecular platform against the epidemic.
In human diseases, it exerts functions through the circ-ZNF609-miRNA-mRNA network, and circ-ZNF609 knockdown or overexpression of miRNA will inhibit the malignant phenotype of cancers. Therefore, knocking down circ-ZNF609 by precise RNA interference (RNAi) or knocking out by CRISPR/Cas9-mediated circRNA knockout (Yang et al., 2018), developing miRNA inhibitors, could serve as potential therapeutic strategies for treatments of multiple human diseases. In comparison with Qian et al. (Qian et al., 2021), we comprehensively discussed the underlying molecular mechanism of circ-ZNF609 in multiple human diseases and expanded the types of the disease in detail. In addition, we discussed future research directions in circ-ZNF609-dysregulated diseases. The possible function of circ-ZNF609 in the prevention of the COVID-19 epidemic was also explored. We hope this study could help reveal the far-reaching clinical significance of circ-ZNF609.
Author Contributions
SBW and JJW wrote and revised the manuscript. ZYW and YYL helped to draft the manuscript. ZXG and YYL participated in the revision of the review. ZJW designed the project. All of the authors read and approved the final manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 81270685 and 81771640), the Project of Nanjing Science and Technology Committee (No. 201605001), and the “333” Project of Jiangsu Province (No. BRA2018083).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Agrawal K., Chauhan N. (2012). Pressure Ulcers: Back to the Basics. Indian J. Plast. Surg. 45, 244–254. 10.4103/0970-0358.101287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alameh M.-G., Weissman D., Pardi N. (2020). Messenger RNA-Based Vaccines against Infectious Diseases. Curr. Top. Microbiol. Immunol. 10.1007/82_2020_202 [DOI] [PubMed] [Google Scholar]
- Almasieh M., Wilson A. M., Morquette B., Cueva Vargas J. L., Di Polo A. (2012). The Molecular Basis of Retinal Ganglion Cell Death in Glaucoma. Prog. Retin. eye Res. 31, 152–181. 10.1016/j.preteyeres.2011.11.002 [DOI] [PubMed] [Google Scholar]
- Aufiero S., Reckman Y. J., Pinto Y. M., Creemers E. E. (2019). Circular RNAs Open a New Chapter in Cardiovascular Biology. Nat. Rev. Cardiol. 16, 503–514. 10.1038/s41569-019-0185-2 [DOI] [PubMed] [Google Scholar]
- Bao L., You B., Shi S., Shan Y., Zhang Q., Yue H., et al. (2018). Metastasis-associated miR-23a from Nasopharyngeal Carcinoma-Derived Exosomes Mediates Angiogenesis by Repressing a Novel Target Gene TSGA10. Oncogene 37, 2873–2889. 10.1038/s41388-018-0183-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratin A., Paganin M., Gaffo E., Dal Molin A., Roels J., Germano G., et al. (2020). Large-scale Circular RNA Deregulation in T-ALL: Unlocking Unique Ectopic Expression of Molecular Subtypes. Blood Adv. 4, 5902–5914. 10.1182/bloodadvances.2020002337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P., Jain R. K. (2000). Angiogenesis in Cancer and Other Diseases. Nature 407, 249–257. 10.1038/35025220 [DOI] [PubMed] [Google Scholar]
- Chen L.-L. (2020). The Expanding Regulatory Mechanisms and Cellular Functions of Circular RNAs. Nat. Rev. Mol. Cell Biol. 21, 475–490. 10.1038/s41580-020-0243-y [DOI] [PubMed] [Google Scholar]
- Chen L.-L., Yang L. (2015). Regulation of circRNA Biogenesis. RNA Biol. 12, 381–388. 10.1080/15476286.2015.1020271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Smith-Cohn M., Cohen A. L., Colman H. (2017). Glioma Subclassifications and Their Clinical Significance. Neurotherapeutics 14, 284–297. 10.1007/s13311-017-0519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.-P., Chan A. T. C., Le Q.-T., Blanchard P., Sun Y., Ma J. (2019). Nasopharyngeal Carcinoma. Lancet 394, 64–80. 10.1016/S0140-6736(19)30956-0 [DOI] [PubMed] [Google Scholar]
- Chua M. L. K., Wee J. T. S., Hui E. P., Chan A. T. C. (2016). Nasopharyngeal Carcinoma. Lancet 387, 1012–1024. 10.1016/S0140-6736(15)00055-0 [DOI] [PubMed] [Google Scholar]
- Cohen P. A., Jhingran A., Oaknin A., Denny L. (2019). Cervical Cancer. Lancet 393, 169–182. 10.1016/S0140-6736(18)32470-X [DOI] [PubMed] [Google Scholar]
- Colloca L., Ludman T., Bouhassira D., Baron R., Dickenson A. H., Yarnitsky D., et al. (2017). Neuropathic Pain. Nat. Rev. Dis. Prim. 3, 17002. 10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K. S., Edwards D. K., Leist S. R., Abiona O. M., Boyoglu-Barnum S., Gillespie R. A., et al. (2020). SARS-CoV-2 mRNA Vaccine Design Enabled by Prototype Pathogen Preparedness. Nature 586, 567–571. 10.1038/s41586-020-2622-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Fu J., Luan J., Qi H., Jiao C., Ran M., et al. (2020). CircZNF609 Is Involved in the Pathogenesis of Focal Segmental Glomerulosclerosis by Sponging miR-615-5p. Biochem. Biophysical Res. Commun. 531, 341–349. 10.1016/j.bbrc.2020.07.066 [DOI] [PubMed] [Google Scholar]
- Dagenais G. R., Leong D. P., Rangarajan S., Lanas F., Lopez-Jaramillo P., Gupta R., et al. (2020). Variations in Common Diseases, Hospital Admissions, and Deaths in Middle-Aged Adults in 21 Countries from Five Continents (PURE): a Prospective Cohort Study. Lancet 395, 785–794. 10.1016/S0140-6736(19)32007-0 [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer H. V., Levin L. A. (2015). Glaucoma as a Neurodegenerative Disease. J. Neuro-Ophthalmology 35, S22–S28. 10.1097/wno.0000000000000293 [DOI] [PubMed] [Google Scholar]
- DeSantis C. E., Ma J., Goding Sauer A., Newman L. A., Jemal A. (2017). Breast Cancer Statistics, 2017, Racial Disparity in Mortality by State. CA a cancer J. Clin. 67, 439–448. 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- Devaux Y. (2017). Transcriptome of Blood Cells as a Reservoir of Cardiovascular Biomarkers. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1864, 209–216. 10.1016/j.bbamcr.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Devesa S. S., Bray F., Vizcaino A. P., Parkin D. M. (2005). International Lung Cancer Trends by Histologic Type: Male:Female Differences Diminishing and Adenocarcinoma Rates Rising. Int. J. Cancer 117, 294–299. 10.1002/ijc.21183 [DOI] [PubMed] [Google Scholar]
- Di X., Jin X., Li R., Zhao M., Wang K. (2019). CircRNAs and Lung Cancer: Biomarkers and Master Regulators. Life Sci. 220, 177–185. 10.1016/j.lfs.2019.01.055 [DOI] [PubMed] [Google Scholar]
- Ding L., Wang R., Shen D., Cheng S., Wang H., Lu Z., et al. (2021). Role of Noncoding RNA in Drug Resistance of Prostate Cancer. Cell Death Dis. 12. 10.1038/s41419-021-03854-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Li H., Lu F., Zhang S., Tang J. (2021). Circular RNA ZNF609 Promotes the Malignant Progression of Glioma by Regulating miR-1224-3p/PLK1 Signaling. J. Cancer 12, 3354–3366. 10.7150/jca.54934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S., Zhang P., Ren W., Yang F., Du C. (2020). Circ-ZNF609 Accelerates the Radioresistance of Prostate Cancer Cells by Promoting the Glycolytic Metabolism through miR-501-3p/HK2 Axis. Cmar Vol. 12, 7487–7499. 10.2147/cmar.S257441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G., de Zeeuw P., Simons M., Carmeliet P. (2015). Endothelial Cell Metabolism in Normal and Diseased Vasculature. Circ. Res. 116, 1231–1244. 10.1161/circresaha.116.302855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egas-Bejar D., Huh W. W. (2014). Rhabdomyosarcoma in Adolescent and Young Adult Patients: Current Perspectives. Adolesc. Health Med. Ther. 5, 115–125. 10.2147/AHMT.S44582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi S., Azari A. A., Safapour S. (2017). Therapeutic Approaches for Corneal Neovascularization. Eye Vis. (Lond) 4, 28–10. 10.1186/s40662-017-0094-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flammer J., Konieczka K., Bruno R. M., Virdis A., Flammer A. J., Taddei S. (2013). The Eye and the Heart. Eur. heart J. 34, 1270–1278. 10.1093/eurheartj/eht023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R., Gao G. (2020). Anti-antioxidant Impacts of circZNF609 Silence in HaCaT Cells through Regulating miR-145. Artif. Cells, Nanomedicine, Biotechnol. 48, 384–392. 10.1080/21691401.2019.1709863 [DOI] [PubMed] [Google Scholar]
- Gu Q., Hou W., Shi L., Liu H., Zhu Z., Ye W. (2021). Circular RNA ZNF609 Functions as a Competing Endogenous RNA in Regulating E2F Transcription Factor 6 through Competitively Binding to microRNA-197-3p to Promote the Progression of Cervical Cancer Progression. Bioengineered 12, 927–936. 10.1080/21655979.2021.1896116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K. (2005). Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 352, 1685–1695. 10.1056/nejmra043430 [DOI] [PubMed] [Google Scholar]
- Harbeck N., Gnant M. (2017). Breast Cancer. Lancet 389, 1134–1150. 10.1016/s0140-6736(16)31891-8 [DOI] [PubMed] [Google Scholar]
- He Y., Huang H., Jin L., Zhang F., Zeng M., Wei L., et al. (2020). CircZNF609 Enhances Hepatocellular Carcinoma Cell Proliferation, Metastasis, and Stemness by Activating the Hedgehog Pathway through the Regulation of miR-15a-5p/15b-5p and GLI2 Expressions. Cell Death Dis. 11, 358. 10.1038/s41419-020-2441-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Xuan H., Glažar P., Latini C., Heizler K., Haase J., Hett R., et al. (2020). Comprehensive Analysis of Translation from Overexpressed Circular RNAs Reveals Pervasive Translation from Linear Transcripts. Nucleic Acids Res. 48, 10368–10382. 10.1093/nar/gkaa704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho-Xuan H., Lehmann G., Glazar P., Gypas F., Eichner N., Heizler K., et al. (2020). Gene Expression Signatures of a Preclinical Mouse Model during Colorectal Cancer Progression under Low-Dose Metronomic Chemotherapy. Cancers 13, 49. 10.3390/cancers13010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Zhang W., Shao Z. (2019). Prognostic and Diagnostic Significance of circRNAs Expression in Lung Cancer. J. Cell. Physiology 234, 18459–18465. 10.1002/jcp.28481 [DOI] [PubMed] [Google Scholar]
- Jeck W. R., Sorrentino J. A., Wang K., Slevin M. K., Burd C. E., Liu J., et al. (2012). Circular RNAs Are Abundant, Conserved, and Associated with ALU Repeats. Rna 19, 141–157. 10.1261/rna.035667.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Zhao W., Zhang Z., Liu W. (2019). Silencing Circular RNA circZNF609 Restrains Growth, Migration and Invasion by Up-Regulating microRNA-186-5p in Prostate Cancer. Artif. Cells, Nanomedicine, Biotechnol. 47, 3350–3358. 10.1080/21691401.2019.1648281 [DOI] [PubMed] [Google Scholar]
- Kenny S. E., Tam P. K. H., Garcia-Barcelo M. (2010). Hirschsprung's Disease. Seminars Pediatr. Surg. 19, 194–200. 10.1053/j.sempedsurg.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Kim E., Kim Y. K., Lee S.-J. V. (2021). Emerging Functions of Circular RNA in Aging. Trends Genet. 37, 819–829. 10.1016/j.tig.2021.04.014 [DOI] [PubMed] [Google Scholar]
- Kolakofsky D. (1976). Isolation and Characterization of Sendai Virus DI-RNAs. Cell 8, 547–555. 10.1016/0092-8674(76)90223-3 [DOI] [PubMed] [Google Scholar]
- Kristensen L. S., Andersen M. S., Stagsted L. V. W., Ebbesen K. K., Hansen T. B., Kjems J. (2019). The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 20, 675–691. 10.1038/s41576-019-0158-7 [DOI] [PubMed] [Google Scholar]
- Legnini I., Di Timoteo G., Rossi F., Morlando M., Briganti F., Sthandier O., et al. (2017). Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 66, 22–37. e29. 10.1016/j.molcel.2017.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. K., Chen C., Liu J. Y., Shi J. Z., Liu S. P., Liu B., et al. (2017). Long Noncoding RNA MRCCAT1 Promotes Metastasis of Clear Cell Renal Cell Carcinoma via Inhibiting NPR3 and Activating P38-MAPK Signaling. Mol. Cancer 16, 111–114. 10.1186/s12943-017-0681-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Sun D., Pu W., Wang J., Peng Y. (2020). Circular RNAs in Cancer: Biogenesis, Function, and Clinical Significance. Trends Cancer 6, 319–336. 10.1016/j.trecan.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Li L., Luo Y., Zhang Y., Wei M., Zhang M., Liu H., et al. (2020). CircZNF609 Aggravates Neuropathic Pain via miR-22-3p/ENO1 axis in CCI Rat Models. Gene 763, 145069. 10.1016/j.gene.2020.145069 [DOI] [PubMed] [Google Scholar]
- Li M., Li Y., Yu M. (2020). CircRNA ZNF609 Knockdown Suppresses Cell Growth via Modulating miR-188/ELF2 Axis in Nasopharyngeal Carcinoma. Ott Vol. 13, 2399–2409. 10.2147/ott.S234230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Huang C., Bao C., Chen L., Lin M., Wang X., et al. (2015). Exon-intron Circular RNAs Regulate Transcription in the Nucleus. Nat. Struct. Mol. Biol. 22, 256–264. 10.1038/nsmb.2959 [DOI] [PubMed] [Google Scholar]
- Liang B., Li M., Deng Q., Wang C., Rong J., He S., et al. (2020). CircRNA ZNF609 in Peripheral Blood Leukocytes Acts as a Protective Factor and a Potential Biomarker for Coronary Artery Disease. Ann. Transl. Med. 8, 741. 10.21037/atm-19-4728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G.-B., Li X.-Z., Zeng S., Liu C., Yang S.-M., Yang L., et al. (2018). Regulation of the Master Regulator FOXM1 in Cancer. Cell Commun. Signal 16, 57. 10.1186/s12964-018-0266-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Zhan W., Tian B., Luo Y., Gu F., Li R. (2020). Circular RNA ZNF609 Promoted Hepatocellular Carcinoma Progression by Upregulating PAP2C Expression via Sponging miR-342-3p. Ott Vol. 13, 7773–7783. 10.2147/OTT.S253936 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liberti M. V., Locasale J. W. (2016). The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 41, 211–218. 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Yao M.-D., Li C.-P., Shan K., Yang H., Wang J.-J., et al. (2017). Silencing of Circular RNA-Znf609 Ameliorates Vascular Endothelial Dysfunction. Theranostics 7, 2863–2877. 10.7150/thno.19353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Rybakina E. G., Korneva E. A., Noda M. (2018). Effects of Derinat on Ischemia-Reperfusion-Induced Pressure Ulcer Mouse Model. J. Pharmacol. Sci. 138, 123–130. 10.1016/j.jphs.2018.08.013 [DOI] [PubMed] [Google Scholar]
- Liu Q., Cui W., Yang C., Du L.-P. (2021). Circular RNA ZNF609 Drives Tumor Progression by Regulating the miR-138-5p/SIRT7 axis in Melanoma. Aging 13, 19822–19834. 10.18632/aging.203394 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liu S., Yang N., Jiang X., Wang J., Dong J., Gao Y. (2021). FUS‐induced Circular RNA ZNF609 Promotes Tumorigenesis and Progression via Sponging miR‐142‐3p in Lung Cancer. J. Cell Physiol. 236, 79–92. 10.1002/jcp.29481 [DOI] [PubMed] [Google Scholar]
- Liu Z., Pan H. M., Xin L., Zhang Y., Zhang W. M., Cao P., et al. (2019). Circ-ZNF609 Promotes Carcinogenesis of Gastric Cancer Cells by Inhibiting miRNA-145-5p Expression. Eur. Rev. Med. Pharmacol. Sci. 23, 9411–9417. 10.26355/eurrev_201911_19433 [DOI] [PubMed] [Google Scholar]
- Liu Z., Liu F., Wang F., Yang X., Guo W. (2021). CircZNF609 Promotes Cell Proliferation, Migration, Invasion, and Glycolysis in Nasopharyngeal Carcinoma through Regulating HRAS via miR-338-3p. Mol. Cell Biochem. 476, 175–186. 10.1007/s11010-020-03894-5 [DOI] [PubMed] [Google Scholar]
- Memczak S., Jens M., Elefsinioti A., Torti F., Krueger J., Rybak A., et al. (2013). Circular RNAs Are a Large Class of Animal RNAs with Regulatory Potency. Nature 495, 333–338. 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- Mobaraki M., Abbasi R., Omidian Vandchali S., Ghaffari M., Moztarzadeh F., Mozafari M. (2019). Corneal Repair and Regeneration: Current Concepts and Future Directions. Front. Bioeng. Biotechnol. 7. 10.3389/fbioe.2019.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J., Bahnson R. R., Boston B., Busby J. E., D'Amico A., Eastham J. A., et al. (2010). Prostate Cancer. J. Natl. Compr. Canc Netw. 8, 162–200. 10.6004/jnccn.2010.0012 [DOI] [PubMed] [Google Scholar]
- Nicolet B. P., Engels S., Aglialoro F., van den Akker E., von Lindern M., Wolkers M. C. (2018). Circular RNA Expression in Human Hematopoietic Cells Is Widespread and Cell-type Specific. Nucleic acids Res. 46, 8168–8180. 10.1093/nar/gky721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Chen G., Zhu Z., Shen Z., Du C., Zang R., et al. (2017). Circular RNA ZNF609 Functions as a Competitive Endogenous RNA to Regulate AKT3 Expression by Sponging miR-150-5p in Hirschsprung's Disease. Oncotarget 8, 808–818. 10.18632/oncotarget.13656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro D. G., Kohmoto R., Fujita Y., Gardner T. W., Padovani-Claudio D. A. (2016). Bioelectric Impact of Pathological Angiogenesis on Vascular Function. Proc. Natl. Acad. Sci. U.S.A. 113, 9934–9939. 10.1073/pnas.1604757113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Li Y., Li R., Yang T., Jia R., Ge Y. Z. (2021). circ‐ZNF609: A Potent circRNA in Human Cancers. J. Cell Mol. Med. 25, 10349–10361. 10.1111/jcmm.16996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu L., Yi Z., Shen Y., Lin L., Chen F., Xu Y., et al. (2022). Circular RNA Vaccines against SARS-CoV-2 and Emerging Variants. Cell S0092-8674, 00394. 10.1016/j.cell.2022.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rini B. I., Campbell S. C., Escudier B. (2009). Renal Cell Carcinoma. Lancet 373, 1119–1132. 10.1016/S0140-6736(09)60229-4 [DOI] [PubMed] [Google Scholar]
- Rong D., Lu C., Zhang B., Fu K., Zhao S., Tang W., et al. (2019). CircPSMC3 Suppresses the Proliferation and Metastasis of Gastric Cancer by Acting as a Competitive Endogenous RNA through Sponging miR-296-5p. Mol. Cancer 18, 25. 10.1186/s12943-019-0958-6 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rosenberg A. Z., Kopp J. B. (2017). Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 12, 502–517. 10.2215/cjn.05960616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi F., Legnini I., Megiorni F., Colantoni A., Santini T., Morlando M., et al. (2019). Circ-ZNF609 Regulates G1-S Progression in Rhabdomyosarcoma. Oncogene 38, 3843–3854. 10.1038/s41388-019-0699-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak-Wolf A., Stottmeister C., Glažar P., Jens M., Pino N., Giusti S., et al. (2015). Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 58, 870–885. 10.1016/j.molcel.2015.03.027 [DOI] [PubMed] [Google Scholar]
- Salzman J., Gawad C., Wang P. L., Lacayo N., Brown P. O. (2012). Circular RNAs Are the Predominant Transcript Isoform from Hundreds of Human Genes in Diverse Cell Types. PLoS One 7, e30733. 10.1371/journal.pone.0030733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. (1976). Viroids Are Single-Stranded Covalently Closed Circular RNA Molecules Existing as Highly Base-Paired Rod-like Structures. Proc. Natl. Acad. Sci. U.S.A. 73, 3852–3856. 10.1073/pnas.73.11.3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadendorf D., van Akkooi A. C. J., Berking C., Griewank K. G., Gutzmer R., Hauschild A., et al. (2018). Melanoma. Lancet 392, 971–984. 10.1016/S0140-6736(18)31559-9 [DOI] [PubMed] [Google Scholar]
- Shelar S., Shim E.-H., Brinkley G. J., Kundu A., Carobbio F., Poston T., et al. (2018). Biochemical and Epigenetic Insights into L-2-Hydroxyglutarate, a Potential Therapeutic Target in Renal Cancer. Clin. Cancer Res. 24, 6433–6446. 10.1158/1078-0432.ccr-18-1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Hu Y., Lou J., Yin S., Wang W., Wang Y., et al. (2019). CircRNA-0044073 I-s U-pregulated in A-therosclerosis and I-ncreases the P-roliferation and I-nvasion of C-ells by T-argeting miR-107. Mol. Med. Rep. 19, 3923–3932. 10.3892/mmr.2019.10011 [DOI] [PubMed] [Google Scholar]
- Shi X., Wang B., Feng X., Xu Y., Lu K., Sun M. (2020). circRNAs and Exosomes: A Mysterious Frontier for Human Cancer. Mol. Ther. - Nucleic Acids 19, 384–392. 10.1016/j.omtn.2019.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Jia X., Xu J. (2020). The New Function of circRNA: Translation. Clin. Transl. Oncol. 22, 2162–2169. 10.1007/s12094-020-02371-1 [DOI] [PubMed] [Google Scholar]
- Sitarz R., Skierucha M., Mielko J., Offerhaus J., Maciejewski R., Polkowski W. (2018). Gastric Cancer: Epidemiology, Prevention, Classification, and Treatment. Cmar Vol. 10, 239–248. 10.2147/CMAR.S149619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Guo W., Shen J. K., Mankin H. J., Hornicek F. J., Duan Z. (2015). Rhabdomyosarcoma: Advances in Molecular and Cellular Biology. Sarcoma 2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Zuo Y., Wang J., Zhang M. Q., Malhotra A., Mayeda A. (2006). Characterization of RNase R-Digested Cellular RNA Source that Consists of Lariat and Circular RNAs from Pre-mRNA Splicing. Nucleic Acids Res. 34, e63. 10.1093/nar/gkl151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó G. T., Mahiny A. J., Vlatkovic I. (2022). COVID-19 mRNA Vaccines: Platforms and Current Developments. Mol. Ther. 30 (5), 1850–1868. 10.1016/j.ymthe.2022.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson D. W., Dinger M. E. (2016). Endogenous microRNA Sponges: Evidence and Controversy. Nat. Rev. Genet. 17, 272–283. 10.1038/nrg.2016.20 [DOI] [PubMed] [Google Scholar]
- Tong H., Zhao K., Wang J., Xu H., Xiao J. (2019). CircZNF609/miR-134-5p/BTG-2 axis Regulates Proliferation and Migration of Glioma Cell. J. Pharm. Pharmacol. 72, 68–75. 10.1111/jphp.13188 [DOI] [PubMed] [Google Scholar]
- Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J., Jemal A. (2015). Global Cancer Statistics, 2012. CA A Cancer J. Clin. 65, 87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- Vea A., Llorente-Cortes V., de Gonzalo-Calvo D. (2018). Circular RNAs in Blood. Circ. RNAs, 119–130. 10.1007/978-981-13-1426-1_10 [DOI] [PubMed] [Google Scholar]
- Verduci L., Tarcitano E., Strano S., Yarden Y., Blandino G. (2021). CircRNAs: Role in Human Diseases and Potential Use as Biomarkers. Cell Death Dis. 12, 468. 10.1038/s41419-021-03743-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva A. (2019). Hepatocellular Carcinoma. N. Engl. J. Med. 380, 1450–1462. 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- Wang F., Li X., Jia X., Geng L. (2021). CircRNA ZNF609 Knockdown Represses the Development of Non-small Cell Lung Cancer via miR-623/FOXM1 Axis. Cmar Vol. 13, 1029–1039. 10.2147/CMAR.S282162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.-J., Liu C., Shan K., Liu B.-H., Li X.-M., Zhang S.-J., et al. (2018). Circular RNA-Znf609 Regulates Retinal Neurodegeneration by Acting as miR-615 Sponge. Theranostics 8, 3408–3415. 10.7150/thno.25156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lin Y., Jiang D. H., Yang X., He X. G. (2021). CircRNA ZNF609 Promotes Angiogenesis in Nasopharyngeal Carcinoma by Regulating miR ‐145/STMN1 axis. Kaohsiung J. Med. Sci. 37, 686–698. 10.1002/kjm2.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Long B., Liu F., Wang J.-X., Liu C.-Y., Zhao B., et al. (2016). A Circular RNA Protects the Heart from Pathological Hypertrophy and Heart Failure by Targeting miR-223. Eur. Heart J. 37, 2602–2611. 10.1093/eurheartj/ehv713 [DOI] [PubMed] [Google Scholar]
- Wang K., Sun Y., Tao W., Fei X., Chang C. (2017). Androgen Receptor (AR) Promotes Clear Cell Renal Cell Carcinoma (ccRCC) Migration and Invasion via Altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 Signals. Cancer Lett. 394, 1–12. 10.1016/j.canlet.2016.12.036 [DOI] [PubMed] [Google Scholar]
- Wang S., Xue X., Wang R., Li X., Li Q., Wang Y., et al. (2018). CircZNF609 Promotes Breast Cancer Cell Growth, Migration, and Invasion by Elevating p70S6K1 via Sponging miR-145-5p. Cmar Vol. 10, 3881–3890. 10.2147/CMAR.S174778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu J., Ma J., Sun T., Zhou Q., Wang W., et al. (2019). Exosomal circRNAs: Biogenesis, Effect and Application in Human Diseases. Mol. Cancer 18, 116–126. 10.1186/s12943-019-1041-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M., Wick W., Aldape K., Brada M., Berger M., Pfister S. M., et al. (2015). Glioma. Nat. Rev. Dis. Prim. 1, 15017. 10.1038/nrdp.2015.17 [DOI] [PubMed] [Google Scholar]
- Wu L., Xia J., Yang J., Shi Y., Xia H., Xiang X., et al. (2018). Circ-ZNF609 Promotes Migration of Colorectal Cancer by Inhibiting Gli1 Expression via microRNA-150. J. buon 23, 1343–1349. [PubMed] [Google Scholar]
- Wu P., Zhang D., Geng Y., Li R., Zhang Y. (2020). Circular RNA-Znf609 Regulates Corneal Neovascularization by Acting as a Sponge of miR-184. Exp. Eye Res. 192, 107937. 10.1016/j.exer.2020.107937 [DOI] [PubMed] [Google Scholar]
- Wu W., Wei N., Shao G., Jiang C., Zhang S., Wang L. (2019). circZNF609 Promotes the Proliferation and Migration of Gastric Cancer by Sponging miR-483-3p and Regulating CDK6. Ott Vol. 12, 8197–8205. 10.2147/OTT.S193031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M.-S., Ai Y., Wilusz J. E. (2020). Biogenesis and Functions of Circular RNAs Come into Focus. Trends Cell Biol. 30, 226–240. 10.1016/j.tcb.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M.-S., Wilusz J. E. (2019). An Improved Method for Circular RNA Purification Using RNase R that Efficiently Removes Linear RNAs Containing G-Quadruplexes or Structured 3′ Ends. Nucleic Acids Res. 47, 8755–8769. 10.1093/nar/gkz576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Zhang J., Song C. (2019). CircRNA ZNF609 Functions as a Competitive Endogenous RNA to Regulate FOXP4 Expression by Sponging miR‐138‐5p in Renal Carcinoma. J. Cell. Physiology 234, 10646–10654. 10.1002/jcp.27744 [DOI] [PubMed] [Google Scholar]
- Yang J., Meng X., Pan J., Jiang N., Zhou C., Wu Z., et al. (2018). CRISPR/Cas9-mediated Noncoding RNA Editing in Human Cancers. RNA Biol. 15 (1), 35–43. 10.1080/15476286.2017.1391443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.-y., Zhang P.-y. (2017). Gastric Cancer: Somatic Genetics as a Guide to Therapy. J. Med. Genet. 54, 305–312. 10.1136/jmedgenet-2016-104171 [DOI] [PubMed] [Google Scholar]
- Zhang X., Xue H., Zhou P., Liu L., Yu J., Dai P., et al. (2019). RETRACTED: Angelica Polysaccharide Alleviates Oxidative Response Damage in HaCaT Cells through Up-Regulation of miR-126. Exp. Mol. Pathology 110, 104281. 10.1016/j.yexmp.2019.104281 [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhao Y., Kong P., Han M., Li B. (2019). Expression of circZNF609 Is Down-Regulated in Colorectal Cancer Tissue and Promotes Apoptosis in Colorectal Cancer Cells by Upregulating P53. Med. Sci. Monit. 25, 5977–5985. 10.12659/MSM.915926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang Y. (2021). Circular RNAs in Hepatocellular Carcinoma: Emerging Functions to Clinical Significances. Front. Oncol. 11. 10.3389/fonc.2021.667428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Zhang X.-O., Chen T., Xiang J.-F., Yin Q.-F., Xing Y.-H., et al. (2013). Circular Intronic Long Noncoding RNAs. Mol. Cell 51, 792–806. 10.1016/j.molcel.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Zhu L., Liu Y., Yang Y., Mao X. M., Yin Z. D. (2019). CircRNA ZNF609 Promotes Growth and Metastasis of Nasopharyngeal Carcinoma by Competing with microRNA-150-5p. Eur. Rev. Med. Pharmacol. Sci. 23, 2817–2826. 10.26355/eurrev_201904_17558 [DOI] [PubMed] [Google Scholar]
- Zuo Y., Shen W., Wang C., Niu N., Pu J. (2020). Circular RNA Circ-Znf609 Promotes Lung Adenocarcinoma Proliferation by Modulating miR-1224-3p/ETV1 Signaling. Cmar Vol. 12, 2471–2479. 10.2147/CMAR.S232260 [DOI] [PMC free article] [PubMed] [Google Scholar]