Abstract
Background/Aim: RAB27A and RAB27B are involved in exosome secretion. To date, there have been many attempts to elucidate the roles of RAB27A and RAB27B in the prognosis of various cancer types. The association of RAB27A and RAB27B expression with the clinical and pathological features was evaluated in patients with stomach cancer.
Materials and Methods: A total of 360 patients who had undergone surgery for stomach cancer between January 1999 and December 2007 at Gyeongsang National University were enrolled in the study. Disease-free survival (DFS) and disease-specific survival (DSS) were compared according to immunohistochemistry of tumor samples. RAB27A and RAB27B mRNA and protein were also extracted from four stomach cancer cell lines using quantitative polymerase chain reaction and western blotting.
Results: Strong nuclear RAB27A expression in tumor samples was statistically significantly correlated with lymph node metastasis. Cytoplasmic RAB27B expression was related to poor disease-free survival and its combined cytoplasmic and membranous expression was related to disease-specific survival of patients with different histopathological types of stomach cancer. High RAB27A expression and high RAB27B expression was found in four stomach cancer cell blocks. Among the four cell lines, NCI-N87 exhibited the lowest relative mRNA density and HS746T exhibited the highest relative protein density for both RAB27A and RAB27B.
Conclusion: RAB27A and RAB27B expression may help predict lymph node metastasis and survival of patients with gastric cancer.
Keywords: RAB27A, RAB27B, lymph node metastasis, gastric cancer
Stomach cancer is the fifth most common cancer, accounting for more than 5.7% of all cancer diagnoses worldwide (1). According to the World Cancer Research Fund and American Institute for Cancer Research, South Korea had the highest rate of stomach cancer in 2018, followed by Mongolia (1). The prognosis of gastric cancer varies greatly depending on the stage, being very good in the case of mucosal- or submucosal-limited early gastric cancer, while advanced gastric cancer has a 5-year survival rate of 30% (2). Since it is best to detect and treat gastric cancer as early as possible, the role of biomarkers in predicting the prognosis and metastasis of gastric cancer is important.
RABs are members of the Ras small GTPase superfamily that play an important role in vesicle trafficking in cells. RAB27A and RAB27B are particularly known to be involved in exosome secretion (3-11). Tumor cells secrete exosomes with intra-exosomal cargos, including tumor-suppressor genes, proteins, and other substances that might affect metastatic properties. To date, there have been many studies regarding the prognostic role of RAB27A and RAB27B through immunostaining methods in various cancer (12-18). In a previous study, we confirmed that RAB27A expression was strongly positive in a large group of patients with clear-cell renal cell carcinoma (CCRCC) (13). Negative RAB27A expression was significantly associated with poor prognosis of CCRCC (13). In data from Ostrowski et al., RAB27A and RAB27B were shown to have different roles in human vascular endothelial cells, maintaining different subcellular distributions in those cells (8). In addition, Fukuda et al. and Pfeffer et al. suggested that RAB27A and RAB27B are involved in sequential exosome secretion in combination with different effector proteins, synaptotagmin-like protein 4-A (SLP4-A) and synaptotagmin-like protein homologoue lacking C2 domains-C (SLAC2-B), respectively (19,20).
In this study, we evaluated the association of RAB27A and RAB27B expression with the clinical and pathological features of patients with stomach cancer. In addition, we confirmed the existence of RAB27A and RAB27B in stomach cancer cell lines using semi-quantitative polymerase chain reaction (semi-qPCR), western blotting, and cell block.
Materials and Methods
Patients and clinicopathological data. A total of 360 consecutive patients who had undergone surgery for stomach cancer between January 1999 and December 2007 at the Gyeongsang National University Hospital, Jinju, Republic of Korea, were enrolled for the study. Representative hematoxylin and eosin-stained slides from these patients were re-examined by two pathologists. Electronic medical records were reviewed, and clinical and pathological information, including age, sex, pathological tumor differentiation and histology, T-stage, and N-stage, were collected. The stages of stomach cancer were determined according to the eighth edition guidelines of the American Joint Committee on Cancer (21). This study was approved by the Institutional Review Board of the Gyeongsang National University Hospital (GNUH-2019-02-016).
Tissue microarray (TMA) construction and immunohistochemistry. Representative hematoxylin and eosin-stained glass slides containing intratumoral lesions were examined. A core (3 mm in size) was collected from the invasive tumor front of each representative paraffin block and was transplanted into recipient TMA blocks. Immunohistochemical staining was carried out using an automated immunostainer (Benchmark Ultra, Ventana Medical Sys tems Inc., Tucson, AZ, USA) with the following monoclonal antibodies: Anti-RAB27A (1:50 dilution, ab55667; Abcam, Cambridge, MA, USA) and anti-RAB27B (1:100 dilution, PA5-54096: Thermo Fisher Scientific, Waltham, MA, USA).
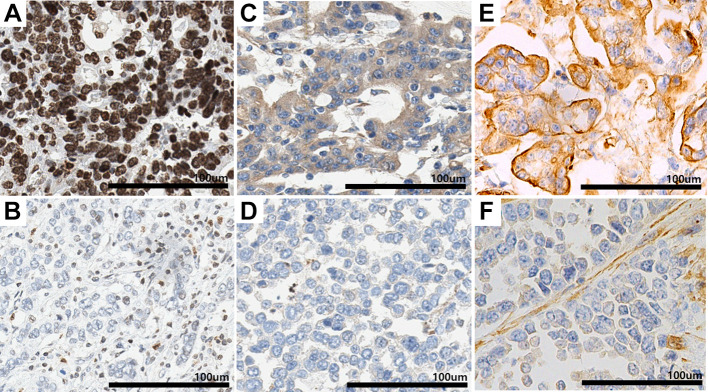
Evaluation of RAB27A and RAB27B expression in human tissue. The immunohistochemical staining patterns of RAB27A and RAB27B were evaluated on each of the 296 cores of the TMA blocks of patients with stomach cancer. Strong nuclear staining for RAB27A and distinct cytoplasmic or membranous staining for RAB27B were considered. The intensity of the stained tumor cells was scored as follows: Unstained: 0, weakly stained: 1, moderately stained: 2, and strongly stained: 3. Patients were then divided into two groups for comparison. For RAB27A expression, scores of 1 were considered low, and 2 and 3 were considered high. For RAB27B expression, scores of 0 were considered low, and scores of 1, 2, and 3 were considered high. Stomach glandular epithelial cells and stromal cells were compared as positive controls for RAB27A and RAB27B. Representative microscopic images are presented in Figure 1.
Figure 1. Representative images of RAS oncogene family members RAB27A and RAB27B immunostaining patterns. A: Strong and distinct nuclear staining of adenocarcinoma sample with high RAB27A expression. B: Weak nuclear staining of an adenocarcinoma sample with low RAB27A expression. C: Diffuse strong cytoplasmic staining of adenocarcinoma sample with high cytoplasmic RAB27B expression. D: Negative cytoplasmic expression of adenocarcinoma sample with low RAB27B cytoplasmic expression. E: Uniformly intense membranous staining of adenocarcinoma sample with high membranous RAB27B expression. F: Adenocarcinoma sample with low membranous RAB27B expression.
Statistical analysis. The relationships between RAB27A and RAB27B expression and pathological or clinical data were evaluated by Pearson’s chi-square test and Fisher’s exact test. Disease-free (DFS) and disease-specific (DSS) survival were evaluated by univariate and multivariate Cox proportional hazard regression models. DFS was defined as the duration from the date of surgery to the date of cancer relapse, and DSS was defined as the duration from the date of surgery to the date of death. p-Values less than 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS ver. 24.0 (IBM Corp., Ar monk, NY, USA).
Cell culture. The human stomach cell lines MKN-45 (adenocarcinoma, intestinal type), HS746T (carcinoma, metastasis to lung), NCI-N87 (carcinoma, metastasis to liver), and SNU-601 (signet-ring cell carcinoma) were purchased from the Korean Cell Line Bank, Seoul, Republic of Korea. HS746T cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Grand Ιsland, NY, USA), and the MKN-45, NCI-N87, and SNU-601 cell lines were cultured in RPMI 1640 (Gibco). Both media were supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin (Corning, Corning, NY, USA), and cell lines were incubated at 37˚C under an atmosphere containing 5% CO2.
Semi-qPCR. Once the cells reached 70% confluence, RNA was extracted from stomach cell lines using TRIzol (Qiagen, Germantown, MD, USA). Total RNA was quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA) The prepared RNA (1 μg) was reverse-transcribed to cDNA using the Maxime RT PreMix Kit (iNtRON, Burlington, MA, USA). Equal amounts of synthesized cDNA (1 μg) were used to carry out semi-qPCR using Maxime PCR PreMix kit (iNtRON, Burlington, MA, USA). RAB27A (#P196767; Bioneer, Oakland, CA, USA) and RAB27B (#P119176; Bioneer) primers were added. PCR was performed in 20 μl using a thermocycler (Biometra, Uberlingen, Germany) with the following PCR program: pre-denaturation for 2 min at 94˚C, denaturation for 20 s at 94˚C, annealing for 10 s at 58˚C, extension for 20 s at 72˚C, and a final elongation for 2 min at 72˚C. PCR was performed for 40 cycles. PCR products were analyzed by electrophoresis on a 1.5% agarose gel, and band intensity was measured directly on a gel documentation system (Bio–Rad, Hercules, CA, USA) and quantified relative to that of glyceraldehyde 3-phosphate dehydrogenase.
Western blot analysis. Once the cells reached 70% confluence, proteins were extracted from the harvested cells using RIPA lysis buffer (Thermo Fisher Scientific Waltham, MA, USA) containing protease inhibitor cocktail (Thermo Fisher Scientific). The total protein concentration of each cell lysate was measured by the Bradford method using bovine serum albumin as a standard. Equal amounts of protein lysates (45 μg) were loaded onto denaturing polyacrylamide gels and then transferred to a nitrocellulose membrane. The primary antibodies used for immunoblotting were anti-RAB27A (diluted 1:2,000; cat. ab55667; Abcam) and anti-RAB27B (diluted 1:2,000; cat. PA5-54096, Thermo Fisher Scientific), followed by horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were detected by enhanced chemiluminescence reaction (Thermo Fisher Scientific). Digital chemiluminescence images were captured and quantitatively analyzed by Fusion solo (Vilber, Marne-la-Vallee, France).
Cell block construction and immunohistochemistry. Once the cells had reached 70% confluence, they were harvested and mixed with an equal volume of plasma. To minimize cell loss, 1 ml of 70% ethanol was added, and a pellet was acquired by centrifugation. The semi-clotted pellet was completely fixed in 1 ml of 95% ethanol for 3 hours. Histological processing was performed on the pellet. Paraffin sections of 4 μm were obtained for staining. The same primary antibodies were used as for western blotting as described above, followed by horseradish peroxidase-conjugated secondary antibodies. The immunohistochemical staining pattern of RAB27A and RAB27B were evaluated on each of the cell block as for tissue microarray as described above.
Results
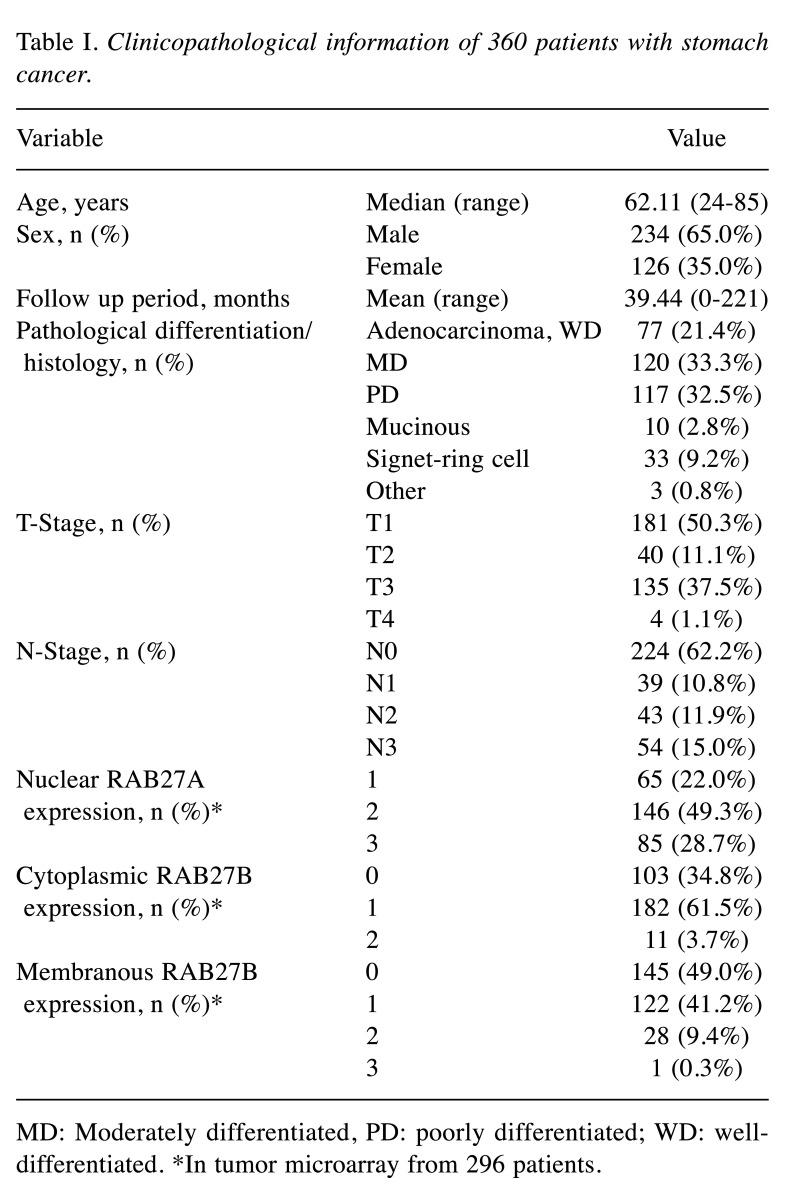
Clinicopathological information of the patients. A total of 360 patients with stomach cancer were enrolled in this study. The clinical and pathological variables of the patients were analyzed for their relationship with RAB27A and RAB27B expression. (Table I). The mean age of the patients was 62.11 years. Among them, 234 (65.0%) were males, and 126 (35.0%) were female. All patients enrolled in the study were South Korean. In addition, this was a representative cohort which included impartial distribution of pathological differentiation, histological pattern, T-stage, and N-stage of stomach cancer patients as described in Table I.
Table I. Clinicopathological information of 360 patients with stomach cancer.
MD: Moderately differentiated, PD: poorly differentiated; WD: welldifferentiated. *In tumor microarray from 296 patients.
RAB27A and RAB27B expression in gastric cancer. A total of 296 TMA cores (64 cores had tissue loss), were evaluated for the expression of RAB27A and RAB27B using immunohistochemical staining (Table I). Overall, 78% (score 2 or 3: 231/296) of patients had high expression of RAB27A, and RAB27B expression was generally high (cytoplasmic, score 1, 2: 65%; membranous, score 1-3: 51%).
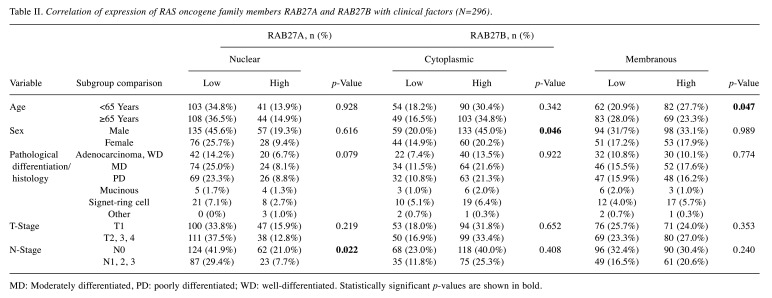
Correlation of RAB27A and RAB27B expression with clinicopathological data. The association of RAB27A and RAB27B expression with clinicopathological data is shown in Table II. High nuclear RAB27A expression was statistically significantly correlated with N0 stage (p=0.022) and was marginally significantly associated with pathological differentiation and histology of gastric cancer (p=0.079). High cytoplasmic RAB27B expression was significantly associated with male sex (p=0.046), and high RAB27B membrane expression was significantly associated with age less than 65 years (p=0.047). However, neither cytoplasmic nor membranous RAB27B expression was related to the N-stage. However, when cases were limited to RAB27B-high cases (250 cases, either RAB27B cytoplasmic or membranous staining pattern) out of all cases (296 cases) of gastric cancer, high RAB27A expression was significantly positively correlated with lymph node metastasis (p=0.005).
Table II. Correlation of expression of RAS oncogene family members RAB27A and RAB27B with clinical factors (N=296).
MD: Moderately differentiated, PD: poorly differentiated; WD: well-differentiated. Statistically significant p-values are shown in bold.
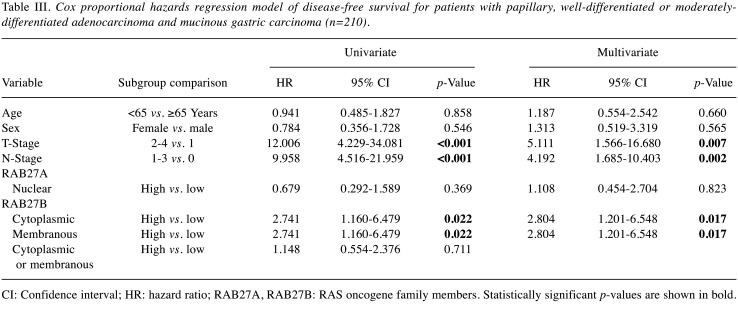
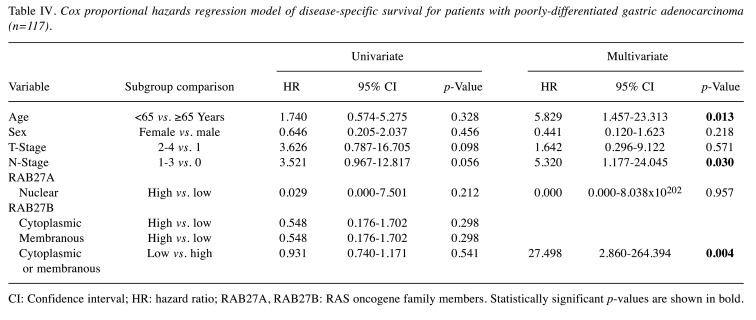
Correlation between RAB27A and RAB27B expression and survival data. To determine whether RAB27A and RAB27B expression might be independent prognostic markers, univariate and multivariate Cox proportional analysis was performed. We divided patients with stomach cancer into different histopathological groups. In the group of 210 patients with papillary, well-differentiated or moderately-differentiated adenocarcinomas and mucinous carcinomas, high cytoplasmic and membranous expression of RAB27B was significantly associated with poor DFS (hazard ratio=2.804, 95% confidence interval=1.201-6.548, p=0.017) in multivariate analysis (Table III). In the group of 117 patients with poorly-differentiated adenocarcinoma, low cytoplasmic or membranous expression of RAB27B was significantly associated with poor DSS (hazard ratio=27.498, 95% confidence interval=2.860-264.394, p=0.004) in multivariate analysis (Table IV).
Table III. Cox proportional hazards regression model of disease-free survival for patients with papillary, well-differentiated or moderatelydifferentiated adenocarcinoma and mucinous gastric carcinoma (n=210).
CI: Confidence interval; HR: hazard ratio; RAB27A, RAB27B: RAS oncogene family members. Statistically significant p-values are shown in bold.
Table IV. Cox proportional hazards regression model of disease-specific survival for patients with poorly-differentiated gastric adenocarcinoma (n=117).
CI: Confidence interval; HR: hazard ratio; RAB27A, RAB27B: RAS oncogene family members. Statistically significant p-values are shown in bold.
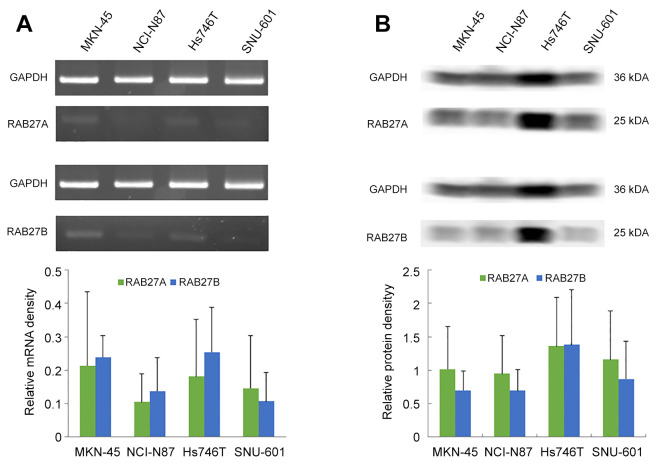
RAB27A and RAB27B expression were identified in stomach cancer cell lines. The mRNA levels of RAB27A and RAB27B were estimated from total mRNA extracted from the human stomach cancer cell lines MKN-45, HS746T, NCI-N87, and SNU-601 using semi-qPCR (Figure 2A). Among the four cell lines, NCI-N87 exhibited the lowest relative mRNA density for both RAB27A and RAB27B. Western blotting was used to determine the protein levels of RAB27A and RAB27B in the stomach cancer cell lines (Figure 2B). Among the four cell lines, HS746T exhibited the highest relative protein density for both RAB27A and RAB27B.
Figure 2. mRNA and protein expression of RAS oncogene family members RAB27A and RAB27B in stomach cancer cell lines MKN-45, NCI-N87, HS746T and SNU-601. A: Total mRNA extracted from human stomach cancer cell lines was used to determine the mRNA levels of RAB27A and RAB27B relative to those of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) by quantitative polymerase chain reaction. B: Protein levels of RAB27A and RAB27B in stomach cancer cell lines were determined by western blotting. Data are the mean±standard deviation from three independent experiments.
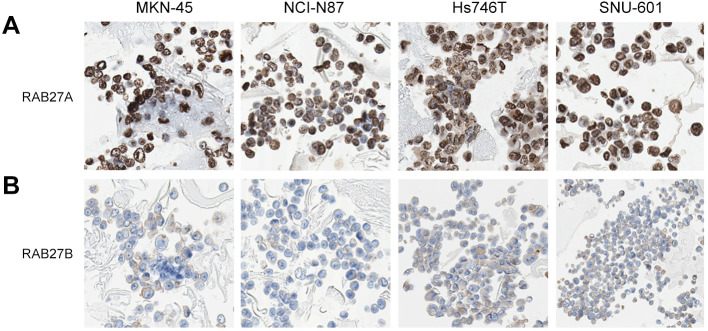
RAB27A and RAB27B expression were identified in the cell blocks of stomach cancer cell lines. RAB27A expression was high in all cell blocks of the four stomach cancer cell lines, with a strong nuclear staining pattern (Figure 3A). In contrast, the pattern for RAB27B staining was weakly cytoplasmic or incomplete membranous in all cell blocks of four stomach cancer cell lines (Figure 3B).
Figure 3. Expression of RAS oncogene family members RAB27A and RAB27B in cell blocks of four stomach cancer cell lines. Staining considered as high expression for RAB27A was nuclear and strongly positive in all cell blocks of four stomach cancer cell lines. In contrast, high RAB27B expression exhibited weakly positive and cytoplasmic or incomplete membranous staining patterns in all cell blocks of four stomach cancer cell lines.
Discussion
To date, there have been many studies regarding the prognostic role of RAB27A and RAB27B expression through immunohistochemical staining methods in various cancer types (12-18). In clear cell renal cell carcinoma renal cell carcinoma, we confirmed that RAB27A expression was strongly positive in a large group of patients. Negative RAB27A expression was significantly correlated with poor prognosis (13). Dong et al. evaluated the association of RAB27A and RAB27B expression with the clinical and pathological features of hepatocellular carcinoma (12). They divided patients into four groups according to RAB27A and RAB27B expression (RAB27A+/RAB27B+, RAB27A+/RAB27B−, RAB27A−/RAB27B+, RAB27A−/ RAB27B−); however, RAB27A and RAB27B expression were not significantly correlated with the clinical and pathological findings. But their analyses did reveal that patients with RAB27A+/RAB27B+ tumors had significantly reduced overall survival compared with those with RAB27A−/RAB27B− hepatocellular carcinoma (12). Additionally, the results from their unpublished data on gastric cancer and colorectal cancer revealed that the tumor staining of RAB27A and RAB27B was weaker than that of surrounding tissue and was not correlated to the patient survival. The staining pattern of RAB27A, RAB27B differs depending on the cancer type and classification of the staining pattern used in different studies. In order to use RAB27A and RAB27B as prognostic biomarkers for stomach cancer, we examined their expression in samples of stomach cancer from patients and compared it in the metastatic stomach cancer cell lines. In addition, we evaluated the relationship between the expression of RAB27A, RAB27B and the patient survival according to specific histological patterns.
In our study, expression of RAB27A was nuclear, and of high intensity in most cases (70%). In contrast, expression of RAB27B was cytoplasmic or membranous, with weaker staining patterns. Strong RAB27A expression was statistically significantly correlated with lymph node metastasis. RAB27B expression alone was not related to lymph node metastasis; however, when cases were limited to RAB27B-high cases, high RAB27A expression was associated with lymph node metastasis (p=0.005). This result is in line with previous data by Ostrowski et al., RAB27A and RAB27B have different roles in human vascular endothelial cells, maintaining different subcellular distributions in those cells (8). In our previous study with clear cell renal cell carcinoma renal cell carcinoma, negative RAB27A expression was associated with poor DSS in multivariate analysis. We hypothesized that when secretory RAB, RAB27A, is reduced in cancer, multivesicular bodies inside cancer cells are enlarged (8) and degraded together with lysosomes (20), losing their tumor-suppressor genes inside the cargo thereby changing their metastatic potential (13). In contrast, when RAB27A is strongly expressed, exosomes might be actively secreted from multivesicular bodies inside cancer cells (3,6,7,10). During exosomal secretion, it is thought that the metastatic properties of the tumor itself may change due to the active secretion of the exosomal cargo, including microRNAs, long noncoding RNAs, and other proteins (7,22).
To the best of our knowledge, this is the first study to evaluate RAB27B expression and prognosis of patients with stomach cancer with different histopathological features using survival data. It is believed that the differences in the prognosis of patients with gastric cancer according to the histopathological type and RAB27B expression might be due to the functional roles of exosomes, such as cellular uptake, and delivery (23). Secreted exosomes can affect other cancer cells or cells of the tumor microenvironment, including the extracellular matrix, lymphocytes, endothelial cells, and other stromal cells by cell to cell communication (23-25). In our study, the group with poorly-differentiated adenocarcinoma, in which cancer cells displayed non-cohesive or diffusely scattered patterns of growth, cell uptake of exosomes and their cargos might be reduced, and contact with other cells or factors of the tumor microenvironment described above may have increased, thus affecting metastatic properties. A treatment approach exploiting the mechanism of RAB27B-associated exosome secretion might be required considering the different histopathological patterns of stomach cancer cells.
Cell experiments showed different mRNA and protein expression levels of RAB27A and RAB27B in stomach cancer cell lines (Figure 2). Among the four stomach cancer cell lines, HS746T (carcinoma, metastasis to lung) showed the highest protein relative density for both RAB27A and RAB27B. It is meaningful to confirm the existence of RAB27A and RAB27B mRNA and protein in stomach cancer cell lines to determine whether they affect tumor metastatic potential; their role may be further investigated through knockdown or forced-expression experiments.
A limitation of this study is that we did not evaluate RABs other than RAB27A and RAB27B, nor their effector proteins which may affect exosome trafficking within the cancer cells. However, the most powerful secretory RAB protein, i.e., RAB27A and RAB27B-related proteins (20), are clearly worthy of further study. Proteins that inhibit or increase the secretion of exosomes by controlling RAB27A have been found, and treatment based on this is anticipated. For example, wild-type WW and C2 domain containing 1 (WWC1; KIBRA) was shown to increase exosome secretion through blocking RAB27A proteasome degradation (26).
In conclusion, strong nuclear RAB27A expression is related to lymph node metastasis in gastric cancer. Considering RAB27B expression in different histopathological types of gastric cancer may help to predict prognosis.
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Authors’ Contributions
HJA and DHS wrote the article. JSL, JWW, MHK and JMN conducted the experiments. HJA, JSL, JWW, MHK, JMN and DHS designed the study and interpreted the data. HJA and DHS prepared the figures and conducted the statistical analysis.
Acknowledgements
The Authors express special thanks to Gyung Hyuck Ko, Jeong-Hee Lee, and Dong Chul Kim. This work was supported by biomedical research institute fund (GNUHBRIF-2020-0003) from the Gyeongsang National University Hospital.
References
- 1.Chan DSM, Abar L, Cariolou M, Nanu N, Greenwood DC, Bandera EV, McTiernan A, Norat T. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control. 2019;30(11):1183–1200. doi: 10.1007/s10552-019-01223-w. [DOI] [PubMed] [Google Scholar]
- 2.Karimi P, Islami F, Anandasabapathy S, Freedman ND, Kamangar F. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700–713. doi: 10.1158/1055-9965.EPI-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukuda M. Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci. 2008;65(18):2801–2813. doi: 10.1007/s00018-008-8351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J Biochem. 2005;137(1):9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda M. Rab27 and its effectors in secretory granule exocytosis: a novel docking machinery composed of a Rab27.effector complex. Biochem Soc Trans. 2006;34(Pt 5):691–695. doi: 10.1042/BST0340691. [DOI] [PubMed] [Google Scholar]
- 6.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91(1):119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, Bui KH, Fristrup N, Christensen EI, Nordentoft I, Morth JP, Jensen JB, Pedersen JS, Beck M, Theodorescu D, Borre M, Howard KA, Dyrskjøt L, Ørntoft TF. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74(20):5758–5771. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 8.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 1-13. [DOI] [PubMed] [Google Scholar]
- 9.Seabra MC, Mules EH, Hume AN. Rab GTPases, intracellular traffic and disease. Trends Mol Med. 2002;8(1):23–30. doi: 10.1016/s1471-4914(01)02227-4. [DOI] [PubMed] [Google Scholar]
- 10.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10(8):513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 11.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2(2):107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 12.Dong WW, Mou Q, Chen J, Cui JT, Li WM, Xiao WH. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J Gastroenterol. 2012;18(15):1806–1813. doi: 10.3748/wjg.v18.i15.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An HJ, Song DH, Koh HM, Ko GH, Lee JH, Kim DC, Yang JW, Kim MH, Seo DH, Jang SM, Lee JS. RAB27A is an independent prognostic factor in clear cell renal cell carcinoma. Biomark Med. 2019;13(4):239–247. doi: 10.2217/bmm-2018-0336. [DOI] [PubMed] [Google Scholar]
- 14.Koh HM, Kim DC, Kim YM, Song DH. Prognostic role of macrophage migration inhibitory factor expression in patients with squamous cell carcinoma of the lung. Thorac Cancer. 2019;10(12):2209–2217. doi: 10.1111/1759-7714.13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren P, Yang XQ, Zhai XL, Zhang YQ, Huang JF. Overexpression of Rab27B is correlated with distant metastasis and poor prognosis in ovarian cancer. Oncol Lett. 2016;12(2):1539–1545. doi: 10.3892/ol.2016.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H, Wang Q, Wang X, Zhu H, Zhang S, Wang W, Wang Z, Huang J. Correlation between RAB27B and p53 expression and overall survival in pancreatic cancer. Pancreas. 2016;45(2):204–210. doi: 10.1097/MPA.0000000000000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong W, Cui J, Yang J, Li W, Wang S, Wang X, Li X, Lu Y, Xiao W. Decreased expression of Rab27A and Rab27B correlates with metastasis and poor prognosis in colorectal cancer. Discov Med. 2015;20(112):357–367. [PubMed] [Google Scholar]
- 18.Bao J, Ni Y, Qin H, Xu L, Ge Z, Zhan F, Zhu H, Zhao J, Zhou X, Tang X, Tang L. Rab27b is a potential predictor for metastasis and prognosis in colorectal cancer. Gastroenterol Res Pract. 2014;2014:913106. doi: 10.1155/2014/913106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic. 2013;14(9):949–963. doi: 10.1111/tra.12083. [DOI] [PubMed] [Google Scholar]
- 20.Pfeffer SR. Two Rabs for exosome release. Nat Cell Biol. 2010;12(1):3–4. doi: 10.1038/ncb0110-3. [DOI] [PubMed] [Google Scholar]
- 21.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. Springer. 2017. AJCC Cancer Staging Manual, Eighth Edition. [Google Scholar]
- 22.Dickman CT, Lawson J, Jabalee J, MacLellan SA, LePard NE, Bennewith KL, Garnis C. Selective extracellular vesicle exclusion of miR-142-3p by oral cancer cells promotes both internal and extracellular malignant phenotypes. Oncotarget. 2017;8(9):15252–15266. doi: 10.18632/oncotarget.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 24.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 25.Ghayad SE, Rammal G, Ghamloush F, Basma H, Nasr R, Diab-Assaf M, Chelala C, Saab R. Exosomes derived from embryonal and alveolar rhabdomyosarcoma carry differential miRNA cargo and promote invasion of recipient fibroblasts. Sci Rep. 2016;6:37088. doi: 10.1038/srep37088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song L, Tang S, Han X, Jiang Z, Dong L, Liu C, Liang X, Dong J, Qiu C, Wang Y, Du Y. KIBRA controls exosome secretion via inhibiting the proteasomal degradation of Rab27a. Nat Commun. 2019;10(1):1639. doi: 10.1038/s41467-019-09720-x. [DOI] [PMC free article] [PubMed] [Google Scholar]