Abstract
Allogeneic blood or marrow transplantation (allo-BMT) remains the only treatment for chronic lymphocytic leukemia (CLL) with curative potential. Although post-transplantation cyclophosphamide (PTCy) reduces allo-BMT toxicity by decreasing the risk of graft-versus-host disease (GVHD), its effect on CLL allo-BMT outcomes is unknown. We studied 64 consecutive patients with CLL who underwent nonmyeloablative (NMA) haploidentical allo-BMT at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center. In this cohort, the 4-year overall survival was 52% (95% confidence interval [CI], 40% to 68%), and progression-free survival was 37% (95% CI, 26% to 54%). Six patients experienced engraftment failure. PTCy prophylaxis was associated with a modest cumulative incidence of 1-year grade II-IV acute GVHD (27%; %95% CI, 15% to 38%) and %%%2-year chronic GVHD (17%; 95% CI, 7% to 26%). We demonstrate that NMA haploidentical allo-BMT with PTCy is a safe and effective treatment option.
Keywords: Chronic lymphocytic leukemia, Haploidentical, Allogeneic blood or marrow, transplantation, Post-transplantation, cyclophosphamide, Graft-versus-host-disease
INTRODUCTION
Chronic lymphocytic leukemia (CLL), the most common adult leukemia in United States, has a rapidly evolving treatment paradigm. Two phase III clinical trials have demonstrated that inhibition of Burton’s tyrosine kinase with ibrutinib as a first-line agent results in extended progression-free survival (PFS) compared with standard chemoimmunotherapy in both younger [1] and older patients [2]. Similar PFS improvement was noted in a phase III trial with the first-line use of the B cell lymphoma 2 (BCL2) inhibitor venetoclax [3]. Outcomes have also improved for patients with relapsed/refractory CLL failing first-line treatment with the use of phosphatidylinositol 3-kinase (PI3K) inhibitor idelalisib [4] or venetoclax [5]. The advent of these novel pathway inhibitors (Pis) have reduced the number of patients undergoing allogeneic blood or marrow transplantation (allo-BMT) for CLL across Europe and the United States [6,7]. However, allo-BMT remains the sole CLL treatment modality with proven curative potential. Allo-BMT is recommended in patients with CLL who progress after chemoimmunotherapy and PI therapy, in agreement with current guidelines [7-9]. Unfavorable risk chemoimmunotherapy-resistant CLL patients who are responsive to PI therapy should continue with the PI or may be offered allo-BMT in certain circumstances. Although there is some agreement regarding the decision to proceed with allo-BMT after patients have achieved at least a partial treatment response, whether depth of response before allo-BMT affects survival is unclear.
Only a minority of patients have a matched sibling donor (MSD), and the remaining patients rely on matched unrelated donors (MUDs) or HLA-haploidentical donors [10]. Recently, the number of patients undergoing haploidentical allo-BMT has increased substantially, with the advent of new graft-versus-host disease (GVHD) prophylaxis strategies, such as post-transplantation cyclophosphamide (PTCy), that reduce the risk of GVHD complications [10-12]. However, almost all CLL allo-BMT studies published to date used HLA-identical donors, and information on CLL patients undergoing haploidentical allo-BMT is limited. The European Society of Blood and Marrow Transplantation (EBMT) retrospective study of 2589 HLA-matched allo-BMT in patients with CLL demonstrated a 5-year %overall survival (OS) of 45%, %event-free survival of 35%, and %nonrelapse mortality (NRM) of 36% [13,14]. Similarly, the German GCLLSG CLL3X trial of 90 recipients of MSD and MUD allo-BMT found an %OS of 65%,% event-free survival of 42%, and %NRM of 23% at 4 years [15,16]. Subsequently, the Dana-Farber group reported a superior 5-year OS of 63% associated with a reduced-intensity conditioning regimen, compared with 49% with myeloablative conditioning regimens [17]. The sole report of haploidentical allo-BMT for CLL published to date with a 5-year OS of 38% and PFS of 31% in 117 patients with CLL [18]. Although the outcomes seem to be significantly worse with haploidentical allo-BMT compared with HLA matched allo-BMT, only 38% of the haploidentical allo-BMT recipients received PTCy GVHD prophylaxis; therefore, the outcomes of haploidentical transplantation with PTCy in CLL remain unclear. Here we report the outcomes of 64 consecutive CLL patients who underwent nonmyeloablative (NMA) haploidentical allo-BMT with PTCy GVHD prophylaxis at the Sidney Kimmel Comprehensive Cancer Center (SKCCC) at Johns Hopkins Hospital.
METHODS
Patients
On receipt of Institutional Review Board approval, we queried the SKCCC transplantation database for patients with CLL who underwent haploidentical allo-BMT with PTCy GVHD prophylaxis between January 1, 2005, and August 30, 2018. Medical records including clinical notes and pathology, radiology, and laboratory reports were reviewed; data were locked in March 2019. Cytogenetic study data were available for 62 patients (96.8%), and IGHV mutation status was available for 47 patients (73.4%). In accordance with National Comprehensive Cancer Network guidelines, 17p deletion, 11q deletion, complex karyotype (≥3 chromosomal abnormalities), and unmutated IGHV were considered unfavorable prognostic features [8]. Pre-allo-BMT remission status was assessed according to International Workshop on CLL guidelines [19]. All patients underwent pre-allo-BMT bone marrow biopsy. The decision to perform allo-BMT was made by the treating physician and generally based on the presence of unfavorable features, an aggressive disease course, and patient’s preference and candidacy for allo-BMT. As discussed below, after 2013, patients with >20% CLL marrow cellularity did not meet our institutional standard for NMA allo-BMT.
Allo-BMT with PTCy
All patients received a NMA conditioning regimen consisting of fludarabine, cyclophosphamide, and 200 cGy total body irradiation (TBI), as described previously [20]. Before 2013, all patients received unmanipulated bone marrow. Because of a high rate of graft failure, after 2013, some patients received G-CSF-stimulated marrow, and more recently, all patients received G-CSF-mobilized peripheral blood allografts. Patients received PTCy (i.v. 50 mg/kg/day) [21] on days +3 and +4, along with additional GVHD prophylaxis with mycophenolate mofetil between days +5 and +35 and tacrolimus or sirolimus between days +5 and +180. The development of GVHD prompted additional treatment according to institutional guidelines, as described previously [22]. Our review included patients undergoing allo-BMT as standard of care and as part of allo-BMT research protocols.
Disease Status and Clinical Outcome Definitions
OS was defined as the time from allo-BMT to death from any cause or censoring at the last follow-up date. PFS was defined as the time from allo-BMT to CLL relapse/progression or death from any cause, or censoring at the last follow-up date for relapse-free patients. NRM was defined as death from any cause unrelated to CLL relapse. NRM was considered a competing event when estimating the cumulative incidence (CuI) of relapse and vice versa. For the CuI of GVHD, graft failure was a competing event. Death without chronic GVHD (cGVHD) was an additional competing event when estimating the Cul of cGVHD. Neutrophil and platelet recovery times were defined as the interval between allograft infusion and first of 3 consecutive days with >500 neutrophils/μL and the first of 3 consecutive days with >20,000 platelets/μL respectively. Patients were classified as donor T cell engrafted if ≥5% donor cells were detected in peripheral blood CD3+ compartment at day +60 or beyond. Engraftment failure was defined as <5% donor chimerism +in the CD3+ peripheral blood compartment at any point beyond day +60. In T cell-engrafted patients, full engraftment was defined as ≥95% donor T cells, whereas ≥5% to %94% donor T cells was considered mixed chimerism. Modified Keystone criteria and National Institutes of Health consensus criteria were used to diagnose and score acute GVHD (aGVHD) and cGVHD [23,24].
Statistical Analysis
The primary endpoint for this study was OS. Patient characteristics and clinical variables were summarized using descriptive statistics. Kaplan-Meier estimators were reported for OS and PFS outcomes. Univariate Cox proportional hazard models were applied to test the associations between patient characteristics and OS and PFS outcomes. In addition, we constructed time-varying covariates based on GVHD status (GVHDt measures GVHD occurrence as a time-varying covariate) to assess the associations between outcomes (OS/PFS) and occurrence of GVHD. The CuIs of relapse, NRM, and GVHD were reported, and the Fine and Gray regression model [25] was applied for univariate analysis in these outcomes. Results of univariate analyses within the lower mortality risk group are reported as exploratory findings. All analyses were carried out with R version 3.6.0, using the “survival” and “cmprsk” packages (R Foundation for Statistical Computing, Vienna, Austria). All reported Pvalues are 2-sided, and P <.05 is considered to indicate statistical significance.
RESULTS
Patient and Allo-BMT Characteristics
Between January 2005 and August 2018, 64 consecutive patients with CLL underwent haploidentical allo-BMT at SKCCC (Table 1). The median age was 59 years (range, 26 to 74 years). Four patients (6.2%) with Richter’s transformation underwent allo-BMT after first-line treatment Twenty patients (31.2%) underwent allo-BMT after second-line treatment, and 40 patients (62.5%) underwent allo-BMT after 3 or more lines of treatment for relapsed and/or refractory disease. Chemoimmunotherapy regimens included single-agent rituximab (18 patients; 28.1%) and alemtuzumab (14 patients; 21.8%); bendamustine and rituximab (BR) (24 patients; 37.5%); fludarabine, cyclophosphamide, and rituximab (FCR) (25 patients; 39%); rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) (11 patients; 17.1%); and rituximab, ifosfamide, carboplatin, and etoposide (R-ICE) (4 patients; 6.2%). Ibrutinib (22 patients; 34.3%) was the most common PI used, followed by venetoclax (12 patients; 18.7%) and idelalisib (3 patients; 4.6%). Cytogenetic study data were available for 62 patients (96.8%), and IGHV mutation status was available for 47 patients (73.4%). Fifty-six patients (87.5%) had unfavorable prognostic features defined by the presence of del (17p), del (11q), a complex karyotype, or an unmutated IGHV sequence. Before allo-BMT, the majority of patients were treatment-responsive, including 16 patients (25%) in complete remission and 44 patients (68.7%) in partial remission (PR); the remaining 4 patients (6.2%) had stable disease. The allo-BMT characteristics are summarized in Table 2. Female donors served as the allograft source for 17 male patients. Thirty-three patients (51.5%) received bone marrow allografts, 4 patients (6.2%) received G-CSF-mobilized bone marrow grafts, and the remaining 27 patients (42.2%) received peripheral blood stem cell grafts. The median CD34+ and CD3+ bone marrow allograft doses were 4.8 × 106 cells/kg body weight and 4.1 × 107 cells/kg, respectively. The median CD34+ and CD3+ peripheral blood allograft doses were 9.7 × 106 cells/kg and 31.3 × 107 cells/kg, respectively.
Table 1.
Patient Characteristics
| Characteristic | Value |
|---|---|
| Age at allo-BMT, yr, median (range) | 59 (26-74) |
| Sex, n (%) | |
| Male | 46 (71.8%) |
| Female | 18 (28.1%) |
| Time from diagnosis to allo-HCT, mo, median (range) | 67 (8-234) |
| Number of previous treatments, n (%) | |
| 1 | 4 (6.2%) |
| 2 | 20 (31.2%) |
| ≥3 | 40 (62.5%) |
| Previous therapies, n (%) | |
| Chemoimmunotherapy | 61 (95.3%) |
| Ibrutinib | 22 (34.3%) |
| Idelalisib | 3 (4.6%) |
| Venetoclax | 12 (18.7%) |
| Cytogenetic studies, n (%) | |
| 17p deletion | 28 (43.7%) |
| 11q deletion | 26 (40.6%) |
| 13q deletion | 33 (51.5%) |
| Trisomy 12 | 5 (7.8%) |
| Complex karyotype (≥3 chromosomal abnormality) | 10 (15.6%) |
| Cytogenetics unknown | 2 (3.1%) |
| IGHV mutation status, n (%) | |
| IGHV unmutated | 40 (62.5%) |
| IGHV mutated | 7 (10.9%) |
| Status unknown | 17 (26.5%) |
| Unfavorable versus neutral or favorable risk, n (%) | |
| Unfavorable risk (presence of 17p or 11q deletion or complex karyotype or unmutated IGHV) | 56 (87.5%) |
| Neutral or favorable risk (absence of 17p, 11q deletion, complex karyotype, and mutated IGHV) | 4 (6.2%) |
| Incomplete data for risk assessment | 4 (6.2%) |
| Pre-allo-BMT remission status, n (%) | |
| Complete remission | 16 (25%) |
| Partial remission | 44 (68.7%) |
| Stable disease | 4 (6.2%) |
| Patients with CLL marrow cellularity ≥20%, n (%)% | 8 (12.5%) |
Table 2.
Transplantation Characteristics
| Characteristic | Value% |
|---|---|
| Number of transplantations (%) | 64 (100) |
| Transplantation type: NMA conditioning, HLA haploidentical, n (%) | 64 (100%) |
| Donor age, yr, median (range) | 36 (14-66) |
| Female donor to male recipient, n (%) | 17 (26.5%) |
| Allograft source, n (%) | % |
| Bone marrow | 33 (51.5) |
| G-CSF-mobilized bone marrow | 4 (6.2%) |
| Peripheral blood | 27 (42.2%) |
| Peripheral blood total cell graft dose, × 108/k8, median (IQR) | 5.6 (4.1-8.5) |
| CD34 cells, × 106/kg, median (IQR) | |
| Bone marrow/G-CSF-mobilized bone marrow | 4.8 (3.4-6.3) |
| Peripheral blood | 9.7 (6.9-10.0) |
| CD3 cells, × 107/kg, median (IQR) | |
| Bone marrow/G-CSF-mobilized bone marrow | 4.1 (3.0-5.6) |
| Peripheral blood | 31.3 (23.4-38.7) |
| Count recovery time, d, median (range) | |
| Days to neutrophil count recovery | 16 (11-56) |
| Days to platelet count recovery | 24 (11-95) |
| Engraftment/chimerism at day +60, n (%)* | |
| Chimerism full (≥95% donor) | 41 (64%) |
| Chimerism mixed (5-94% donor) | 15 (23.4%) |
| Chimerism (<5% donor) | 6 (9.3%) |
Engraftment not tested in 2 patients who died before day +60.
Engraftment and GVHD
The median time to neutrophil recovery was 16 days (range, 11 to 56 days), and the median time to platelet recovery was 24 days (range, 11 to 95 days), which is comparable to our past experience with haploidentical BMT (Table 2). Two patients died before the day +60 chimerism analysis. On day +60 chimerism analysis, 41 patients (64%) showed full (≥95%) donor chimerism, 15 patients (23.4%) had mixed (5% to 94%)% donor chimerism, and 6 patients (9.3%) failed to engraft. Among the 6 patients with engraftment failure (<5% donor chimerism), 5 patients (83.3%) had ≥20% marrow CLL involvement before allo-BMT (Supplementary Table S1). None of the patients with engraftment failure were in complete remission, 4 patients (66.6%) were in PR, and 2 patients (33.3%) had stable disease.
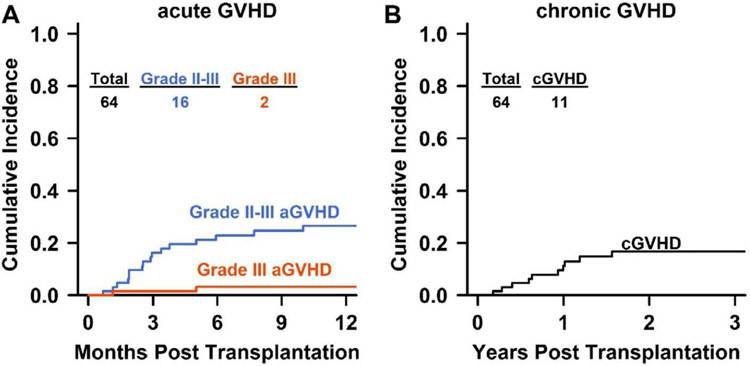
Because of the high rate of nonengraftment seen in patients with ≥20% CLL marrow involvement, only patients with <20% CLL marrow involvement were considered for allo-BMT after 2013. In patients with <20% marrow CLL involvement, peripheral blood- and bone marrow-derived allografts produced similar percentages of full and mixed donor chimerism (Supplementary Table S2). The 1-year CuI of grade II-IV aGVHD was 27% (95% confidence interval [CI], 15% to 38%), and that of grade III-IV aGVHD was %%%3% (95% CI, 1% to 8%). No patients experienced grade IV aGVHD. The CuI of cGVHD at 2 years was 17 % (95% CI, 7% to 26%), with 6 of the 11 patients with cGVHD requiring treatment (Figure 1). Univariate analysis showed that receipt of peripheral blood allografts was associated with an elevated risk of grade II-IV aGVHD (subdistribution hazard ratio [HR], 1.70; 95% CI, 1.04 to 2.78) compared with bone marrow-derived allografts (Supplementary Table S3). Other features, including patient age at allo-BMT, donor age, and female donor-male recipient, did not appear to affect the risk of aGVHD, although the small number of patients might have obscured small influences. Our analysis also did not reveal an elevated cGVHD risk with peripheral blood allografts, but our small sample size was underpowered to detect such an effect (Supplementary Table S3).
Figure 1.
Cumulative incidence of aGVHD (A) and cGVHD (B). Grade II-III aGVHD is shown in blue; grade III aGVHD, in red. No patient had grade IV aGVHD. The curves were truncated at 12 months for aGVHD and 3 years for cGVHD.
Relapse and NRM
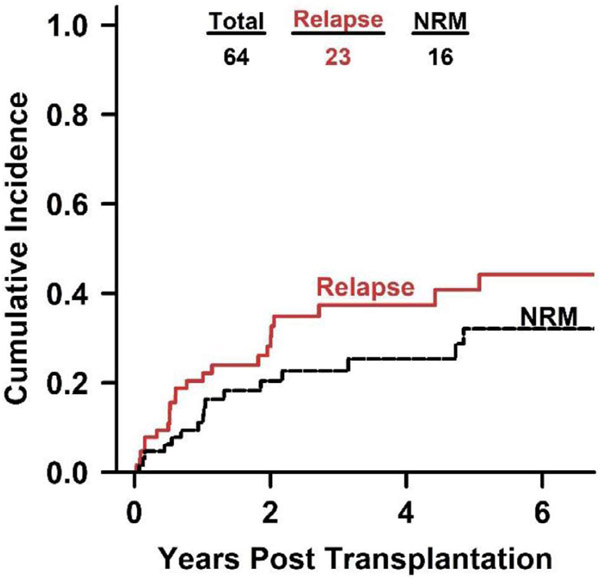
We found a CuI of relapse of 36% (95% CI, 23% to 49%) and an NRM of 24% (95% CI, 13-36%) (Figure 2) at 3 years. None of the 8 patients with ≥20% marrow CLL involvement was alive at 4 years post-transplantation, with 4 patients dying from progressive disease and 4 patients dying from NRM (fungal or bacterial infection). Causes of NRM in the 12 patients with <20% CLL marrow involvement were infection (7 patients: 58.3%), cardiovascular disease (3 patients; 25%), and fatal bleeding events (2 patients; 16.6%).
Figure 2.
Cumulative incidence of relapse and NRM after haploidentical allo-BMT with PTCy. The curves were truncated at year 6.5.
Survival
For all 64 patients, the median duration of follow-up was 4.4 years (range, 26 days to 10.4 years) based on the reverse Kaplan-Meier method. The 4-year OS was 52% (95% CI, 40-68%), and the 4-year PFS was 37% (95% CI, 26% to 54%) (Figure 3). The 56 patients with <20% marrow CLL involvement before undergoing allo-BMT had a 4-year OS of 61% (95% CI, 48% to 78%), a 4-year PFS of 43% (95% CI, 30% to 61%), and a median OS of 4.8 years (95% CI, 3.5 to NA (not applicable)) (Supplementary Figure S1). Univariate Cox regression analysis demonstrated that donor age, stem cell source, IGHV mutation status, or grade II-III aGVHD did not affect risk of progression or survival. Del (17p) was associated with reduced OS (HR, 2.23; 95% CI, 1.06 to 4.68) without a statistically significant elevated risk of progression (HR, 1.67; 95% CI, .89 to 3.14) (Table 3), but failed to achieve statistical significance when limited to patients with <20% marrow CLL (HR, 1.68; 95% CI, .71 to 3.96) (Supplementary Table S4).
Figure 3.
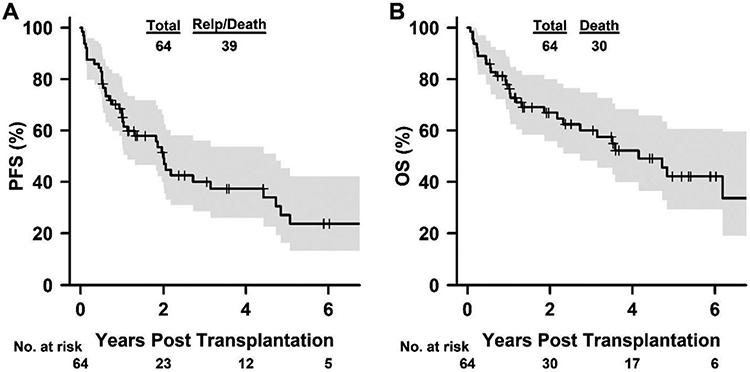
Kaplan-Meier curves for PFS (A) and OS (B) after haploidentical allo-BMT with PTCy. The curves were truncated at year 6.5.
Table 3.
Univariate Risk Factor Analysis for PFS and OS
| PFS | OS | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Age at BMT, ≥60 yr versus <60 yr | 1.22 (.65-2.29) | .54 | 1.63 (.79-3.36) | .19 |
| Stem cell source, peripheral blood versus bone marrow/G-CSF mobilized bone marrow | .80 (.39-1.63) | .54 | .74 (.32-1.62) | .42 |
| Donor age, ≥40 yr versus <40 yr | 1.24 (.64-2.38) | .52 | 1.05 (.50-2.23) | .89 |
| Female donor to male recipient, yes versus no | 1.06 (.53-2.12) | .87 | 1.51 (.72-3.18) | .28 |
| Cytogenetics | ||||
| 17p deletion versus no 17p deletion | 1.67 (.89-3.14) | .11 | 2.23 (1.06-4.68) | .03 |
| 11q deletion versus no 11q deletion | .99 (.52-1.88) | .98 | .95 (.45-1.97) | .88 |
| 13q deletion versus no 13q deletion | .59 (.31-1.11) | .10 | .55 (.26-1.14) | .11 |
| Trisomy 12 versus no trisomy 12 | 1.20 (.36-3.99) | .77 | .90 (.21-3.80) | .89 |
| Complex karyotype (≥3 chromosomal abnormalities) versus no complex karyotype | 1.32 (.57-3.03) | .51 | .96 (.33-2.79) | .94 |
| IGHV status: mutated versus unmutated | 1.66 (.62-4.46) | .31 | 1.75 (.57-5.35) | .33 |
| aGVHD(t), grade II-IV* versus none/grade I | .63 (.26-1.53) | .31 | 1.08 (.43-2.69) | .87 |
| Presence of chronic GVHD(t), yes versus no | .80 (.27-2.32) | .68 | 1.20 (.40-3.64) | .75 |
| Pretransplantation treatment | 1.34 (.62-2.91) | .46 | 1.06 (.42-2.67) | .90 |
| Ibrutinib versus chemoimmunotherapy | ||||
| Ibrutinib/idelalisib/venetoclax versus chemoimmunotherapy | .83 (.41-1.68) | .61 | .75 (.32-1.73) | .50 |
| Allo-BMT year: 2005-2013 versus 2014-2018 | .94 (.47-1.88) | .86 | .72 (.32-1.62) | .42 |
No patient had grade IV aGVHD. aGVHD(t) measures aGVHD occurrence as a time-varying covariate.
The year of allo-BMT did not affect outcome; univariate analysis showed no survival difference between patients undergoing allo-BMT between 2005 and 2013 and those undergoing allo-BMT between 2014 and 2018. Twenty-six patients received a single oral PI agent or a combination of agents (ibrutinib, venetoclax, or idelalisib) before allo-BMT, including 12 patients treated with ibrutinib alone. We found no survival difference between patients receiving PI therapy pre-allo-BMT compared with chemoimmunotherapy regimens (Table 3). Based on the effectiveness of PI drugs in treating CLL, recent guidelines suggest classifying patients as high risk I (abnormal TP53, chemoimmunotherapy-resistant but PI-responsive) or high risk II (resistant to both chemoimmunotherapy and a PI). Allo-BMT is favored for high risk II patients, because they have exhausted both chemoimmunotherapy and PI options, and can be considered for select high risk I patients with low predicted transplantation-related mortality [9,26]. We did not observe a survival difference between the 8 high risk I patients and the 10 high risk II patients in our cohort at a median follow-up of 13 years (Supplementary Figure S2).
DISCUSSION
Our findings suggest that haploidentical allo-BMT with PTCy in patients with <20% CLL marrow involvement produces outcomes similar to those previously reported with HLA-matched allo-BMT [14,16,18]. As seen in other diseases using PTCy after haploidentical allo-BMT [27-30], we found a low incidence of GVHD, with a 1-year CuI of grade II-IV aGVHD of 27% (95% CI, 15% to 38%) and a 2-year CuI of cGVHD of 17% (95% CI, 7% to 26%)]. Haploidentical allo-BMT induces intense bidirectional alloreactivity, leading to GVHD or graft failure [31,32]. Although such interventions as T cell depletion is associated with increased graft failure [33], higher pre-allo-BMT TBI dose [34,35] and PTCy prophylaxis [36-38] facilitate engraftment. Among donor variables affecting engraftment, a major ABO mismatch reduced engraftment in the EBMT report [39], but bone marrow or peripheral blood stem cell grafts did not affect outcomes or engraftment in patients with lymphoid malignancies [40,41]. The 6 patients in our cohort with engraftment failure received identical TBI doses and PTCy prophylaxis similar to the remaining 58 patients, and only 1 %of the 6 patients with engraftment failure (16.6%) had a major ABO mismatch. However, the 6 patients did have less-responsive disease, as reflected by their elevated marrow CLL %involvement (≥20%) and higher rates of PR and stable disease. Although it is generally accepted that patients with CLL should undergo allo-BMT while treatment-responsive [6], the response threshold that allows engraftment has not yet been well defined. Our data suggest that CLL marrow involvement of <20% is permissive for engraftment in patients undergoing NMA haploidentical allo-BMT and provides long-term disease-free survival.
Unfavorable cytogenetics or IGHV mutation status was associated with no statistically significant increased risk of post-allo-BMT disease progression or reduced survival in patients with <20% marrow CLL involvement. Although this study was underpowered to exclude the impact of unfavorable cytogenetics on post-allo-BMT survival, our results demonstrate that such high-risk features should not be considered a contraindication for allo-BMT. Our findings are reminiscent of the results of the CLL3X trial [15,16] which showed no survival difference in del (17p) or del (11q) CLL patients and also in patients with multiple myeloma [42,43], in whom high-risk cytogenetics did not affect post-allo-BMT survival. Because of the high graft failure rate in our CLL patients before 2013, we switched to peripheral blood allografts in 2014. Unfortunately, we have no data on the use of mobilized peripheral blood allografts for patients with >20% CLL marrow involvement, because we now exclude these patients from allo-BMT. However, in patients with <20% CLL marrow involvement, the use of mobilized peripheral blood as an allograft source did not appear to impact engraftment or survival but was associated with an increased risk of aGVHD compared with bone marrow. This finding is also consistent with the results of a large study comparing peripheral blood and bone marrow as stem cell sources in haploidentical transplantation with PTCy [40].
CLL treatment options have improved in both front-line [1-3,44,45] and relapsed settings [4,5], likely reducing the need for allo-BMT in this disease. As a result, the role of allo-BMT in the CLL treatment paradigm is becoming less clear, and is being increasingly relegated to patients with disease refractory to multiple agents, including Pis, such as ibrutinib and venetoclax. However, a significant percentage of patients with relapsed/refractory CLL experience disease progression while receiving PI therapy [46-48], and allo-BMT remains one of the few viable treatment options in this setting. We observed no survival difference between patients receiving pre-allo-BMT chemoimmunotherapy or PIs, including single agent ibrutinib or a combination of ibrutinib, venetoclax, and idelalisib. This suggests that the pre-allo-BMT depth of response affects survival, but the pre-allo-BMT treatment regimen does not. The majority of our patients had unfavorable risk factors, and collectively our data show that haploidentical allo-BMT with PTCy in CLL with <20% marrow involvement is a safe treatment option carrying a low risk of serious GVHD and other toxicities.
Limitations of this study include its retrospective nature and lower statistical power due to the limited number of events. We observed disease progression in more than one-half of our patients with <20% marrow CLL involvement at 4 years, and similar disease progression was observed by the EBMT CLL allotransplantation study [14] and the German GCLLSG CLL3X report [16]. Post-BMT maintenance therapy to decrease relapse has been established for oral tyrosine kinase inhibition in Philadelphia chromosome-positive acute lymphocytic leukemia [49]. Whether post-allo-BMT Burton's tyrosine kinase, PI3K, or BCL2 inhibition by PI drugs will improve disease progression and survival remains unclear, and we are currently testing such strategies prospectively.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the nurses, physicians, bone marrow transplantation service members, transplantation coordinator’s office, and staff at the cell therapy laboratory for the exceptional clinical care they provided.
Financial disclosure: This study was supported by the National Institutes of Health, National Cancer Institute grants P01 CA015396 and P30 CA06973 (to R.J.J.).
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
SUPPLEMENTARY MATERIALS
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.bbmt.2019.11.008.
REFERENCES
- 1.Shanafelt TD, Wang XV, Kay NE, et al. Ibrutinib-rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N Engl J Med. 2019;381:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379:2517–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer K, Al-Sawaf O, Bahlo J, et al. Venetoclax and obinutuzumab in patients with CLL and coexisting conditions. N Engl J Med. 2019;380:2225–2236. [DOI] [PubMed] [Google Scholar]
- 4.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetodax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–1120. [DOI] [PubMed] [Google Scholar]
- 6.Gribben JG. How and when I do allogeneic transplant in CLL Blood. 2018;132:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharfan-Dabaja MA, Kumar A, Hamadani M, et al. Clinical practice recommendations for use of allogeneic hematopoietic cell transplantation in chronic lymphocytic leukemia on behalf of the guidelines committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2016;22:2117–2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comphrehensive Cancer Network. NCCN Guidelines®. Chronic lymphocytic leukemia/small lymphocytic lymphoma. 2019. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed 10 August 2019.
- 9.Duarte RF, Labopin M, Bader P, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019;54:1525–1552. [DOI] [PubMed] [Google Scholar]
- 10.Elmariah H, Fuchs EJ. Post-transplantation cyclophosphamide to facilitate HLA-haploidentical hematopoietic cell transplantation: mechanisms and results. Semin Hematol. 2019;56:183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–1316. [DOI] [PubMed] [Google Scholar]
- 12.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125:3024–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Gelder M, de Wreede L Henseler A, et al. Long-term follow-up data support the curative potential of allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia: a retrospective analysis from the chronic malignancies working party of the EBMT. Blood. 2014:124:2561. [Google Scholar]
- 14.van Gelder M, de Wreede LC, Bornhäuser M, et al. Long-term survival of patients with CLL after allogeneic transplantation: a report from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2017;52:372–380. [DOI] [PubMed] [Google Scholar]
- 15.Dreger P, Döhner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. [DOI] [PubMed] [Google Scholar]
- 16.Krämer I, Stilgenbauer S, Dietrich S, et al. Allogeneic hematopoietic cell transplantation for high-risk CLL: 10-year follow-up of the GCLLSG CLL3X trial. Blood. 2017;130:1477–1480. [DOI] [PubMed] [Google Scholar]
- 17.Brown JR, Kim HT, Armand P, et al. Long-term follow-up of reduced-intensity allogeneic stem cell transplantation for chronic lymphocytic leukemia: prognostic model to predict outcome. Leukemia. 2013;27:362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Gorkom G, van Gelder M. Eikema DJ, et al. Outcomes of haploidentical stem cell transplantation for chronic lymphocytic leukemia: a retrospective study on behalf of the chronic malignancies working party of the EBMT. Bone Marrow Transplant. 2018;53:255–263. [DOI] [PubMed] [Google Scholar]
- 19.Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131:2745–2760. [DOI] [PubMed] [Google Scholar]
- 20.O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. [DOI] [PubMed] [Google Scholar]
- 21.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115:3224–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 24.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Dreger P, Ghia P, Schetelig J, et al. High-risk chronic lymphocytic leukemia in the era of pathway inhibitors: integrating molecular and cellular therapies. Blood. 2018;132:892–902. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh N, Karmali R, Rocha V, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors versus HLA-matched sibling donors: a Center for International Blood and Marrow Transplant Research analysis.J Clin Oncol. 2016;34:3141–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez C, Gayoso J, Canals C, et al. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for hodgkin lymphoma: a registry study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation.J Clin Oncol. 2017;35:3425–3432. [DOI] [PubMed] [Google Scholar]
- 30.Devillier R, Legrand F, Rey J, et al. HLA-matched sibling versus unrelated versus haploidentical related donor allogeneic hematopoietic stem cell transplantation for patients aged over 60 years with acute myeloid leukemia: a single-center donor comparison. Biol Blood Marrow Transplant. 2018;24:1449–1454. [DOI] [PubMed] [Google Scholar]
- 31.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. [DOI] [PubMed] [Google Scholar]
- 32.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings.J Clin Oncol. 1997;15:1767–1777. [DOI] [PubMed] [Google Scholar]
- 33.Aversa F, Tabilio A, Velardi A, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998;339:1186–1193. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic hematopoietic stem-cell transplantation for sickle cell disease. N Engl J Med. 2009;361:2309–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolaños-Meade J, Gamper C, Cooke KR, Jones RJ, Brodsky RA. Curative allogeneic bone marrow transplantation (Allo-BMT) for severe hemoglobin-opathies no longer requires matched donors or the ability to tolerate myeloablative conditioning. Presented at: American Society for Blood and Marrow Transplantation 2018 BMT Tandem Meetings. February 21-25. Salt Lake City, UT; 2018. [Google Scholar]
- 36.Luznik L, Engstrom LW, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. Biol Blood Marrow Transplant. 2002;8:131–138. [DOI] [PubMed] [Google Scholar]
- 37.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98:3456–3464. [DOI] [PubMed] [Google Scholar]
- 38.Aversa F, Terenzi A, Tabilio A, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447–3454. [DOI] [PubMed] [Google Scholar]
- 39.Canaani J, Savani BN, Labopin M, et al. Impact of ABO incompatibility on patients' outcome after haploidentical hematopoietic stem cell transplantation for acute myeloid leukemia - a report from the Acute Leukemia Working Party of the EBMT. Haematologica. 2017;102:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bashey A, Zhang MJ, McCurdy SR, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017;35:3002–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Donnell PV, Eapen M, Horowitz MM, et al. Comparable outcomes with marrow or peripheral blood as stem cell sources for hematopoietic cell transplantation from haploidentical donors after non-ablative conditioning: a matched-pair analysis. Bone Marrow Transplant. 2016;51:1599–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roos-Weil D, Moreau P, Avet-Loiseau H, et al. Impact of genetic abnormalities after allogeneic stem cell transplantation in multiple myeloma: a report of the Société Française de Greffe de Moelle et de Thérapie-Cellulaire. Haematologica. 2011;96:1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghosh N, Ye X, Tsai HL, et al. Allogeneic blood or marrow transplantation with post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis in multiple myeloma. Biol Blood Marrow Transplant. 2017;23:1903–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shanafelt TD, Wang V, Kay NE, et al. A randomized phase III study of ibrutinib (PCI-32765)-based therapy vs. standard fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy in untreated younger patients with chronic lymphocytic leukemia (CLL): a trial of the ECOG-ACRIN Cancer Research Group (E1912). Presented at: 60th ASH Annual Meeting. December 1-4. San Diego, CA; 2018. [Google Scholar]
- 45.Jain N, Keating M, Thompson P, et al. Ibrutinib and venetoclax for first-line treatment of CLL. N Engl J Med. 2019;380:2095–2103. [DOI] [PubMed] [Google Scholar]
- 46.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts AW, Ma S, Kipps TJ, et al. Efficacy of venetoclax in relapsed chronic lymphocytic leukemia is influenced by disease and response variables. Blood. 2019;134:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharman JP, Coutre SE, Furman RR, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37:1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giebel S, Czyz A, Ottmann O, et al. Use of tyrosine kinase inhibitors to prevent relapse after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a position statement of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Cancer. 2016;122:2941–2951. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.