Abstract
Chimeric antigen receptors (CARs) recently gained momentum in cancer treatment due to their ability to promote T-cell mediated responses to a specific tumor-associated antigen. CARs are part of the adoptive cell transfer (ACT) strategies that utilize patients' T lymphocytes, genetically engineered to kill cancer cells. However, despite the therapy's success against blood-related malignancies, treating solid tumors has not reached its fullest potential yet. The reasons include the complex suppressive tumor microenvironment, mutations on cancer cells' target receptors, lethal side-effects, restricted trafficking into the tumor, suboptimal persistence in vivo and the lack of animal models that faithfully resemble human tumor's immunological responses. Currently, rodent models are used to investigate the safety and efficacy of CAR therapies. However, these models are limited in representing the human disease faithfully, fail to predict the adverse treatment events and overestimate the efficacy of the therapy. On the other hand, spontaneously developed tumors in dogs are more suited in CAR research and their efficacy has been demonstrated in a number of diseases, including lymphoma, osteosarcoma and mammary tumors. The present review discusses the design and evolution of CARs, challenges of CAR in solid tumors, human and canine clinical trials and advantages of the canine model.
Keywords: chimeric antigen receptors therapy, immunotherapy, solid tumor, hematological malignancies, canine, dog
1. Introduction
Treatment of cancer by standard methods, surgery, radiation, and chemotherapy, is less effective in advanced-stage disease and causes numerous side effects. Consequently, researchers are in the quest to explore the possibility of developing more effective, less toxic therapy. Recently immunotherapy has emerged as a sound approach that includes immune checkpoint inhibitors, T-cell transfer therapy, monoclonal antibodies, vaccines, and immune system modulators. The most studied type of immunotherapy is T-cell transfer therapy or adoptive cell transfer (ACT). ACT is the collection and the use of patients' immune cells to treat their cancer. Currently, there are a few types of ACT-based therapies, tumor-infiltrating lymphocytes (TIL), engineered T cell receptor (TCR), natural killer (NK) cells, iNKT cells, Chimeric antigen receptors (CAR) T-cell (1), and γδT cells (2). TIL uses T cells around or in a patient's tumor tissues. These T cells are collected, and the best that recognizes and kills the tumor ex-vivo is selected, expanded, and adoptively transferred back to the patient to eliminate tumor cells. TCR or transduced T-cell is the genetic engineering of T-cells to express new specific TCR to recognize tumors ex vivo. NK cells therapy depends on the immune system's activation against abnormal cells. Unlike TLs, NK cell receptors interact with target cells independent of antigen processing and presentation. γδ T cells are T cells that express a unique TCR composed of one γ-chain and one δ-chain (3,4). In CAR T cell therapy, the T lymphocytes undergo modification with a receptor based on a recognition sequence of an antibody, called CAR, a non-MHC restricted receptor, to attach to specific proteins (antigens) on cancer cells' surface ex-vivo. The T cells in CAR therapy have an improved ability to attack and kill the cancer cells compared to T cells in TIL therapy (5). In these therapies, the lymphocyte undergoes modification via plasmids or viral vectors, such as adenovirus, retrovirus, or lentivirus (6).
CAR T therapy showed promising success in treating malignant blood diseases such as acute lymphoblastic leukemia (ALL) and diffused-large B cell lymphoma (DLBCL) in children and young adults. Therefore, the FDA authorized cluster of differentiation 19 (CD19) specific CAR T cell therapies for these diseases. Tisagenlecleucel (Kymriah™) against ALL and DLBCL for children/young adults, Axicabtagene ciloleucel (Yescarta™) against adult non-Hodgkin lymphoma (NHL) and DLBCL, Brexucabtagene autoleucel (Tecartus™) for relapsed or refractory (R/R) mantle cell lymphoma (MCL) treatments (7-10), and most recently, Breyanzi (lisocabtagene maraleucel) for the treatment of relapsed or refractory (R/R) large B cell lymphomas (LBCL) in adult patients (11).
However, these CAR T therapies have limited success in solid tumors. CAR T cells treatment directed against antigens such as vascular endothelial growth factor receptor 2 (VEGF-R2), CD171, folate receptor alpha, disialoganglioside GD2, human epidermal growth factor receptor 2, mesothelin, EGFRvIII, or carbonic anhydrase IX, in patients with solid tumors failed to produce similar beneficial outcomes as seen in blood-related malignancies (12).
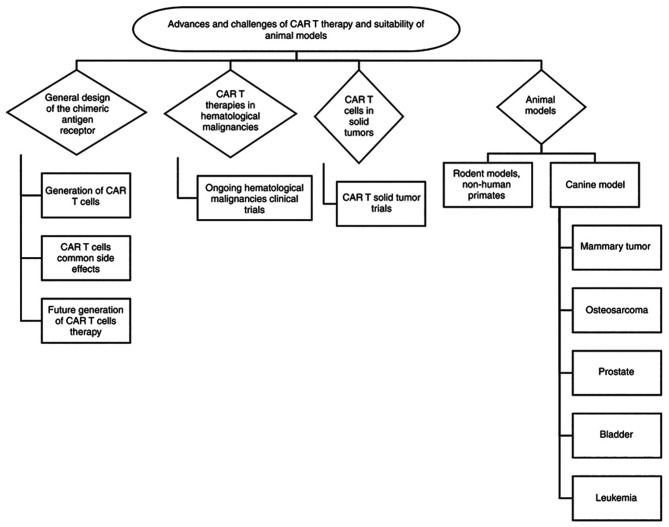
Translating successful CAR T-cell therapies to solid tumors requires overcoming several barriers, including identifying an ideal tumor-associated antigen to target and overcome antigen expression heterogeneity, addressing the tumor-suppressive microenvironment, and employing a preclinical model that faithfully represents the disease. The review collected data using PubMed, Google Scholar and other publicly available databases and discusses the design and evolution of CARs and the challenges facing CAR therapies in solid tumors. Also, it discusses the advantages and disadvantages of preclinical animal models emphasizes the advantages of using the canine model (Fig. 1).
Figure 1.
Schematic diagram of the advances and challenges of CAR-T cell therapy in animal models. General design of the chimeric receptor, trials and differences between hematological malignancies and solid tumors are discussed in the present review paper. In addition, comparison between different animals used as pre-clinical models are discussed presenting their potential translational impact in CAR-T cell development. CAR, chimeric antigen receptors.
2. General design of the CARs
The discovery of the CARs started around the 1980s. Several factors are essential for CAR T cell therapy to be effective, such as recruitment, activation, expansion, and persistence of bioengineered T cells at the tumor site. Even though ~41 years have passed since the first CAR T cell's creation, some essential components of its structure remained the same (13). However, these components have undergone numerous modifications to enhance CAR T therapeutic capabilities over the years. The structure consists of four components: the ectodomain (the domain of a membrane protein outside the cytoplasm) a hinge, the transmembrane domain, and the intracellular signaling endodomain. Each domain has a specific function and optimal molecular design. The extracellular domain, the target-binding domain, is usually a single-chain variable fragment (scFv) of the antigen-binding region of a monoclonal antibody's light and heavy chain. It recognizes any antigen and binds targets with high affinity. The hinge connects the extracellular antigen-binding domain to the intracellular signaling domains and regulates the extracellular domain flexibility, facilitating the migration and binding capacity to tumor cell receptors. The length and composition of the hinge can affect antigen binding and signal through the CAR. Generally, the hinge domain consists of amino acid sequences from CD8, CD28, IgG1, or IgG4. The transmembrane domains anchor the CAR in the T cell membrane. It consists of a hydrophobic alpha helix that spans the membrane, such as CD3ζ, CD28, CD4, or CD8α. The primary function of the transmembrane domain is to stabilize the CAR. The endodomain domain (intracellular signaling domain) comprises of the activation domain, a TCR-derived CD3ζ-derived immunoreceptor tyrosine-based activation motifs, and intracellular costimulatory domains derived from CD28 or 4-1BB (CD137) (14,15). The first CAR generations with CD3-ζ transmembrane domains suffered detachment from the surface of T cells. Consequently, CAR T structure is subjected to modification with a well-balanced transmembrane domain composed of the CD4, CD8, or CD28 molecules (16). Antigen-specific T cell activation, in nature, requires three signals to gain full functionality that enables proliferation, differentiation, and survival. Co-stimulation plays a vital role in the CAR T-cell functionality as it triggers the T-cell immune response against foreign antigens. The absence of co-stimulation can enter T cells in a state of anergy, leading to its unresponsiveness to antigen binding (17). Unfortunately, cancer cells promote co-stimulatory-ligand deficient environments generating unfavorable antitumor responses. Therefore, CAR T is designed with various costimulatory molecules to overcome the tumor cell suppressing environment. The conserved region of a CD3-ζ domain, the immunoreceptor tyrosine-based activation motifs (ITAMs), carries out signaling transduction pathways on CAR T cells to build sufficient T cell activation (18).
Also, CARs function without relying on the major histocompatibility complex (MHC), allowing it to target various antigens without antigen presentation for activation since activated with the single-chain Fv domain interaction with the targeted TAA (19) The MHC independence is an essential feature of CAR design since the tumor microenvironment consistently down-regulates the MHC complexes.
3. Generations of CAR T cells
Although CAR T therapy can lead to long-lasting remissions for some patients with very advanced malignant disease, it can cause severe and fatal side effects such as cytokines storm and neurological problems, including termer, delirium, and seizures. Therefore, scientists modified CAR T cells to create safe and more effective therapy by building on the CAR T cell's original components and information gained from clinical trials. These include:
CAR 1st generation
It consists of a single-chain variable fragment (scFv) ectodomain and a TCR-derived signaling CD3-ζ constant region representing the endodomain fragment. These 1st generation CAR cannot maintain the CAR stable on the T cell membrane and T cell activation for a considerable amount of time (20).
CAR second and third generations
The second and the third generation compared to the 1st generation were modified to enhance the receptor cohesion toward the lymphocyte surface, thus allowing optimal functionality. As a result, these CARs generations have one (2nd generation) or two (3rd generation) costimulatory signals that augment T cell proliferation, differentiation, and survival despite the effect of tumor-suppressing environments (17).
CAR 4th generation
The fourth generation compared to 2nd and 3rd generation CAR, create a robust immune attack to eliminate the tumor before they re-generate or mutate. The 4th generation CAR T cells redirected for universal cytokine killing (TRUCK), has the same structure and physiology as the 2nd and the 3rd CAR generations with a slight genotypic difference (20). These TRUCKs contain a nuclear factor of the activated T cells (NFAT), codifying a transgenic cytokine. NFATs are found in T cells and play a crucial role in cytokine expression. TRUCKs deliver a considerable amount of IL-12 on the tumor site stimulating T cells and recruiting other immunological cells to target tumor cells not recognized by the (svFc) fragment of a CAR (21).
CAR 5th generation
The 5th generation have the same structure as the second generation of CARs, but they contain a truncated cytoplasmic IL-2 receptor β-chain domain with a binding site for the transcription factor STAT3. The antigen-specific activation of this receptor simultaneously triggers TCR (through the CD3ζ domains), costimulatory (CD28 domain), and cytokine (JAK-STAT3/5) signaling required physiologically to drive full T cell activation and proliferation.
4. CAR T-cell therapies common side effects
CAR-based therapy's common side effects are the body's immunological defense impulses triggered by the T cell artificial receptor. These autoimmune consequences can affect the patient's prognosis and disease outcomes. The most common side effects include.
Cytokine release syndrome (On-target on-tumor toxicity)
One of the most frequent setbacks in using CAR T therapies is releasing proinflammatory cytokines into the body or cytokine release syndrome (CRS) due to excessive antigen-CAR T cell engagement. These cytokines are small proteins that act as cell messengers to help direct the body's immune response. Increased cytokine levels lead to chronic inflammation throughout the body, which can be harmful and interfere with several body functions. CRS is characterized by increased serum levels of cytokines, fever, diarrheas, hypotension, hypoxemia, low blood pressure, and organ dysfunctions. Most patients have a mild CRS form, but it may be severe or life-threatening in some individuals due to organ failure. The severity of CRS depends upon the disease burden. Generally, splitting the initial dose and strictly monitoring the vital parameters can mitigate the risk. Also, treating specific symptoms to lower the immune response, such as tocilizumab and siltuximab, interferes with IL-6 or corticosteroids to help reduce inflammatory and immune response (22).
Immune effector cell-associated neurotoxicity syndrome (ICANS)
Although CAR T neurotoxicity is the most common side effect, its pathophysiology is not entirely understood. Recent studies suggested that blood-brain barrier disfunction (BBB) causes CAR T cells' infiltration into the cerebrospinal fluid (23). Symptoms include confusion, myoclonus, seizures, delirium, aphasia, memory loss, and coma (8,9,22). Neurotoxic issues are reported in patients within the first two months of CAR T treatment lasting between 6-17 days, depending on the type of blood cancer treated and the specific drug-infused (24). Trials studying GD2 in treating neuroblastoma with high-affinity GD2 specific CAR T and ERBB2 with ERBB specific CAR T for metastatic colorectal cancer found it to cause severe neurotoxicity and multi-organ failure, respectively (25,26).
On-target toxicities (On-target off-tumor toxicity)
On-target off-tumor effect arises in patients with target antigens expressed on both tumors and healthy tissues. The condition was first noticed in patients who experienced uncommon reductions of healthy B-lymphocytes, B-cell aplasia, in trials utilizing a CD-19 specific CAR T cell due to the binding of the engineered T cells to both CD-19 malignant and healthy B cell (27,28). Similarly, low-level ERBB2, CAIX, and CEACAM5 expression on healthy lung, liver, and gastrointestinal epithelia resulted in deadly toxicities in these organs (25,29). Thus, it is crucial to know the background expression of the target antigen in healthy tissues to determine whether its levels are over the threshold that may cause toxicity and the potential severity.
Off-target toxicity
Off-target toxicity occur when CAR T cells attack an antigen other than those for which the CAR T was meant to bind or activate themselves independently from their specificity. The risk of off-target toxicity occurs due to the inherited CAR T makeup (23). For example, patients treated with CAR T-anti-HER2/neu. CAR T-anti-HER2/neu carries IgG1-derived CH2CH3 domain as an extracellular spacer which can interact with the Fc receptor expressed on innate immune cells and, as a result, lead to antigen-independent activation (29).
5. Future generations of CAR T therapy
Even though treatment with CAR-T cells has produced remarkable clinical responses with specific subsets of B cell leukemia or lymphoma, a number of challenges (mentioned above) limit the therapeutic efficacy of CAR-T cells in solid tumors and hematological malignancies. However, researchers are working restlessly to overcome these limitations by pursuing various new CAR concepts and models to generate the next generation of CAR therapies. These concepts include:
The bispecific adaptor platform
Among numerous platforms to improve CAR T therapy, the adaptor CAR platforms have received much attention and immense research. The platform separates the tumor-targeting and signaling moieties of conventional CARs resulting in a system consisting of an adaptor CAR or Universal CAR and soluble, tumor-specific adaptor molecules. The universal CAR construct contains cytoplasmic activation domains in conventional CAR and an extracellular single-chain variable fragment (scFv) that recognizes fluorescein (anti-FITC CAR T cell. The bispecific adapter molecule comprises fluorescein linked to a tumor-specific ligand. Such an adaptor brings the CAR T cell to the tumor cell triggering CAR T-cell activation and the subsequent destruction of the cancer cell-the omission of the bispecific adapter prevents CAR T-cell engagement with the cancer cell and the tumor cell killing. A cocktail of orthogonal fluorescein-linked bispecific adapters in which each fluorescein-linked adapter is attached to a unique tumor-specific ligand capable of binding one of the cancer cell's antigens could be prepared (30,31). Developing this platform improves conventional CAR T cells' flexibility, tumor specificity, and controllability (32).
Dual CAR T-cells
Despite the great successes with Tisagenlecleucel and Axicabtagene, Anti-CD19 chimeric CAR T cell, therapy in leukemia, up to 60% of patients relapse due to CD19 antigen loss. A new approach to overcoming antigen loss targets more than one antigen on cancer cells, such as autologous CD19/CD22 CAR T cell therapy, which demonstrated to be safe and had anti-leukemic activity in patients with relapsed/refractory B-ALL (33).
Dominant-negative receptor CAR T cells
In addition to the target antigen scFv, dominant-negative receptor CAR T cells are transduced with an additional co-inhibitory receptor that controls inhibitory signals sent by the tumor milieu to the T cell. Those receptors include PD-1 and TGF-βRII (34,35). Other upregulated receptors when the T cell is exhausted, and potential candidates for this type of method are CTLA-4, TIM-3, and TIGIT.
Off-the-shelf CAR T cells
These Off-the-shelf CAR T cells are a third-party, healthy donor-derived alternative. Because the preparation of autologous CAR T cells takes time, the patient needs to be stable to withdraw their T cells by leukapheresis; pre-made CAR T cells offer a ready-to-use therapy for advance stage cancer patients.
6. CAR T therapies in hematological malignancies
The FDA gave authorization for five CART therapies up to date. The first four therapies utilize slightly different methods of genetic engineering to transform the patient's T cells into CAR-T cells. However, all therapies produced CAR T cells that bind to the cluster differentiation 19 (CD19) protein on the B-cell surface.
The first approved CAR-T therapy is tisagenlecleucel (Kymriah; Novartis), approved in August 2017. In this therapy, the T cells are induced by a vector that encodes a second-generation CAR with scFv, derived from the CD19-specific monoclonal antibody FMC63 and the costimulatory domain from 4-1BB and CD3ζ. The therapy is indicated to treat acute lymphoblastic leukemia, the most common cause of cancer-related deaths among children in the USA age 25 or younger (36).
The second FDA-approved CAR T therapy is Axicabtagene ciloleucel (Yescarta™), developed by Kite, a Gilead Science, Inc company, in October 2017. In this therapy, patient-derived T cells are transduced using a gamma-retroviral vector expressing a second-generation CAR that targets CD19. Yescarta is created from CD3+ enriched autologous T cells, while Kymriah is generated from autologous CD4/CD8 T-cell. The therapy works similarly to Kymriah but is indicated for treating adults with certain non-Hodgkin lymphomas, including diffuse large B-cell lymphoma (37).
The third FDA-approved CART therapy is brexucabtagene autoleucel (Tecartus), on July 24, 2020, developed by Kite Pharma to treat relapsed or refractory (R/R) mantle cell lymphoma (MCL), which is a form of non-Hodgkin lymphoma occurring in cells from the ‘mantle’ zone of the lymph node. It is aggressive cancer that primarily affects men 60 years and over. Tecartus is similar to Yescarta in generation and CAR structure. It is the first and only CAR-T cell therapy for adult patients suffering from R/R mantle cell lymphoma (38).
In February 2021, the FDA approved the fourth CAR T therapy, Lisocabtagene maraleucel (Breyanzi®; Bristol Myers Squibb). Breyanzi® is indicated for adult patients with relapsed or refractory large B-cell lymphoma, including diffuse large B-cell lymphoma (DLBCL) not otherwise specified (including DLBCL arising from indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B after two or more lines of systemic therapy.
However, these treatments caused two potentially fatal side effects: neurologic toxicity and cytokine release syndrome (CRS). CRS occurred in 94% of patients; 13% experienced symptoms that required aggressive treatment or were considered life-threatening in the phase II ZUMA-1 trial (11,39).
Recently, in March 2021, FDA approved the first B-cell maturation agent (BCMA)-directed CAR T cell therapy, idecabtagene vicleucel (Abecma®) developed by Bristol Myers Squibb. It is indicated for relapsed or refractory multiple myeloma treatment after four or more prior lines of therapy (40). BCMA is a member of the tumor necrosis factor superfamily and only expressed by some B cells, normal plasma cells, and malignant plasma cells and not expressed by hematopoietic stem cells and normal essential non-hematopoietic tissues (41).
Ongoing hematological malignancies clinical trials
Currently, numerous trials used CAR T cells against different hematological malignancies: A Phase I clinical trial (NCT03778346) against Refractory/Recurrent Multiple Myeloma using BCMA-7x19 CAR-T cells by Wenzhou Medical University. The CAR-T cell targets BCMA antigens and expresses IL-7 and CCL19. This design provides superior T cells differentiation, migration, expansion, and tumor killing. Both patients enrolled achieved complete remission (CR) and very good partial response (VGPR) with a response of over 12 months. Side effects included Grade 1 cytokine release syndrome one month after the first infusion. A Phase II clinical trial evaluated the efficacy and safety of anti-CD19 CAR-T cells alone or in combination with anti-B cell maturation antigen CAR-T cells therapy against relapsed/refractory multiple myeloma. The disease targeted immunoglobulin D (IgD) multiple myeloma, a rare subtype with a worse prognosis. A total of 7 patients enrolled in the trial. Six achieved stringent complete remissions (CR), and one with extracellular disease achieved minimal response (MR) 60 days after the first infusion.
Clinical trials conducted by Kite Pharma, Inc., the developers of Yescarta™, are currently underway to demonstrate safety and clinical benefits to patients with R/R Indolent Non-Hodgkin Lymphoma (iNHL). ZUMA-5 is a Phase II multicenter trial in which participants receive an infusion of axi-cel CAR-T cells (2x106 cells/kg). The participants included 124 patients with follicular lymphoma (FL) and 22 with marginal zone lymphoma (MZL). Out of the evaluated 104 patients, the ORR was 92%, with a CR of 76% after a 17.5-month follow-up. FL patients (n=84) responded with an ORR of 94% and CR of 80% compared to the MZL patients (n=20) with 85% ORR and a 60% CR.
Three different clinical trials ELIANA (NCT02435849), ENSIGN (NCT02228096), and B2101J (NCT01626495), tested Kymriah™ (Novartis Pharmaceuticals Corp.) in CD19-positive R/R B cell acute lymphoblastic leukemia. The patients of all three trials experienced a minimum of 69-95% overall remission rates (ORR) with durable remission. A Phase I clinical trial using m971 anti-CD22 CAR-T cells targeting R/R B-cell ALL patients previously received an infusion of CD19 CAR-T cells. Even though CD19 CAR T has impressive results treating ALL patients, some patients relapse. The trial consisted of two cohorts of patients with R/R Large B cell lymphoma (n=9) and patients with R/R B-cell ALL (n=6) that undergo allogeneic hematopoietic stem cell transplant. Patients that experienced R/R Large B cell lymphoma received an infusion of 1x106 (n=3) and 3x106 cells/kg (n=6), while all R/R B-cell ALL received 1x106 cells/kg. Large B cell lymphoma patients experienced ORR of 78% and CR of 56%. Five of the R/R B-cell ALL patients were minimal disease negative in the 28 days, while all subjects except one experienced relapse. Flow cytometry analysis showed that ALL patients downregulate CD22, promoting relapse.
7. CAR T cells in solid tumors
T cell therapy's potential to induce successful immunological responses in patients with solid tumors has been demonstrated in immune checkpoint therapy (42) and TIL and TCR therapies in melanoma, sarcoma, cholangiocarcinoma, and breast cancer in a few patients (43), suggesting T cells can eliminate solid tumors under adequate condition. However, few CAR-T cell therapy attempts have been reported in glioblastoma and neuroblastoma (44,45). The Key challenges posed to CAR T cell therapy success in solid tumors can be described in three steps: finding, entering, and surviving in the tumor. These challenges include the lack of tumor-specific target antigens and tumor cell heterogeneity, CAR T cell trafficking/infiltration towards tumor sites, T cell inhibitory signals in solid tumors, physical barriers in the solid tumor microenvironment, and the immunosuppressive microenvironment (26,46,47).
Antigen selection and heterogeneity in solid tumors
Target selection in solid tumors is a major hurdle in implementing CAR T-cell therapy against solid tumors. Also, in contrast with hematological malignancies, where the surface antigen expression is uniform and intense, solid tumor cells rarely express uniformly one specific antigen, and even when present, the levels may be quite variable (47). The antigen is also more common to be enriched on tumors and at low levels on healthy tissues, increasing the potential risk of significant on-target off-tumor toxicity. Almost all currently targeted TAAs for solid tumors display this heterogeneity, including CEA, ERBB2, EGFR, GD2, mesothelin, MUC1, and PSMA. The lack of antigen specificity and the acceptance of low levels of the target antigen on normal tissues have led to a number of catastrophic events. A patient with metastatic colon cancer died after receiving an infusion of CAR T cells targeted to the HER2 (ERBB2) antigen (48). Another patient died from encephalitis when infused with a high-affinity anti-GD2 CAR for neuroblastoma (49). CAR targets used for the treatment of solid malignancies include:
Prostate-specific membrane antigen (PSMA). PSMA is a Glutamate carboxypeptidase 2, a type II membrane protein highly expressed on most prostate-cancer cells and tumor-associated neovasculature of numerous solid tumors (50).
Mesothelin (MSLN). MSLN is a protein present in malignant pleural mesothelioma, ovarian, pancreatic, and lung cancers. Also, mesothelin is expressed on non-transformed peritoneal, pleural and pericardial mesothelial cells (51).
Fibroblast activation protein-α (FAP). FAP is a type-II transmembrane serine protease expressed almost exclusively in pathological conditions including fibrosis, arthritis, and cancer, where explicitly expressed on cancer-associated stromal cells present in epithelial cancers (52).
Epidermal growth factor receptor (EGFR). EGFR is a transmembrane protein that serves as receptors for numerous epidermal growth factor families of extracellular protein ligands. Different human tumors, including non-small cell lung cancer (NSCLC), breast, head, neck, gastric, colorectal, esophageal, prostate, bladder, renal, pancreatic, and ovarian cancers, express EGFR. EGFR signaling causes increased proliferation, decreased apoptosis, and enhanced tumor cell motility and neo-angiogenesis.
Carcinoembryonic antigen (CEA). CLA are glycosylphosphatidylinositol (GPI) cell-surface-anchored glycoproteins, characterized as members of the CD66 cluster of differentiation. These proteins serve as functional colon carcinoma L-selectin and E-selectin ligands (53). Currently, CEA-targeted CAR T cell is used to treat patients with liver metastases that are positive for CEA expression.
The human epidermal (HER2). HER2 is a receptor tyrosine-protein kinase member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. HER2 is expressed on epithelial cells in the gastrointestinal, respiratory, reproductive, and urinary tract, and it is amplification or over-expression on breast cancer denote aggressive types of breast cancer (54).
CAR T trafficking in solid tumors
In hematological malignancies, infused CAR T Cells and tumor cells co-circulate in the blood and have a higher propensity to migrate to similar areas such as bone marrow and lymph nodes. On the other hand, CAR T cells in solid tumors encounter a number of hurdles, including difficulty migrating to and adequately penetrating the tumor, binding to receptors, and completing their cytotoxic function. Chemokines, such as CXCL12 and CXCL5, secreted by the tumor inhibit T-cell migration into the tumor. In some instances, the chemokine receptors on T cells do not adequately match the tumors' chemokine signature, resulting in little migration to the tumor site. For example, it has been shown that T cells genetically modified to express CXCR2 migrate towards tumor cells expressing CXCL1. Chemokines secreted by the tumor's stroma, the chemokine repertoire in the tumor location, and the local ‘normal’ cytokine milieu also affect the CAR T cell movement and migration. Furthermore, solid tumor stroma sends chemokines signals that mismatch the chemokine-receptors on T cells' surface, resulting in dysregulation and cancer progression (55).
T cell inhibitory signals in solid tumors
Endogenous suppressive signal and their upregulation reduce CAR T cells' therapeutic ability. Intrinsic inhibitory T cells and upregulation inhibitory receptors CTLA-4/PD-1 may cause T cell exhaustion and prevent T cell persistence by interacting with ligands overexpressed on tumor cells.
Physical barriers in the solid tumor microenvironment
Physical barriers generated by excessive tumor-stromal density favors tumor progression and aggressiveness. The physical barriers that affect CAR T cell function in solid tumors include:
Hypoxia. Abnormal vascularization and rapidly growing tumor cells limit the amount of oxygen (hypoxia) in the tumor. Hypoxia impacts CAR-T cells' attributes by decreasing CAR-T cells' expansion ability, blocking their differentiation into effector memory cells, and enriching the cultures with T cells with a central memory cell phenotype (56). Also, abnormal hypoxia-derived tumor vessels affect T cell adhesion and extravasation towards the solid tumor. Additionally, abnormalities of blood vessels, known as high endothelial venules (HEV), compromise immune cell trafficking to the tumor (47,57).
Extracellular matrix. Peritumoral extracellular matrix (ECM) collagen fibers limit T cell access to tumors, and it is known that tumors with high collagen density present lower levels of infiltrating T cells.
Tumor vasculature. The tumor's core exhibits immature vessel formation, leading to low permeability (46).
Fibroblasts. Other non-immune cells that enhance tumorigenesis are stromal cells, such as cancer-associated fibroblast (CAF) (47). The cells are involved in the secretion of pro-tumorigenic molecules contributing to tumor vasculature and anti-inflammatory reaction to immune cells (47,57). In addition, fibroblast differentiation can express activation makers that support matrix degradation and remodeling (46).
Tumor microenvironment
The immunosuppressive nature of the tumor microenvironment plays an essential role in tumor survival, metastatic progression, and influences immunotherapies' outcomes (57). Numerous suppressive immune cells and molecular factors in the tumor microenvironment can block CAR T cell's antitumor immune function. These immune cells include immune suppressor cells, such as Tregs, myeloid-derived suppressor cells, and tumor-associated macrophages. In contrast, molecular factors include cytokines and soluble factors associated with immunosuppression, such as TGF-β and IL-10, promoting T cell anergy by indirect contact. Another factor known to condition the antitumor effect of T cells in solid tumors is soluble factors such as transforming growth factor B (TGF-β) and vascular endothelial growth factors (VEGF) secreted mainly by stromal and tumor cells (47). TGF-β can also be secreted by regulatory T cells (Tregs), platelets, macrophages, and fibroblasts to suppress T cell proliferation and effect function (25). Evidence suggests that it promotes Treg maturation and modulate CD8+ effector cell function (26,58).
CAR T solid tumors trials
The accomplishments surrounding CAR T-cell-based therapies hinge on their success in hematological diseases; however, for the reasons mentioned above, much work is needed to sure their success in solid tumors (59).
The CAR T cells' persistence in the stromal micro-environment was the main setback in two clinical trials targeting neuroblastoma and ovarian tumors. Neuroblastoma CARs were generated with the use of EBV-specific cytotoxic T lymphocytes (EBV-CTLs) and activated T cells (ATCs) targeting GD2(45). Although both engineered T cells were found to circulate the system at higher concentrations demonstrating improved functionality for CAR-T cell therapy purposes, only three out of eleven patients with active disease completed remission (45).
Furthermore, few clinical trials used CAR T-EGFR to treat biliary tract cancers (BTC), cholangiocarcinomas, and gallbladder carcinomas that express EGFR. The results reported that out of 19 patients, one achieved complete remission and ten stable diseases, concluding that CAR T-EGFR treatment was a safe and promising strategy for EGFR-positive advanced biliary tract cancers (60) Also, trials targeted carcinoembryonic antigens (CEA), utilizing CAR T-CEA. CEA is overexpressed in lung, gastrointestinal, and breast cancers and is used as a tumor marker for cancer patients' diagnosis and prognosis (61) In this Phase I trial, a total of 8 patients with CEA-positive liver metastases were included, of which 4 have more than ten metastatic foci in the liver. Patients received treatment with anti-CEA CAR T cells via hepatic arterial infusions. In addition to CAR T cell infusion, half of the patients received IL-2 cytokine. The trial results indicated that patients experienced no fatal side effects or adverse unpredictable outcomes and that patients tolerated very well the anti-CEA CAR-T therapy with or without IL2 administration (62).
8. Animal models
Preclinical animal testing requires using a relevant animal model that truly represents the human disease and can elicit a biological response similar to what would happen in humans. However, the preclinical model used in testing the safety and efficacy of CAR T cell therapy fell short to adhere to the standard due to variability in cross-species reactivity to non-human target antigens and, therefore, difficult to identify potential adverse events in humans and often offer a false sense of safety.
Rodent models
Before testing new therapeutic approaches in human patients for clinical trial purposes, safety and efficacy are usually assessed pre-clinically in animal models such as mice, zebrafish, among others. Rodent models have been critical for understanding pathways, identifying tumor-target antigens, and understanding the tumor physiology and the microenvironment (63). However, despite rodent models' role in preclinical trials, which led to numerous breakthroughs in modern medicine, it has a number of limitations. For example, among drugs that showed strong efficacy and inhibited tumor growth in mice, only 11% are approved for human use by FDA. Furthermore, side effects seen in humans were not observed in mice (64).
Also, rodent models do not appropriately portray the complex microenvironment relationship between the immune cells and tumor cells (65). These animals do not develop spontaneous tumors. Their living condition, which is pathogen-free, impacts their immune system flora (64). Thus, rodents do not produce ‘normal’ immune cell lines found in humans or animals exposed to natural environments, resulting in the same immune milieu between them and identical gene sequence composition. Therefore, studies using animals with none functioning immune systems have limited translational impact. In the case of toxicities involving immune system signaling, brain swelling after CAR T cells therapy is not detectable in studies using immunodeficient mice. All these mentioned factors make rodent models less trustworthy and raise questions regarding whether their contribution is sufficient to use them as preclinical models.
Non-human primate model
Of all the animal models mentioned, the one that more accurately resembles the human genetic composition are the non-human primates. Although similar, these models are not adequate for comparative studies since they experience low spontaneous cancer rates (64), high maintenance, and ethical regulation surrounding these models. Taraseviciute et al studied how neurotoxicities can affect the non-primate model, rhesus macaque, after transferring autologous CD20-specific CAR T cells. The group demonstrated that CD20 CAR and non-CAR T cells infiltrate and accumulate in the cerebrospinal fluid (CSF) and brain parenchyma, causing high levels of proinflammatory cytokines in the CSF and pan-encephalitis (66).
Canine model
Unlike the rodent models, dogs develop spontaneous tumors that resemble human disease in morphology, molecular aspects, and genetic behavior (67). Also, dogs have intact immune systems with considerable similarities to humans' immune milieu because dogs and humans cohabitate in the same household, therefore, sharing the same environmental risk factors (64). Furthermore, the genetic diversity displayed by different dog breeds provides an ideal tool that enriches the preclinical studies by providing similar challenges seen in humans' studies from different ethnic groups. Also, cancer is the number one cause of death in dogs (63). All hematological malignancies and solid tumors in dogs are similar to human diseases. These included mammary tumors (breast), osteosarcoma, prostate, bladder cancer, and leukemia.
Canine mammary tumors
Studies revealed that spontaneous invasive mammary carcinomas are closely similar in pathology, epidemiology, and immunohistochemical characterization with human breast cancers (68). Commonly overexpressed estrogen and progesterone hormone receptors, the conglomeration of similar tumor-infiltrating lymphocyte ratios, and homologous cancer risk factors such as obesity and age are similar between humans and canines' tumors (64,69) Clinical outcomes after tumor progression are closely related to these two species. Furthermore, molecular markers such as the nuclear protein Ki-67, the p53 tumor suppressor gene, and the BCRA genes provide valuable information regarding both species' prognosis status (70). Clinical trials using canine CAR T therapy in canine mammary tumors are not initiated yet. However, CAR T cell therapies' benefits in humans breast cancer have been explored over the last years. The following trials are ongoing and centered on improving the safe dose and uncovering the different effects (good and bad). Phase I trials are ongoing targeting HER2+ breast cancer (NCT04650451 and NCT03740256) in patients with advanced-stage III (NCT04650451) or metastatic (stage IV) (NCT04650451 and NCT03740256) cancer with no other treatment option available using BPX-603 and HER2 specific CAR T cells, respectively. City of Hope Medical Center conducted a trial using HER2 specific CAR T cells targeting HER2+ breast cancer cells (NCT03696030) in patients with brain or leptomeningeal metastases. Two trials (NCT02414269 and NCT02792114) at the Memorial Sloan Kettering Cancer Center are ongoing targeting Mesothelin in patients with metastatic (stage IV) breast cancer that spread to the pleura (iCasp9M28z CAR T-cells-Phase I/II) and HER2-cells (Mesothelin CAR T cells-Phase I), respectively. Tmunity Therapeutics using CART-TnMUC1 (NCT04025216) in patients with triple-negative and ER-low, HER2-breast cancer with TnMUC1 positive cells. Minerva Biotechnology Corporation conducts a trial targeting MUC1* (NCT04020575) utilizing huMNC2-CAR44 CAR T cells in patients with metastatic (stage IV) breast cancer. Fred Hutchinson Cancer Research Center conducts a phase I trial on triple-negative and ER-low breast cancer (NCT02706392), targeting ROR1 positive cells. Lastly, patients that received a minimum of two therapies for advanced cancer expressing GD2 antigen are carried on by Baylor's College of Medicine (NCT03635632) using a C7R-GD2 CAR T cell.
Canine osteosarcoma
Canine develops osteosarcoma (OSA) at a much higher rate than humans (71), serving as a remarkable model for developing treatments and overcoming the numerous challenges in solid tumor therapies. There are a number of similarities between the canine and humans concerning this disease. The tumor location, the pattern of metastasis, genetic drivers of the disease, and response to therapy are similar in both species. Canine OSA is a spontaneous, naturally occurring disease as in humans. Canine OSA has aggressive biology and an increased rate of metastasis, and the animal often dies within six months, and almost 96% of dogs with OSA perish from the disease. Canine trials or in-vitro experiments related to osteosarcoma are scarce in the literature. Mata et al (65) developed a CAR-T cell targeting HER2 overexpressing tumor cells in-vitro. Canine and human-derived transmembrane and signaling domains were tested on tumor cells, demonstrating little to no difference in tumor suppression (65) On the other hand, Baylor College of Medicine is conducting a Phase I clinical trial (NCT03635632) in human patients with relapsed or refractory osteosarcoma with increased expression of GD2 antigen utilizing C7R-GD2 CAR-T cells. The National Cancer Institute (NCI) has completed a Phase I clinical targeting GD2 positive solid tumors with anti-GD2 CART cells in children and young adults that suffer osteosarcoma (NCT02107963), no final data has been posted yet.
Canine prostate cancer
Canines are a few animal models that develop spontaneous prostate cancer as humans (72,73). Both dogs and humans share similar risk factors, including advanced age, low mortality rates, clinical outcomes, and prostate gland functionality, suggesting that these animals may be ideal models for future clinical trials (72-74). Unfortunately, a lack of prostate cancer screening in canine augments the malignancy's mortality rate and aggressiveness, thus not allowing proper treatment strategies (74,75). On the other hand, human screening methods have strengthened over the last few years, enabling rapid diagnosis (76). ACT therapy for prostate cancer has been developed mainly in humans. CAR-T cells against TCRγ chain alternative reading frame protein (TARP), prostate stem cell antigen (PSCA), and prostate-specific membrane antigen (PSMA) were developed and used to suppress tumor growth in vitro (77-80). Phase I clinical trials are currently conducted in patients with castrate-resistance prostate cancer targeting PSMA with doses of CART-PSMA-TGFβRDN, LIGHT-PSMA CART P-PSMA-101 CART cells (NCT03089203, NCT04053062, and NCT04249947). The City of Hope Medical Center conducted another trial against metastatic castration-resistance prostate cancer, targeting the PSCA antigen's overexpression with anti-PSCA-4-1BB/TCRζ-CD19 CART cells (NCT03873805). Phase I/II clinical trial (NCT02744287), sponsored by Bellicium Pharmaceuticals, PSCA-CART (BPX-601), is currently used to treat patients with previously treated advanced tumors, including metastatic prostate and metastatic castrate-resistance prostate cancer. Finally, the First Affiliated Hospital of Chengdu Medical College targeted EpCAM positive prostate cancer with an EpCAM-specific CART cell (NCT03013712), a second-generation CAR (CD28/CD3ζ) targeting PSMA.
Canine bladder cancer
Invasive Urinary bladder cancer (InvUC), Invasive transitional cell carcinoma (TCC), and invasive urothelial carcinoma (UC) are three different subtypes of bladder cancer spontaneously developed in canines that resemble ‘humans’ malignancies (79,81,82). Similarities in clinical outcomes, histological features, and progression sites make canines straightforward compared to humans (79). Canine trials or CAR-T generations are not seen in literature, but human clinical trials are currently under investigation. A Phase I/II clinical trial, conducted by Shenzen Geno-Immune Medical Institute (NCT03185468), is currently evaluating the safety and efficacy of a 4SCART-PSMA CART cell against PSMA-expressing bladder cancer.
Canine leukemias
As mentioned above, preclinical trials driven with canine models could represent an enormous step in adoptive T cell therapy development. Unfortunately, preclinical trials using canine models are scarce in the scientific literature. The few clinical trials available are primarily performed in B cell lymphomas. Panjwani conducted a trial in patients with B cell lymphomas, targeting the CD20 antigen. The study concluded the need for stable CAR T cell expression and that further studies must be performed (83). Nonetheless, the second trial showed stable CAR T transduction using lentiviral vectors (84). Their CD20-BB-ζ CAR T cell, alongside cytokines IL7 and IL5, proved to be durable and antigen-specific against DLBCL. Non-Hodgkin's Lymphoma (NHL) is the most common cancer in dogs, and the most common sub-type is Diffuse Large B-Cell Lymphoma (DLBCL). While combinations of chemotherapy agents lead to clinical remission in ~75% of dogs, most dogs relapse within six to nine months of standard treatment, a statistic that has remained unchanged for the past 30 years. An urgent need exists for new therapies for canine lymphoma. Furthermore, evaluating these new therapies in pet dogs with naturally occurring cancer may also provide vital information to advance novel therapies for individuals.
9. Conclusions
The remarkable progress that adoptive immunotherapy has experienced these past years, especially in blood-related cancers, provides optimism for CARs therapy. Trials of CAR T in leukemia and lymphomas had shown positive outcomes, with some cases experiencing mild side effects. Notwithstanding, trials conducted in solid tumors represent a daunting task to achieve. Tumor microenvironment, CARs tracking and duration, and the various toxicities experienced by a number of patients represent significant setbacks that need addressing. The animal model that faithfully resembles humans is another milestone in this endeavor. Up to date, all preclinical studies of CAR T safety and efficacy are conducted in mice, including syngeneic, transgenic, and xenograft, and humanized mouse models to represent humans' immune responses and diseases to test the safety and efficacy of CART therapy. However, these models fell short in representing the disease and its adverse effect. The dog's importance is recently recognized as a preclinical model for cancer CAR T therapy because of its human physiology, immune responses, and disease similarities. The development of reagents and the use of the dogs in clinical trials will help advance the CAR T therapy field for both species.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SIM conceived and outlined the review. XERC, WL and SIM wrote the manuscript and edited it. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Wang Z, Guo Y, Han W. Current status and perspectives of chimeric antigen receptor modified T cells for cancer treatment. Protein Cell. 2017;8:896–925. doi: 10.1007/s13238-017-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilones KA, Aryankalayil J, Demaria S. Invariant NKT cells as novel targets for immunotherapy in solid tumors. Clin Dev Immunol. 2012;2012(720803) doi: 10.1155/2012/720803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Niu C, Cui J. Gamma-delta (γδ) T cells: Friend or foe in cancer development? J Transl Med. 2018;16(3) doi: 10.1186/s12967-017-1378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chmielewski M, Abken H. TRUCKs: The fourth generation of CARs. Expert Opin Biol Ther. 2015;15:1145–1154. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 6.Izsvák Z, Hackett PB, Cooper LJN, Ivics Z. Translating sleeping beauty transposition into cellular therapies: Victories and challenges. Bioessays. 2010;32:756–767. doi: 10.1002/bies.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng PP, Kros JM, Li J. Approved CAR T cell therapies: Ice bucket challenges on glaring safety risks and long-term impacts. Drug Discov Today. 2018;23:1175–1182. doi: 10.1016/j.drudis.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, Jäger U, Jaglowski S, Andreadis C, Westin JR, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 11.Mullard A. FDA approves fourth CAR-T cell therapy. Nat Rev Drug Discov. 2021;20(166) doi: 10.1038/d41573-021-00031-9. [DOI] [PubMed] [Google Scholar]
- 12.Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S. Teaching an old dog new tricks: Next-generation CAR T cells. Br J Cancer. 2019;120:26–37. doi: 10.1038/s41416-018-0325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charrot S, Hallam S. CAR-T Cells: Future perspectives. Hemasphere. 2019;3(e188) doi: 10.1097/HS9.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, Nagase F, Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 15.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11:855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharpe AH, Abbas AK. T-cell costimulation-biology, therapeutic potential, and challenges. N Engl J Med. 2006;355:973–975. doi: 10.1056/NEJMp068087. [DOI] [PubMed] [Google Scholar]
- 18.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5(22) doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fesnak AD, June CH, Levine BL. Engineered T cells: The promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: Chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev. 2014;257:83–90. doi: 10.1111/imr.12125. [DOI] [PubMed] [Google Scholar]
- 21.Pegram HJ, Lee JC, Hayman EG, Imperato GH, Tedder TF, Sadelain M, Brentjens RJ. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood. 2012;119:4133–4141. doi: 10.1182/blood-2011-12-400044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter BD, Jacobson CA. CAR T-Cell associated neurotoxicity: Mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019;111:646–654. doi: 10.1093/jnci/djz017. [DOI] [PubMed] [Google Scholar]
- 23.Sun S, Hao H, Yang G, Zhang Y, Fu Y. Immunotherapy with CAR-Modified T Cells: Toxicities and overcoming strategies. J Immunol Res. 2018;2018(2386187) doi: 10.1155/2018/2386187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yáñez L, Sánchez-Escamilla M, Perales MA. CAR T cell toxicity: Current management and future directions. Hemasphere. 2019;3(e186) doi: 10.1097/HS9.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fucà G, Reppel L, Landoni E, Savoldo B, Dotti G. Enhancing chimeric antigen receptor T-cell efficacy in solid tumors. Clin Cancer Res. 2020;26:2444–2451. doi: 10.1158/1078-0432.CCR-19-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, Ye Z, Qian Q. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15:2548–2560. doi: 10.7150/ijbs.34213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davila ML, Brentjens RJ. CD19-Targeted CAR T cells as novel cancer immunotherapy for relapsed or refractory B-cell acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2016;14:802–808. [PMC free article] [PubMed] [Google Scholar]
- 28.Dotti G. The other face of chimeric antigen receptors. Mol Ther. 2014;22:899–900. doi: 10.1038/mt.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hombach A, Hombach AA, Abken H. Adoptive immunotherapy with genetically engineered T cells: Modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Ther. 2010;17:1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 30.Lee YG, Marks I, Srinivasarao M, Kanduluru AK, Mahalingam SM, Liu X, Chu H, Low PS. Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors. Cancer Res. 2019;79:387–396. doi: 10.1158/0008-5472.CAN-18-1834. [DOI] [PubMed] [Google Scholar]
- 31.Darowski D, Kobold S, Jost C, Klein C. Combining the best of two worlds: Highly flexible chimeric antigen receptor adaptor molecules (CAR-adaptors) for the recruitment of chimeric antigen receptor T cells. mAbs. 2019;11:621–631. doi: 10.1080/19420862.2019.1596511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arndt C, Fasslrinner F, Loureiro LR, Koristka S, Feldmann A, Bachmann M. Adaptor CAR platforms-next generation of T Cell-based cancer immunotherapy. Cancers (Basel) 2020;12(1302) doi: 10.3390/cancers12051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai H, Wu Z, Jia H, Tong C, Guo Y, Ti D, Han X, Liu Y, Zhang W, Wang C, et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(30) doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen N, Morello A, Tano Z, Adusumilli PS. CAR T-cell intrinsic PD-1 checkpoint blockade: A two-in-one approach for solid tumor immunotherapy. Oncoimmunology. 2016;6(e1273302) doi: 10.1080/2162402X.2016.1273302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kloss CC, Lee J, Zhang A, Chen F, Melenhorst JJ, Lacey SF, Maus MV, Fraietta JA, Zhao Y, June CH. Dominant-Negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther. 2018;26:1855–1866. doi: 10.1016/j.ymthe.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Makita S, Yoshimura K, Tobinai K. Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci. 2017;108:1109–1118. doi: 10.1111/cas.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reagan PM, Friedberg JW. Axicabtagene ciloleucel and brexucabtagene autoleucel in relapsed and refractory diffuse large B-cell and mantle cell lymphomas. Future Oncol. 2021;17:1269–1283. doi: 10.2217/fon-2020-0291. [DOI] [PubMed] [Google Scholar]
- 39.FDA approves second CAR T-cell therapy. Cancer Discov. 2018;8:5–6. doi: 10.1158/2159-8290.CD-NB2017-155. [DOI] [PubMed] [Google Scholar]
- 40.Mullard A. FDA approves first BCMA-targeted CAR-T cell therapy. Nat Rev Drug Discov. 2021;20(332) doi: 10.1038/d41573-021-00063-1. [DOI] [PubMed] [Google Scholar]
- 41.Novak AJ, Darce JR, Arendt BK, Harder B, Henderson K, Kindsvogel W, Gross JA, Greipp PR, Jelinek DF. Expression of BCMA, TACI, and BAFF-R in multiple myeloma: A mechanism for growth and survival. Blood. 2004;103:689–694. doi: 10.1182/blood-2003-06-2043. [DOI] [PubMed] [Google Scholar]
- 42.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guedan S, Ruella M, June CH. Emerging cellular therapies for cancer. Annu Rev Immunol. 2019;37:145–171. doi: 10.1146/annurev-immunol-042718-041407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016;375:2561–2569. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118:6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vignali D, Kallikourdis M. Improving homing in T cell therapy. Cytokine Growth Factor Rev. 2017;36:107–116. doi: 10.1016/j.cytogfr.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 47.Martinez M, Moon EK. CAR T cells for solid tumors: New strategies for finding, infiltrating, and surviving in the tumor microenvironment. Front Immunol. 2019;10(128) doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richman SA, Nunez-Cruz S, Moghimi B, Li LZ, Gershenson ZT, Mourelatos Z, Barrett DM, Grupp SA, Milone MC. High-Affinity GD2-Specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res. 2018;6:36–46. doi: 10.1158/2326-6066.CIR-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6 (Suppl 10):S13–S18. [PMC free article] [PubMed] [Google Scholar]
- 51.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. Eur J Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39:783–803. doi: 10.1007/s10555-020-09909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hammarström S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 54.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 55.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berahovich R, Liu X, Zhou H, Tsadik E, Xu S, Golubovskaya V, Wu L. Hypoxia selectively impairs CAR-T cells in vitro. Cancers (Basel) 2019;11(602) doi: 10.3390/cancers11050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliver AJ, Lau PKH, Unsworth AS, Loi S, Darcy PK, Kershaw MH, Slaney CY. Tissue-Dependent tumor microenvironments and their impact on immunotherapy responses. Front Immunol. 2018;9(70) doi: 10.3389/fimmu.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newick K, O'Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 59.Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo Y, Feng K, Liu Y, Wu Z, Dai H, Yang Q, Wang Y, Jia H, Han W. Phase I study of chimeric antigen receptor modified T cells in patients with EGFR-positive advanced biliary tract cancers. Clin Cancer Res. 2018;24:1277–1286. doi: 10.1158/1078-0432.CCR-17-0432. [DOI] [PubMed] [Google Scholar]
- 61.Lee JH, Lee SW. The roles of carcinoembryonic antigen in liver metastasis and therapeutic approaches. Gastroenterol Res Pract. 2017;2017(7521987) doi: 10.1155/2017/7521987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katz SC, Burga RA, McCormack E, Wang LJ, Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al. Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA+ liver metastases. Clin Cancer Res. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nat Rev Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
- 64.Park JS, Withers SS, Modiano JF, Kent MS, Chen M, Luna JI, Culp WTN, Sparger EE, Rebhun RB, Monjazeb AM, et al. Canine cancer immunotherapy studies: Linking mouse and human. J Immunother Cancer. 2016;4(97) doi: 10.1186/s40425-016-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mata M, Vera JF, Gerken C, Rooney CM, Miller T, Pfent C, Wang LL, Wilson-Robles HM, Gottschalk S. Toward immunotherapy with redirected T cells in a large animal model: Ex vivo activation, expansion, and genetic modification of canine T cells. J Immunother. 2014;37:407–415. doi: 10.1097/CJI.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taraseviciute A, Tkachev V, Ponce R, Turtle CJ, Snyder JM, Liggitt HD, Myerson D, Gonzalez-Cuyar L, Baldessari A, English C, et al. Chimeric antigen receptor T cell-mediated neurotoxicity in nonhuman primates. Cancer Discov. 2018;8:750–763. doi: 10.1158/2159-8290.CD-17-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Steenbeek FG, Hytönen MK, Leegwater PA, Lohi H. The canine era: The rise of a biomedical model. Anim Genet. 2016;47:519–527. doi: 10.1111/age.12460. [DOI] [PubMed] [Google Scholar]
- 68.Abadie J, Nguyen F, Loussouarn D, Pena L, Gama A, Rieder N, Belousov A, Bemelmans I, Jaillardon L, Ibisch C, Campone M. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res Treat. 2018;167:459–468. doi: 10.1007/s10549-017-4542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Queiroga FL, Raposo T, Carvalho MI, Prada J, Pires I. Canine mammary tumours as a model to study human breast cancer: Most recent findings. In Vivo. 2011;25:455–465. [PubMed] [Google Scholar]
- 70.Buishand FO, Kik M, Kirpensteijn J. Evaluation of clinico-pathological criteria and the Ki67 index as prognostic indicators in canine insulinoma. Vet J. 2010;185:62–67. doi: 10.1016/j.tvjl.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 71.Siobhan S, Dunning MD, de Brot S, Grau-Roma L, Mongan NP, Rutland CS. Comparative review of human and canine osteosarcoma: Morphology, epidemiology, prognosis, treatment and genetics. Acta Vet Scand. 2017;59(71) doi: 10.1186/s13028-017-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leroy BE, Northrup N. Prostate cancer in dogs: Comparative and clinical aspects. Vet J. 2009;180:149–162. doi: 10.1016/j.tvjl.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Sun F, Báez-Díaz C, Sánchez-Margallo FM. Canine prostate models in preclinical studies of minimally invasive interventions: Part I, canine prostate anatomy and prostate cancer models. Transl Androl Urol. 2017;6:538–546. doi: 10.21037/tau.2017.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McEntee M, Isaacs W, Smith C. Adenocarcinoma of the canine prostate: Immunohistochemical examination for secretory antigens. Prostate. 1987;11:163–170. doi: 10.1002/pros.2990110207. [DOI] [PubMed] [Google Scholar]
- 75.Sorenmo KU, Goldschmidt M, Shofer F, Goldkamp C, Ferracone J. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Vet Comp Oncol. 2003;1:48–56. doi: 10.1046/j.1476-5829.2003.00007.x. [DOI] [PubMed] [Google Scholar]
- 76.Yu H, Pan J, Guo Z, Yang C, Mao L. CART cell therapy for prostate cancer: Status and promise. Onco Targets Ther. 2019;12:391–395. doi: 10.2147/OTT.S185556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hillerdal V, Nilsson B, Carlsson B, Eriksson F, Essand M. T cells engineered with a T cell receptor against the prostate antigen TARP specifically kill HLA-A2+ prostate and breast cancer cells. Proc Natl Acad Sci USA. 2012;109:15877–15881. doi: 10.1073/pnas.1209042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morgenroth A, Cartellieri M, Schmitz M, Günes S, Weigle B, Bachmann M, Abken H, Rieber EP, Temme A. Targeting of tumor cells expressing the prostate stem cell antigen (PSCA) using genetically engineered T-cells. Prostate. 2007;67:1121–1131. doi: 10.1002/pros.20608. [DOI] [PubMed] [Google Scholar]
- 79.Fulkerson CM, Dhawan D, Ratliff TL, Hahn NM, Knapp DW. Naturally occurring canine invasive urinary bladder cancer: A complementary animal model to improve the success rate in human clinical trials of new cancer drugs. Int J Genomics. 2017;2017(6589529) doi: 10.1155/2017/6589529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma Q, Gomes EM, Lo AS, Junghans RP. Advanced generation anti-prostate specific membrane antigen designer T cells for prostate cancer immunotherapy. Prostate. 2014;74:286–296. doi: 10.1002/pros.22749. [DOI] [PubMed] [Google Scholar]
- 81.Knapp DW, Ramos-Vara JA, Moore GE, Dhawan D, Bonney PL, Young KE. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55:100–118. doi: 10.1093/ilar/ilu018. [DOI] [PubMed] [Google Scholar]
- 82.Dhawan D, Paoloni M, Shukradas S, Choudhury DR, Craig B, Ramos-Vara JA, Hahn N, Bonney PL, Khanna C, Knapp DW. Comparative gene expression analyses identify luminal and basal subtypes of canine invasive urothelial carcinoma that mimic patterns in human invasive bladder cancer. PLoS One. 2015;10(e0136688) doi: 10.1371/journal.pone.0136688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Panjwani MK, Smith JB, Schutsky K, Gnanandarajah J, O'Connor CM, Powell DJ Jr, Mason NJ. Feasibility and safety of RNA-transfected CD20-specific chimeric antigen receptor T cells in dogs with spontaneous B cell lymphoma. Mol Ther. 2016;24:1602–1614. doi: 10.1038/mt.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panjwani MK, Atherton MJ, MaloneyHuss MA, Haran KP, Xiong A, Gupta M, Kulikovsaya I, Lacey SF, Mason NJ. Establishing a model system for evaluating CAR T cell therapy using dogs with spontaneous diffuse large B cell lymphoma. OncoImmunology. 2019;9(1676615) doi: 10.1080/2162402X.2019.1676615. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.