Abstract
We present a case report of a fetal diagnosis of cystic fibrosis after ultrasound abnormalities. After delivery, a type 3A intestinal atresia was diagnosed. Segmental enterectomy with end‐to‐end anastomosis was performed. This case report highlights the diagnosis complexity of a fetal intestinal atresia associated with cystic fibrosis.
Keywords: cystic fibrosis, intestinal atresia, meconium peritonitis, prenatal diagnosis
We document and association between intestinal atresia and CF. In a setting of CF and absence of intestinal passage in the first days of life should have a low threshold to conduct a differential diagnosis with intestinal atresia.
1. INTRODUCTION
Cystic fibrosis is one of the most severe chronic disease affecting multiple organ systems with autosomal recessive transmission. The estimated incidence is 1:3000 in Caucasian individuals and is associated with a carrier frequency of 1/25–1/35. 1 , 2 , 3
It is caused by pathogenic mutations in a gene on chromosome 7, specifically on the gene encoding the CF transmembrane conductance regulator, or CFTR, which functions as a chloride channel on the apical membrane of epithelial cells. 4 The mutation presents variations between ethnic and geographic groups and shows phenotypic heterogeneity. 2 , 3 , 4 , 5
CF is responsible for chronic pulmonary disease as result of systematic obstruction and infection, as well as exocrine pancreatic insufficiency (diarrhea and malnutrition), loss of salt, and obstructive azoospermia syndrome. 4 According to the Cystic Fibrosis Foundation 2018 Registry Report, the average life expectancy is 47,4 years for those born in 2018. 6 CF also has a considerable impact in the public health systems; consequently, prevention from disease through carrier identification, genetic counseling, and prenatal diagnosis remains the most realistic approach to reduce its burden. 7
Prenatal diagnosis is recommended to the couples known to be CFTR mutation carriers and in cases of ultrasound fetal anomalies such as meconium peritonitis, hyperechogenic and/or bowel dilatation, intraabdominal calcifications and meconium ileus (MI). 7 Intestinal affection is usually the first manifestation, sometimes in the prenatal period.
Bowel meconial obstruction leads to meconium ileus that is present in 10–20 percent of newborns with CF. Conversely, 80–90 percent of infants with meconium ileus have CF. 8 Meconium ileus is the most common cause of meconial peritonitis in fetuses with CF. 9
In this clinical case, we present a case of prenatal diagnosis of CF associated with intestinal atresia.
2. CASE DESCRIPTION
We report a case of a thirty‐two‐year‐old caucasian woman in her fourth pregnancy, first pregnancy with the current male partner, non‐consanguine couple. The previous pregnancies were uneventful. She had no relevant medical history. Gestational diabetes was diagnosed in the first trimester. First and second trimester ultrasound were normal.
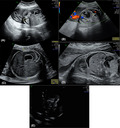
An ultrasound performed at 28 weeks identified intestinal dilation of 20 mm, echogenic bowel, linear calcifications throughout the peritoneum and ascites suggestive of meconium peritonitis (Figure 1A–E). Estimated fetal weight (EFW) was in the 80th percentile for the gestational age. Although these morphological alterations were isolated and the fetal estimated weight (EFW) adequate, genetic evaluation has become pertinent in order to exclude genetic diseases, as CF. Amniocentesis was performed and genetic panel associated with CF analyzed. The study was based in polymerase chain reaction (PCR) gene amplification and fragment analysis using the CF‐EU2V1 kit (Elucigene Diagnostics®). The following CFTR gene mutations were studied: CFTRdele2,3, E60X, P67L, G85E, 394delTT, 444delA, R117C, R117H, Y122X, 621+1G>T, 711+1G>T, L206W, 1078delT, R334W, R347P, R347H, A455E, I507del, F508del, 1677delTA, V520F, 1717‐1G>A, G542X, S549R(T>G), S549N, G551D, R553X, R560T, 1811+1.6kbA>G, 1898+1G>A, 2143delT, 2184delA, 2347delG, W846X, 2789+5G>A, Q890X, 3120+1G>A, 3272‐26A>G, R1066C, Y1092X(C>A), M1101K, D1152H, R1158X, R1162X, 3659delC, 3849+10kbC>T, S1251N, 3905insT, W1282X, and N1303K. This panel does not exclude all existing mutations; however, it corresponds to 81.6% of detectable mutations in the Portuguese population. 10 Pathogenic variants c.1521_1523del p.(Phe508del) and c.1624G>T p.(Gly542*) were identified in heterozygosity in the CFTR gene, what allowed to diagnose CF.
FIGURE 1.

28th week ultrasound with intraabdominal image (A) intestinal dilatation without vascular structures; (B) image showing abdominal circumference with visible stomach, without evidence of double bubble sign; (C) Subdiaphragmatic ascites; (D) Hyperechogenic bowel
Ultrasound evaluation at 31 weeks fetal growth was in 53rd percentile and previous anomalies remained as previously described. Due to poor glycemic control, oral metformin was instituted at 31 weeks of gestation.
At 32nd week, the patient was referred to a level IV maternal care obstetric department. 11 Ultrasound features remained stable during the evaluations—intestinal dilatation with a larger diameter of 20 mm with a wall thickness of 2.4 mm (Figure 2A–D). No other morphological findings were objectified.
FIGURE 2.

Ultrasound at 32 weeks of gestation. (A) Intestinal dilatation with increased wall thickness; (B) maximum dilatation diameter; (C) intestinal dilatation; (D) intestinal dilatation at sagittal view
At 36th week, a fetal growth crossed centiles (> 2 quartiles on growth centiles) and cerebroplacental ratio below 5th centile in doppler flowmetry and elective pregnancy termination was decided. A cesarean section was performed at 37 weeks due to persistent breech fetal presentation. A female newborn was delivered with a birthweight of 2500 g and 8/10/10 Apgar score admitted to neonatal intensive care unit.
Placenta anatomopathological examination revealed maternal vascular malperfusion with an infarction hematoma.
On the second day of life, the newborn presented absence of intestinal transit and started persistent vomiting and hematemesis. The abdomen was distended and bloated. Abdominal ultrasound revealed marked dilation of the intestinal loops with endoluminal fluid measuring 25 mm in diameter. Extraluminal material compatible with meconium peritonitis was described. In the third day of life, the feces were colorless and with mucus and the newborn kept the vomiting and the bloated abdomen. N‐acetylcysteine was administered, and pancreatic enzyme supplementation was initiated. The contrast radiography revealed a microcolon with a concentration of contrast solution in the distal ileum, without air‐fluid levels. The newborn was kept on a nil per os, and nasogastric tube was placed. Spontaneous gastrointestinal function absence until 7th day of life and maintenance of obstruction in X‐ray lead to the decision of surgical approach. Type 3A intestinal atresia, disproportion of the size of the intestinal loops and adherence of the distal segment to the abdominal wall were found. Segmental enterectomy with end‐to‐end anastomosis was performed after a copious wash of intestinal loops. The procedure resulted in a 95 cm small intestine with a consecutive a small bowel syndrome. No anastomosis dehiscence occurred in postoperative period. Newborn showed a slow progression to normal gastrointestinal tract function.
3. DISCUSSION
The clinical manifestation of CF in this case were intestinal dilation, hyperechogenic bowel, linear calcifications throughout the peritoneum and ascites suggestive of intrauterine intestinal rupture and subsequent meconium peritonitis. Hyperechogenic bowel occurs in 3%–8% of fetuses with CF but can be identified in 0.2%–1.8% of all fetuses from the 2nd trimester onwards (1, 3). CF prenatal presentation included also meconium ileus with intestinal dilatation. 2 , 5 Other reported findings, not present in our case, are intrabdominal calcifications and persistent no visualization of the fetal gallbladder. 2 , 5
Due to the clinical suspicion of cystic fibrosis, a directed genetic study to CF was performed. The confirmation of diagnosis allowed to offer optimized health care, namely delivery programmed in a level IV of maternal care center.
MI was not observed previously to 28th week pregnancy ultrasound, however, is reported as first manifestation of CF in neonatal period. Nevertheless, in 10%–15% of cases, other situations could occur and should be a part of differential diagnosis as prematurity, intestinal atresia, and pancreatic disorders. 12 After rupture, meconial peritonitis resulting from MI and the jejunoileal atresia/stenosis resulted in ultrasound findings of linear calcifications throughout the peritoneum and ascites. Jejunoileal atresia/stenosis as MI are among the main causes of neonatal intestinal obstruction and should be part of different diagnosis workup. In our clinical case, both causes were present, in a fetus with CF diagnosis.
Intestinal atresia manifested with typical symptoms in the postnatal period. Features of neonatal intestinal obstruction include abdominal distention, vomiting, and failure to pass meconium within the first 48 h of life, 12 being the proximal jejunum or distal ileum are the most common levels of obstruction (6). Some studies described an association of CF and jejunal or ileal atresia of 8%. 13 For this reason, testing and counseling for CF, despite not being the most common cause of atresia, should be considered (6). Also, in the presence of MI described in intestinal atresia, the diagnosis suspicion increases and CF can be implicated.
Post‐natal conduction can be guided by prenatal findings. In fact, the severity of MI will establish the urge for surgical intervention. The majority of infants present with failure to pass meconium abdominal distension, and feeding intolerance in the first day of life. 12 When intraabdominal calcifications are the only manifestation, conservative management is a possibility; however, when associated with other complications, the risk of intervention demand increases. 2 Bowel obstruction on ultrasound is also a surgery predictor. 1
When the obstruction is associated with CF or multiple atresia, the outcome can be poor. Our clinical case presents an association of two clinical conditions that could predict a worse prognosis.
This possible confounding factor may delay the diagnosis of an intestinal atresia requiring surgical treatment in newborns with CF. Moreover, the timely diagnosis of this complication is essential for an appropriate surgical management in the postnatal period.
4. CONCLUSION
The association between CF and intestinal atresia is documented and highlighted in our clinical case. The absence of intestinal passage in this setting with suspected bowel obstruction should promote an image and differential diagnostic workup. Therefore, surgical approach can be conducted to minimize postnatal morbidity associated with small intestinal resection and poor weight progression. We highlight the importance of recalling jejunoileal atresia as an associated diagnosis in newborns diagnosed with CF in prenatal period.
AUTHOR CONTRIBUTIONS
Sara Cunha was responsible for the conception and drafting of the work. Sara Cunha, Cátia Rasteiro, Manuela Silva, and Célia Araújo were responsible for the acquisition, analysis, and interpretation of information contained within the manuscript. Sara Cunha, Cátia Rasteiro, Mariana Dias, and Carla Ramalho contributed for the design of the work, to revise and to approve the final manuscript. All authors approved the final version to be published and ensured that aspects related to the integrity/accuracy are accomplished.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICAL APPROVAL
Ethics committee approved the final manuscript before publication.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
ACKNOWLEDGMENT
None.
Cunha SB, Rasteiro C, Silva M, Dias M, Araújo C, Ramalho C. Cystic fibrosis and jejunoileal atresia: A clinical case. Clin Case Rep. 2022;10:e05869. doi: 10.1002/ccr3.5869
Funding information
No funding
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Muller F, Simon‐Bouy B, Girodon E, Monnier N, Malinge MC, Serre JL. Predicting the risk of cystic fibrosis with abnormal ultrasound signs of fetal bowel: results of a French molecular collaborative study based on 641 prospective cases. Am J Med Genet. 2002;110(2):109‐115. [DOI] [PubMed] [Google Scholar]
- 2. Peter Callen MN, Scoutt L, Feldstein V. Ultrasonography in Obstetrics and Gynecology, 6th ed. Sauders Elsevier; 2008. [Google Scholar]
- 3. Carlyle BE, Borowitz DS, Glick PL. A review of pathophysiology and management of fetuses and neonates with meconium ileus for the pediatric surgeon. J Pediatr Surg. 2012;47(4):772‐781. [DOI] [PubMed] [Google Scholar]
- 4. del Ciampo IR, Oliveira TQ, del Ciampo LA, et al. Early manifestations of cystic fibrosis in a premature patient with complex meconium ileus at birth. Rev Paul Pediatr. 2015;33(2):241‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Diana W, Bianchi TMC, D'Alton ME, Malone FD. Fetology: Diagnosis And Management Of The Fetal Patient. 2nd ed. [Google Scholar]
- 6. Foundation CF . Patient Registry: Annual Data Report. 2018. [Google Scholar]
- 7. Hadj Fredj S, Ouali F, Siala H, et al. Prenatal diagnosis of cystic fibrosis: 10‐years experience. Pathol Biol (Paris). 2015;63(3):126‐129. [DOI] [PubMed] [Google Scholar]
- 8. Gorter RR, Karimi A, Sleeboom C, Kneepkens CM, Heij HA. Clinical and genetic characteristics of meconium ileus in newborns with and without cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;50(5):569‐572. [DOI] [PubMed] [Google Scholar]
- 9. Chandra R, Kesavan A. Current treatment paradigms in pediatric short bowel syndrome. Clin J Gastroenterol. 2018;11(2):103‐112. [DOI] [PubMed] [Google Scholar]
- 10. Programme WHOHG . The Molecular Genetic Epidemiology of Cystic Fibrosis : Report of a Joint Meeting of WHO/IECFTN/ICF(M)A/ECFS. World Health Organization; 2004. [Google Scholar]
- 11. Levels of Maternal Care . Obstetric care consensus no, 9. Obstet Gynecol. 2019;134(2):e41‐e55. [DOI] [PubMed] [Google Scholar]
- 12. Lahiri T, Sullivan JS, Sartorelli KH, Murphy JJ. Delayed presentation of meconium ileus in an infant with cystic fibrosis. Pediatrics. 2020;146(4):e20193717. [DOI] [PubMed] [Google Scholar]
- 13. Siersma CL, Rottier BL, Hulscher JB, Bouman K, van Stuijvenberg M. Jejunoileal atresia and cystic fibrosis: don't miss it. BMC Res Notes. 2012;5:677‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


