Abstract
Myxococcus xanthus fibrils are cell surface-associated structures composed of roughly equal amounts of polysaccharide and protein. The level of M. xanthus polysaccharide production under different conditions in the wild type and in several mutants known to have alterations in fibril production was investigated. Wild-type exopolysaccharide increased significantly as cells entered the stationary phase of growth or upon addition of Ca2+ to growing cells, and the polysaccharide-induced cells exhibited an enhanced capacity for cell-cell agglutination. The activity of the key gluconeogenic pathway enzyme phosphoenolpyruvate carboxykinase (Pck) also increased under these conditions. Most fibril-deficient mutants failed to produce polysaccharide in a stationary-phase- or Ca2+-dependent fashion. However, regulation of Pck activity was generally unimpaired in these mutant strains. In an stk mutant, which overproduces fibrils, polysaccharide production and Pck activity were constitutively high under the conditions tested. Polysaccharide production increased in most fibril-deficient strains when an stk mutant allele was present, indicating that these fibril-deficient mutants retained the basic cellular components required for fibril polysaccharide production. In contrast to other divalent cations tested, Sr2+ effectively replaced Ca2+ in stimulating polysaccharide production, and either Ca2+ or Sr2+ was required for fruiting-body formation by wild-type cells. By using transmission electron microscopy of freeze-substituted log-phase wild-type cells, fibril material was observed as a cell surface-associated layer of uniform thickness composed of filaments with an ordered structure.
The fruiting bacterium Myxococcus xanthus has a complex life cycle that is characterized by a variety of multicellular behaviors (16, 35). The most obvious of these behaviors is the production of fruiting bodies, which are multicellular spore-filled aggregates formed on solid surfaces in response to nutrient depletion. In addition, groups of M. xanthus cells exhibit at least two other forms of coordinated multicellular movement, rippling and social motility (S motility). This multicellular life cycle clearly involves extensive cell-cell communication and a variety of intercellular contact-mediated interactions.
Bacteria produce a wide variety of exopolysaccharides that are used to cope in various ways with the external environment. Exopolysaccharides are important in bacterial infections of animals and plants, where these components may help the bacteria to evade host defenses or to adhere to appropriate surfaces, and they are essential for the establishment of bacterial biofilms in a variety of ecological settings (10, 27, 29). M. xanthus produces exopolysaccharide-containing structures called fibrils which are found on the cell surface (16). Fibrils also contain a large amount of protein (approximately equal to the amount of polysaccharide) and appear to consist of a polysaccharide backbone decorated with several abundant protein species. Similar structures appear to be present on the cell surface of another myxobacterium, Stigmatella aurantiaca (9).
Mutants with alterations in fibril production have been useful in understanding the role of fibrils in social behavior. Two major groups of fibril-deficient mutants have been described: the S-motility and Cds mutants. S-motility mutants were isolated based on defects in social motility, one of the two genetically defined systems involved in gliding motility in M. xanthus (20). Among this group, the dsp mutants have been shown to be particularly fibril deficient (1, 11, 34). The other mutant group, the Cds group, was identified based on the failure of mutant colonies to bind the fluorescent dye calcofluor white, a trait that has been associated with the loss of exopolysaccharide (30). While at least some S-motility mutants may also fail to bind calcofluor white, the Cds mutants retain the capacity for S motility and continue to produce pili, appendages associated with S motility in M. xanthus and generally not found in S-motility mutants (23, 29). Fibril-deficient mutants are generally unable to agglutinate in a liquid medium, form multicellular fruiting aggregates, or produce fibrils that can be observed by electron microscopy. The hypothesis that fibrils function in agglutination and developmental aggregation is supported by the observation that purified fibrils rescue these defects in a fibril-deficient dsp mutant (8). A mutant which has an enhanced capacity to produce fibrils has also been identified (11). This mutant has a transposon insertion at the stk locus. In contrast to the properties of fibril-deficient cells, stk mutant cells adhere tightly to each other and to solid substrates. stk mutation has been shown to suppress the defects in agglutination and calcofluor white binding found in several S-motility mutants (11). While mutant analysis has suggested important roles for fibrils in the social interactions of M. xanthus, much remains to be determined about the specific roles of these structures.
There is also relatively little information on the genes involved in fibril production or on the environmental factors that influence fibril biosynthesis. One environmental factor which may be involved is intercellular contact, since fibrils have been observed primarily associated with groups of cells in close contact (3). Studies with S. aurantiaca have indicated that calcium ions induce fibril production and agglutination in that organism (9, 18). Fibril biosynthesis involves not only the production, transport, and assembly of fibrils from the polysaccharide and protein components but also the production of the monosaccharide building blocks. This is because M. xanthus has not been shown to utilize exogenous monosaccharides, and gluconeogenesis must be employed to meet the requirement for fibrillar polysaccharide production (6, 38). Fibril polysaccharide appears to contain substantial quantities of galactose, glucosamine, glucose, rhamnose, and xylose (4). The importance of glucosamine, glucose, and xylose in fibril function is indicated by the observation that these three sugars are effective at inhibiting the agglutination of M. xanthus cells (4). The identification of a gene which may encode one of the fibril proteins has recently been reported (36). There is also evidence that the physical structure of fibrils is altered during development, suggesting that fibrils may function dynamically during the bacterial life cycle (5).
We have investigated the regulation of polysaccharide production and the activity of a gluconeogenic enzyme in wild-type M. xanthus cells and in different mutants previously shown to have alterations in the levels of observable fibrils. Both polysaccharide production and gluconeogenic enzyme activity were found to be regulated in response to the growth phase of cells and by Ca2+. Analysis of polysaccharide production in mutant strains suggested that the polysaccharide produced was largely fibril associated and that many mutants have defects in the regulation of fibril polysaccharide.
MATERIALS AND METHODS
M. xanthus strains and culture conditions.
M. xanthus DK1622 was used as the wild-type strain, and the other strains used in this study are listed in Table 1. M. xanthus cultures were grown in Casitone-yeast extract (CYE) medium (7). When required, kanamycin and oxytetracycline were added at concentrations of 50 and 25 μg/ml, respectively. To prepare concentrated cell suspensions for monitoring polysaccharide and phosphoenolpyruvate carboxykinase (Pck) activity during stationary phase, log-phase cells at a density of approximately 4 × 108 to 5 × 108 cells/ml were harvested by centrifugation (8,000 × g, 10 min) and suspended in fresh CYE medium at a density of 5 × 109 cells/ml. The concentrated cell suspensions were incubated at 30°C with vigorous agitation, and samples were removed at various times for determination of the carbohydrate and Pck levels. Fruiting-body formation was analyzed in submerged culture by a modification of the method described by Kuner and Kaiser (25, 37). Wild-type cells growing exponentially in CYE medium were collected by centrifugation and washed with 10 mM MOPS (morpholinepropanesulfonic acid) (pH 7.2) buffer. The cells were suspended in the same buffer containing 4 mM CaCl2, MgCl2, or SrCl2 at densities ranging from 2 × 108 to 8 × 108 cells per ml. The developing cells were incubated in 24-well tissue culture plates at 30°C. These cultures were photographed with an inverted microscope after 96 h.
TABLE 1.
M. xanthus strains used in this study
| Straina | Genotypeb | Phenotypec | Derivation or referenced |
|---|---|---|---|
| DK1622 | Wild type | 23 | |
| DZF1 | sglA1 | S− | 14 |
| LS1102 | stk (Ω1907) | Stk | 11 |
| DK3088 | sglA1 stk | 11 | |
| JD300 | esg (Ω258) | Cds | 37 |
| JD509 | esg stk | Mx4 (Ω258 to LS1102) | |
| DK3468 | dsp-1680 | S− | 33 |
| LS1111 | dsp-1680 stk | 11 | |
| DK3481 | sgl-2234 | S− | 33 |
| LS1118 | sgl-2234 stk | 11 | |
| DK3482 | tgl-3114 | 33 | |
| SR53 | Ω53 | Cds | 30 |
| JD510 | stk Ω53 | Mx4 (Ω53 to LS1102) | |
| SR171 | Ω171 | Cds | 30 |
| JD511 | stk Ω171 | Mx4 (Ω171 to LS1102) | |
| SR200 | Ω200 | Cds | 30 |
| JD512 | stk Ω200 | Mx4 (Ω200 to LS1102) |
All strains except DZF1 are derived from DK1622.
All stk mutations are the Ω1907 Tn5 insertion allele with the kanamycin resistance gene of Tn5 replaced with a tetracycline resistance gene by in situ substitution (2). All esg mutations are the Ω258 Tn5 insertion allele. The mutations in the Cds strains (SR53, SR171, and SR200) are Tn5 or Tn5lac insertions.
The S− phenotype refers to the loss of S (group) motility. The Cds phenotype refers to the loss of calcofluor white binding by an S-motile strain. Both S− and Cds mutants are fibril deficient. Stk mutant cells adhere to each other and have a large amount of fibril material. The phenotypes of the double-mutant strains are described in Results.
The double-mutant strains constructed as part of this study were produced by using Mx4 transduction to transfer transposon insertion alleles with the associated kanamycin resistance marker into the tetracycline-resistant stk mutant LS1102.
Mx4 transduction.
Myxophage Mx4 transductions were performed by the method of Rhie and Shimkets (32) with the M. xanthus stk Tn5-132 insertion mutant strain LS1102 as the recipient. This strain contains a modified version of Tn5 in which the transposon-associated kanamycin resistance gene was replaced with a tetracycline resistance gene (2). Mx4 phage propagated on kanamycin-resistant Tn5 or Tn5lac (24) Cds insertion mutant strains were added to recipient LS1102 cells at a multiplicity of infection of 0.5. The recipient cells were prepared by pelleting log-phase cells and resuspending them in 0.01 M Tris-HCl buffer (pH 7.5) containing the salts mixture used in 17P medium (7). The infected cells were incubated for several hours at 30°C before being plated in top agar on CYE plates containing kanamycin (50 μg/ml). Kanamycin-resistant transductants were uniformly found to also be oxytetracycline resistant and to be double transposon insertion mutants.
Polysaccharide measurement.
The amount of anthrone-reactive material was determined by a method based on the Molish test described by Dische (13, 19) with glucose as the standard. This assay detects the simple pentoses, hexoses, and heptoses that are present in sulfuric acid-hydrolyzed cell samples. The cells to be assayed were first washed in 10 mM MOPS buffer (pH 7.0) and then suspended in the same buffer before being disrupted by sonication. The pelletable carbohydrate was assayed following centrifugation of sonicated cell extracts at 14,000 × g for 15 min. Cell growth was monitored with a Klett-Summerson colorimeter with the red filter. A reading of 100 Klett units corresponds to a cell density of approximately 9 × 108 cells/ml. The protein concentration in crude cell extracts was determined by using the bicinchoninic acid Protein Assay Reagent Kit (Pierce Chemicals) with bovine serum albumin as the standard.
Assay of Pck activity in cell extracts.
Phosphoenolpyruvate carboxykinase (Pck) activity was routinely assayed by using concentrated cell suspensions (5 × 109 cells/ml) prepared as described above. Samples (1 ml) of the concentrated cells were removed from the incubation flasks, and the cells were harvested by centrifugation for 10 min at 8,000 × g. The cell pellets were then washed with KH2PO4-K2HPO4 buffer (0.1 M, pH 7.0) and resuspended in 2 ml of the same buffer. Cells were disrupted by sonication on ice with a Branson Sonifier, and the cell debris was removed by centrifugation for 15 min at 14,000 × g. The supernatant (crude extract) was used for measurement of Pck activity. Pck mediates the first reaction in the gluconeogenic pathway and catalyzes a reversible reaction to convert oxaloacetate (OAA) to phosphoenolpyruvate (PEP). Attempts to measure PEP-forming Pck activity were not satisfactory due to a competing reaction which utilizes OAA to form pyruvate in the crude extracts, and the reverse reaction (PEP to OAA) was utilized. Enzyme activity was measured spectrophotometrically at room temperature by monitoring the disappearance of NADH at 340 nm. The 1-ml reaction mixture contained 100 mM imidazole-HCl (pH 6.6), 50 mM NaHCO3, 2.5 mM PEP, 1.25 mM ADP, 2 mM MnCl2, 2 mM glutathione, 0.25 mM NADH, 3 IU of malate dehydrogenase, and 100 μl of crude cell extract (22). The endogenous rate of ADP-independent NADH oxidation in the crude cell extract was measured so that it could be subtracted from the ADP-dependent value to estimate the level of Pck activity (26). One unit of activity is defined as the amount of enzyme that catalyzes the oxidation of 1 nmol of NADH/min/mg of extract protein. Both ADP-dependent and PEP-dependent measurements have been used for the measurement of Pck activity. Although PEP-dependent assays resulted in higher levels of Pck activity, more consistent results were obtained with ADP-dependent assays. Therefore, the latter method was employed in this study. The activity of phosphoenolpyruvate carboxylase, which converts PEP to OAA by using CO2 and ATP, was not significant under our assay conditions.
Agglutination assay.
Agglutination was measured by a modification of a method described previously (34). Cells grown in CYE medium to a density of 5 × 108 cells/ml were collected by centrifugation, washed with 10 mM MOPS buffer (pH 6.8), and suspended to a density of 9 × 108 cells/ml (100 Klett units) in agglutination buffer (10 mM MOPS, 1 mM MgCl2, 1 mM CaCl2 [pH 6.8]). The cell suspensions were incubated at room temperature without shaking, and changes in turbidity were measured at 625 nm.
TEM.
M. xanthus strains were grown to mid-log phase in CYE broth, harvested by centrifugation, and concentrated to 2 × 109 cells/ml in 10 mM Tris-HCl (pH 7.6). Cells were prepared for transmission electron microscopy (TEM) by spray-freezing freeze substitution (SFFS). This procedure has been described in detail previously (17). The cell suspensions were sprayed with an airbrush (20 lb/in2) into transfer baskets made from nylon mesh with a fiber spacing of 5 μm (Small Parts, Inc., Miami Lakes, Fla.). The transfer baskets were submerged in liquid propane (−183°C) during the spraying of the samples, and liquid nitrogen was used to liquefy and maintain the temperature of the propane. The airbrush was equipped with a needle valve that was adjusted to produce a sample droplet size of approximately 40 to 50 μm. After freezing, the mesh baskets containing the frozen samples were drained and transferred through a series of anhydrous acetone rinses at −85°C. The freeze-substituted cell samples were then brought to room temperature gradually over a 10-h period. These samples were postfixed with 2% OsO4 for 3 h, rinsed in water, and dehydrated in a series of graded acetone washes. At this point the samples were embedded with EmBed 812 resin (Electron Microscopy Sciences, Fort Washington, Pa.). Thin sections of the embedded samples were applied to Formvar-coated grids and then stained successively with saturated uranyl acetate and Reynold’s lead acetate. The sections were viewed by TEM with a JEOL 2000 electron microscope at an accelerating voltage of 100 kV.
RESULTS
Stationary-phase polysaccharide induction.
The M. xanthus esg locus has been shown to encode the E1α and E1β components of a branched-chain ketoacid dehydrogenase, and an esg mutant is pleiotropically defective in a variety of properties found in both growing and developing cells (14, 30, 37). The esg locus appears to be involved with an intercellular signaling system which functions to control developmental gene expression and coordinate multicellular activities (15). During studies of an M. xanthus esg mutant, we observed that esg stationary-phase cultures differed dramatically in appearance from those of the wild-type parental strain DK1622. While stationary-phase wild-type cells in broth culture formed aggregates that were associated with a copious amount of viscous extracellular polysaccharide material, esg mutant cells remained dispersed, with little extracellular material apparent. This difference in the cultures was not readily apparent in log-phase cultures. The morphology of older colonies of the esg strain on agar plates also differed greatly from that of the wild type, with the esg colonies having a much smoother appearance. Both the colony morphology and the stationary-phase culture differences between the esg and wild-type strains were not apparent in the DZF1 background, which was used in an earlier study (37).
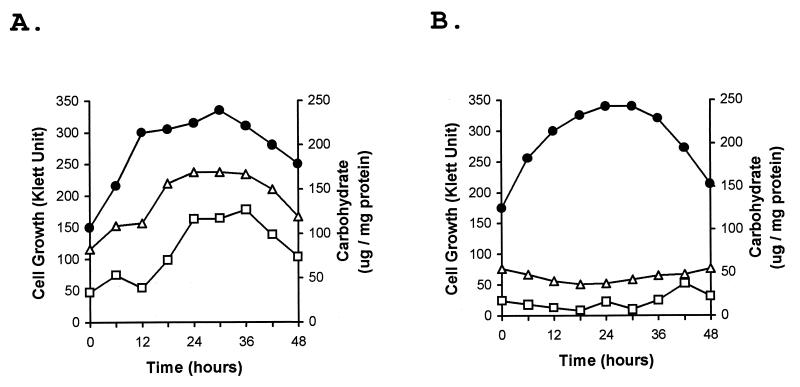
To document the differences in the properties of the two strains that were apparent visually, the polysaccharide contents of the wild-type and esg strains during vegetative growth were determined by using a simple carbohydrate assay. The specific carbohydrate content of the wild-type culture increased greatly as the cells entered stationary phase, rising from 75 to 160 μg of carbohydrate/mg of protein (Fig. 1A). Much of the carbohydrate appeared to be in the form of polysaccharide, since more than 80% of the carbohydrate in a 36-h sonicated cell extract could be removed by low-speed centrifugation and about 90% of the cell extract carbohydrate could be precipitated with ethanol. The esg cells, which grew at a rate similar to that of the wild type, failed to exhibit increased polysaccharide production (Fig. 1B). Polysaccharide induction by the wild-type strain was also observed with concentrated cell suspensions. Optimal induction was observed in fresh CYE medium with log-phase cells concentrated to 5 × 109 cells/ml (500 Klett units). Under these conditions, the maximum wild-type polysaccharide content was observed after about 6 to 8 h of incubation. Under these conditions, the maximum amount of polysaccharide produced was somewhat less than that found associated with cells which had gradually entered stationary phase during batch culture (Table 2), but the use of concentrated cell suspensions was found to be a convenient method for the characterization of M. xanthus growth-phase-dependent polysaccharide production.
FIG. 1.
Specific carbohydrate contents of wild-type and esg mutant cells during growth. Wild-type M. xanthus (DK1622) and an esg mutant (JD300) were grown in CYE broth shaker culture. At the indicated times, samples were removed from the wild-type culture (A) and the esg culture (B) for determination of cell-associated carbohydrate and protein contents. The total specific carbohydrate content values (micrograms per milligram of total cellular protein) were determined by using the sonicated cell extracts (triangles) or with the carbohydrate content of a pellet fraction of the extracts that was obtained by low-speed centrifugation (squares). Cell growth was monitored with a Klett-Summerson colorimeter (circles). Results are shown only for the portion of the growth curve during which cells were leaving log phase and entering stationary phase.
TABLE 2.
Induction of polysaccharide and Pck activity in mutant M. xanthus strainsa
| Strainb | Specific carbohydrate content (μg of carbohydrate/mg of protein)
|
Pck sp act (nmol/min/mg of protein)
|
||
|---|---|---|---|---|
| Log | Stationary | Log | Stationary | |
| DK1622 (WT) | 72 (5) | 127 (9) | 8.1 (1.8) | 18.1 (2.5) |
| LS1102 (stk) | 153 (11) | 169 (13) | 18.6 (3.0) | 17.6 (2.7) |
| DZF1 (sglA1) | 64 (2) | 60 (2) | 6.3 (2.0) | 13.6 (2.8) |
| JD300 (esg) | 61 (3) | 56 (2) | 6.7 (1.6) | 15.2 (3.2) |
| JD509 (esg stk) | 122 (9) | 119 (11) | 12.3 (2.7) | 12.1 (2.3) |
| DK3468 (dsp) | 66 (3) | 67 (2) | 7.6 (2.0) | 22.3 (4.6) |
| LS1111 (dsp stk) | 81 (4) | 74 (3) | 7.6 (1.6) | 25.2 (5.2) |
| DK3481 (sgl) | 73 (3) | 70 (2) | 8.3 (2.2) | 16.8 (3.7) |
| LS1118 (sgl stk) | 130 (8) | 134 (6) | 13.9 (3.0) | 13.8 (3.2) |
| DK3482 (tgl) | 57 (2) | 57 (3) | 5.0 (1.5) | 21.0 (3.8) |
| SR53 (Cds) | 62 (3) | 60 (2) | 5.3 (1.7) | 16.1 (3.3) |
| JD510 (Cds stk) | 134 (10) | 136 (12) | 14.3 (2.6) | 14.1 (2.9) |
| SR171 (Cds) | 67 (2) | 69 (3) | 7.6 (2.1) | 18.4 (3.2) |
| JD511 (Cds stk) | 91 (5) | 94 (8) | 11.6 (3.1) | 12.1 (2.9) |
| SR200 (Cds) | 60 (2) | 57 (2) | 5.9 (1.7) | 12.7 (2.5) |
| JD512 (Cds stk) | 70 (3) | 68 (3) | 6.5 (1.8) | 14.2 (2.0) |
All measurements were made by using concentrated cell suspensions obtained from log-phase CYE cultures of the various strains. The determinations of specific carbohydrate content and Pck activity were from cell suspensions either immediately after concentration (Log) or after 6 h of incubation in CYE shaker culture (Stationary). The values reported for the specific carbohydrate content and Pck activity are the averages of measurements from three independent experiments. The standard errors are shown in parentheses.
Strains are indicated by strain numbers (Table 1) with genotypic and/or phenotypic designations in parentheses.
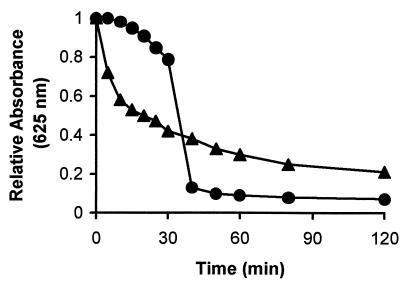
Fibrils are extracellular structures in M. xanthus that are composed of similar amounts of polysaccharide and protein. These structures have been associated with the ability of cells to agglutinate and settle from suspension during incubation in MOPS-Ca2+ agglutination buffer. To determine if stationary-phase wild-type cells with a high polysaccharide content behaved as if they had an increased fibril content, these cells were tested in the M. xanthus agglutination assay. Log-phase wild-type cells are normally used in the agglutination assay, and these cells agglutinate well in comparison with a variety of fibril-deficient mutants. However, the stationary-phase cells agglutinated much more rapidly than log-phase cells (Fig. 2). Microscopic examination of the agglutinated stationary-phase cells indicated that aggregates were formed in which the cells were less closely associated than in log-phase cell aggregates (data not shown). This observation may explain why the absorbance values for the stationary-phase cells remained somewhat higher during prolonged incubation than the values observed with log-phase cells. The rapid agglutination indicated that the stationary-phase cells behaved functionally as if they had an increased fibril content and suggests that the induced polysaccharide was associated with fibrils. When the polysaccharide-deficient esg mutant cells were tested, both log- and stationary-phase cells agglutinated poorly and there was no indication of any growth phase-dependent change in the capacity of cells to agglutinate (data not shown).
FIG. 2.
Agglutination by polysaccharide-induced M. xanthus wild-type cells. The absorbance of wild-type cells was monitored during incubation in agglutination buffer. The wild-type cells used in the assay were obtained from a growing culture (circles) or from a polysaccharide-induced stationary-phase culture (triangles). Decreases in absorbance are associated with the agglutination of cells during incubation in the buffer.
Polysaccharide induction in M. xanthus mutants.
Several M. xanthus mutant strains have been reported to be deficient in fibril production. These mutants include the Cds and the S-motility mutants. Scanning electron microscopy has failed to detect fibrils in these mutants, and all of these strains agglutinated poorly compared to the wild type. These mutants are also deficient in binding dyes like Congo red and calcofluor white, which are known to bind to extracellular polysaccharide. Several of these mutants were tested for stationary-phase induction of polysaccharide (Table 2). As was already shown, the wild-type strain DK1622 had a strong induction of polysaccharide during stationary phase, while there was no detectable induction of polysaccharide in the esg strain. Four S-motility mutants (DZF1 and the dsp, sgl, and tgl mutants) and three Cds mutants (SR53, SR171, and SR200) also failed to induce polysaccharide production. Besides failing to induce polysaccharide production, all of these mutant strains grew dispersed throughout stationary phase in CYE broth culture and failed to produce the viscous cell-associated material that was readily apparent in the wild-type cultures. It was also significant that the log-phase mutant cells were generally observed to have lower-than-wild-type levels of polysaccharide. The specific carbohydrate values for most of the mutants ranged from 57 to 67 μg/mg of protein, compared with the wild-type level of 72 μg/mg of protein.
Mutation of the M. xanthus stk locus results in cells with a higher-than-normal level of fibrils. stk mutant cells also exhibit a variety of properties that are likely to result from fibril overproduction, including clumping of cells during growth in broth culture, rapid agglutination, and formation of colonies in which cells adhere tightly to each other and the agar surface. stk mutation has also been shown to restore fibril production in several fibril-deficient mutant strains. The effect of stk mutation on polysaccharide production was tested in the wild type and several of the fibril-deficient mutants. The stk mutant (LS1102) produced a very high level of polysaccharide both during the log and stationary phases of growth (Table 2). This level was double the log-phase value observed for wild-type cells and significantly greater than the wild-type stationary-phase-induced level. The stk cells adhered to each other, forming small multicellular aggregates during growth which were very difficult to dissociate.
Double mutants were constructed by transducing transposon insertion alleles from fibril-deficient strains into the stk mutant. The double mutants generally displayed increased levels of polysaccharide in the log phase, and the level did not increase significantly during the stationary phase (Table 2). The constitutive level of synthesis varied from strain to strain but was never as high as that found in the stk mutant (LS1102). The double-mutant strains were also generally similar to the stk mutant in that many cell aggregates were observed during vegetative growth. Only the dsp stk (LS1111) and SR200 stk (JD512) double mutants failed to exhibit the general phenotypic characteristics of the stk mutant; these strains contained reduced levels of polysaccharide (Table 2) and grew dispersed in liquid culture like the parental dsp and SR200 strains. It was shown previously that LS1111 (dsp stk) failed to agglutinate or produce fibrils (11).
Pck activity during polysaccharide induction.
M. xanthus does not utilize exogenous sugars and is apparently completely dependent on gluconeogenesis for the production of hexoses and pentoses for polysaccharide production (6, 38). The evolutionarily conserved enzyme Pck converts the tricarboxylic acid cycle intermediate OAA to PEP, an important initial step in gluconeogenesis. Pck activity in log- and stationary-phase cell extracts prepared from the wild type and several mutant strains was measured (Table 2). In wild-type cells (DK1622), Pck activity increased more than twofold as cells entered stationary phase and began producing high levels of polysaccharide. Stationary-phase Pck activity also increased from two- to fourfold in all of the fibril-deficient mutants that were tested. In the stk mutant (LS1102), however, a different activity pattern was observed. The level of activity found in log-phase extracts was high, and activity did not increase during stationary phase. The constitutive level of activity in the stk mutant extracts was similar to that found in stationary-phase wild-type extracts. Two patterns of activity were observed in double mutants containing the stk transposon insertion allele and insertion mutations causing polysaccharide deficiency. Several double mutants, including the esg stk mutant (JD509), exhibited constitutive levels of Pck activity like the stk mutant strain. However, the levels of Pck activity in the double mutants were significantly lower than that found in the stk mutant. The other pattern of activity was observed in the dsp stk (LS1111) and SR200 stk (JD512) strains. In these strains there was a pattern of Pck activity that was similar to that observed in the dsp and SR200 strains, in which Pck activity increased as cells entered stationary phase.
Calcium-induced agglutination, polysaccharide production, and developmental aggregation.
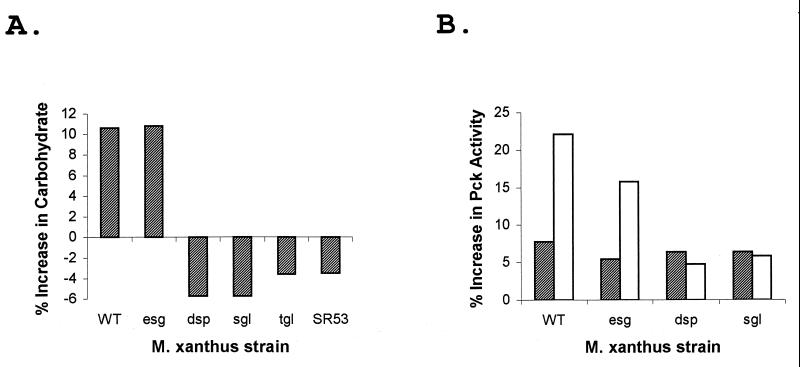
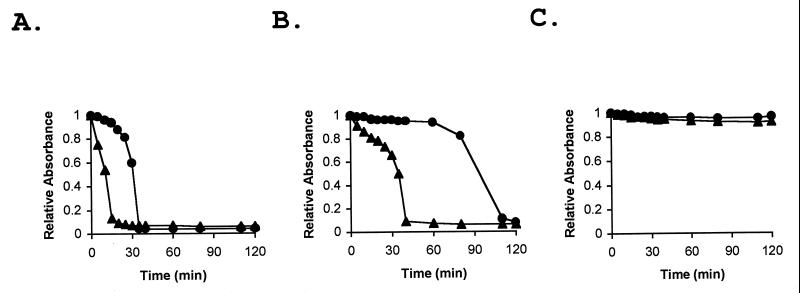
Studies with the myxobacterium S. aurantiaca have shown that fibrils are also produced in response to Ca2+ (9). When Ca2+ was added to log-phase wild-type M. xanthus cells growing in CYE medium, the cells began to stick together, forming multicellular clumps. The wild-type cells were tested for polysaccharide content and Pck activity after 2 h of incubation with Ca2+. The Ca2+-treated cells had about a 10% greater polysaccharide content than untreated cells and about threefold higher Pck activity (Fig. 3). Several of the fibril-deficient mutant strains were also tested for Ca2+-induced polysaccharide production (Fig. 3A). The polysaccharide content of esg (JD300) cells increased by about 10%, a relative increase similar to that observed for the wild type, but no Ca2+ induction was observed with the four other fibril-deficient mutants tested. These mutants were the dsp (DK3468) mutant, the sgl (DK3481) and tgl (DK3482) S-motility mutants, and the Cds mutant SR53. A similar pattern was observed for Pck activity, where a Ca2+-induced increase in activity was observed with the esg strain but not with the dsp and sgl mutants (Fig. 3B). Ca2+-induced wild-type, esg, and dsp cells were also tested in the agglutination assay. The Ca2+-induced wild-type and esg cells agglutinated much more rapidly than uninduced cells (Fig. 4A and B). However, Ca2+-induced dsp cells failed to agglutinate and behaved like the uninduced cells (Fig. 4C). These results suggest that M. xanthus, like S. aurantiaca, responds to Ca2+ by producing fibrils.
FIG. 3.
Effect of Ca2+ on polysaccharide production and Pck activity. The M. xanthus wild-type strain DK1622 (WT) and fibril-deficient mutant strains were grown in CYE medium. CaCl2 was added to the growth medium to a final concentration of 4 mM, and the cells were incubated for 2 h. Crude extracts from Ca2+-treated cells were assayed for specific carbohydrate content and Pck specific activity, and these values were compared with the values determined for untreated cell extracts. (A) The following strains were tested for specific carbohydrate content: WT, JD300 (esg), DK3468 (dsp), DK3481 (sgl), DK3482 (tgl), and SR53. The data presented are from one experiment that was representative of three experiments performed. The assays were performed in triplicate, with standard errors of less than 2%. (B) The following strains were tested for Pck specific activity: WT, JD300 (esg), DK3468 (dsp), and DK3481 (sgl). The Pck activity in Ca2+-treated cells (open bars) was compared with the activity in untreated cells (hatched bars). The percent increases reported (from a representative experiment) are relative to the activity in cell extracts measured prior to the 2-h incubation period. The values reported are the averages from Pck assays performed in triplicate, with standard errors of less than 3%.
FIG. 4.
Ca2+-induced changes in M. xanthus agglutination. Growing M. xanthus cells in CYE medium were treated with 4 mM CaCl2 for 2 h. Ca2+-treated and untreated cells were harvested by centrifugation and transferred to agglutination buffer as described in Materials and Methods. The agglutination of Ca2+-treated WT (DK1622) (A), esg (JD300) (B), and dsp (DK3468) (C) cells (triangles) was compared with that of untreated cells (circles).
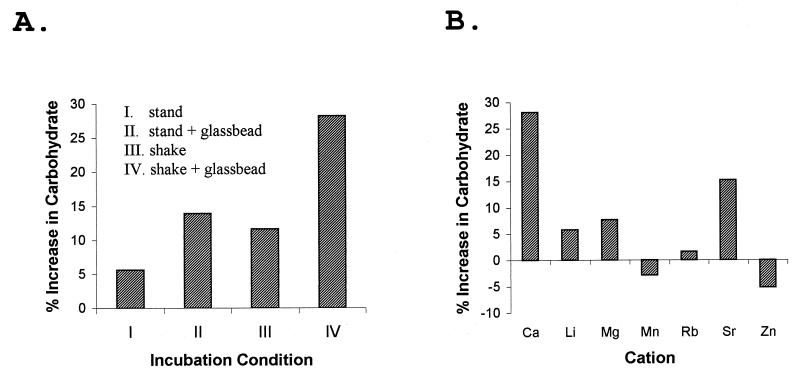
The effect of calcium on M. xanthus cells in a low-nutrient environment was also investigated. Incubation of wild-type cells in agglutination buffer, a MOPS-CaCl2 buffer, has been shown to result in fibril production in an energy-dependent process (33). Wild-type cells incubated in MOPS-CaCl2 buffer were examined for the accumulation of polysaccharide. The specific polysaccharide content was found to increase by about 5% during a standard 2-h incubation without agitation (Fig. 5A, condition I). With incubation of the cells in the same buffer either in the presence of small glass beads (Fig. 5A, condition II) or with rotary agitation (Fig. 5A, condition III), the level of polysaccharide production was found to increase 10 to 15% during the incubation. Finally, incubation with both glass beads and agitation (condition IV) resulted in a nearly 30% increase in the specific polysaccharide content. The glass beads presumably increased the surface area available for contact with the cells. Contact with a solid surface is a factor which has been implicated in fibril production (3). Agitation of the culture obviously helped to maintain the oxygen level in the buffer and may have helped the cells to produce energy which could be used for polysaccharide synthesis, but agitation also would be expected to diminish the contact of the cells with a solid surface. Thus, agitation and use of the glass beads would be expected to counteract each other. The observed additive effect of these treatments on polysaccharide production was surprising.
FIG. 5.
Divalent cation-mediated induction of polysaccharide production in buffer. (A) M. xanthus wild-type cells (DK1622) were harvested from the growth medium and suspended in Ca-MOPS (10 mM MOPS [pH 6.8], 4 mM CaCl2). The cell suspensions were incubated for 2 h at 30°C under the indicated conditions before being tested for specific carbohydrate content. The incubation conditions were as follows: I, incubation without agitation; II, incubation without agitation and with glass beads added; III, incubation with rapid rotary agitation; and IV, incubation with glass beads and rotary agitation. The results shown are from an experiment that was representative of three experiments performed. (B) M. xanthus wild-type cells were suspended in MOPS buffer with the indicated divalent cation present at a concentration of 4 mM. The cell suspensions were then incubated for 2 h with glass beads and rapid rotary agitation (condition IV) before the cells were assayed for the specific carbohydrate content. The percent increase is relative to the specific polysaccharide content of the batch of wild-type cells at the beginning of the incubation.
Having established conditions which supported vigorous polysaccharide production in a simple buffer (Fig. 5A, condition IV), we investigated the divalent cation requirement for polysaccharide production by wild-type cells in more detail. The cells responded to Ca2+ concentrations of from 0.5 to 8.0 mM with similar increases in the specific polysaccharide content (data not shown). The abilities of other divalent cations to substitute for Ca2+ in polysaccharide induction were also examined. Mg2+, Mn2+, Zn2+, Li2+, Sr2+, and Rb2+ were tested at a concentration of 4 mM (Fig. 5B). Sr2+ supported about 50% of the Ca2+-induced level of polysaccharide production (15% induction). Smaller effects (6 to 8% induction) were observed with Mg2+ and Li2+. No clear effect was found with the remaining divalent cations, i.e., Mn2+, Zn2+, and Rb2+.
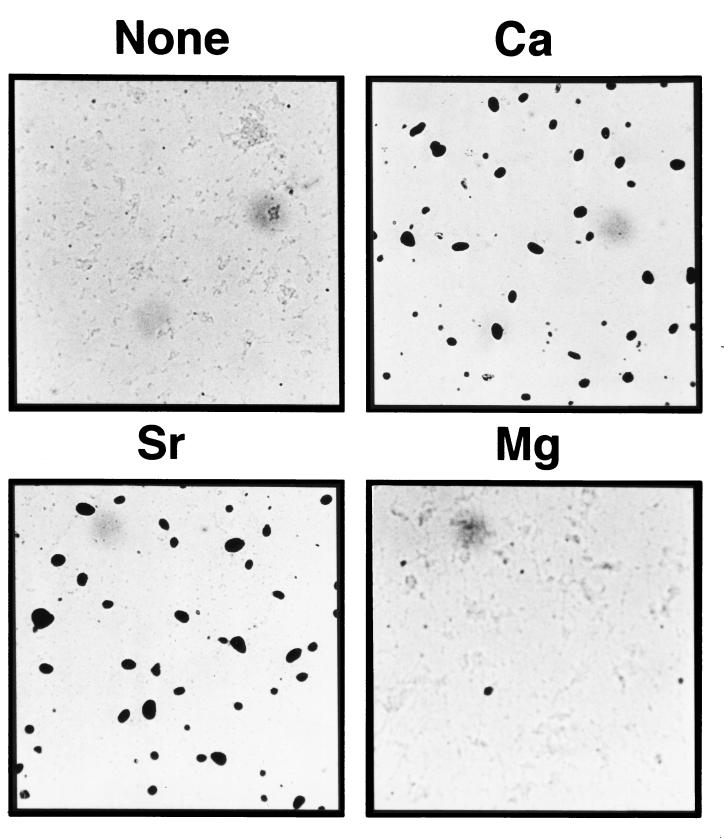
Ca2+ is required by M. xanthus cells for fruiting-body formation on a plastic surface during incubation in MOPS buffer (submerged culture development) (25). Under these conditions, the cells first form a biofilm of cells which adheres to the plastic surface before aggregation and fruiting-body formation occur. The abilities of different divalent cations to substitute for Ca2+ in submerged culture development were examined. As shown in Fig. 6, an Sr2+-containing buffer supported fruiting-body formation to about the same extent as with Ca2+, but the formation of fruiting aggregates in the presence of Mg2+ was only slightly better than that in the control culture lacking a divalent cation. No fruiting-body formation was observed with the other divalent cations (Li2+, Zn2+, Mn2+, and Rb2+) that were tested (data not shown). In general, the ability of buffers with various divalent cations to support development was correlated with the ability of the buffers to support polysaccharide induction.
FIG. 6.
Effect of divalent cations on M. xanthus fruiting-body formation. Wild-type M. xanthus cells (DK1622) were tested for the formation of fruiting bodies in MOPS buffer without a divalent cation (None) or with 4 mM CaCl2, MgCl2, or SrCl2. The cells were suspended in buffer at an initial density of 4 × 108 cells/ml. Large spore-filled fruiting bodies were formed in the presence of Ca2+ and Sr2+. The cultures were photographed with an inverted microscope after 96 h of incubation at 30°C.
Visualization of fibrils associated with log-phase M. xanthus cells.
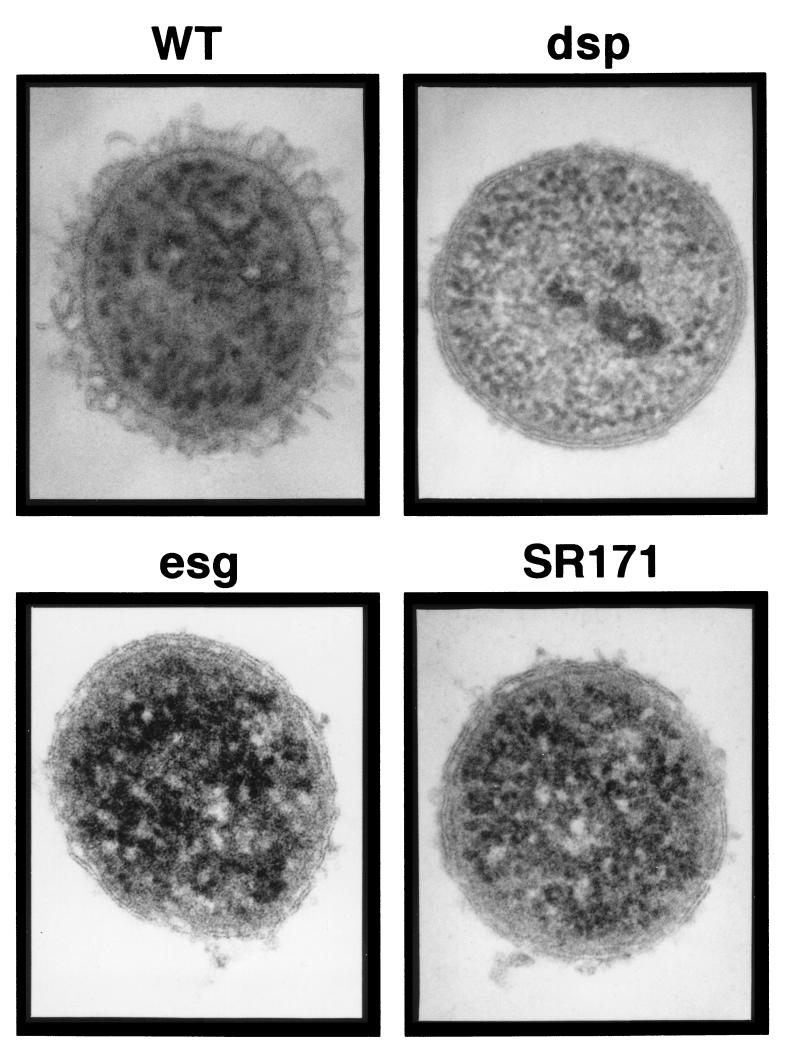
The data presented in Table 2 and the results of an earlier study (30) indicated that the specific carbohydrate content (total carbohydrate/total cellular protein) determined for log-phase wild-type cells was higher than the values determined for most of the fibril-defective mutants. For example, the carbohydrate content determined for log-phase esg cells was 61 μg/mg, while the value for wild-type cells was 72 μg/mg. These observations suggested that the growing wild-type cells have a significant polysaccharide-containing cellular structure that is not found in the mutant strains. The most likely polysaccharide-containing structural component to account for these observations is, of course, the fibrils themselves. However, fibrils have not previously been detected in association with individual log-phase cells from broth culture and have primarily been observed forming intercellular connections in groups of cells. In this study, M. xanthus cells were prepared for examination by TEM with SFFS (17) in an attempt to preserve the cell surface structure of log-phase cells. Thin sections of wild-type cells prepared by this procedure were examined and compared with those of fibril-deficient mutants (Fig. 7). A surface layer was readily apparent on the wild-type cells. This layer was external to the gram-negative double membrane and was composed of individual filaments which were 10 nm in diameter and 60 to 100 nm in length. These structures were evenly distributed over the cell surface. Although the surface structures observed by this technique were different in appearance from any surface component observed previously, the material appeared to be fibrillar, since this layer was absent from several fibril-deficient mutants that were examined. One of these mutants was the dsp mutant (DK3468), which has been studied in some detail and has been shown to lack a detectable level of fibril material. In this mutant, the gram-negative double membrane could be easily observed, and there was no detectable cell surface material outside the outer membrane (Fig. 7). Two other fibril-deficient mutants, the esg mutant (JD300) and a Cds mutant (SR171), had a few small clumps of extracellular material, but the clumps did not appear to be organized into the regular structures that were observed associated with wild-type cells. SFFS analysis of stationary-phase wild-type cells with a high polysaccharide content was not possible, because it was difficult to disperse cells prepared under these conditions.
FIG. 7.
TEM of thin sections of freeze-substituted M. xanthus cells. Log-phase M. xanthus cells were removed from the growth medium and prepared for examination by TEM with the SFFS procedure as described in Materials and Methods. The M. xanthus strains examined were the wild type (WT) (DK1622) and the dsp (DK3468), esg (JD300), and SR171 mutants. The diameter of the cross sections of M. xanthus cells was 0.7 to 0.8 μm, and the thickness of the cell surface-associated layer observed with wild-type cells was approximately 80 nm.
DISCUSSION
Myxobacterial cells produce an extracellular matrix referred to as fibrils. By using scanning electron microscopy, fibrils connecting M. xanthus cells can be observed, and these structures appear to play a central role in the multicellular activities displayed by this organism (3, 4). For example, fibrils mediate the agglutination of cells incubated in solution, and fibrils appear to play an important role in the aggregation of cells that is required for fruiting-body formation (8, 30). Fibrils may also allow M. xanthus cells to form communal mats (biofilms) in aqueous environments and associate firmly with solid substrates (25). Although the detailed features of fibril structure have not been elucidated, fibril material has been isolated and appears to consist of a polysaccharide backbone which is associated with a roughly equal amount of protein (4). Several abundant protein species are associated with fibrils (5).
In this study we have investigated the regulation of exopolysaccharide production in M. xanthus. In wild-type cells, exopolysaccharide production was induced in response to the entry into the stationary phase of growth and by the addition of Ca2+ to cells under a variety of conditions. Our observations are consistent with the hypothesis that all or most of the induced polysaccharide is fibril polysaccharide. These observations include the following: (i) stationary-phase- or Ca2+-induced cells agglutinated more rapidly than uninduced cells, (ii) the extent of developmental aggregation of wild-type cells in the presence of different divalent cations was correlated with the degree of induced polysaccharide production under developmental conditions, and (iii) mutants previously shown to have defects in fibril production and developmental aggregation also exhibited alterations in polysaccharide induction.
Two groups of M. xanthus mutants have been shown to be deficient in fibril production. One of these groups, the social motility (S-motility) mutants, have defects involving the movement of groups of cells. Among the S-motility mutants, those with defects at the dsp locus have been shown to be the most severely defective in fibril production (11, 33). The other group of fibril-deficient mutants is the Cds mutants (30). Mutants with the Cds phenotype include esg strains, which are believed to be defective in cell-cell communication (15). When members of each group were tested for stationary-phase induction of polysaccharide production, all were found to be deficient. Despite the failure of these mutants to produce stationary-phase-induced polysaccharide, there was an increase in the activity of the key gluconeogenic enzyme Pck under these conditions. The pattern of Pck activity activation was similar to that observed with the wild type. The increase in the activity of Pck presumably allows an increase in the flow of carbon from tricarboxylic acid cycle intermediates to the polysaccharide biosynthetic apparatus in these cells. These results suggest that the block in fibril polysaccharide biosynthesis in these mutant strains occurs in the later stages of the gluconeogenesis pathway or in the biosynthetic steps associated with the polymerization of the sugars into polysaccharide.
Several of these fibril-deficient mutants were also tested for Ca2+ induction of polysaccharide in a rich growth medium. In this case the different mutants were not uniform in their response. Polysaccharide induction by the dsp, sgl, tgl, and SR53 (a Cds mutant) strains tested was not observed, and no increase in Pck activity was observed. However, in the esg mutant, polysaccharide induction was observed and there was an associated increase in Pck activity. While the amount of polysaccharide produced by the esg mutant did not reach wild-type levels, the percent increase in polysaccharide content exhibited by the mutant was similar to that exhibited by the wild type. This was possible because the esg cells had a lower polysaccharide content during log-phase growth before the addition of Ca2+. The polysaccharide produced by Ca2+ treatment of wild-type and esg cells behaved functionally like fibril material in that the induced cells exhibited increased rates of agglutination. Fibril production in response to Ca2+ addition has been demonstrated for another myxobacterium, S. aurantiaca (9), but this response had not been previously described for M. xanthus.
The ability of Ca2+ to stimulate fibril production helps to explain the requirement for Ca2+ in agglutination and in development. As noted above, evidence has accumulated for fibrils being the mediators of the cell-cell contacts that occur during agglutination and also for being essential structural components for developmental aggregation. This evidence includes the demonstration that developmental aggregation could be rescued in the fibril-deficient dsp mutant by the addition of fibril material isolated from wild-type cells (8) and the observation that the Cds mutants that were most strongly deficient in polysaccharide production were also the most severely defective in fruiting-body formation (30). The ability of Sr2+ to effectively substitute for Ca2+ in supporting development in submerged culture could also be explained by the effectiveness of Sr2+ in inducing fibril polysaccharide production. Other divalent cations, like Mg2+, Mn2+, Li2+, or Rb2+, had little capacity to substitute for Ca2+ in the aggregation assay and had little or no effect on polysaccharide production. Factors besides the growth phase and Ca2+ appear to play a role in fibril polysaccharide production. Although these factors have not been rigorously established, they may include oxygen availability and/or the contact of cells with a solid substrate (Fig. 5). In Pseudomonas aeruginosa production of the extracellular polysaccharide alginate, a polysaccharide involved in biofilm formation, has been found to be stimulated by cell contact with a solid surface (12, 21). The induction of polysaccharide production in the P. aeruginosa system is also accompanied by an increase in polysaccharide biosynthetic gene expression. It will be interesting to determine if a similar regulatory response is found in M. xanthus.
Polysaccharide production and activation of Pck activity were also investigated with an stk mutant, a mutant previously shown to produce increased levels of fibril material (11). This mutant showed a constitutively high polysaccharide content which did not increase in response to stationary phase, and Pck levels were uniformly high during log or stationary phase.
Dana and Shimkets (11) demonstrated that the phenotypic effect of stk mutation was epistatic to the fibril-deficient phenotype caused by several mutations in S-motility genes; that is to say, double mutants with mutations in the stk locus and in S-motility genes exhibited a phenotype like that of the stk mutant. One exception to this pattern was observed, i.e., an stk dsp double mutant which displayed the fibril-deficient phenotype. We reexamined this genetic relationship by testing polysaccharide induction and Pck activity in several double-mutant strains. The results of this study correlated well with those of the earlier study in that most of the double mutants examined showed constitutively elevated polysaccharide and Pck activity levels. The levels of polysaccharide and Pck activity were not as high in the double mutants as in the stk mutant, indicating that there was some effect of the various fibril deficiency mutations on the overall phenotype of the double-mutant strains. However, cells of the double mutants generally adhered tightly to one another during growth in liquid medium or as colonies on agar plates, which are distinctive phenotypes displayed by the stk mutant and associated with enhanced fibril production. Our results indicate that stk mutation causes increased fibril polysaccharide production in most of the fibril-deficient mutants that have previously been identified. Apparently these fibril-deficient mutants have retained the capacity to produce fibril polysaccharide. In two cases the fibril deficiency phenotype of mutants was found to be epistatic to the Stk phenotype. Consistent with what was previously reported (11), a dsp stk double mutant displayed properties very similar to those of the dsp mutant, including the inability to exhibit a growth phase-dependent induction of polysaccharide production. One of the Cds mutants, SR200, also exhibited a phenotype that was epistatic to the Stk phenotype.
Clearly, fibril production in M. xanthus is the focus of extensive regulation. A model for the genetic control of fibril production has been proposed by Dana and Shimkets (11). The work presented in this paper allows us to propose a more detailed and somewhat modified version of the earlier model. Based on epistasis studies with stk, the genes involved in fibril production can be placed in two classes: class I genes regulate fibril production in response to environmental conditions, and class II genes are directly involved in fibril component production and/or assembly of the fibrils. The class I genes may regulate the expression or activity of the class II genes. Mutants defective in class I genes include many S-motility and Cds mutants. These mutants are deficient in fibril production but have retained the basic capacity to produce fibrillar polysaccharide. This capacity was evident in the stk genetic background, in which the S-motility or Cds mutation did not prevent the production of relatively high levels of fibril polysaccharide. The class I mutants are proposed to be defective in a regulatory pathway(s) which connects the perception of environmental conditions, such as nutrient depletion or the level of external Ca2+, to the activation of fibril production. Most of the class I mutants that have been examined are defective in both the growth phase and Ca2+ induction of fibril production, but the esg mutant and the Cds mutant SR171 (which is defective in a locus marked by transposon insertion Ω171) (31) were found to be defective only in the growth phase induction response. These results suggest that the esg locus and the Ω171 locus function specifically in a branch of the pathway involved in growth phase activation. The esg locus has been shown to be defective in the regulation of multicellular development, and these studies implicate esg function in the regulation of nondevelopmental growth phase-related functions as well. Mutants with defects in the class II genes retain the fibril-deficient phenotype even in the stk genetic background and may have lost the capacity to produce fibrillar polysaccharide. Class II genes may encode proteins directly involved in fibril polysaccharide biosynthesis. The dsp and SR200 (containing transposon insertion Ω200) mutant strains appeared to have defects in class II genes, and, based on earlier results (11), the sgl-3119 mutant may also belong to this group. The stk gene product seems to function to limit fibril production. Many of the stk fibril-defective double mutants displayed polysaccharide levels that were intermediate between the levels found in the individual stk and fibril-deficient mutants. This result suggests that the role of stk in the regulation of fibril production is complex and that there may be multiple regulatory pathways which modulate the level of fibril production in response to a variety of environmental conditions. While stk mutants generally had high levels of Pck activity, the stk locus does not appear to directly control the level of Pck, since enzyme activity and regulation were normal in the stk dsp and stk Ω200 double mutants.
Many S-motility mutants have been shown to be defective in the production of pili, and genetic analysis has shown that the M. xanthus pili belong to the type IV family (39). This being the case, it was puzzling that a number of the S-motility mutants were also shown to be defective in fibril polysaccharide production. Recent work on the P. aeruginosa type IV pilus system has demonstrated a role for pilin subunits in a general pathway for the secretion of several extracellular proteins (28). These extracellular proteins include both pilus structural components and bacterial virulence factors with no connection to pilus biogenesis. If M. xanthus pilus components are involved in the translocation of proteins required for fibril polysaccharide synthesis, then the connection between pili and fibril production may be explained.
The application of the SFFS technique to the observation of M. xanthus cells by TEM has provided a new high-resolution view of fibril structure. By using this approach, an extracellular surface layer was observed associated with log-phase wild-type cells but not with several fibril-deficient mutants. The absence of a structurally significant fibrillar polysaccharide layer in these mutants was initially suggested by the observation that most fibril-deficient mutants had lower log-phase polysaccharide levels than the wild type (Table 2) (30). The fibrils observed associated with the freeze-substituted cells were highly ordered in structure, with a diameter of 10 nm and a length of roughly 60 to 100 nm. These fibrils were much more regular in structure than those observed by scanning electron microscopy or by TEM of negatively stained material. The highly ordered structure was also unusual for a bacterial extracellular structure composed of polysaccharide. Presumably the association of fibril polysaccharide with a specific group of proteins (5) is responsible for this remarkable regularity of structure. Fibrils have not previously been observed in association with growing cells, but wild-type cells removed from growth medium agglutinate rapidly when suspended in buffer. These results suggest that fibrils are normal components of the M. xanthus cell surface and that these fibrils may be used under appropriate conditions to form the cell-cell connections between agglutinating cells. Attempts to determine the structure of the cell surface material associated with growth phase- or Ca2+-induced cells by using the SFFS technique have been hampered by the technical difficulty of working with these cells. In the future it may be possible to modify the SFFS procedure to improve the analysis of these cells.
The M. xanthus fibrils are complex in structure and appear to play dynamic roles in the various intercellular interactions displayed by this organism during growth and development. The work described in this paper argues that fibril production is subject to extensive regulation and that understanding the regulatory processes involved will be an important aspect of studies on fibril structure and function. This work sets the stage for the detailed analysis of the genes involved in the regulation and production of fibrils.
ACKNOWLEDGMENTS
We thank Larry Shimkets for providing mutant strains and for helpful comments. Greg Stout from the Noble Electron Microscopy Laboratory at the University of Oklahoma provided expert technical assistance.
Financial support from the Oklahoma Center for the Advancement of Science and Technology (OCAST) and an NSF EPSCoR grant is gratefully acknowledged.
REFERENCES
- 1.Arnold J W, Shimkets L J. Cell surface properties correlated with cohesion in Myxococcus xanthus. J Bacteriol. 1988;170:5771–5777. doi: 10.1128/jb.170.12.5771-5777.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avery L, Kaiser D. In situ transposon replacement and isolation of a spontaneous tandem genetic duplication. Mol Gen Genet. 1983;191:99–109. doi: 10.1007/BF00330896. [DOI] [PubMed] [Google Scholar]
- 3.Behmlander R M, Dworkin M. Extracellular fibril and contact-mediated cell interactions in Myxococcus xanthus. J Bacteriol. 1991;173:7810–7821. doi: 10.1128/jb.173.24.7810-7820.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behmlander R M, Dworkin M. Biochemical and structural analysis of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994;176:6295–6303. doi: 10.1128/jb.176.20.6295-6303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behmlander R M, Dworkin M. Integral proteins of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994;176:6304–6311. doi: 10.1128/jb.176.20.6304-6311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretscher A P, Kaiser D. Nutrition of Myxococcus xanthus, a fruiting myxobacterium. J Bacteriol. 1978;133:763–768. doi: 10.1128/jb.133.2.763-768.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campos J M, Geisselsoder J, Zusman D R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 8.Chang B Y, Dworkin M. Isolated fibrils rescue cohesion and development in the dsp mutant of Myxococcus xanthus. J Bacteriol. 1994;176:7190–7196. doi: 10.1128/jb.176.23.7190-7196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang B-Y, White D. Cell surface modifications induced by calcium ion in the myxobacterium Stigmatella aurantiaca. J Bacteriol. 1992;174:5780–5787. doi: 10.1128/jb.174.18.5780-5787.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 11.Dana J R, Shimkets L J. Regulation of cohesion-dependent cell interactions in Myxococcus xanthus. J Bacteriol. 1993;175:3636–3647. doi: 10.1128/jb.175.11.3636-3647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies D G, Chakrabarty A M, Geesey G G. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl Environ Microbiol. 1993;59:1181–1186. doi: 10.1128/aem.59.4.1181-1186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dische Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953;204:983–997. [PubMed] [Google Scholar]
- 14.Downard J, Ramaswamy S V, Kil K-S. Identification of esg, a genetic locus involved in cell-cell signaling during Myxococcus xanthus development. J Bacteriol. 1993;175:7762–7770. doi: 10.1128/jb.175.24.7762-7770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Downard J, Toal D. Branched-chain fatty acids—the case for a novel form of cell-cell signaling during Myxococcus xanthus development. Mol Microbiol. 1995;16:171–175. doi: 10.1111/j.1365-2958.1995.tb02290.x. [DOI] [PubMed] [Google Scholar]
- 16.Dworkin M. Recent advances in the social and developmental biology of the myxobacteria. Microbiol Rev. 1996;60:70–102. doi: 10.1128/mr.60.1.70-102.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields S D, Stout G W, Russell S D. Spray-freezing freeze substitution (SFFS) of cell suspensions for improved preservation of ultrastructure. Microsc Res Technol. 1997;38:315–328. doi: 10.1002/(SICI)1097-0029(19970801)38:3<315::AID-JEMT12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Gilmore D F, White D. Energy-dependent cell cohesion in myxobacteria. J Bacteriol. 1985;161:113–117. doi: 10.1128/jb.161.1.113-117.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanson R S, Phillips J A. Chemical composition. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 328–364. [Google Scholar]
- 20.Hartzell P L, Youderian P. Genetics of gliding motility and development in Myxococcus xanthus. Arch Microbiol. 1995;164:309–323. doi: 10.1007/BF02529977. [DOI] [PubMed] [Google Scholar]
- 21.Hoyle B D, Williams L J, Costerton J W. Production of mucoid exopolysaccharide during development of Pseudomonas aeruginosa biofilms. Infect Immun. 1993;61:777–780. doi: 10.1128/iai.61.2.777-780.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jetten M S M, Sinskey A J. Characterization of phosphoenolpyruvate carboxykinase from Corynebacterium glutamicum. FEMS Microbiol Lett. 1993;111:183–188. [Google Scholar]
- 23.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroos L, Kaiser D. Construction of Tn5lac, a transposon that fuses lacZ expression to endogenous promoters, and its introduction into Myxococcus xanthus. Proc Natl Acad Sci USA. 1984;81:5816–5820. doi: 10.1073/pnas.81.18.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuner J M, Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982;151:458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane M D, Chang H C, Miller R S. Phosphoenolpyruvate carboxykinase from pig liver mitochondria. Methods Enzymol. 1969;13:270–277. [Google Scholar]
- 27.Leigh J A, Coplin D L. Exopolysaccharides in plant-bacterial interactions. Annu Rev Microbiol. 1992;46:307–346. doi: 10.1146/annurev.mi.46.100192.001515. [DOI] [PubMed] [Google Scholar]
- 28.Lu H M, Motley S T, Lory S. Interactions of the components of the general secretion pathway: role of Pseudomonas aeruginosa type IV pilin subunits in complex formation and extracellular protein secretion. Mol Microbiol. 1997;25:247–259. doi: 10.1046/j.1365-2958.1997.4561818.x. [DOI] [PubMed] [Google Scholar]
- 29.Moxon E R, Kroll J S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- 30.Ramaswamy S, Dworkin M, Downard J. Identification and characterization of Myxococcus xanthus mutants deficient in calcofluor white binding. J Bacteriol. 1997;179:2863–2871. doi: 10.1128/jb.179.9.2863-2871.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramaswamy, S., and J. Downard. Unpublished results.
- 32.Rhie H G, Shimkets L J. Developmental bypass suppression of Myxococcus xanthus csgA mutations. J Bacteriol. 1989;171:3268–3276. doi: 10.1128/jb.171.6.3268-3276.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimkets L J. Correlation of energy-dependent cell cohesion with social motility in Myxococcus xanthus. J Bacteriol. 1986;166:837–841. doi: 10.1128/jb.166.3.837-841.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimkets L J. Role of cell cohesion in Myxococcus xanthus fruiting body formation. J Bacteriol. 1986;166:842–848. doi: 10.1128/jb.166.3.842-848.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimkets L J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith R S, Dworkin M. A mutation that affects fibril protein, development, cohesion and gene expression in Myxococcus xanthus. Microbiology. 1997;143:3683–3692. doi: 10.1099/00221287-143-12-3683. [DOI] [PubMed] [Google Scholar]
- 37.Toal D R, Clifton S W, Roe B A, Downard J. The esg locus of Myxococcus xanthus encodes the E1α and E1β subunits of a branched-chain keto acid dehydrogenase. Mol Microbiol. 1995;16:177–189. doi: 10.1111/j.1365-2958.1995.tb02291.x. [DOI] [PubMed] [Google Scholar]
- 38.Watson B F, Dworkin M. Comparative intermediary metabolism of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1968;96:1465–1473. doi: 10.1128/jb.96.5.1465-1473.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu S S, Kaiser D. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]