Abstract
Rationale
Sepsis survivors experience adverse outcomes including high rates of postdischarge mortality and rehospitalization. Given the heterogeneity of the condition, using a person-centered framework to identify subtypes within this population with different risks of postdischarge outcomes may optimize postsepsis care.
Objectives
To classify individuals into subtypes and assess the association of subtypes with 30-day rehospitalization and mortality.
Methods
We conducted a retrospective observational study between January 2014 and October 2017 among 20,745 patients admitted to one of 12 southeastern U.S. hospitals with a clinical definition of sepsis. We used latent class analysis to classify sepsis survivors into subtypes, which were evaluated against 30-day readmission and mortality rates using a specialized regression approach. A secondary analysis evaluated subtypes against readmission rate for ambulatory care–sensitive conditions.
Results
Among 20,745 patients, latent class analysis identified five distinct subtypes as the optimal solution. Clinical subtype was associated with 30-day readmission, with the subtype existing poor health with severe illness and complex needs after discharge demonstrating highest risk (35%) and the subtype low risk, barriers to care demonstrating the lowest risk (9%). Forty-seven percent of readmissions in the subtype poor functional status were for ambulatory care–sensitive conditions, whereas 17% of readmissions in the subtype previously healthy with severe illness and complex needs after discharge, barriers to care were for ambulatory care–sensitive conditions. Subtype was significantly associated with 30-day mortality: highest in for existing poor health with severe illness and complex needs after discharge (8%) and lowest for low risk, barriers to care (0.1%).
Conclusions
Sepsis survivors can be classified into subtypes representing nuanced constellations of characteristics, with differential 30-day mortality and readmission risk profiles. Predischarge classification may allow an individualized approach to postsepsis care.
Keywords: sepsis, survivor, hospital readmission, mortality, phenotype
Each year approximately 14 million sepsis survivors encounter increased long-term mortality and morbidity across functional, cognitive, and psychological domains (1–4). The limitations of current postsepsis care strategies are reflected by increased mortality risk and high rates of healthcare use, for example, a 90-day hospital readmission rate of 40%, resulting in more than $3 billion in preventable costs (5–7). These findings prompted a 2017 World Health Organization resolution to address the needs of sepsis survivors (8).
Randomized controlled trials (RCTs) have generally been unsuccessful in improving postsepsis outcomes (2). In one RCT, a multicomponent primary care management intervention delivered to sepsis survivors did not improve its primary endpoint (9). Another RCT demonstrated that a multicomponent nurse navigator intervention reduced a composite outcome of 30-day mortality and readmission, but there was notable risk-based heterogeneity in treatment response (10). Limited success in reducing the burden of morbidity and high healthcare use for sepsis survivors may be due to the heterogeneity of the syndrome, characterized by diverse pathophysiological mechanisms, preexisting health trajectories, illness courses, and recovery milieus (11, 12). An intervention may be effective in only a subpopulation of the overall cohort of patients enrolled in a clinical trial. Hence, the 2018 colloquium of the International Sepsis Forum named “limited data on how to identify patients most likely to benefit from interventions” as one of the urgent research gaps for sepsis survivors (13).
Modeling multiple risks to predict postsepsis outcomes typically involves a variable-centered framework, such as multiple regression or cumulative risk indices. However, these approaches often assume that all individuals at a certain level of the predictor are at equal risk for an adverse outcome, regardless of other risk factors or individual characteristics and that the relationship between a risk factor and an outcome is the same across the entire population.
In contrast, a person-centered framework assumes that adverse events after sepsis are the result of multiple interacting factors. Whereas variable-centered analyses describe the “average sepsis survivor,” person-centered analyses identify sets of characteristics that describe distinguishable subgroups of sepsis survivors. The person-centered approach is ideal for studying sepsis survivorship when the goals are 1) to understand how constellations of multiple, interacting risk factors are associated with postsepsis outcomes and 2) to identify groups of sepsis survivors who are most likely to benefit from specific targeted interventions.
Latent class analysis (LCA) is an increasingly used person-centered approach (14, 15). In LCA, each patient’s latent class membership is unknown and inferred from a set of categorical items. This approach allows the analysis and interpretation of higher order interactions among risk factors than what can be accomplished using typical regression methods. In addition, LCA focuses on identifying subgroups characterized by particular combinations of risks, and the risk factors are not treated as interchangeable. LCA typically yields many fewer latent risk classes than the number of observed risk profiles, allowing a parsimonious description of risks and limiting statistical challenges attributed to sparseness.
We hypothesize that among the heterogeneous population of sepsis survivors, latent, currently uncharacterized, clinically distinct subtypes exist that have differential risk of clinically important outcomes. Identifying these subtypes of sepsis survivors is an important step toward elucidating pathophysiological features, predicting outcomes parsimoniously, and guiding treatment selection. In this study, we leveraged data from an existing cohort of sepsis survivors to examine whether readily available electronic health record (EHR) variables could effectively classify sepsis survivors into clinical subtypes, describe the phenotype of these groups, and evaluate whether the subtypes explain variation in 30-day readmission and mortality.
Partial results have been submitted as an abstract to the Society of Critical Care Medicine annual meeting for consideration for presentation in February 2022 in San Juan, Puerto Rico.
Methods
We identified patients hospitalized with sepsis at 1 of 12 hospitals between January 2014 and October 2017. Adapting clinical criteria for suspected sepsis from defined guidelines (16), we included adults (≥18 years of age) presenting to the emergency department who met the following criteria: 1) oral/parenteral antibiotic or bacterial culture ordered within 24 hours of emergency department presentation and a) culture drawn first, antibiotics ordered within 48 hours, or b) antibiotics ordered first, culture ordered within 48 hours; and 2) organ dysfunction, defined as at least one of the following within the first 24 hours of presentation: a) lactate ≥ 2 mmol/L, b) platelets < 100, c) bilirubin ≥ 2 mg/dl, d) mean arterial pressure < 70 mm Hg, e) creatinine > 2 mg/dl, or f) mechanical ventilation. We excluded patients who did not survive to discharge and those transferred from other hospitals because of potential lack of data on disease course before transfer. We also excluded patients with full do-not-resuscitate orders in the first 24 hours of admission to avoid confounding by treatment limitations and because mortality and readmissions may not be the most important outcomes for patients with non–longevity-focused care goals (17). For patients with multiple sepsis hospitalizations, we included only the first eligible encounter during the study period.
Variable Selection and Reduction
We identified potentially relevant health indicators from the EHR system and Atrium Health enterprise data warehouse (EDW). Data are readily captured variables including patient demographic information, medication prescriptions, healthcare use, medical diagnoses, vital signs, laboratory results, procedures, and discharge disposition.
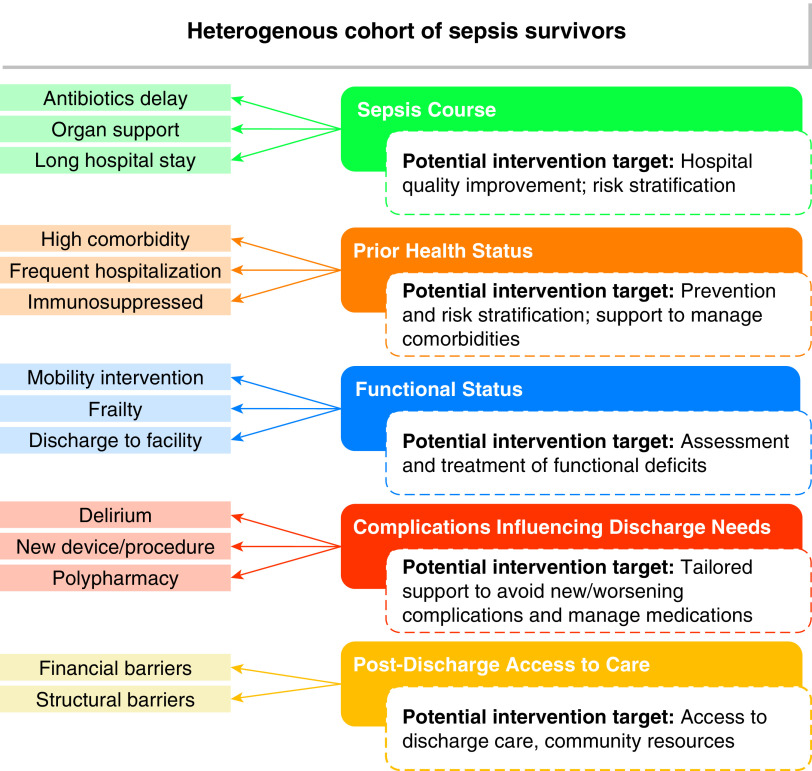
From thousands of discrete features available in the EHR, we generated a limited analytic data set guided by existing evidence. We selected variables known or hypothesized to be associated with poor postsepsis outcomes, grouped by potential for targeted intervention (Figure 1). We excluded basic demographic variables (age, sex, and race and ethnicity) and cohort-defining variables (e.g., Sequential Organ Failure Assessment score) from the variable selection process. We further excluded variables that were rare (<1% of cohort; e.g., absolute neutrophil count ⩽ 1,000) or highly correlated with included variables. Final decisions about included variables were made by consensus of all authors, resulting in a final set of 14 informative variables for our LCA (see Table E1 in the online supplement for variable definitions and collection). Variables were dichotomized to maximize interpretability on the basis of commonly applied thresholds or the distribution within the population.
Figure 1.
Conceptual domains and key indicators for developing sepsis survivor latent phenotypes.
Outcome Measures
The primary outcome was a dichotomous outcome of experiencing hospital readmission within 30 days after index hospital discharge. Hospital readmissions were captured from healthcare use data in the EDW. All inpatient or observation-status readmissions to any Atrium Health facility (i.e., more than 40 hospitals) were counted toward the readmission outcome. As a secondary outcome, hospital readmissions were further classified as potentially avoidable or not, using the Agency for Healthcare Research and Quality’s definition for ambulatory care–sensitive conditions, which is based on principal diagnosis codes (18). We also assessed 30-day postdischarge mortality outcomes, captured through the EDW, with verification ascertained via the monthly Social Security Administration Limited Access Death Master File data feed.
Analysis
We used LCA, a type of mixture modeling, to identify unobserved (latent) subtypes within our heterogeneous population of sepsis survivors (14). We included 14 dichotomous clinical indicators from five conceptual domains: sepsis course, prior health status, functional status, complications influencing discharge needs, and barriers to postdischarge care. Applying an exploratory approach without prespecifying the hypothesized number of expected classes, we sequentially derived LCA models containing an increasing number of classes and accounting for clustering at the hospital level. Selection of the most appropriate latent class model was determined using a combination of the Akaike and Bayesian information criteria, probability of class assignment (class separation), entropy, class prevalence (favoring models with classes >10% of the sample population), and clinical interpretability (19). Lower values for the Akaike and Bayesian information criteria indicate a better balance of model fit and parsimony, and higher values for entropy indicate higher classification utility (20). After model selection, we used the Bolck-Croon-Hagenaars stepwise procedure to evaluate the association between sepsis survivor subtypes and hospital readmission at 30 days (21). We repeated the Bolck-Croon-Hagenaars approach for secondary outcomes, hospital readmission for ambulatory care–sensitive conditions, and postdischarge mortality at 30 days. Descriptive analyses were performed using SAS Enterprise Guide v7.1 (SAS Institute), and heatmaps reflecting the distribution of class-defining indicators, ±1 standard deviation from the overall mean, across the candidate subtypes were visualized using Microsoft Excel. All LCA models were estimated using Latent GOLD 6.0 software (Statistical Innovations Inc.).
Results
Sample Characteristics
A total of 20,745 sepsis survivors were included, with a median age of 60 years; 52% were female, 27% were Black, and 68% were White (Table 1). Patients had a median Charlson comorbidity score of 5 (interquartile range, 3–8) and experienced a median of 2 organ failures (interquartile range, 1–3) during sepsis hospitalization. Within 30 days of hospital discharge, 724 (4%) died and 4,614 (22%) experienced readmission.
Table 1.
Description of hospital survivors with sepsis (N = 20,745)
| n (%) or Median (IQR) | |
|---|---|
| Age, yr | 60 (48–70) |
| Sex | |
| Female | 10,878 (52.4) |
| Male | 9,867 (47.6) |
| Race | |
| Black | 5,488 (26.5) |
| White | 14,096 (68.0) |
| Other | 1,161 (5.6) |
| CCI | 5 (3–8) |
| BMI | |
| Underweight | 888 (4.3) |
| Normal weight | 5,705 (27.5) |
| Overweight | 5,634 (27.2) |
| Obese | 8,518 (41.1) |
| Count of failed organs | 2 (1–3) |
| Admitted to ICU | 8,186 (39.5) |
| Hospital LOS, d | 7 (5–11) |
| Discharge location | |
| Home | 11,611 (55.6) |
| Home with health services | 4,372 (21.1) |
| SNF | 3,531 (17.0) |
| LTACH or rehabilitation | 888 (4.3) |
| Other acute hospital | 343 (1.7) |
| Outcomes | |
| 30-d readmission | 4,614 (22.2) |
| 30-d mortality after discharge | 724 (3.5) |
Definition of abbreviations: BMI = body mass index; CCI = Charlson Comorbidity Index; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; LTACH = long-term acute care hospital; SNF = skilled nursing facility.
Identification of Sepsis Survivor Subtypes
We considered models with one to six latent classes and selected the five-class model as optimal. Model fit statistics showed improving fit with increasing number of classes (see Table E2), with a plateau toward the five-class model. Increasing classes above five led to an increase in class assignment uncertainty and the emergence of classes comprising <8% of patients. The five-class model included class proportions between 14% and 28%, with high mean class probabilities (76–87%), and had the strongest interpretation.
Class-Defining Features of Sepsis Survivor Subtypes
Figure 2 shows the relative proportion of each indicator across the subtypes. On the basis of the distribution of these features, we named the five classes as follows: 1) low risk, barriers to care; 2) previously healthy with severe illness and complex needs after discharge, barriers to care; 3) multimorbidity; 4) poor functional status; and 5) existing poor health with severe illness and complex needs after discharge. The subtype low risk, barriers to care made up 24% of the cohort and was characterized by a high frequency of Medicaid or self-pay status and a low frequency of multimorbidity, organ support, and inpatient mobilization therapy. The subtype previously healthy with severe illness and complex needs after discharge, barriers to care made up 14% of the cohort, characterized by a high frequency of Medicaid or self-pay status; high frequencies of organ support, delirium, prolonged hospital stay, new device or wound acquisition, and polypharmacy; but a low frequency of multimorbidity. The subtype poor functional status comprised 14% of the cohort and had high frequencies of frailty and discharge to a post–acute care facility, with a low frequency of prolonged hospital stay. The multimorbidity subtype made up 28% of the cohort, characterized by high frequencies of comorbid conditions, immunosuppressed status, and frequent prior hospitalization and low frequencies of organ support, prolonged hospital stay, new device or wound acquisition, and frailty. Finally, the subtype existing poor health with severe illness and complex needs after discharge had high frequencies of comorbid conditions, frequent prior hospitalization, prolonged hospital stay, organ support, delirium, new device or wound acquisition, inpatient mobilization therapy, polypharmacy, and discharge to a post–acute care facility, with a low frequency of Medicaid or self-pay status.
Figure 2.
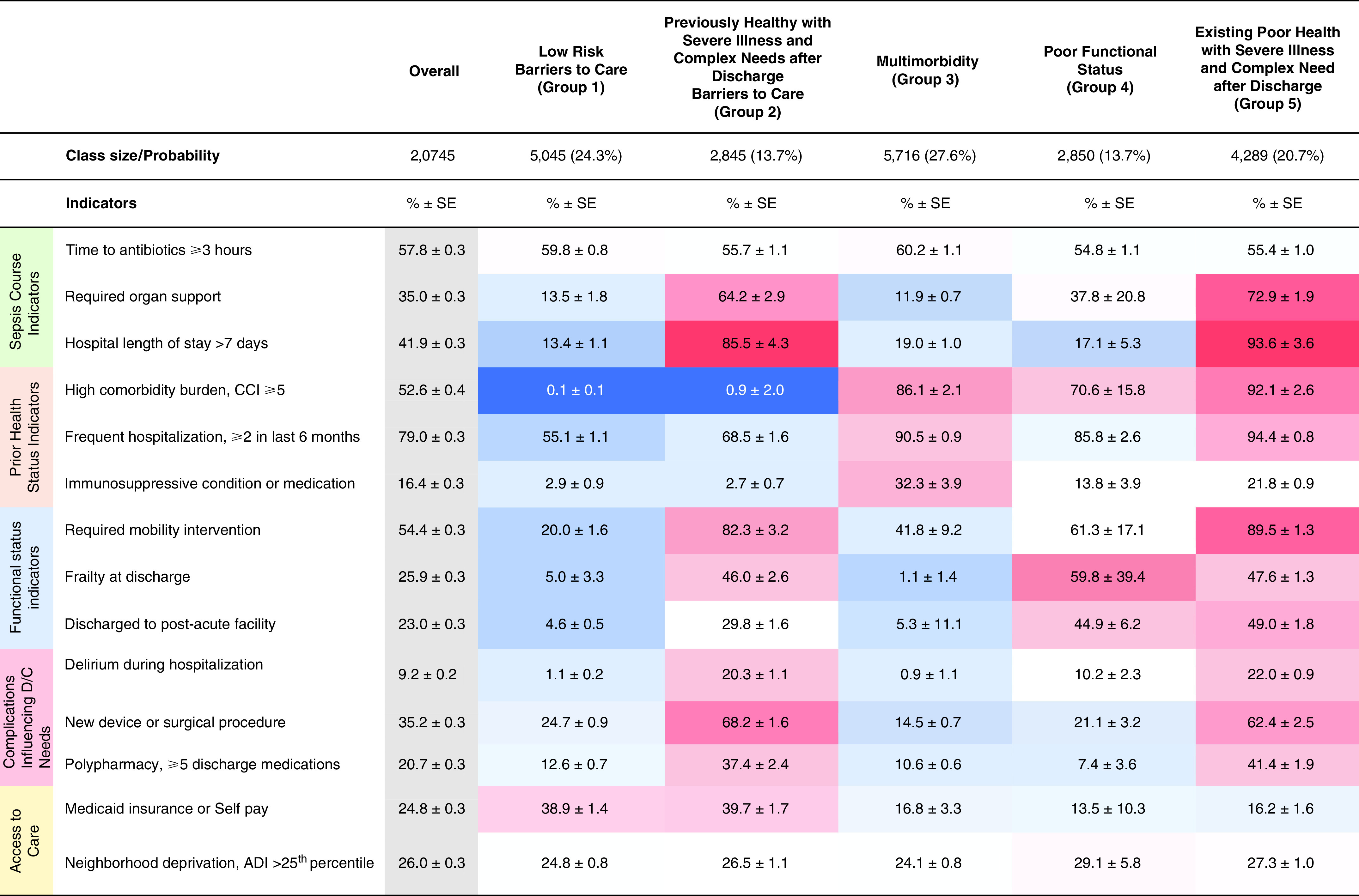
Heatmap displaying the standardized mean values for each variable across groups. The heatmap is shaded according to the proportion of each indicator in the five latent classes. Values represent a relative increase (red) or decrease (blue) from the mean of the proportion in the overall cohort, with darker gradients depicting larger relative differences (i.e., up to ±1 standard deviation from the mean). ADI = Area Deprivation Index; CCI = Charlson Comorbidity Index; D/C = discharge; SE = standard error.
Table 2 shows clinical characteristics of the five subtypes. The subtype low risk, barriers to care was younger on average and the subtype poor functional status was older on average compared with the other subtypes, but age was widely distributed in all groups. The multimorbidity subtype had a higher proportion of patients with malignancy, and the subtype poor functional status had a higher proportion of patients with dementia compared with other subtypes.
Table 2.
Distribution of individual characteristics across the five-class model
| Characteristic | Overall | Low Risk, Barriers to Care (Group 1) | Previously Healthy with Severe Illness and Complex Needs after Discharge, Barriers to Care (Group 2) | Multimorbidity (Group 3) | Poor Functional Status (Group 4) | Existing Poor Health with Severe Illness and Complex Needs after Discharge (Group 5) |
|---|---|---|---|---|---|---|
| Age at admission, yr, mean ± SE | 58.2 ± 10.53 | 45.1 ± 26.51 | 50.4 ± 35.12 | 63.2 ± 22.14 | 68.8 ± 40.33 | 65.3 ± 21.97 |
| Sex, % ± SE | ||||||
| Female | 52.4 ± 0.35 | 57.9 ± 0.78 | 47.8 ± 1.10 | 49.5 ± 0.77 | 54.1 ± 1.30 | 51.8 ± 0.87 |
| Male | 47.6 ± 0.35 | 42.1 ± 0.78 | 52.2 ± 1.10 | 50.5 ± 0.77 | 45.9 ± 1.30 | 48.2 ± 0.87 |
| Race, % ± SE | ||||||
| Black | 26.5 ± 0.31 | 23.4 ± 0.67 | 25.5 ± 0.96 | 25.9 ± 0.68 | 28.5 ± 1.18 | 30.2 ± 0.80 |
| White | 68.0 ± 0.32 | 65.9 ± 0.75 | 67.6 ± 1.03 | 69.9 ± 0.71 | 70.3 ± 1.20 | 66.5 ± 0.82 |
| Other | 5.6 ± 0.16 | 10.8 ± 0.48 | 6.9 ± 0.55 | 4.3 ± 0.31 | 1.2 ± 0.39 | 3.3 ± 0.31 |
| Coexisting conditions, % ± SE | ||||||
| CHF | 19.3 ± 0.26 | — | — | 28.6 ± 0.69 | 27.4 ± 1.14 | 37.2 ± 0.84 |
| COPD | 36.7 ± 0.32 | 13.1 ± 0.61 | 12.3 ± 0.86 | 52.4 ± 0.77 | 45.5 ± 1.30 | 53.9 ± 0.87 |
| Dementia | 4.4 ± 0.14 | — | — | 2.7 ± 0.29 | 13.6 ± 0.79 | 8.8 ± 0.49 |
| Diabetes | 37.6 ± 0.31 | 9.2 ± 0.57 | 7.5 ± 0.82 | 53.1 ± 0.77 | 49.9 ± 1.30 | 62.2 ± 0.85 |
| Metastatic cancer | 4.2 ± 0.13 | — | — | 10.4 ± 0.44 | 0.6 ± 0.43 | 5.7 ± 0.39 |
| Nonmetastatic cancer | 13.1 ± 0.22 | — | — | 31.5 ± 0.69 | 4.1 ± 0.76 | 18.7 ± 0.66 |
| Renal disease | 24.6 ± 0.27 | — | — | 39.5 ± 0.74 | 32.6 ± 1.20 | 44.7 ± 0.86 |
| Liver disease | 16.9 ± 0.35 | 3.0 ± 0.35 | 3.8 ± 0.52 | 32.5 ± 0.65 | 13.8 ± 0.89 | 24.5 ± 0.68 |
| Site of infection, % ± SE | ||||||
| Respiratory | 38.0 ± 0.33 | 27.9 ± 0.72 | 48.6 ± 1.10 | 36.5 ± 0.74 | 38.1 ± 1.30 | 44.9 ± 0.87 |
| Genitourinary | 36.4 ± 0.33 | 40.0 ± 0.77 | 28.2 ± 1.00 | 34.7 ± 0.74 | 49.4 ± 1.30 | 31.3 ± 0.82 |
| Bacteremia, site unspecified | 8.5 ± 0.19 | 7.8 ± 0.42 | 11.8 ± 0.69 | 6.8 ± 0.38 | 3.9 ± 0.60 | 12.4 ± 0.56 |
| Abdominal | 3.5 ± 0.13 | 4.5 ± 0.33 | 1.0 ± 0.25 | 6.5 ± 0.37 | 1.0 ± 0.36 | 1.9 ± 0.24 |
| Wound/soft tissue | 3.6 ± 0.13 | 7.5 ± 0.40 | 4.3 ± 0.43 | 2.0 ± 0.22 | — | 3.2 ± 0.29 |
| Other | 9.9 ± 0.21 | 12.3 ± 0.51 | 6.2 ± 0.54 | 13.4 ± 0.22 | 7.6 ± 0.71 | 6.4 ± 0.44 |
| Infection severity, mean ± SE | ||||||
| Maximum WBC, 103/ml | 14.9 ± 5.53 | 15.4 ± 11.51 | 17.3 ± 19.33 | 12.7 ± 12.46 | 14.6 ± 19.04 | 15.7 ± 15.09 |
| Maximum temperature, °F | 100.3 ± 1.24 | 100.4 ± 2.79 | 100.6 ± 4.38 | 100.1 ± 2.65 | 100.1 ± 4.33 | 100.4 ± 3.19 |
| Maximum respiratory rate, breaths/min | 28.3 ± 8.26 | 26.6 ± 13.84 | 32.9 ± 40.14 | 26.2 ± 14.74 | 29.0 ± 33.01 | 29.7 ± 18.77 |
| Minimum SBP, mm Hg | 94.2 ± 13.27 | 97.8 ± 26.76 | 86.1 ± 43.68 | 101.4 ± 29.11 | 90.6 ± 50.38 | 88.3 ± 37.25 |
Definition of abbreviations: CHF = congestive heart failure; COPD = chronic obstructive pulmonary disease; SBP = systolic blood pressure; SE = standard error; WBC = white blood cell count.
Association between Subtype Membership and 30-Day Outcomes
We found significant differences (Figure 3) among subtypes and 30-day readmission, with the highest proportion of readmissions in the subtype existing poor health with severe illness and complex needs after discharge (35%) and the lowest proportion of readmissions in the subtype low risk, barriers to care (9%). In the analysis of readmissions for ambulatory care–sensitive conditions, the subtype poor functional status had the highest proportion of readmissions for ambulatory care–sensitive conditions (47%) and the subtype previously healthy with severe illness and complex needs after discharge, barriers to care had the lowest proportion of readmissions for ambulatory care–sensitive conditions (17%). We also found significant differences among subtypes and 30-day mortality, with the highest proportion of deaths in the group with existing poor health with severe illness and complex needs after discharge (8%) and the lowest proportion of deaths in the group with low risk, barriers to care (0.1%). Key distinguishing characteristics of each subtype, overall prevalence, and outcomes are summarized in Table 3.
Figure 3.
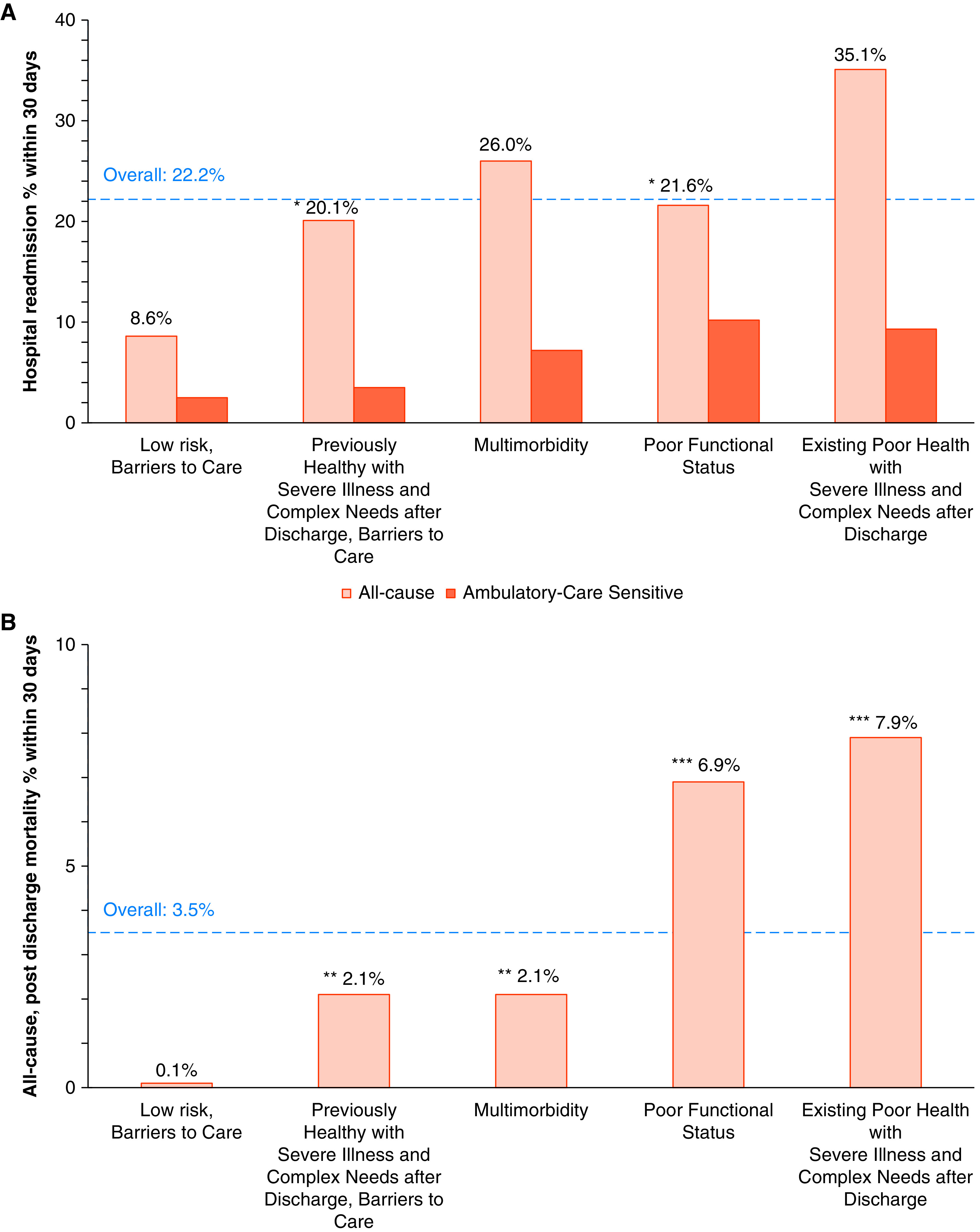
(A and B) Comparison of 30-day hospital readmission (A) and mortality outcomes (B) for five-class model. P < 0.01 for all pairwise comparisons unless otherwise indicated. *P = 0.29, **P = 0.99, and ***P = 0.19 for paired comparisons.
Table 3.
Summary description of five-class model key findings
| Low Risk, Barriers to Care (Group 1) | Previously Healthy with Severe Illness and Complex Needs after Discharge, Barriers to Care (Group 2) | Multimorbidity (Group 3) | Poor Functional Status (Group 4) | Existing Poor Health with Severe Illness and Complex Needs after Discharge (Group 5) | |
|---|---|---|---|---|---|
| Class size | 5,045 (24.3%) | 2,845 (13.7%) | 5,716 (27.6%) | 2,850 (13.7%) | 4,289 (20.7%) |
| Class-defining variables | • Financial barriers to care • Few comorbidities • Infrequent organ support |
• Financial barriers to care • Required organ support • Delirium • Long hospital stay • Frail at discharge • Acquired new device/wound • Polypharmacy at discharge |
• Immunosuppressed • High comorbidity • Frequent hospitalization • Infrequent organ support |
• Frail at discharge • High functional needs • Discharge to facility |
• High comorbidity • Frequent hospitalization • Required organ support • Delirium • Long hospital stay • Acquired new device/wound • High functional needs |
| Baseline characteristics | Younger Female GU, SSTI infections |
Younger Male Respiratory, bloodstream infections |
Older Malignancy Abdominal infections |
Older Dementia GU infections |
Older Respiratory, bloodstream infections |
| Mortality, % ± SE | 0.1 ± 0.12 | 2.1 ± 0.35 | 2.1 ± 0.25 | 6.9 ± 0.63 | 7.9 ± 0.46 |
| Readmission, % ± SE | 8.6 ± 0.48 | 20.1 ± 0.88 | 26.0 ± 0.67 | 21.6 ± 1.11 | 35.1 ± 0.82 |
| Proportion of readmissions for ACS | 29.1% | 17.4% | 27.7% | 47.2% | 26.5% |
| Potential target intervention | Readmission rate low but second highest for ACS readmission: addressing healthcare access or community support may prevent readmission or index admission | Readmissions may be appropriate/necessary; specialized care program such as post-ICU clinic or sepsis navigator may be able to address high-acuity postdischarge needs. Addressing healthcare access may result in reduced severity at presentation | Specialty care follow-up and attention to interaction between sepsis and underlying comorbidities may reduce readmissions | Nearly half of readmissions potentially avoidable (good metric to track); geriatrics or physiatry/rehabilitation referral, infection prevention practices, ascertain goals of care | Focus on goal-concordant care; palliative care involvement, multicomponent recovery interventions likely required |
Definition of abbreviations: ACS = ambulatory care–sensitive condition; GU = genitourinary; ICU = intensive care unit; SE = standard error; SSTI = skin and soft tissue infection.
Discussion
Our study of more than 20,000 sepsis survivors identified five clinical subtypes with distinct profiles and clinically meaningful differences in risk for 30-day rehospitalization and mortality. These findings provide empirical support to the hypothesized and clinically observed heterogeneity of sepsis survivorship. The subtypes were identified using readily available EHR data obtained at the time of patient discharge, increasing their relevance for real-time decision making in the management of sepsis survivors at hospital discharge. Our results provide several important insights into the clinical impact of sepsis survivor subtypes and key differences to help inform individualized clinical care delivery and the design of clinical trials to test targeted interventions aimed at reducing postsepsis adverse events.
First, the crossover of characteristics among subtypes underscores that class membership reflects nuanced combinations and interactions of routinely measured sociodemographic factors and disease severity elements that cannot be represented by any one or a simple combination of observable features. The unique clustering patterns of characteristics provide a novel, foundational construct for understanding sepsis survivorship heterogeneity and outcome risk. Importantly, this approach has significant advantages over traditional variable-centered approaches. For example, a regression model including 14 variables and their interactions would be difficult to specify with adequate power, and multicollinearity among risk factors may mask important relationships. A cumulative risk index approach may accurately predict outcomes, but it assigns equal weight to each risk factor, assuming that the risk factors are interchangeable and do not interact. Thus, these approaches are not ideal to guide the development of interventions that target specific important risk factors.
Our LCA modeling approach allows the identification of subgroups with specific constellations of risk factors that can be targeted for intervention. For example, the subtype poor functional status may be an important group to target for additional postdischarge support, as this group had high mortality and readmissions rates, with nearly half of readmissions potentially preventable (i.e., for ambulatory care–sensitive conditions). Targeted interventions for this group of older, frail patients with high rates of dementia could include specialty geriatrics consultation (22), functional rehabilitation and support (23), and infection prevention practices. The subtype existing poor health with severe illness and complex needs after discharge may benefit from being prioritized in the allocation of scarce specialized resources, such as post–intensive care unit clinics or specialty palliative care (24–26). If our subtypes are replicated in other settings, they will enable predictive enrichment to strategies to improve the suitability of patients to receive resource-intensive therapies and to refine enrollment in clinical trials (27).
Strengths and Limitations
Strengths of this study include the large sample size, which enhanced our ability to detect clinically distinguishable subtypes. Our data were collected from a robust sepsis database with few missing data. Furthermore, the cohort comprised patients from 12 diverse hospitals, which lends external validity to the findings. In the analytic model predicting outcomes from class membership, we applied a rigorous, best-practice approach to adjust for the known estimate bias due to classification error, which is often overlooked by other investigators using latent class methods (28). Importantly, we were able to create a parsimonious model using readily available clinical data to assign patients to subtypes.
Our study has important limitations. We did not include biomarkers of immune response, such as high-sensitivity C-reactive protein or soluble programmed death ligand 1 as class-defining variables. Although differences in biomarker profile may contribute to heterogeneity among sepsis survivors (29), we aimed to identify subtypes that could be distinguished on the basis of readily accessible data to aid in real-time decision making, and current biomarker-guided strategies have neither improved outcomes in sepsis nor enhanced entry criteria for clinical trials (11). We did not have access to other potentially informative variables, such as postsepsis mental health or cognitive status, although we did include delirium as a hospital-course factor that is associated with subsequent neuropsychiatric disorders after discharge (30). Finally, although our health system maintains a large market share and we had access to readmission outcomes from more than 40 hospitals, some measurement error may exist when readmissions occurred at other hospitals.
Conclusions
Our results provide empirical evidence that sepsis survivors constitute distinct clinical subtypes. These findings provide a foundation for future studies to apply and test interventions targeted to the unique needs of each subtype.
Footnotes
Supported by the National Library of Medicine of the National Institutes of Health under award number R21LM013373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: S.P.T. and M.A.K. conceptualized and designed the work. S.-H.C. and M.A.K. acquired and prepared data for the work. S.P.T., B.C.B., and M.A.K. contributed to analysis and interpretation of data for the work. R.B. contributed to data collection and interpretation. S.P.T. and M.A.K. prepared the original draft of the work, and all authors contributed to revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, et al. International Forum of Acute Care Trialists Assessment of global incidence and mortality of hospital-treated sepsis: current estimates and limitations. Am J Respir Crit Care Med . 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 2. Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA . 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc . 2012;60:1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yende S, Austin S, Rhodes A, Finfer S, Opal S, Thompson T, et al. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med . 2016;44:1461–1467. doi: 10.1097/CCM.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S. Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA . 2017;317:530–531. doi: 10.1001/jama.2016.20468. [DOI] [PubMed] [Google Scholar]
- 6. Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med . 2015;43:738–746. doi: 10.1097/CCM.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med . 2014;190:62–69. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority—a WHO resolution. N Engl J Med . 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 9. Schmidt K, Worrack S, Von Korff M, Davydow D, Brunkhorst F, Ehlert U, et al. SMOOTH Study Group Effect of a primary care management intervention on mental health-related quality of life among survivors of sepsis: a randomized clinical trial. JAMA . 2016;315:2703–2711. doi: 10.1001/jama.2016.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor SP, Murphy S, Rios A, McWilliams A, McCurdy L, Chou S, et al. Effect of a multicomponent sepsis transition and recovery program on mortality and readmissions after sepsis Crit Care Med [online ahead of print] 16 Sep 2021; DOI: 10.1097/CCM.0000000000005300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prescott HC, Calfee CS, Thompson BT, Angus DC, Liu VX. Toward smarter lumping and smarter splitting: rethinking strategies for sepsis and acute respiratory distress syndrome clinical trial design. Am J Respir Crit Care Med . 2016;194:147–155. doi: 10.1164/rccm.201512-2544CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scicluna BP, Baillie JK. The search for efficacious new therapies in sepsis needs to embrace heterogeneity. Am J Respir Crit Care Med . 2019;199:936–938. doi: 10.1164/rccm.201811-2148ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prescott HC, Iwashyna TJ, Blackwood B, Calandra T, Chlan LL, Choong K, et al. Understanding and enhancing sepsis survivorship: priorities for research and practice. Am J Respir Crit Care Med . 2019;200:972–981. doi: 10.1164/rccm.201812-2383CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermunt JK, Magidson J.Latent class cluster analysisHagenaars JA, McCutcheon AL, editors. Applied latent class analysis. Cambridge, UK: Cambridge University Press; 200289–106. [Google Scholar]
- 15. Lanza ST. Latent class analysis for developmental research. Child Dev Perspect . 2016;10:59–64. doi: 10.1111/cdep.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rhee C, Zhang Z, Kadri SS, Murphy DJ, Martin GS, Overton E, et al. CDC Prevention Epicenters Program Sepsis surveillance using adult sepsis events simplified eSOFA criteria versus sepsis-3 Sequential Organ Failure Assessment criteria. Crit Care Med . 2019;47:307–314. doi: 10.1097/CCM.0000000000003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Halpern SD, Temel JS, Courtright KR. Dealing with death as an outcome in supportive care clinical trials. JAMA Intern Med . 2021;181:895–896. doi: 10.1001/jamainternmed.2021.1816. [DOI] [PubMed] [Google Scholar]
- 18.Agency for Healthcare Research and Quality. Quality indicator user guide: Prevention Quality Indicators (PQI) composite measures, v2021. Rockville, MD: Agency for Healthcare Research and Quality; 2021. [updated 2021 July; accessed 2021 Aug 7]. Available from: https://qualityindicators.ahrq.gov/Downloads/Modules/PQI/V2021/PQI_Composite_Measures.pdf [Google Scholar]
- 19.Nylund KL, Asparouhov T, Muthén BO.Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study Struct Equ Modeling 200714535–569.. [Google Scholar]
- 20. Celeux G, Soromenho G. An entropy criterion for assessing the number of clusters in a mixture model. J Classif . 1996;13:195–212. [Google Scholar]
- 21. Bolck A, Croon MA, Hagenaars JA. Estimating latent structure models with categorical variables: one-step versus three-step estimators. Polit Anal . 2004;12:3–27. [Google Scholar]
- 22. Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med . 2013;11:48. doi: 10.1186/1741-7015-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chao PW, Shih CJ, Lee YJ, Tseng CM, Kuo SC, Shih YN, et al. Association of postdischarge rehabilitation with mortality in intensive care unit survivors of sepsis. Am J Respir Crit Care Med . 2014;190:1003–1011. doi: 10.1164/rccm.201406-1170OC. [DOI] [PubMed] [Google Scholar]
- 24. Sevin CM, Bloom SL, Jackson JC, Wang L, Ely EW, Stollings JL. Comprehensive care of ICU survivors: Development and implementation of an ICU recovery center. J Crit Care . 2018;46:141–148. doi: 10.1016/j.jcrc.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haines KJ, McPeake J, Hibbert E, Boehm LM, Aparanji K, Bakhru RN, et al. Enablers and barriers to implementing ICU follow-up clinics and peer support groups following critical illness: the Thrive collaboratives. Crit Care Med . 2019;47:1194–1200. doi: 10.1097/CCM.0000000000003818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Courtright KR, Jordan L, Murtaugh CM, Barrón Y, Deb P, Moore S, et al. Risk factors for long-term mortality and patterns of end-of-life care among Medicare sepsis survivors discharged to home health care. JAMA Netw Open . 2020;3:e200038. doi: 10.1001/jamanetworkopen.2020.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stanski NL, Wong HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol . 2020;16:20–31. doi: 10.1038/s41581-019-0199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bray BC, Lanza ST, Tan X. Eliminating bias in classify-analyze approaches for latent class analysis. Struct Equ Modeling . 2015;22:1–11. doi: 10.1080/10705511.2014.935265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, et al. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open . 2019;2:e198686. doi: 10.1001/jamanetworkopen.2019.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brown KN, Soo A, Faris P, Patten SB, Fiest KM, Stelfox HT. Association between delirium in the intensive care unit and subsequent neuropsychiatric disorders. Crit Care . 2020;24:476. doi: 10.1186/s13054-020-03193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]