Abstract
Background:
Childhood asthma carries significant morbidity.
Aim/Objectives:
Aim of the study was to compare efficacy of 2 commonly used therapies for asthma control in children with asthma.
Methods:
This was a 1-year, prospective cohort study at a tertiary care children's hospital. Patients were referred by their primary care physicians (PCPs) for asthma control. All patients were on low-dose inhaled corticosteroids (ICSs) at baseline. They were either switched to medium-dose ICS (ICS group) or medium-dose ICS and long-acting beta agonist (ICS+LABA group). Results were compared over time and between both groups.
Results:
Our cohort included 163 children (ages 2–18 years) with mean age of 5.62 ± 3.61 years. Mean Asthma Control Test (ACT) score at baseline was 15.9 ± 5.4. Mean ACT and percent predicted forced expiratory volume in one second improved (P < 0.0001 for both) in both groups. Median emergency department visits, short courses of oral steroids, and unscheduled PCP visits for acute asthma significantly decreased (P < 0.001 for all) in both groups. Similarly, days/month with wheezing, nighttime cough, and missed school days significantly decreased in both groups (P < 0.001 for all). Patients in ICS group were more likely to fail to achieve asthma control compared to patients in ICS+LABA group.
Conclusion:
Our study suggests that in children with uncontrolled asthma on low-dose ICS, switching to either medium-dose ICS or medium-dose ICS+LABA resulted in better symptom control, ACT improvement, and less asthma exacerbations over time. ICS+LABA had the additional benefit of less risk of treatment failure when compared to medium-dose ICS.
Keywords: asthma, children, ICS
Introduction
Asthma is a major public health problem worldwide, which, when uncontrolled, can severely limit patient's daily life.1–3 The long-term goals of asthma management are to achieve good control of symptoms and to minimize future risk to the patient, including exacerbations.2
National Heart Lung and Blood Institute (Expert Panel Report-3) (NHLBI (EPR-3)) asthma guideline (2007) for step 2 at all ages prefers low-dose inhaled corticosteroid (ICS) as maintenance treatment.3 For step 3 in children 12 years and older and adults, preferred choices are low-dose ICS+long-acting beta agonist (LABA) or medium-dose ICS, and for children 5–11 years, low-dose ICS+ either LABA or leukotriene receptor antagonist (LTRA) or medium-dose ICS, while in children younger than 5 years, preferred is medium-dose ICS.3 Data suggest that for adult or adolescent patients not previously using controller treatment, a combination of low-dose ICS and LABAs as the initial maintenance controller treatment reduces symptoms and improves lung function compared with low-dose ICS alone. However, it is more expensive and does not further reduce the risk of exacerbations compared with ICS alone.4 Number of recent studies5–9 lead to changes in Global Initiative for Asthma (GINA) guidelines. Recent updated GINA guidelines (2020)2 for step 2 in adults and children older than 12 years prefers either low-dose ICS or low-dose ICS+LABA. It also prefers as-needed low-dose ICS+LABA as reliever. In children 5–11 years old, preferred for step 2 is low-dose ICS, and for step 3, it is either low-dose ICS+LABA or medium-dose ICS. In children younger than 5 years, preferred is low-dose ICS, and for step 3, it is medium-dose ICS. Neither guideline recommend LABA as preferred in children younger than 5 years of age. For step 2, current GINA guidelines also recommend (as alternative) as-needed low-dose ICS with as-needed short-acting beta agonist (SABA) in all ages in patient with milder asthma.8
Both NHLBI (EPR-3)3 and GINA2 asthma guidelines recommend that if asthma control is not adequate on low-dose ICS, it is recommended to step up therapy (step 3) after checking for common problems such as inhaler technique, adherence, persistent allergen exposure, and comorbidities.2,3 There are limited data on children as most of the studies are in adults with some adolescents in those studies. Bisgaard et al. have shown that in children (4–11 years old) on low-dose ICS for uncontrolled asthma, ICS+LABA fixed combination was not better than increased ICS dose in reducing asthma exacerbation. They also revealed that use of ICS+LABA both as maintenance and as-needed symptom relief reduced the exacerbation rate compared to both fixed-dose combination and higher fixed-dose ICS alone in children with asthma.9
As there are not enough studies on use of ICS+LABA in children younger than 6 years of age, they are not commonly used by clinicians in younger children. In our study, most of the patients were <12 years of age. Their asthma was not controlled on low-dose ICS. Primary objective of this study was to compare, in pragmatic study design, between 2 treatment options, namely increasing ICS dose to medium-dose ICS versus medium-dose ICS+LABA in single fixed-dose inhaler in children. We monitored outcomes over the 1-year follow-up period in both groups. We monitored acute care need (hospital admissions, emergency department [ED] visits, and urgent care visits), school days missed, lung function tests (pulmonary function test [PFT]), asthma control test (ACT), and symptom scores (number of days/months of albuterol use, wheezing, nighttime cough, and exercise-related symptoms). Improvement was noted for duration of the study and between groups.
Methods
Design
This was a 1-year, prospective cohort study at a tertiary care children's hospital. Patients were referred by their primary care for asthma control. Results were compared over time and between 2 groups of patients. Total number of patients was 163. All these patients were receiving a total daily dose of ICS between 160 and 200 μg/day divided into 2 doses/day. One group (ICS group) (n = 106, 65%) had patients in which the dose of ICS was increased to medium dose (320–440 μg/day divided bid) and second group (n = 57, 35%) had patients in which not only ICS dose was increased to medium dose (320–440 μg/day divided bid) but also a LABA was added as a single fixed-dose combination inhaler (ICS+LABA group). None of the patients received as-needed ICS or as-needed ICS+LABA therapy. NHLBI (EPR-3) guidelines were followed. At each visit, patients/families completed a questionnaire, including the ACT and acute care needs since their last clinic visit, including ED, urgent care visits, unscheduled primary care physician (PCP) visits for acute care, short courses of oral steroids, missed school days, and mean symptom scores (mean number of days/month with wheezing, nighttime cough, and exercise limitations) and mean number of days/month with albuterol use for rescue.
Participants
After receiving approval from our local Institutional Review Board (IRB11-00174), children with the diagnosis of persistent asthma referred by local primary care practices, between January 2012 and December 2015, were enrolled in our pediatric asthma clinics. Inclusion criteria were children, age 2 to 18 years, with persistent asthma. NHLBI (EPR-3) guidelines were followed. All patients were on low-dose ICS (step 2), but their asthma was not controlled. Patients were still having asthma symptoms (cough/wheezing) more than 2 days/week requiring SABAs. Primary caregivers who could communicate in English accompanied the children. Exclusion criteria included children less than 2 years or >18 years old, children without uncontrolled persistent asthma, children with coexistent morbidities such as prematurity, cystic fibrosis, sickle cell disease, or any other pulmonary disease, and children with developmental delay or congenital anomalies or cardiac issues. History of atopic disease (eczema or environmental allergies) was not collected. Patients with intermittent asthma (asthma symptoms of cough/wheezing less than 2 days/week during daytime and less than 2 nights/month, short-acting albuterol use of less than 2 times/week, and no hospital admission or ED visits for acute asthma in last 1 year) were not included in this study. Treatment failure was defined as increased day to day symptoms (cough and wheezing) and/or acute care need (hospital admission, ED, or urgent care visit) when medications needed to be changed because of failure to control asthma. Written consent and assent were waived by the IRB.
Intervention
Instruments: demographic and clinical data form
Families were asked to complete a questionnaire at each visit, which included demographics, asthma-related symptoms, and acute care needs since the prior visit. Demographic and clinical data included age at diagnosis, age at first visit, duration of symptoms, gender, race/ethnicity, family history of asthma, second-hand smoking exposure, and pets in the home. Asthma severity was categorized as mild persistent, moderate persistent, or severe persistent. Clinical data included acute care need questions completed by caregivers or patients if they were 12 years or older at each visit, and included the number of hospital admissions, ED visits, urgent care visits, primary care physician visits for acute symptoms, school days missed, and number of short courses of oral steroids since the last visit. Data also included Asthma Control Test™ (ACT),10 which was completed by caregivers for children ages 4–11 years or by patients 12 years or older to evaluate asthma control over the previous 4 weeks. Low scores indicated poor asthma control. Symptom score data included the number of days/month albuterol was used for acute asthma symptoms, experienced daytime wheezing, nighttime cough, and/or exercise-related limitations since last visit. In children above 5 years of age, PFTs were conducted and percent predicted forced expiratory volume in 1 s (ppFEV1), percent predicted forced expiratory flow between 25% and 75% FVC exhaled (ppFEF25–75), and forced expiratory volume in 1 s/forced vital capacity (FEV1/FVC) were also measured at follow-up visits.
Data were collected at the first clinic visit (baseline), and then at the 3-, 6-, 9-, and 12-month follow-up visits, as determined by the provider. Missing data values were not included.
Statistical analysis
Baseline data were summarized using frequencies and percentages if categorical, means and standard deviations (SD) if continuous and normally distributed, and medians with interquartile ranges if continuous and skewed. Linear mixed-effects models were used to evaluate longitudinal change in ACT and PFT outcomes over 1 year. Generalized linear mixed-effects model with Poisson link was used for analysis of longitudinal outcomes that are count data (ED visit, urgent care visit, primary care visit for acute care, short courses of oral steroids, school days missed, mean number of days/month with wheeze, nighttime cough, exercise-related symptoms, and albuterol use for acute care). To determine whether the trajectory of outcomes differed by treatment groups, a time to treatment group interaction was assessed. Treatment failure was defined as when medications needed to be changed because of failure to control asthma. Time to treatment failure was summarized using Kaplan Meier plots and compared across treatment groups using the log-rank test. The proportion of treatment failures between treatment groups was compared utilizing the χ2 test. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Demographic and clinical asthma indicators
Overall, our cohort included 163 patients. Among them, there were 26 patients with mild persistent asthma (16%), 112 patients with moderate persistent asthma (69%), and 25 had severe asthma (15%) at the time of initial evaluation. The mean age was 5.62 ± 3.61 years. In our cohort, 99 children (60%) were between 2 and 6 years of age, 52 children (32.5%) children were between 6 and 11 years, and only 12 children (7.5%) were >12 years of age. There were 96 males (59%). One hundred two patients were Caucasian (63%), 45 were African American (27.6%), 13 were Hispanic (8%), and remaining 3 were Asian Americans. One hundred twenty-five patients had a family history of asthma (77%). Fifty-one patients had active second-hand smoke exposure (31%) and pet exposure was found in 87 patients (53%) (Table 1).
Table 1.
Patient Characteristics and Baseline Outcomes in Total Cohort and Subgroups
| Variables | Overall |
ICS |
ICS+LABA |
P |
|---|---|---|---|---|
| N = 163 | N = 106 | N = 57 | ||
| Asthma severity | <0.0001 | |||
| Mild | 26 (16%) | 25 (23%) | 1 (2%) | |
| Moderate | 112 (69%) | 74 (70%) | 38 (67%) | |
| Severe | 25 (15%) | 7 (7%) | 18 (31%) | |
| Demographics | ||||
| Age (years) | 5.6 ± 3.6 | 4.4 ± 2.6 | 8.4 ± 4.0 | <0.0001 |
| Male | 96 (59%) | 68 (64%) | 28 (49%) | 0.06 |
| Caucasian | 102 (63%) | 71 (67%) | 31 (54%) | 0.11 |
| Family Hx asthma | 125 (77%) | 84 (79%) | 41 (72%) | 0.29 |
| Smoking exposure | 51 (31%) | 32 (30%) | 19 (33%) | 0.68 |
| Pet exposure | 87 (53%) | 57 (54%) | 30 (53%) | 0.89 |
| Baseline outcomes | ||||
| Asthma control test | 15.9 ± 5.4 | 16.4 ± 5.5 | 14.2 ± 5.1 | 0.02 |
| Hospital admissions | 0 (0, 1) | 0 (0, 0) | 0 (0, 1) | 0.26 |
| Emergency dept visits | 1 (0, 2) | 1 (0, 2) | 1 (0, 2) | 0.51 |
| Urgent care visits | 0 (0, 2) | 0 (0, 2) | 1 (0, 2) | 0.19 |
| Primary care visits | 2 (1, 4) | 2 (1, 5) | 2 (1, 4) | 0.82 |
| School days missed | 1 (0, 5) | 0 (0, 4) | 3 (0, 10) | 0.004 |
| Courses of oral steroids | 2 (0, 3) | 2 (1, 2) | 2 (0, 3) | 0.30 |
| Albuterol use (days/month) | 10.4 ± 9.3 | 9.7 ± 9.3 | 12.4 ± 9.3 | 0.02 |
| Wheezing (days/months) | 10.3 ± 8.7 | 9.1 ± 7.8 | 13.9 ± 9.9 | 0.002 |
| Nighttime symptoms | 12.1 ± 10.2 | 11.1 ± 9.3 | 14.6 ± 11.3 | 0.11 |
| Exercise symptoms | 10.0 ± 11.1 | 9.0 ± 10.9 | 13.3 ± 11.2 | 0.003 |
| PFT variables |
Overall N = 83 |
ICS group N = 39 |
ICS+LABA group N = 44 |
P |
| ppFEV1 | 90.3 ± 16.4 | 92.6 ± 15.6 | 88.20 ± 17.5 | 0.49 |
| FEV1/FVC | 82.7 ± 29.3 | 89.0 ± 28.0 | 77.73 ± 30.7 | 0.57 |
| ppFEF25–75 | 85.7 ± 10.2 | 87.9 ± 9.1 | 83.45 ± 10.9 | 0.26 |
FEV1/FVC, forced expiratory volume in 1 s/forced vital capacity; ICS, inhaled corticosteroids; LABA, long-acting beta agonist; PFT, pulmonary function test; ppFEF25–75, percent predicted forced expiratory flow between 25% and 75% FVC exhaled; ppFEV1, percent predicted forced expiratory volume in 1 s.
All patients (n = 163) were on low-dose ICS at the time of enrollment of study. Among them, 63 (38%) patients were also receiving LTRAs (Montelukast) at the time of enrollment, which was continued. During the study, 27 additional patients were started of LTRA because of continued symptoms or acute asthma exacerbations (10 in ICS+LABA group and 17 in ICS group). Eleven patients were able to discontinue LTRA, among them, 5 in ICS group and 6 in ICS+LABA group.
Over the 1-year follow-up period, in ICS group, because of ongoing symptoms or asthma exacerbations, LABA was added to medium-dose ICS in 20 patients and ICS was switched to different brand in 6 patients. We were able to decrease dose of ICS in 5 patients. During the same time, in ICS+LABA group, total daily dose of ICS was increased in 4 and decreased in 5 patients, and 4 patients were switched to low-dose ICS without LABA.
Mean ACT score at baseline was 15.9 ± 5.4. At initial evaluation (baseline), the median ED visits was 1 (Q1, Q3: 0, 2), acute care to primary care physicians (PCP) was 2 (1, 4), school days missed was 1 (0, 5), and courses of oral steroids/child was 2 (0, 3) during the year before enrollment in the study (Table 1).
Similarly, at time of enrollment (baseline), on average, Albuterol use and wheezing were noted about 10 days/month (10.4 ± 9.3) and (10.3 ± 8.7), respectively. Nighttime cough was reported an average of 12 days/month (12.1 ± 10.2) and exercise-related symptoms were reported 10 days/month (10.0 ± 11.1) (Table 1).
Groups were compared at baseline (initial visit). Patients in the ICS group were younger (4.4 ± 2.6 versus 8.4 ± 4) with less severe asthma. Mild persistent asthma was present in 23% of the ICS group versus 2% in combination group (P < 0.0001). ICS group patients had higher mean ACT scores (16.38 ± 5.5 versus 14.25 ± 5.1, P = 0.02), fewer days/month with wheezing, nighttime cough, and exercise symptoms, and less school days missed compared to patients in ICS+LABA group (P < 0.05 for all) (Table 1).
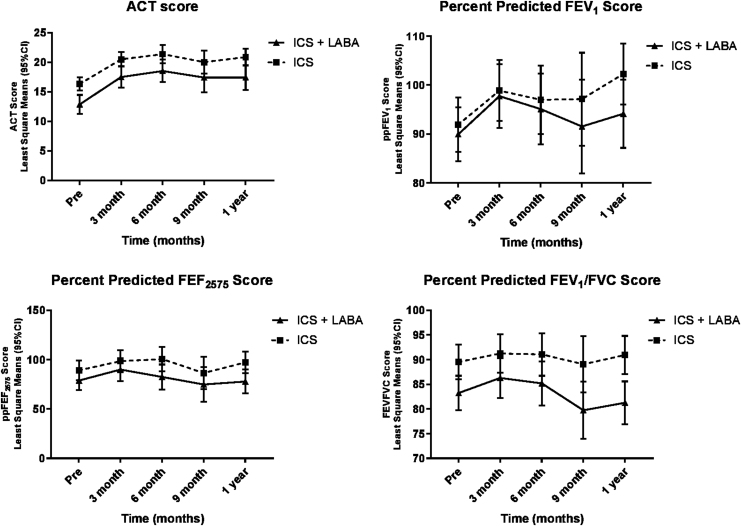
The multivariable linear mixed model for ACT, ppFEV1, and ppFEF25–75 revealed that both groups improved, and the rate of change was similar between both groups (interaction P value not significant), There was a significant change over time (P < 0.05), except for FEV1/FVC. All models were adjusted for age and severity (Fig. 1 and Table 2).
FIG. 1.
Mean change over time between groups in asthma control test and lung functions.
Table 2.
Mean Asthma Control Test and Lung Functions (ppFEV1, ppFEF25–75, and FEV1/FVC) by Treatment Groups Over Time (Mean ± SD)
| Variables | Baseline (mean ± SD) |
3 months (mean ± SD) |
6 months (mean ± SD) |
9 months (mean ± SD) |
1 year (mean ± SD) |
Change over time (P) | Difference between groups over time (P) |
|---|---|---|---|---|---|---|---|
| N = 148 | N = 108 | N = 67 | N = 76 | ||||
| Asthma control test | |||||||
| ICS | 16.4 ± 5.5 | 20.8 ± 4.8 | 21.3 ± 5.3 | 19.2 ± 6.7 | 21.1 ± 4.4 | ||
| ICS+LABA | 14.2 ± 5.1 | 18.5 ± 5.6 | 19.9 ± 6.2 | 16.8 ± 6.4 | 18.2 ± 6.3 | <0.0001 | 0.08 |
| ppFEV1 | |||||||
| ICS | 92.6 ± 15.6 | 96.8 ± 14.2 | 96.1 ± 14.5 | 92.0 ± 12.6 | 101.5 ± 14.5 | ||
| ICS+LABA | 88.2 ± 27.5 | 93.1 ± 10.7 | 89.8 ± 17.1 | 89.8 ± 17.1 | 90.6 ± 16.9 | <0.0001 | 0.59 |
| ppFEF2575 | |||||||
| ICS | 89.0 ± 28.0 | 93.3 ± 22.3 | 104.1 ± 35.9 | 78.2 ± 22.7 | 100.3 ± 28.3 | ||
| ICS+LABA | 77.7 ± 30.7 | 85.6 ± 22.5 | 72.2 ± 23.9 | 78.3 ± 38.4 | 74.7 ± 21.7 | 0.02 | 0.62 |
| FEV1/FVC | |||||||
| ICS | 87.9 ± 9.1 | 88.6 ± 7.9 | 90.1 ± 8.1 | 87.7 ± 12.4 | 90.6 ± 7.7 | ||
| ICS+LABA | 83.4 ± 10.9 | 85.7 ± 8.8 | 82.1 ± 10.4 | 77.7 ± 16.6 | 80.9 ± 9.9 | 0.12 | 0.47 |
SD, standard deviation.
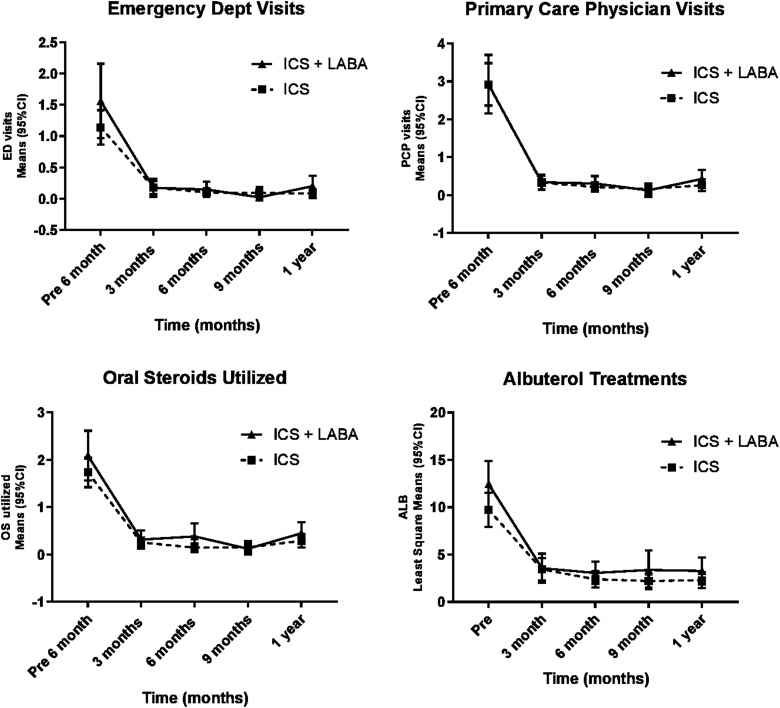
Similarly, there was decrease in the emergency room visits, primary care physician visits, oral steroid use, and albuterol treatments in both groups. Nevertheless, there was no difference in the rate of decrease between the 2 groups (interaction P value not significant) (Fig. 2 and Table 3). A similar pattern was noted in mean number of school days missed/month, nighttime cough, and wheezing. In both groups, there was improvement, but no statistically significant difference in improvement was noted in 1 group over the other (Table 4).
FIG. 2.
Acute care need over time between groups.
Table 3.
Change in Acute Care Need Over Time Between groups (Median [Interquartile Range])
| Variables | Baseline |
3 months |
6 months |
9 months |
1 year |
Change over time (P) | Difference between groups over time (P) |
|---|---|---|---|---|---|---|---|
| N = 163 | N = 106 | N = 137 | N = 127 | N = 124 | |||
| Emergency dept visits | |||||||
| ICS | 1 (0, 2) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | <0.0001 | 0.81 |
| ICS+LABA | 1 (0, 2) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | ||
| Primary care physician visits | |||||||
| ICS | 2 (1, 5) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | <0.0001 | 0.50 |
| ICS+LABA | 2 (1, 4) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | ||
| Oral steroids | |||||||
| ICS | 2 (1, 2) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | <0.0001 | 0.40 |
| ICS+LABA | 2 (0, 3) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 1) | ||
| Albuterol (mean days/month) | |||||||
| ICS | 6 (2, 15) | 2 (0, 4) | 1 (0, 2) | 1 (0, 2) | 1 (0, 3) | <0.0001 | 0.86 |
| ICS+LABA | 10 (5, 16) | 2 (1, 4) | 2 (1, 4) | 2 (0.5, 3) | 2 (0.5, 4) | ||
Table 4.
Symptom Control (School Days Missed, Nighttime Cough, and Wheezing) by Treatment Groups Over Time (Median [Interquartile Range])
| Variables |
Baseline |
3 months |
6 months |
9 months |
1 year |
Change over time (P) | Difference between treatment groups over time (P) |
|---|---|---|---|---|---|---|---|
| days/month | N = 163 | N = 163 | N = 137 | N = 126 | N = 124 | ||
| School days | |||||||
| ICS | 0 (0, 4) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | 0 (0, 0) | ||
| ICS+LABA | 3 (0, 10) | 0 (0, 2) | 0 (0, 2) | 0 (0, 1) | 0 (0, 2) | <0.0001 | 0.47 |
| Nighttime cough | |||||||
| ICS | 8 (4, 15) | 2 (0, 3) | 2 (0, 3) | 2 (1, 2) | 2 (1, 3) | <0.0001 | 0.99 |
| ICS+LABA | 15 (4, 30) | 2 (1, 5.5) | 2 (0, 2) | 2 (0.5, 3) | 2 (1, 4) | ||
| Wheezing | |||||||
| ICS | 7 (4, 12) | 2 (0, 3) | 1 (0, 2) | 1 (0, 2) | 2 (0.5, 2) | ||
| ICS+LABA | 10 (6, 20) | 2 (1, 4) | 2 (1, 4) | 2 (1, 3) | 2 (1, 5) | <0.0001 | 0.40 |
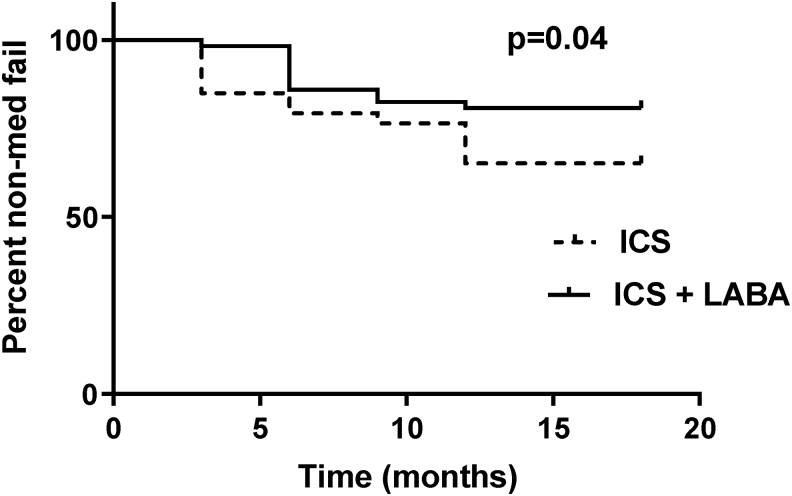
An interesting finding in our study is that patients in ICS group (doubling dose of ICS) were more likely to fail to achieve asthma control compared to patients in ICS+LABA group (Table 5 and Fig. 3). Subsequently, in patients who failed therapy, either dose of ICS was increased or their ICS brand was changed, or they were switched to combination therapy (ICS+LABA) and/or LTRA was added.
Table 5.
Treatment Success/Failure Comparison by Treatment Groups
| Treatment groups | Treatment success |
Treatment failure |
P |
|---|---|---|---|
| N = 115 | N = 48 | ||
| ICS | 69 (60%) | 37 (77%) | 0.04 |
| ICS+LABA | 46 (40%) | 11 (23%) |
FIG. 3.
Kaplan-Meier survival estimates indicating lower treatment failure rate for the ICS+LABA group. ICSs, inhaled corticosteroids; LABA, long-acting beta agonist.
Discussion
Our study focused on children with less than adequately controlled persistent asthma. We compared the difference in outcomes between 2 groups: first group (ICS group) in which dose of ICS was increased to medium dose versus second group (ICS+LABA group) in which not only was the dose of ICS increased to medium dose but also LABA was added in a single fixed combination inhaler.
In our study, with both therapies, we noted significant improvement over time in symptoms (days/month with wheezing, nighttime cough, and exercise-related symptoms), mean ACT score, and lung functions. With both therapies, we also noted significant decrease in asthma exacerbations (days/month of albuterol use for rescue, short courses of oral steroids, visits to ED, urgent care, and primary care physicians for acute asthma). Our results show that asthma control improved with both treatment options and there was no significant difference in outcomes between the groups. However, we noted that among our cohort, patients in ICS group were more likely to experience treatment failure and were more likely to be switched to group with combination therapy (ICS+LABA group). We also noted an inherent bias for patient selection between treatment groups, which was at least partially because of age. Providers prescribed ICS+LABA combination more often when patients were older and had more severe asthma, while medium-dose ICS was chosen more often for children who were either younger (under 6 years of age) or had milder asthma. Even with this limitation, treatment failure was noted more often in the ICS group.
Most of our patients, 151 out of 163 (92.5%), were less than 12 years old. All were already on low-dose ICS (step 2) and their asthma was not controlled. They were switched to step 3. We followed NHLBI (EPR-3) asthma guidelines (2007),3 which in children 5–11 years old prefer either low-dose ICS+LABA or low-dose ICS+LTRA or medium-dose ICS. For children younger than 5 years of age, step 3 preferred is medium-dose ICS, and for step 4 in this group, recommended therapy is ICS+ either LABA or LTRA.3 Thus, in children 5–11 years old, 1 of the preferred option is to increase ICS to medium dose3,11 and in this age group, the effect may be similar to12 or more effective13–15 than adding LABA.3 Recent GINA guidelines (2020)2 have recommended some changes based on few recent studies,5–9 but for children 6–11 years old, for step 3, still prefer either low-dose ICS+LABA or medium-dose ICS. For children 5 years and younger, preferred therapy for step 3 is still medium-dose ICS.
ICSs are the most effective monotherapy for long-term control of asthma in children.16 When ICS alone is insufficient to achieve asthma control, various options may be considered, such as increasing the dose of ICS17 or adding a second drug such as a LABA or a LTRA.18 High use of bronchodilators is associated with increased risk for asthma-related morbidity and overuse of SABAs (1.5–2 canisters/month) may increase risk of near-fatal asthma or even death.19–21 Safety of LABA is also widely debated over the last few years.22 Two previous clinical trials revealed that regular use of LABA might also be a risk factor for near-fatal or fatal asthma,23,24 but in those studies, many patients were not taking ICS as daily asthma medication and were using only LABA for asthma control.23,24 This led to meta-analysis of data comparing LABA versus non-LABA data, which revealed that those using LABA and ICS dispensed separately had higher rate of asthma-related deaths and hospitalization compared to those receiving non-LABA treatment.25 It was also noted that there were no asthma-related deaths or increased rates of hospital admissions when LABA was dispended in a fixed-dose combination with ICS.25,26 It is possible that when dispensed separately, patients may use LABA and be nonadherent with their ICS. These observations led to a large randomized double-blinded trial in adolescents and adults by Stempel et al., which revealed that when used with a fixed-dose combination with ICS, LABA are safe medications and their risk of serious asthma-related events is not higher than those of ICS alone.27 Study also showed that those receiving combination (ICS+LABA) had fewer severe asthma exacerbations than those receiving ICS alone.27
There are several studies supporting the fact that combining a LABA with ICSs leads to greater improvement in the control of symptoms and in lung function than simply doubling the dose of the ICS glucocorticoid.28,29 Pauwels et al.30 in their landmark study have revealed that in adults, adding LABA to ICS is not only safe but also does improve asthma control. In their year-long study, both increased dose of ICS and addition of LABA to low- and high-dose ICS led to a decrease in both mild and severe asthma exacerbations, and most pronounced effect was noted in the group receiving both high-dose ICS and LABA.30 This study revealed again that both increasing dose of ICS and adding LABA can improve asthma control.
Most of the studies comparing ICS with ICS+LABA are in adults or older adolescents. There are limited data on use of LABA in younger children (5–11-year old) with uncontrolled asthma. Two such studies in children with uncontrolled asthma using ICS revealed improvement in lung function and better symptom control with addition of LABA.31,32 In another study in children whose asthma was not adequately controlled with the moderate dose of ICS, addition of LABA was equivalent to doubling the dose of ICS.33 Another study in 4–11-year-old children with uncontrolled asthma on low-dose ICS revealed that the addition of a LABA to the ICS was better when compared to increasing the dose of the ICS or adding an LTRA.34 In a Cochrane meta-analysis, of children with persistent asthma, the addition of LABA to ICS was not associated with a significant reduction in the rate of exacerbations requiring systemic steroids, but it was superior for improving lung function compared with the same or higher doses of ICS.35
In our study, we noted a decrease in treatment failure among the group with ICS+LABA. It is possible that the difference in age between the groups might have influenced the rate of failure. Patients in ICS group, which had a higher failure rate, were significantly younger than those in ICS+LABA group and younger children are at increased risk for more symptoms and asthma exacerbations because of viral infection and day care center use, and some younger children may have less efficiency to properly use ICS or ICS+LABA inhalers. We also noted that there was no significant difference in rate of improvement between 2 groups. However, there was significant difference in age between both groups, which might have impacted result.
Limitations of our study include small sample size, very few adolescent patients, and lack of atopic history (eczema and environmental allergies). Another limitation includes not truly randomized sample by age as there was less use of LABA in younger children as NHLBI (EPR-3) guidelines preferred medium-dose ICS over adding LABA to ICS at younger age group.3 In addition, our study lacked a group with low-dose ICS+LABA. There were many reasons, including younger patients being started on medium-dose ICS before LABA can be added as per NHLBI (EPR-3) guidelines. Other reasons include use of fixed-dose combination inhalers and there are very few, if any, low-dose ICS+LABA fixed-dose combination metered-dose inhalers available on market, which are approved for younger age. There are also limitations placed by insurance companies on using ICS+LABA combination inhalers in younger group and we were not using diskus or any other dry powder delivery devices, but only metered-dose inhalers because of younger cohort. These limitations made it difficult to either have a separate group with low-dose ICS+LABA or to include more younger patients in ICS+LABA group. Given natural history of asthma in children, it is also not possible to quantify role of better adherence or improvement in asthma severity over time.
In summary, we looked at 2 treatment options and noted that both medium dose of ICS and adding LABA to medium dose of ICS were able to control asthma symptoms and acute care needs (asthma exacerbations) over time. However, the rate of improvement between groups was not significantly different. The only benefit we noted of adding LABA to the medium-dose ICS was a decrease in treatment failure among the group with ICS+LABA.
Conclusion
This study suggests that both medium-dose ICS and medium-dose ICS+LABA combination lead to improved mean ACT, mean ppFEV1, and decreased frequency of asthma symptoms and exacerbations. Medium-dose ICS+LABA had the additional benefit of less risk of treatment failure when compared to medium-dose ICS.
Authors' Contributions
Dr. Anas Al-Turki contributed to the literature review, study design, study conduct, data collection, and article preparation. A.S. and Dr. Bai contributed to the study design, data analysis and interpretation, and article preparation. Dr. Sheikh contributed to the study design, study conduct, data collection, and article preparation. He had full access to all of the data in the study and takes responsibility for data integrity, accuracy of the data analysis, and serves as the guarantor of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Global Initiative for Asthma (GINA), Global strategy for asthma management and prevention. Revised 2014. Available at https://ginasthma.org/wp-content/uploads/2019/01/2014-GINA.pdf (accessed December 5, 2019).
- 2. Global Initiative for Asthma. GINA Report, Global Strategy for Asthma Management and Prevention. Available at https://ginasthma.org/wp-content/uploads/2020/06/GINA-2020-report_20_06_04-1-wms.pdf (accessed December 5, 2019).
- 3. Guidelines for the Diagnosis and Management of Asthma (EPR-3). NHLBI. 2007. Available at https://www.nhlbi.nih.gov/files/docs/guidelines/asthsumm.pdf (accessed December 7, 2019).
- 4. Ni Chroinin M, Greenstone I, Lasserson TJ, Ducharme FM. Addition of inhaled long-acting beta2-agonists to inhaled steroids as first line therapy for persistent asthma in steroid-naive adults and children. Cochrane Database System Rev 2009:CD005307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med 2018; 378:1865–1876. [DOI] [PubMed] [Google Scholar]
- 6. Bateman ED, Reddel HK, O'Byrne PM, et al. as-needed budesonide-formoterol versus maintenance budesonide in mild asthma. N Engl J Med 2018; 378:1877–1887. [DOI] [PubMed] [Google Scholar]
- 7. Beasley R, Holliday M, Reddel HK, et al. Novel START study team. Controlled trial of budesonide-formoterol as needed for mild asthma. N Engl J Med 2019; 380:2020–2030. [DOI] [PubMed] [Google Scholar]
- 8. Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomized, double-blind, placebo-controlled trial. Lancet 2011; 377:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bisgaard H, Le Roux P, Bjåmer D, et al. Budesonide/formoterol maintenance plus reliever therapy: a new strategy in pediatric asthma. Chest 2006; 130:1733–1743. [DOI] [PubMed] [Google Scholar]
- 10. Childhood Asthma Control Test. Available at https://www.asthma.com/additional-resources/childhood-asthma-control-test.html (accessed October, 2017).
- 11. Ni Chroinin M, Lasserson TJ, Greenstone I, Ducharme FM. Addition of long-acting beta-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev 2009:CD007949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaessen-Verberne AA, van den Berg NJ, van Nierop JC, et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. Am J Respir Crit Care Med 2010; 182:1221–1227. [DOI] [PubMed] [Google Scholar]
- 13. Bisgaard H. Effect of long-acting beta2 agonists on exacerbation rates of asthma in children. Pediatr Pulmonol 2003; 36:391–398. [DOI] [PubMed] [Google Scholar]
- 14. Verberne AA, Frost C, Duiverman EJ, et al. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. Am J Respir Crit Care Med 1998; 158:213–219. [DOI] [PubMed] [Google Scholar]
- 15. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev 2010:CD005533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev 2012; 2012:CD002314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Adams NP, Bestall JC, Jones P, et al. Fluticasone at different doses for chronic asthma in adults and children. Cochrane Database Syst Rev 2008; 2008:CD003534. [DOI] [PubMed] [Google Scholar]
- 18. Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev 2014:CD003137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ernst P, Habbick B, Suissa S, et al. Is the association between inhaled beta-agonist use and life-threatening asthma because of confounding by severity? Am Rev Respir Dis 1993; 148:75–79. [DOI] [PubMed] [Google Scholar]
- 20. Spitzer WO, Suissa S, Ernst P, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med 1992; 326:501–506. [DOI] [PubMed] [Google Scholar]
- 21. Suissa S, Ernst P, Boivin JF, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med 1994; 149:604–610. [DOI] [PubMed] [Google Scholar]
- 22. Cates CJ, Cates MJ. Regular treatment with salmeterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev 2008; 3:CD006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ 1993; 306:1034–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson HS, Weiss ST, Bleecker ER, et al. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129:15–26. [DOI] [PubMed] [Google Scholar]
- 25. Levenson M. Long-acting beta-agonists and adverse asthma events meta-analysis. Statistical briefing package for Joint Meeting of the Pulmonary-Allergy Drugs Advisory Committee, Drug Safety and Risk Management Advisory Committee and Pediatric Advisory Committee on December 2008. Available at http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4398b1-01-FDA.pdf (accessed October 2016).
- 26. Weatherall M, Wijesinghe M, Perrin K, et al. Meta-analysis of the risk of mortality with salmeterol and the effect of concomitant inhaled corticosteroid therapy. Thorax 2010; 65:39–43. [DOI] [PubMed] [Google Scholar]
- 27. Stempel DA, Raphiou IH, Kral KM, et al. Serious asthma events with fluticasone plus salmeterol versus fluticasone alone. N Engl J Med 2016; 374:1822–1830. [DOI] [PubMed] [Google Scholar]
- 28. FitzGerald JM, Boulet LP, McIvor RA, et al. Asthma control in Canada remains suboptimal: The Reality of Asthma Control (TRAC) study. Can Respir J 2006; 13:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woolcock A, Lundback B, Ringdal N, Jacques LA. Comparison of addition of salmeterol to inhaled steroids with doubling of the dose of inhaled steroids. Am J Respir Crit Care Med 1996; 153:1481–1488. [DOI] [PubMed] [Google Scholar]
- 30. Pauwels RA, Löfdahl CG, Postma DS, et al. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med 1997; 337:1405–1411. [DOI] [PubMed] [Google Scholar]
- 31. Russell G, Williams DA, Weller P, Price JF. Salmeterol xinafoate in children on high dose inhaled steroids. Ann Allergy Asthma Immunol 1995; 75:423–428. [PubMed] [Google Scholar]
- 32. Zimmerman B, D'Urzo A, Berube D. Efficacy and safety of formoterol Turbuhaler when added to inhaled corticosteroid treatment in children with asthma. Pediatr Pulmonol 2004; 37:122–127. [DOI] [PubMed] [Google Scholar]
- 33. Vaessen-Verberne AA, van den Berg NJ, van Nierop JC, et al. Combination therapy salmeterol/fluticasone versus doubling dose of fluticasone in children with asthma. Am J Respir Crit Care Med 2010; 182:1221–1227. [DOI] [PubMed] [Google Scholar]
- 34. Lemanske RF Jr, Mauger DT, Sorkness CA, et al. Step-up therapy for children with uncontrolled asthma receiving inhaled corticosteroids. N Engl J Med 2010; 362:975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chauhan BF, Chartrand C, Ni Chroinin M, et al. Addition of long-acting beta2-agonists to inhaled corticosteroids for chronic asthma in children. Cochrane Database Syst Rev 2015; CD007949. [DOI] [PMC free article] [PubMed] [Google Scholar]