Abstract
Background:
Standard insulin infusion sets (IISs) are to be replaced every 2 to 3 days to avoid complications and diabetic ketosis due to set failure. This pivotal trial evaluated the safety and performance of a new extended-wear infusion set (EIS) when used for 7 days by adults with type 1 diabetes (T1D).
Methods:
This single-arm, nonrandomized trial enrolled adults (18–80 years of age) with T1D, who used their own MiniMed™ 670G system with insulin lispro or insulin aspart and the EIS for up to 7 days, across 12 consecutive wears. Safety endpoints included incidence of serious adverse events (SAEs), serious adverse device effects (SADEs), unanticipated adverse device effects (UADEs), severe hypoglycemia (SevHypo), severe hyperglycemia (SevHyper), diabetic ketoacidosis (DKA), and skin infection. The EIS failure rate due to unexplained hyperglycemia (i.e., suspected occlusion), the overall EIS survival rate, glycemic control outcomes (i.e., A1C, mean sensor glucose and time spent in established glucose ranges), total daily insulin delivered, and satisfaction with the EIS were determined.
Results:
The intention to treat population (n = 259, 48% men, 45.0 ± 14.1 years) wore a total of 3041 EIS devices. No SADE, UADE, or DKA events was reported. Overall rates of SAEs, SevHypo, SevHyper, and skin infection were 3.8, 2.5, 104.1, and 20.1 events per 100 participant-years. The rate of EIS failure due to unexplained hyperglycemia at the end of day 7 was 0.1% (95% confidence interval [CI]: 0.03–0.51) and 0.4% (95% CI: 0.16–1.00) for insulin lispro and aspart use, respectively. Overall EIS survival rate at the end of day 7 was 77.8% (95% CI: 76.2–79.3), glycemic control did not change, and participants reported greater satisfaction with the EIS compared with standard IISs worn before the study (P < 0.001).
Conclusions:
This investigation demonstrates that the EIS, when worn for up to 7 days, was safe and rated with high satisfaction, without adversely affecting glycemic control in adults with T1D.
Clinical Trial Registration number: NCT04113694 (https://clinicaltrials.gov/ct2/show/NCT04113694).
Keywords: Insulin infusion set, Survival rate, Failure rate, Unexplained hyperglycemia, Time in range, Adults
Introduction
Insulin infusion sets (IISs) in traditional continuous subcutaneous insulin infusion (CSII) pump systems deliver rapid-acting insulin analogs from the pump reservoir to the subcutaneous space through a stainless steel needle or a Teflon cannula held on the skin surface by an adhesive patch. Standard IISs should be changed every 2 to 3 days,1–3 regardless of rapid-acting insulin used,4 to prevent or reduce adverse events (e.g., site failure resulting in hyperglycemia, skin inflammation, infection or pain at the IIS site, adhesive patch failure, etc.). However, continuous glucose monitoring (CGM) systems often used in conjunction with CSII pump therapy can function for ≥7 days before a sensor change.
Thus, for users of both CSII and CGM technologies, the combined application and intervals of device change can be burdensome because of numerous adhesive patch removals and increased risk of skin irritation,5,6 skin reactions,5,6 or adhesive tape allergies,7 with minimal days for recovery. Extending the wear duration of IIS devices to closely align with that of CGM sensor change intervals would not only reduce diabetes management burden but also reduce IIS waste and insulin loss associated with more frequent IIS changes.
While early feasibility and pilot IIS studies showed significant device-related adverse events that included increased hyperglycemia and functional or mechanistic issues with IIS use during longer durations of wear,1–3 few randomized studies have reported failure and survival rates when IIS devices are worn out to 7 days.8,9 Overall findings from the latter determined that 7-day wear (irrespective of steel or Teflon cannula) did not adversely influence glycemic control, yet rates of IIS failure due to “unexplained hyperglycemia” (i.e., hyperglycemia >250 mg/dL and a failed correction bolus due to suspected IIS occlusion) ranged from a relatively high 19%9 to 30%.8
Since 2017, Medtronic began designing a new IIS with a longer wear duration and improved performance (i.e., reduced failure/increased survival rate) based on observations that insulin degradation and preservative loss increased the inflammatory response10 and insulin-associated inflammation contributed to standard IIS failure.11 Iterative advancements relative to earlier IIS devices designed for 2 to 3 days of wear included a new H-cap connector and tubing with new fluid path design that improve insulin preservative retention and stability,12 in addition to a new extended-wear adhesive patch that improves skin adherence.13
The aim of this pivotal clinical trial was to validate a new extended-wear infusion set (EIS) as safe and effective when used for up to 7 days in adults managing type 1 diabetes (T1D).
Methods
This prospective, single-arm and nonrandomized trial conducted at 15 investigational centers throughout the United States enrolled individuals 18–80 years of age. Investigators and investigational staff were trained to the protocol and internal review board approvals were obtained before study start. Informed consent was obtained and documented as per the specifications of the United States Food and Drug Administration and in accordance with Title 21 CFR Part 50. All research efforts complied with the principles of the Declaration of Helsinki.
Study devices and participants
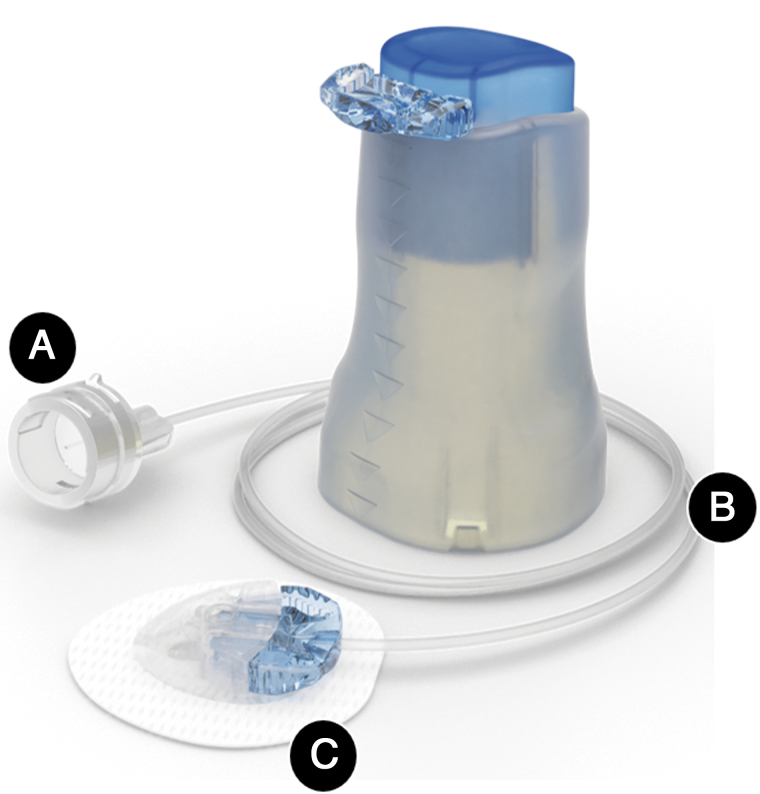
All study participants underwent device training before study start and used their own MiniMed™ 670G system (MiniMed 670G insulin pump and Guardian™ Sensor 3 glucose sensor with the CONTOUR® NEXT LINK 2.4 glucose meter [Ascensia Diabetes Care, Piscataway, NJ]). They were instructed to check their blood glucose (BG) at least four to six times each day (before meals and at bedtime). They were also instructed to check blood ketones using a Precision Xtra™ ketone meter (Abbott, Alameda, CA), if the glucose meter reading was >250 mg/dL, but not if it was within 3 h of a meal. Each participant received 12 EIS devices with 43-inch tubing and accompanied inserter (Fig. 1).
FIG. 1.
Extended infusion set and insertion device The Medtronic extended infusion set (shown with the inserter) has several advancements relative to earlier IISs designed for 2 to 3 days of wear, which include a new (A) H-cap connector and (B) tubing with new fluid path design that improves insulin preservative retention and stability,12 in addition to (C) a new extended-wear adhesive patch that improves skin adherence.13
After each EIS insertion, participants were instructed to wear the EIS for ≥174 h or until infusion set failure. They were to change insulin reservoirs every 174 h, although sets and reservoirs could be replaced independent of each other based on the total daily dose of insulin. Participants were expected to inspect their infusion site daily. If signs of infection (i.e., erythema >1 cm in diameter with warmth, pain, and/or induration) were observed, participants were to call and inform the investigational center. Throughout the trial, participants were to follow their standard routine care for diabetes management.
Criteria for study inclusion were T1D diagnosis greater than 1 year, use of the MiniMed 670G system within 1 year before screening, and willingness to use the Auto Mode function of the system throughout the study. Participants also had to be able and willing to perform study procedures and provide their own insulin lispro (Eli Lilly, Indianapolis, IN) or insulin aspart (NovoNordisk, Plainsboro, NJ). Study exclusion criteria included inability to tolerate the IIS tape adhesive; an infection or any unresolved adverse skin condition (e.g., psoriasis, dermatitis herpetiformis, rash, and Staphylococcus infection) in the area of infusion set placement; an A1C >8.5%, as tested by a National Glycohemoglobin Standardization Program laboratory; an episode of diabetic ketoacidosis (DKA) within 12 months before screening; or a history of one or more episodes of severe hypoglycemia (SevHypo) that resulted in a coma or seizures or required medical assistance within 6 months before screening.
Visit schedule and procedures
The trial comprised a total of seven visits, two of which were by telephone. Informed consent was obtained at Visit 1. Eligibility criterion confirmation and study device training occurred at Visit 2. During Visit 2, participants uploaded their pump system data to the CareLink™ personal software management system and completed a Medtronic intake questionnaire that included Likert-based questions pertaining to prior standard IIS use and satisfaction. Follow-up Visits 3 and 5 involved telephone reminders to complete the at-home upload of pump data, and acetaminophen use and daily logs. Follow-up Visits 4, 6, and 7 comprised pump system upload, collection of acetaminophen and daily logs, and collection of used infusion sets. Each visit also included an inquiry about whether there was any adverse event experienced. Visit 7 included A1C measurement, completion of the exit questionnaire, and study exit.
Safety and effectiveness endpoints
The primary safety endpoints included the incidence rate of serious adverse events (SAEs), serious adverse device effects (SADEs), unanticipated adverse device effects (UADEs), SevHypo, severe hyperglycemia (SevHyper), DKA, and skin infections at the infusion set insertion site. SevHypo was defined as an event requiring the assistance of another individual to actively administer carbohydrate, glucagon, or other resuscitative actions due to participant-altered consciousness. SevHyper was defined as hyperglycemia (BG >250 mg/dL) with blood ketones ≥0.6 mM, urine ketones moderate or large, or symptoms of nausea, vomiting, or abdominal pain. DKA was defined as a BG meter reading >250 mg/dL, arterial pH <7.3, bicarbonate <15 mEq/L, and moderate ketonuria or ketonemia requiring treatment in a medical facility.
The primary effectiveness endpoint was the rate of EIS failure due to unexplained hyperglycemia through day 6 (i.e., 144 h) of EIS wear and a secondary endpoint was the rate of unexplained hyperglycemia through day 7 (i.e., 168 h) of EIS wear. The failure rate was defined as the number of device removals associated with unexplained hyperglycemia divided by the total number of device insertions. Unexplained hyperglycemia (i.e., suspected infusion set occlusion) was defined as a BG meter reading >250 mg/dL (>3 h post-meal) and failure of an insulin pump correction bolus to lower the BG meter reading by ≥50 mg/dL within 60 min after bolus delivery. One additional insulin pump correction bolus was allowed after the initial bolus, as recommended by the bolus calculator.
During this period, BG was to be measured every 60 min and the device could remain in the body if BG improved (lowered by ≥50 mg/dL) within 90 min after the second correction. In the situation of an improved BG and the set not needing to be changed, the event was not considered an adverse event (e.g., unexplained hyperglycemia) or set failure. The failure rate due to both unexplained hyperglycemia and device-related SevHyper (BG >250 mg/dL and ketones ≥0.6 mM) was also determined.
The EIS survival rate was determined based on the number of sets worn for 7 days, excluding those due to study participant incorrect early removal, pump replacement and insulin depletion (i.e., not-for-cause). The not-for-cause analysis was conducted to provide the most accurate report of device performance. A list of the number and percentage of removals, in addition to the reasons associated with each, was created. Analyses included determination of overall survival rates across the 7 days of device wear, as well as the rates when insulin lispro or insulin aspart was used.
Statistical analysis of failure and survival rates and glycemic outcomes
Sample size estimation was based on previously published single-center data.14 Approximately 300 enrolled participants were estimated to ensure that 240 met eligibility criteria, with approximately half using insulin lispro and half using insulin aspart. A sample size of at least 100 for each insulin type would yield power >80% to demonstrate a failure rate due to unexplained hyperglycemia, on wear-day 6 or 7, of ≤20% (with two-sided 0.05 significance level).
Exploratory and descriptive endpoints included survival rate, change in mean A1C from baseline (Visit 1) to end of study (Visit 7, and if within 14 days of the last EIS removal), mean sensor glucose (SG), coefficient of variation of SG (CV), the percentage of time spent at SG ranges (i.e., <54, <70, 70–180, >180, >250, and >300 mg/dL) and total daily insulin dose (TDD) delivered from day 1 of device insertion to the end of day 7 wear. Elapsed days of device wear were based on a 24-h window from the insertion time of each infusion set.
For the Likert-based satisfaction questionnaire developed by Medtronic, participants responded to queries regarding the ease of insertion, comfort and wear duration, time required to change, and convenience of previously worn standard IISs versus the EIS device, before study start and at study end, respectively. The range of response options were Extremely Satisfied―5, Very Satisfied―4, Satisfied―3, Somewhat Satisfied―2, Not Satisfied −1, and Not Sure―0. The satisfaction scores and rates associated with EIS use were compared and analyzed with a Wilcoxon signed-rank test.
Results
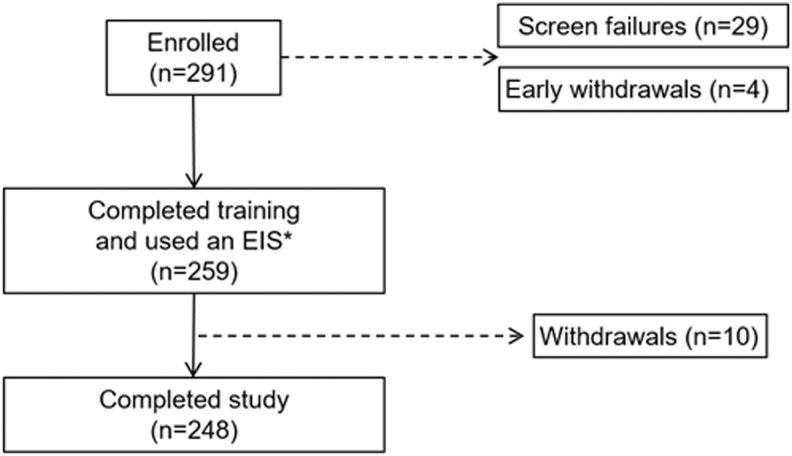
There were 291 adults who enrolled in the trial, 29 screen failures, 4 early withdrawals and 10 withdrawals after, at least, one insertion of an EIS (Fig. 2). The demographics of the intention to treat population (n = 259, 45.0 ± 14.1 years of age, min–max 18.7–77.4 years) who wore a total of 3041 EIS devices (1561 used with insulin lispro and 1480 used with insulin aspart) are listed in Table 1. A total of 248 participants completed the study.
FIG. 2.
Study participant disposition. *One participant wore the extended-wear infusion set (EIS), but did not meet eligibility criteria and was withdrawn from the study and designated a screen failure. EIS.
Table 1.
Study Participant Demographics and Characteristics at Study Start
| Overall (n = 259) | Insulin lispro (n = 132) | Insulin aspart (n = 127) | |
|---|---|---|---|
| Age, years | 45.0 ± 14.1 | 43.4 ± 14.3 | 46.7 ± 13.7 |
| Sex | |||
| Women, n (%) | 134 (51.7) | 68 (51.5) | 66 (52.0) |
| Men, n (%) | 125 (48.3) | 64 (48.5) | 61 (48.0) |
| A1C at screening, %a | 7.2 ± 0.6 | 7.2 ± 0.6 | 7.2 ± 0.6 |
| Weight, kg | 85.6 ± 17.9 | 85.2 ± 16.6 | 85.9 ± 19.3 |
| BMI, kg/m2 | 29.0 ± 5.7 | 29.0 ± 5.6 | 29.0 ± 5.9 |
| Diabetes duration, years | 27.0 ± 13.6 | 25.6 ± 13.7 | 28.4 ± 13.4 |
| Race, n (%) | |||
| White | 240 (92.7) | 118 (89.4%) | 122 (96.1%) |
| African American or Black | 12 (4.6) | 8 (6.1%) | 4 (3.1%) |
| Alaskan/Native American | 1 (0.4) | 1 (0.8%) | 0 (0%) |
| Asian | 4 (1.5) | 3 (2.3%) | 1 (0.8%) |
| Multiracial | 1 (0.4) | 1 (0.8%) | 0 (0%) |
| Other | 1 (0.4) | 1 (0.8%) | 0 (0%) |
Values are shown as mean ± SD, excluding sex and race.
n = 258 participants.
SD, standard deviation.
Safety endpoint evaluation
A summary of safety outcomes during EIS use with insulin lispro and insulin aspart delivery is shown in Table 2. There were three SAEs not related to device or study procedure. One event involved a man who ruptured an Achilles tendon. A second event involved a woman, not wearing an EIS, who lost consciousness at work due to SevHypo, which resolved after emergency medical intravenous glucose treatment. The third event involved a man who lost consciousness due to SevHypo at home and whose BG resolved after emergency medical intravenous glucose treatment. The overall rates of SAEs, SevHypo, SevHyper, and skin infection were 3.8, 2.5, 104.1, and 20.1 events per 100 participant-years. There were no SADE, UADE, or DKA events. Supplementary Table S1 summarizes the device-related safety outcomes during EIS use.
Table 2.
Safety Outcomes During Extended Infusion Set Wear
| Insulin lispro |
Insulin aspart |
|||||
|---|---|---|---|---|---|---|
| Events (n) | Participants (n) | Events per 100 participant-years (n) | Events (n) | Participants (n) | Events per 100 participant-years (n) | |
| Serious adverse events | 2 | 2 | 5 | 1 | 1 | 2.5 |
| Serious adverse device effects | 0 | 0 | 0 | 0 | 0 | 0 |
| Unanticipated adverse device effects | 0 | 0 | 0 | 0 | 0 | 0 |
| Diabetic ketoacidosis events | 0 | 0 | 0 | 0 | 0 | 0 |
| Severe hypoglycemic events | 2 | 2 | 5 | 0 | 0 | 0 |
| Severe hyperglycemic events | 46 | 22 | 114.4 | 37 | 24 | 93.5 |
| Insertion site skin infections | 6 | 5 | 14.9 | 10 | 10 | 25.3 |
| Unexplained hyperglycemia | 2 | 2 | 5 | 6 | 5 | 15.2 |
Effectiveness endpoint evaluation
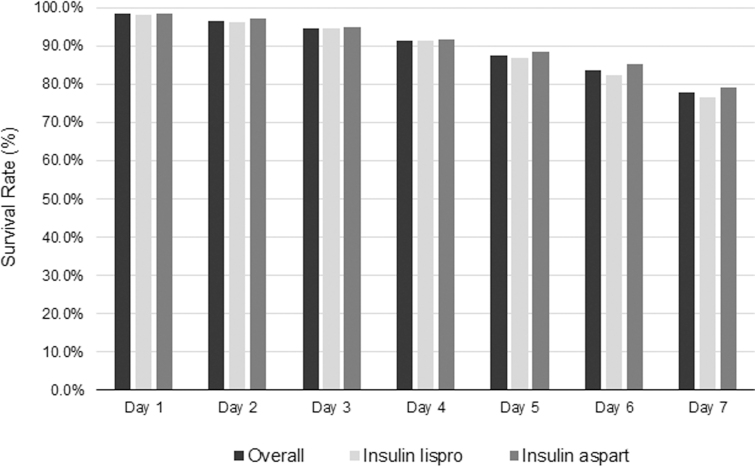
Table 3 shows the rate of EIS failure due to unexplained hyperglycemia and combined unexplained hyperglycemia and device-related SevHyper by end of the sixth and seventh day of wear. The overall survival rates and those for when insulin lispro or insulin aspart was used are shown across each day of EIS wear (Fig. 3). On day 6, the overall rate was 83.8% (95% confidence interval [CI]: 82.4–85.1); on day 7, it was 77.8% (95% CI: 76.2–79.3). The survival rate analysis was based on 2788 of 3041 infusion sets, excluding not-for-cause removals unrelated to the investigational device, where 234 sets were inadvertently removed by the participants early (<174 h) due to time calculation error and 19 sets were removed due to insulin depletion (n = 14) and pump replacement (n = 5). The number of EIS removals and the reasons associated with removals are summarized in Supplementary Table S2.
Table 3.
Rate of Extended Infusion Set Failure Due to Unexplained and Severe Hyperglycemia
| Participants (n) | EIS worn (n) | EIS failure number (n) | EIS failure Rate (%) | |
|---|---|---|---|---|
| End of day 6 | ||||
| Unexplained hyperglycemia | ||||
| Insulin lispro | 132 | 1561 | 1 | 0.06 |
| Insulin aspart | 127 | 1480 | 4 | 0.3 |
| Unexplained hyperglycemia and device-related SevHyper | ||||
| Insulin lispro | 132 | 1561 | 6 | 0.4 |
| Insulin aspart | 127 | 1480 | 11 | 0.7 |
| End of day 7 | ||||
| Unexplained hyperglycemia | ||||
| Insulin lispro | 132 | 1561 | 2 | 0.1 |
| Insulin aspart | 127 | 1480 | 6 | 0.4 |
| Unexplained hyperglycemia and device-related SevHyper | ||||
| Insulin lispro | 132 | 1561 | 17 | 1.1 |
| Insulin aspart | 127 | 1480 | 18 | 1.2 |
The rates of extended infusion set failure due to unexplained hyperglycemia and both unexplained hyperglycemia and device-related SevHyper during insulin lispro and insulin aspart used, by the end of day 6 (primary effectiveness endpoint) and end of day 7 (secondary effectiveness endpoint), are shown.
EIS; SevHyper, severe hyperglycemia.
FIG. 3.
Survival rates of the extended infusion set across days of wear. The survival rates of all extended infusion sets (overall, n = 2788) worn for 7 days are shown, in addition to the survival rates when extended infusion sets were used with insulin lispro (n = 1412) and insulin aspart (n = 1376).
To determine the impact of EIS wear on glycemia, A1C was measured at baseline and compared with the A1C at the end of study. When the EIS was used with either insulin lispro or insulin aspart, A1C changed from 7.2% ± 0.6% to 7.1% ± 0.6% (Δ of −0.1 ± 0.4). An exploratory analysis of glycemic control and TDD across days of EIS wear showed that mean SG, CV, and the percentage of time spent at different glucose ranges aligned with appropriate glycemic control, over time (Table 4). In addition, the amount of TDD delivered remained stable across the 7 days of EIS wear.
Table 4.
Glycemic Outcomes and Total Daily Insulin Delivered Across Extended Infusion Set Days of Wear
| EIS day of wear |
|||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| SG, mg/dL | 150 ± 14.5 | 144.9 ± 12.8 | 146.8 ± 12.6 | 149.6 ± 12.3 | 152.0 ± 12.7 | 154.2 ± 14.6 | 156.4 ± 14.7 |
| CV, % | 35.6 ± 5.7 | 31.0 ± 5.0 | 30.5 ± 5.0 | 30.2 ± 4.7 | 30.3 ± 4.5 | 30.2 ± 4.6 | 30.5 ± 4.7 |
| Percentage of time at SG ranges | |||||||
| <54 mg/dL, % | 1.0 ± 1.3 | 0.6 ± 0.9 | 0.5 ± 0.9 | 0.5 ± 0.8 | 0.4 ± 0.8 | 0.4 ± 0.7 | 0.4 ± 0.7 |
| <70 mg/dL, % | 3.7 ± 3.3 | 2.5 ± 2.5 | 2.1 ± 2.4 | 1.8 ± 2.1 | 1.7 ± 2.0 | 1.6 ± 2.0 | 1.6 ± 1.9 |
| 70–180 mg/dL, % | 71.5 ± 9.2 | 77.7 ± 9.0 | 77.2 ± 9.0 | 75.9 ± 9.1 | 74.3 ± 9.1 | 73.2 ± 10.2 | 71.6 ± 10.8 |
| >180 mg/dL, % | 24.8 ± 9.6 | 19.8 ± 9.1 | 20.7 ± 9.2 | 22.2 ± 9.2 | 24.0 ± 9.3 | 25.1 ± 10.4 | 26.8 ± 11.2 |
| >250 mg/dL, % | 5.8 ± 4.4 | 3.3 ± 3.3 | 3.3 ± 3.3 | 3.7 ± 3.5 | 4.1 ± 3.8 | 4.8 ± 5.0 | 5.2 ± 4.8 |
| >300 mg/dL, % | 1.8 ± 2.0 | 0.7 ± 1.0 | 0.8 ± 1.3 | 0.8 ± 1.3 | 1.0 ± 1.4 | 1.2 ± 2.0 | 1.4 ± 2.0 |
| TDD, units | 54.3 ± 28.6 | 51.4 ± 27.1 | 52.5 ± 28.0 | 53.5 ± 28.5 | 54.3 ± 28.8 | 54.2 ± 28.0 | 53.6 ± 28.0 |
Data are shown as mean ± SD.
For extended infusion sets worn for 7 days (168.9 ± 44.5 h), the continuous glucose monitoring data (N = 258 participants) and TDD delivered (N = 259 participants) are shown for each day of wear.
CV, coefficient of variation of SG; SG, sensor glucose; TDD, total daily insulin dose.
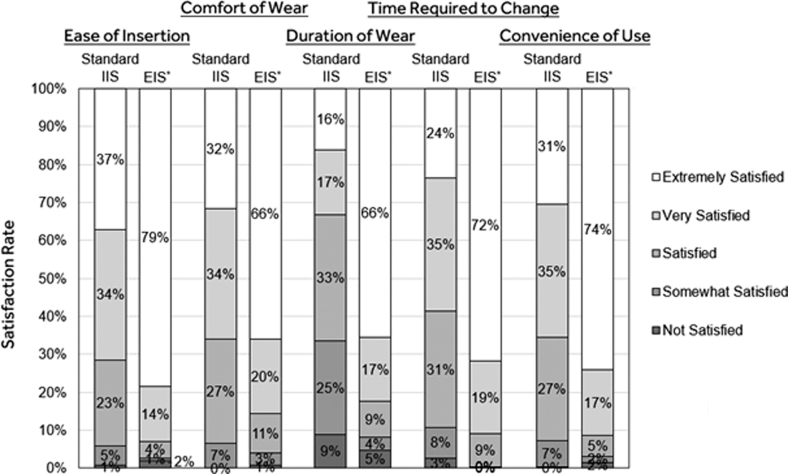
The satisfaction questionnaire results that compared ease of insertion, comfort of wear, duration of wear, time required to change, and convenience of use with previously used standard IISs (e.g., the Minimed Mio™, Quick-Set™, Silhouette™ or Sure-T™) before study start versus with the EIS are listed in Table 5. The satisfaction rates based on the responses captured for each query are shown in Figure 4 and indicate significantly greater satisfaction with EIS versus standard IIS use (P < 0.001).
Table 5.
Participant-Reported Satisfaction with Standard Infusion Set and Extended Infusion Set Use
| Standard IIS (Before study) | EIS (Study end) | P | |
|---|---|---|---|
| Ease of insertion | 4.0 ± 0.9 | 4.7 ± 0.8 | <0.001 |
| Comfort of wear | 3.9 ± 0.9 | 4.5 ± 0.9 | <0.001 |
| Duration of wear | 3.1 ± 1.2 | 4.4 ± 1.1 | <0.001 |
| Time required to change | 3.7 ± 1.0 | 4.6 ± 0.7 | <0.001 |
| Convenience of use | 3.9 ± 0.9 | 4.6 ± 0.8 | <0.001 |
Data are shown as mean ± SD.
The satisfaction questionnaire score results for the standard IIS devices worn before study start versus the EIS, at end of study, are shown for the “ease of insertion,” “comfort of wear,” “duration of wear,” “time required to change,” and “convenience of use” queries. Only participants who completed the questionnaire at both Visit 2 and the end-of-study visit were included in the paired analysis (Wilcoxon signed-rank test). Categorical response options were converted to numerical values: Extremely Satisfied―5, Very Satisfied―4, Satisfied―3, Somewhat Satisfied―2, Not Satisfied −1, Not Sure―0.
IIS, insulin infusion set.
FIG. 4.
Satisfaction rates with standard IIS and extended infusion set use. The mean rates of satisfaction during standard IIS and extended infusion set (EIS) use for the queries of interest (top) are shown. Only participants who completed the questionnaire at both Visit 2 and the end-of-study visit were included in the paired analysis. *P < 0.001 based on the satisfaction score comparison with the previously used standard IIS (Wilcoxon signed-rank test). IIS, insulin infusion set.
Discussion
In this single-arm study, performance of the Medtronic EIS and its impact on glycemic control during use with either insulin aspart or insulin lispro were evaluated in adults with T1D. There was no serious adverse device event, UADE, or episode of DKA. There were two SevHypo events that were not related to the EIS. The rate of SevHyper (BG >250 mg/dL with blood ketones ≥0.6 mM) was 104.1 events per 100 participant-years. The MiniMed 670G system pivotal trial15 used a definition of SevHyper of BG >300 mg/dL and blood ketones >0.6 mM and involved use of a 3-day IIS.
Using the definition of SevHyper in that study and based on an analysis of similarly aged adults (≥18 years), this study had 41.4 events per 100 participant-years, which was similar to the 42.7 events per 100 participant-years in the MiniMed 670G system trial. In this study, there were 16 infusion site skin infections reported by 15 participants (5.8%) that corresponded with 20.1 events per 100 participant-years. This rate was lower than the self-reported rates of 17.4%16 and 28%17 by individuals who completed survey reporting on a prior year of standard IIS use.
The overall EIS survival rate of 77.8% (95% CI: 76.2–79.3) by the end of day 7 was greater than previously reported survival rates of 69% (n = 40 total participants and 80 total steel cannula sets),9 63% (n = 40 total participants, 80 total soft cannula sets),9 43% (n = 20 total participants, 40 total sets in non-lipohypertrophied tissue),18 33% (n = 20 total participants and 40 total sets in lipohypertrophied tissue),18 and 32% (n = 20 total participants and 77 total steel cannula or soft cannula sets)8 for standard IIS devices worn for similar durations of time.
The EIS failure rate due to unexplained hyperglycemia or both unexplained hyperglycemia and device-related SevHyper was quite low (≤2%) and substantially lower than the 19%,9 23%,18 30%,8 and 35%18 reported to be attributable or partly attributable to unexplained hyperglycemia in the studies investigating extended standard IIS wear. It is also important to note that one randomized crossover study investigating intended real-world 3-day use of standard IIS devices reported comparable failure rates (or unplanned IIS changes) of 16%–20% and that ∼40% were due to unexpected high glucose, occlusion alarms, or backflow of insulin.19 The differences in failure rates observed across these studies were likely due to multiple factors, including study participant selection and definitions of infusion set failure.
There were also multiple definitions of a failed correction dose where 1 h8,18 or 60–90 min9 was the elapsed time by when a single correction dose was required to decrease BG by 50 mg/dL. The variability in reporting may, in part, be due to the variability in clinical infusion set failures, where some are partial and/or transient and can be seen frequently when monitoring infusion pressures that do not result in alarm (i.e., silent occlusions).20 In this study, infusion set failure was defined by having one or two failed correction doses over a period of at least 2½ h, to exclude transient occlusions that resolve when given additional time.
Early studies of extended standard IIS wear demonstrated deterioration in glycemic control and significant IIS- or IIS site-related adverse events.1–3 The Schmid et al.'s pilot study of 12 adults (40.3 ± 12.6 years of age) using the MiniMed Comfort infusion set or MiniMed Silhouette infusion set (ConvaTec Unomedical A/S, Lejre, Denmark) reported average BG levels that increased from 135.0 ± 68.4 mg/dL to 208.8 ± 39.6 mg/dL from day 1 to 7 of IIS wear.2 While only a few study participants reached 7-day IIS wear, events resulting in IIS change occurred more frequently after day 3 and impaired insulin absorption resulting in hyperglycemia was significant by day 7.
The follow-up 3-month randomized crossover study1 with 22 adults (39.0 ± 11.0 years of age) using the MiniMed Mio infusion set or Inset II™ infusion set (ConvaTec Unomedical A/S) also reported a trending increase in IIS-related issues that included hyperglycemia, increased frequency of IIS changes (from 73 to 143 changes), increased number of infusion set-related adverse events (from 305 to 517 events), and site reactions (from 1.7 ± 2.2 to 7.5 ± 14.0 events/participant), all of which were significant.
Thus, while early standard IIS device studies confirmed manufacturer indications for 2- to 3-day use due to increased risk of IIS site reactions or compromised glycemic control, more recent studies with greater numbers of study participants have reported minimal or no issues with glycemia over time or fewer adverse events associated with extended IIS wear.8,9,18,19
These different results likely reflect iterative modifications in IIS device performance, IIS insertion devices, and user experience, or a combination of these factors. For instance, Patel et al., indicated that kinking at initial insertion of Teflon-cannula IISs (MiniMed Quick-Set infusion set [Medtronic]) contributed to 15% of the total IIS failure rate, although investigational staff inserted all IIS devices during the study.8 In Freckmann et al.'s study, where the study participants (N = 80 total) inserted their IIS devices (Accu-Chek™ FlexLink infusion set and Accu-Chek FlexLink Plus infusion set [Roche Diabetes Care, Mannheim, Germany]), 18.7% had kinked cannulas.19 While a different Teflon-cannula IIS device (YpsoPump™ Orbit™ soft infusion set [Ypsomed AG, Burgdorf, Switzerland]) was used in Waldenmaier et al.'s study, participants could use an accompanying insertion device, and no issues with kinking at IIS insertion were reported.9
Interestingly, some of the aforementioned studies reported that standard IIS survival appeared to depend heavily on the individual user, as ∼42% of the Patel et al.'s study participants were able to wear an IIS for 6–7 days and 75% of those in Waldenmaier et al.'s study wore at least one standard IIS for 7 days. In this study, and excluding the initial training visit, participants inserted the EIS on their own and the rate of kinking at insertion was a low 0.5% with 99.6% participants being able to wear at least one EIS for 7 days.
Glycemic control and participant-reported satisfaction during EIS use were favorable. Glucose metrics that included mean SG and the percentage of time spent at SG ranges indicated glycemic stability for the 7-day wear duration. Glucose variability was 35.6% ± 5.7% on day 1 and 30.5% ± 4.7% on day 7 and TDD was relatively unchanged (54.3 ± 28.6 U, 53.5 ± 28.5 U, and 53.6 ± 28.0 U on days 1, 4, and 7, respectively). The satisfaction scores regarding device convenience and use and the proportion of participants responding with “very satisfied” and/or “extremely satisfied” were higher for the EIS, when compared with standard IIS devices used before study start.
A significant limitation of this study is the open-label and nonrandomized design without a comparator. The trial also required that study participants use the Auto Mode function of their MiniMed 670G system throughout the course of the study, which may limit the generalizability of glycemic control findings to populations of individuals with T1D using open-loop insulin pump systems. In addition, the study population also included participants, a majority of whom were white, with relatively well-controlled glycemia at study start. However, the Auto Mode function, which included multiple periods of time (some lasting hours) when insulin delivery was suspended to prevent hypoglycemia, served as a more rigorous test of infusion set function than an open-loop system where there is continuous insulin delivery. Overall, the demonstrated survival rate and the failure rate due to unexplained hyperglycemia while wearing the EIS are notable.
For some time, the iterative advancements in IIS devices have aimed toward minimizing adverse events and reducing user burden, as the IIS has long been regarded the “Achilles' heel” of CSII therapy.21 In addition to these findings, a recent pilot study of 22 adults (40.1 ± 14.3 years of age) using varied insulin delivery systems demonstrated a survival rate of 82% at 6.8 days of wear, during use of a novel IIS with multiple perforations within the cannula shaft (i.e., Lantern technology [ConvaTec, Inc., Deeside, United Kingdom]) without significant change in glucose or daily insulin delivered at this time point.22
A recent medical economics Markov Chain Monte Carlo model analysis of standard IIS clinical data8,18 versus this study EIS trial data showed a greater average lifetime of 151.6 versus 66.0 h, resulting in 75 fewer set changes per year, for the EIS.23 Both of these reports of extended infusion set survival and usability are important, as they indicate potential reductions in burden and cost, and contribute toward advancements that may lead to the development of combined infusion set-glucose sensing devices.
Conclusions
Opportunities for IIS technology advancement have not only included extending the duration of IIS wear but also maintaining glycemic control, while mitigating adverse events across wear duration. The Medtronic extended infusion set described in this study was safely worn for up to 7 days by adults without adversely affecting glycemic control and with favorable user satisfaction.
Supplementary Material
Acknowledgments
The authors gratefully thank the study participants and all investigational site personnel for their contribution and efforts made toward the successful conduct of the trial. The authors also thank Emma Pham, MSc, for clinical trial monitoring and article development input.
Author Disclosure Statement
The investigator authors received research support from Medtronic to conduct the study. S.C., G.Z., J.S., V.C., T.L.C., S.W.L., A.S.R., and R.A.V. are Medtronic employees.
Funding Information
This work was funded by Medtronic Diabetes.
Supplementary Material
References
- 1. Pfutzner A, Sachsenheimer D, Grenningloh M, et al. : Using insulin infusion sets in CSII for longer than the recommended usage time leads to a high risk for adverse events: results from a prospective randomized crossover study. J Diabetes Sci Technol 2015;9:1292–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schmid V, Hohberg C, Borchert M, et al. : Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol 2010;4:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thethi TK, Rao A, Kawji H, et al. : Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J Diabetes Complications 2010;24:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerr D, Morton J, Whately-Smith C, et al. : Laboratory-based non-clinical comparison of occlusion rates using three rapid-acting insulin analogs in continuous subcutaneous insulin infusion catheters using low flow rates. J Diabetes Sci Technol 2008;2:450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berg AK, Norgaard K, Thyssen JP, et al. : Skin problems associated with insulin pumps and sensors in adults with type 1 diabetes: a cross-sectional study. Diabetes Technol Ther 2018;20:475–482. [DOI] [PubMed] [Google Scholar]
- 6. Christensen MO, Berg AK, Rytter K, et al. : Skin problems due to treatment with technology are associated with increased disease burden among adults with type 1 diabetes. Diabetes Technol Ther 2019;21:215–221. [DOI] [PubMed] [Google Scholar]
- 7. Heinemann L, Kamann S: Adhesives used for diabetes medical devices: a neglected risk with serious consequences? J Diabetes Sci Technol 2016;10:1211–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patel PJ, Benasi K, Ferrari G, et al. : Randomized trial of infusion set function: steel versus teflon. Diabetes Technol Ther 2014;16:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Waldenmaier D, Zschornack E, Buhr A, et al. : A prospective study of insulin infusion set use for up to 7 days: early replacement reasons and impact on glycemic control. Diabetes Technol Ther 2020;22:734–741. [DOI] [PubMed] [Google Scholar]
- 10. Chattaraj S, Zhang G, Anselmo E, Fusselman JC: Study of insulin stability impact on pump therapy: test model development. Diabetes 2020;69:1012-P. [Google Scholar]
- 11. Chattaraj S, Zhang G, Anselmo E, et al. : The Medtronic extended wear infusion set: determining mechanisms of action. Diabetes Technol Ther 2021;23:A149. [Google Scholar]
- 12. Ilany J, Cohen O, Konvalina N, et al. : Clinical study of a new extended wear infusion set design. Diabetes Technol Ther 2020;22:A-27. [Google Scholar]
- 13. Zhang G, Chattaraj S, Anselmo E, et al. : Assessment of adhesive patches for an extended-wear infusion set. Diabetes 2020;69:986-P. [Google Scholar]
- 14. Buckingham BA, Marcal T, Hoffman L, et al. : Testing a novel infusion set for extended wear duration. Diabetes 2020;69:997-P. [Google Scholar]
- 15. Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408. [DOI] [PubMed] [Google Scholar]
- 16. Pickup JC, Yemane N, Brackenridge A, Pender S: Nonmetabolic complications of continuous subcutaneous insulin infusion: a patient survey. Diabetes Technol Ther 2014;16:145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taleb N, Messier V, Ott-Braschi S, et al. : Perceptions and experiences of adult patients with type 1 diabetes using continuous subcutaneous insulin infusion therapy: results of an online survey. Diabetes Res Clin Pract 2018;144:42–50. [DOI] [PubMed] [Google Scholar]
- 18. Karlin AW, Ly TT, Pyle L, et al. : Duration of infusion set survival in lipohypertrophy versus nonlipohypertrophied tissue in patients with type 1 diabetes. Diabetes Technol Ther 2016;18:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freckmann G, Arndt S, Fiesselmann A, et al. : Randomized cross-over study comparing two infusion sets for CSII in daily life. J Diabetes Sci Technol 2017;11:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gibney M, Xue Z, Swinney M, et al. : Reduced silent occlusions with a novel catheter infusion set (BD FlowSmart): results from two open-label comparative studies. Diabetes Technol Ther 2016;18:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heinemann L, Krinelke L: Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol 2012;6:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lal RA, Hsu L, Zhang J, et al. : Longevity of the novel ConvaTec infusion set with Lantern technology. Diabetes Obes Metab 2021;23:1973–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwa T, Zhang G, Shepard K, et al. : The improved survival rate and cost-effectiveness of a 7-day continuous subcutaneous insulin infusion set. J Med Econ 2021;24:837–845. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.