Abstract
Early HIV viral suppression (VS) improves individual health outcomes and decreases onward transmission. We designed an outpatient clinic protocol to rapidly initiate antiretroviral therapy (ART) in a large Veterans Health Administration (VA) HIV clinic. A pre–post evaluation was performed using a retrospective cohort study design for new diagnoses of HIV infection from January 2012 to February 2020. Time-to-event analyses were performed using the Cox proportional hazards model with the intervention group as the main exposure adjusted for integrase inhibitor usage, baseline viral load, age, gender, and race. Most of the patients were men (historical control: 94.8%, n = 55; Rapid Start: 94.8%, n = 55) and Black or African American persons (historical control: 87.9%, n = 51; Rapid Start: 82.8%, n = 48). More patients initiated treatment with an integrase inhibitor-based regimen in the Rapid Start group (98.3%, n = 57) compared with the historical control group (39.7%, n = 23). Compared with controls, the Rapid Start patients were significantly more likely to achieve VS at any given time during the study period (hazard ratio 2.65; p < 0.001). Median days (interquartile range) from diagnosis to VS decreased from 180.5 (102.5–338.5) to 62 (40–105) (p < 0.001), first appointment to VS decreased from 123 (68.5–237.5) to 45 (28–82) (p < 0.001), referral to first visit decreased from 20 (10–43) to 1 (0–3) (p < 0.001), and from first visit to ART dispense date decreased from 27.5 (3–50) to 0 (0–0) (p = 0.01). Prioritizing immediate ART initiation can compress the HIV care continuum from diagnosis to linkage to VS. Implementation of the Rapid Start Protocol should be considered at all VA facilities providing HIV care.
Keywords: HIV, rapid ART, antiretroviral therapy, same-day therapy, viral suppression, continuum of care
Introduction
Antiretroviral therapy (ART) is now recommended for anyone with HIV, regardless of CD4 count.1 Delays in initiating ART are often associated with subsequent lower rates of viral suppression (VS) and retention in care, as well as earlier development of first AIDS event.2 The rapid ART model, defined as linkage to care and ART treatment initiation as soon as possible after a new positive HIV result, offers an accelerated entry into HIV health care. Rapid ART reduces the time to VS and can improve morbidity and mortality for persons with HIV (PWH).3–13
Based on improved efficacy, safety, and acceptability, the World Health Organization and leading United States guideline committees now recommend early ART initiation.1,14 Structural barriers, stigma, patient disposition toward ART, or provider attitude/comfort can impede rapid initiation of ART.15,16 Rapid initiation of ART is logistically challenging. To achieve rapid or same-day initiation, an individual who is informed of a new HIV diagnosis needs to be immediately linked to care, which often requires an efficient and reliable outpatient clinic with providers who are comfortable with initiating ART during the first visit.
The Veterans Health Administration (VA) is the largest single institutional provider of HIV care in the United States with nearly 60,000 enrolled PWH.17 The VA population is unique—patients recently diagnosed with HIV are likely to receive most of their care within the VA. The VA also has a centralized electronic health record (EHR), allowing for acquisition of national medical information to facilitate clinical care and research. Reports describing rapid ART initiation in a real-world clinical setting among Veteran populations are lacking. For this study, we designed an outpatient clinic protocol to streamline HIV treatment initiation for newly diagnosed HIV and evaluated the effectiveness of the health system intervention.
Methods
Study population
The Atlanta VA Medical Center Infectious Disease Clinic (IDC) is an urban clinic that provides care to more than 1850 PWH annually and is the largest HIV clinic in the VA. Patients primarily reside in northern Georgia, but the clinic also provides care to a few patients who reside in neighboring States.
Study design
This study was a clinic-based pre–post evaluation of an intervention using a retrospective cohort study design among individuals referred to care following diagnosis of HIV infection from January 1, 2012 to February 1, 2020. Follow-up information was current through May 10, 2021. The primary endpoint was time to VS from diagnosis. Secondary endpoints were time to VS from first care visit and time to VS from ART initiation among patients managed in the Rapid Start Protocol (intervention group, from January 2017 to February 2020) compared with the clinic's standard of care in the period before the initiation of the protocol, where universal ART treatment was used (historical controls, from January 2012 to December 2016). Additional endpoints included the percentage of patients achieving VS, time to first attended visit from both diagnosis and separately from HIV clinical referral, and time to ART initiation from diagnosis and separately from first visit, and mortality proportions.
Historical controls were randomly selected from patients newly diagnosed between January 2012 and December 2016. Inclusion criteria for analysis included: (1) new HIV diagnosis within the Atlanta VA Medical Center, (2) treatment initiated at the Atlanta IDC, and (3) resided within the catchment area and did not transfer care in the first 6 months. Patients diagnosed elsewhere, transferred care, or previously received or currently receiving ART at clinic enrollment were excluded from the study. All data were extracted from the VA EHR through the VA Informatics and Computing Infrastructure (VINCI). The study was IRB approved (no. 00068877).
Study intervention
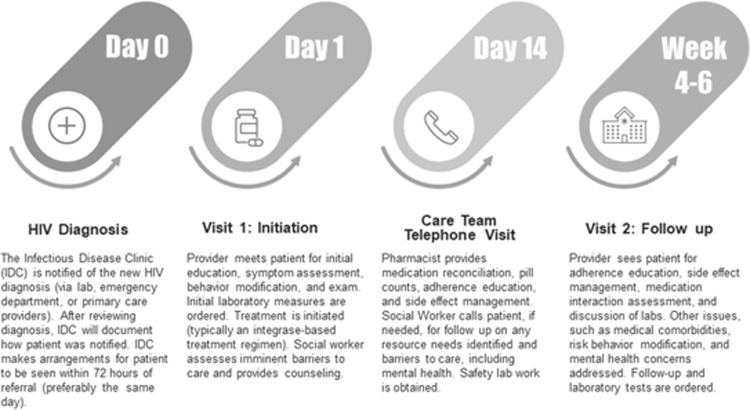
The Rapid Start Protocol at the IDC is illustrated in Fig. 1. The protocol was designed to circumvent aspects of care that traditionally have delayed initiation of treatment, such as preparatory laboratory results (e.g., HIV genotyping), which can take weeks for results to return. In the Rapid Start Protocol, the clinic is notified of a new HIV diagnosis by the laboratory, Emergency Department, or primary care providers. After the diagnosis is reviewed, the clinic documents how the patient was informed. Then, the IDC schedules an appointment for the patient within 72 h of the referral (preferably on the same day of diagnosis). Within the IDC, a multidisciplinary team, including a nurse, scheduler, provider, pharmacist, psychologist, and social worker is responsible for providing care during the first visit. The provider performs an initial assessment, opportunistic infection screening, HIV education, and counseling, including prophylaxis counseling for partners, and sexually transmitted infection screening and counseling. Initial laboratory measures are performed. ART is prescribed during the visit, which is preferably an integrase-based regimen, such as bictegravir, emtricitabine, and tenofovir alafenamide.
FIG. 1.
The rapid start protocol at the Atlanta VA Medical Center IDC. IDC, Infectious Disease Clinic; VA, Veterans Health Administration.
The clinic social worker meets with the patient to assist with partner notification and assess potential barriers to care, such as housing needs, with linkage to further resources. A psychologist embedded in the clinic addresses mental health and substance use concerns. Two weeks later, the care team, including the pharmacist and social worker, has a telephone visit with the patient where side-effect management, adherence education, and counseling are provided. If necessary, follow-up laboratory testing is performed. Between weeks 4 and 6, the patient has a follow-up visit with the provider, where adherence, medication interactions, and comorbidities are addressed; laboratory testing is performed; and recent results are discussed. Further follow-up laboratory tests and visits are scheduled to occur around 6–8 weeks later.
Measures
Demographic, sociobehavioral characteristics, indicators of clinical care, ART prescribed, and virologic outcomes were abstracted from both the EHR and the corporate data warehouse through May 1, 2021, allowing at least 12 months of follow-up for all cases included in the study. These measures were compared between Rapid Start intervention and historical control groups. Date of HIV diagnosis was defined as the earliest confirmed reactive HIV antibody or antigen/antibody test or detectable HIV RNA. The first care visit was defined as the first visit with a prescribing provider at the Atlanta IDC. ART initiation was defined as the date ART was dispensed for the patient (either the pharmacy dispense or mail-out date). HIV VS was defined as a viral load <200 copies/mL. Suppression was defined at this threshold to allow for consistency across time periods since assay quantification has improved over time and to remain consistent with prior publications.
Achieving VS was defined as ever having an HIV-1 RNA <200 copies/mL during the study period (or at least a 12-month window). The following time (in days) intervals were calculated: from diagnosis to referral; from diagnosis and separately from referral (1) to first visit, (2) to ART initiation, and (3) to VS; and from first care visit to ART initiation and separately to VS. Other HIV continuum of care data such as engagement in care (defined as having kept an appointment within 6 months of dataset closure) and annual retention in care (defined as at least two encounters within the 12-month measurement year) were assessed. A medical encounter was defined as a medical visit with a provider having prescribing privileges or an HIV viral load test since clinic enrollment and thereafter.
Statistical analysis
Descriptive statistics characterized the population overall and by the intervention group. Categorical characteristics were compared between groups using Pearson's chi-squared test or Fisher's exact test. Continuous variables were compared between groups with Student's t tests. For all analyses p ≤ 0.05 was considered statistically significant.
The primary analysis consisted of a Cox proportional hazards model of time from diagnosis to VS, with the intervention group as the main exposure. The model was adjusted for baseline viral load, integrase inhibitor usage, age, gender, and race. Kaplan–Meier curves were created illustrating the proportions of patients achieving VS over time. A sensitivity analysis was performed by including excluded patients that had insufficient follow-up time in the main Cox model to ensure results were robust. Secondary analyses also included the Cox proportional hazards model for time from first appointment (visit) to VS and time from ART prescription to VS. SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R Studio (R Core Team, Vienna, Austria) were used for data management and statistical analysis.
Results
One hundred sixteen patients were included in the analysis. Eleven patients (three intervention patients and eight control patients) were excluded due to insufficient follow-up duration (<3 months) secondary to transferring care or moving out of the catchment area but were examined in the sensitivity analysis. Demographic, sociobehavioral, and clinical characteristics are presented in Table 1. Most of the patients were men (historical control: 94.8%, n = 55; Rapid Start: 94.8%, n = 55) and Black or African American persons (historical control: 87.9%, n = 51; Rapid Start: 82.8%, n = 48). Intervention and control groups were comparable regarding demographic, sociobehavioral, and baseline clinical characteristic data without significant differences observed, other than baseline CD4+ T cell counts.
Table 1.
Demographics, Psychosocial and Clinical Characteristics Among Patients Newly Enrolling at the Atlanta Veterans Health Administration Medical Center Infectious Disease Clinic During January 2012–February 2020
| Characteristics n (%) or median (IQR) | Overall n = 116 | Pre-rapid start controls n = 58 | Rapid start n = 58 | Between-group comparison, p |
|---|---|---|---|---|
| Sociodemographic | ||||
| Age at diagnosis, year | 44 (33–52) | 45 (31–52) | 44 (34–51) | 0.74 |
| Gender | 1.00 | |||
| Male | 110 (94.8) | 55 (94.8) | 55 (94.8) | |
| Female | 6 (5.2) | 3 (5.2) | 3 (5.2) | |
| Race | 0.60 | |||
| Black or African American | 99 (85.3) | 51 (87.9) | 48 (82.8) | |
| White | 17 (14.7) | 7 (12.1) | 10 (17.2) | |
| Ethnicity | 1.00 | |||
| Hispanic/Latino | 1 (0.86) | 0 (0) | 1 (1.7) | |
| Not | 115 (99.1) | 58 (100) | 57 (98.3) | |
| Mental health diagnosis | 95 (81.9) | 44 (75.9) | 51 (87.9) | 0.15 |
| Anxiety | 53 (45.7) | 23 (39.7) | 30 (51.7) | 0.26 |
| Bipolar | 5 (4.3) | 3 (5.2) | 2 (3.4) | 1.00 |
| Depression | 81 (69.8) | 37 (63.8) | 44 (75.9) | 0.22 |
| PTSD | 54 (46.5) | 24 (41.4) | 30 (51.7) | 0.35 |
| Schizophrenia | 3 (2.6) | 2 (3.4) | 1 (1.7) | 1.00 |
| Othera | 41 (35.3) | 17 (29.3) | 24 (41.4) | 0.24 |
| Substance use | 51 (44.0) | 23 (39.7) | 28 (48.3) | 0.45 |
| Alcohol | 33 (28.4) | 14 (24.1) | 19 (32.8) | 0.41 |
| Cannabis | 29 (25) | 15 (25.9) | 14 (24.1) | 1.00 |
| Cocaine | 14 (12.1) | 6 (10.3) | 8 (13.8) | 0.78 |
| Opioid | 1 (0.86) | 1 (1.7) | 0 (0) | 1.00 |
| Tobacco | 27 (23.3) | 13 (22.4) | 14 (24.1) | 1.00 |
| Hallucinogen | 2 (1.7) | 1 (1.7) | 1 (1.7) | 1.00 |
| Stimulant | 9 (7.8) | 4 (6.9) | 5 (8.6) | 1.00 |
| Baseline clinical characteristics | ||||
| BMI | 26.1 (22.6–29.4) | 25.9 (22.7–28.7) | 26.8 (22.5–29.8) | 0.85 |
| Median baseline CD4 cell count (cells/μL) | 393 (224–615) | 330 (141–551) | 408 (312–669) | 0.01 |
| Median baseline CD4 cell percentage | 23 (13–30) | 20 (9–31) | 24 (18–30) | 0.14 |
| Median baseline HIV RNA VL | 61,415 (9896–138,559) | 68,560 (15,140–222,484) | 41,890 (4689–135,035) | 0.82 |
| Creatinine clearanceb | 100.5 (85.3–118.5) | 100.5 (83.6–116.5) | 100.9 (87.2–120.3) | 0.30 |
| HBsAb, positive | 60 (51.7) | 25 (43.1) | 35 (60.3) | 0.09 |
| HBsAg, positive | 4 (96.5) | 3 (5.2) | 1 (1.7) | 0.62 |
| Hepatitis C Ab, positive | 6 (5.2) | 4 (6.9) | 2 (3.4) | 0.68 |
Other includes attention deficit disorder, obsessive–compulsive disorder, psychosis, adjustment disorder, or substance use disorder.
Cockcroft–Gault formula, adjusted for obesity.
BMI, body mass index; HBsAb, hepatitis B surface antibody; HBsAg, hepatitis B surface antigen; IQR, interquartile range; PTSD, posttraumatic stress disorder; RNA, ribonucleic acid; VL, viral load.
Nearly 82% (n = 95) of the population had a mental health diagnosis during the observation period, most commonly depression (69.8%, n = 81), posttraumatic stress disorder (46.5%, n = 54), and anxiety (45.7%, n = 53). Substance use was reported in 44% of patients, most commonly alcohol (28.4%, n = 33), tobacco (23.3%, n = 27), and marijuana (25%, n = 29). The median [interquartile range (IQR)] baseline CD4+ T cell count was 330 (141–551) cells/μL in the control group and 408 (312–669) cells/μL in the Rapid Start group, with an overall median T cell count of 393 (224–615) cells/μL (p = 0.01).
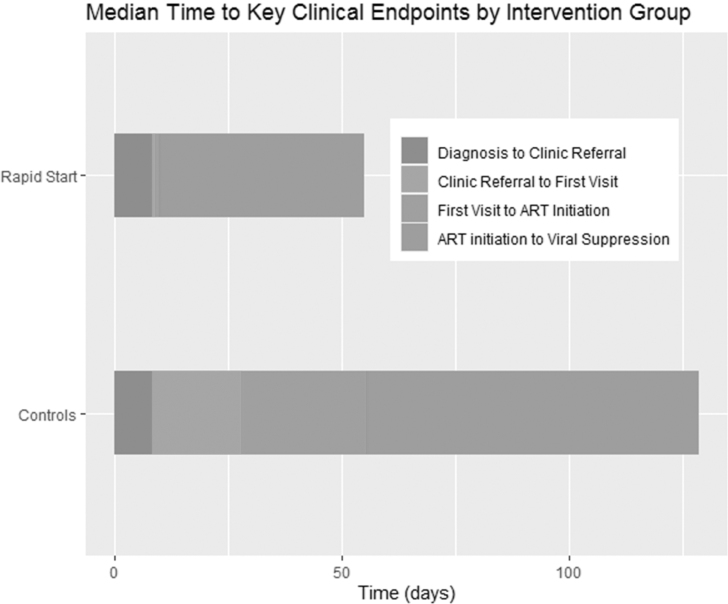
The median times to VS for the historical controls and Rapid Start group are shown in Fig. 2. The median (IQR) time to VS from diagnosis decreased from 180.5 days (102.5–338.5) in the control group to 62 days (40–105) (p < 0.001) in the Rapid Start group. The median (IQR) time to VS from first appointment decreased from 123 days (68.5–237.5) to 45 days (28–82) (p < 0.001). The median (IQR) time from ART initiated to first VS decreased from 73 days (34.5–156) to 45 days (28–82) (p = 0.21). Two patients in the control group did not achieve VS, whereas all patients in the Rapid Start group achieved VS in the 12 months following ART initiation. More deaths were seen in the control group (n = 6), compared with the Rapid Start group (n = 2).
FIG. 2.
Median time to key clinical endpoints by intervention group.
The median (IQR) time from diagnosis to clinical referral was 8 days (3–30) in the control group and 8 days (6–13) in the Rapid Start group (p = 0.04). The median time (IQR) from referral to first attended clinic appointment was reduced from 20 days (10–43) in the control group to 1 (0–3) in the Rapid Start group (p < 0.001). The median (IQR) time from diagnosis date to ART dispense date decreased from 68.5 days (36–169) in the control group to 12 days (8–20) in the Rapid Start group (p < 0.001). The median (IQR) time from clinic referral to the ART dispense date decreased from 48.5 days (29–165) in the control group to 2 days (1–5) in the Rapid Start group (p = 0.002). The median (IQR) time from first attended visit to the ART dispense date decreased from 27.5 days (3–50) in the control group to 0 days (0–0) in Rapid Start group (p = 0.01), indicating ART was typically initiated on the same day as the first clinic visit.
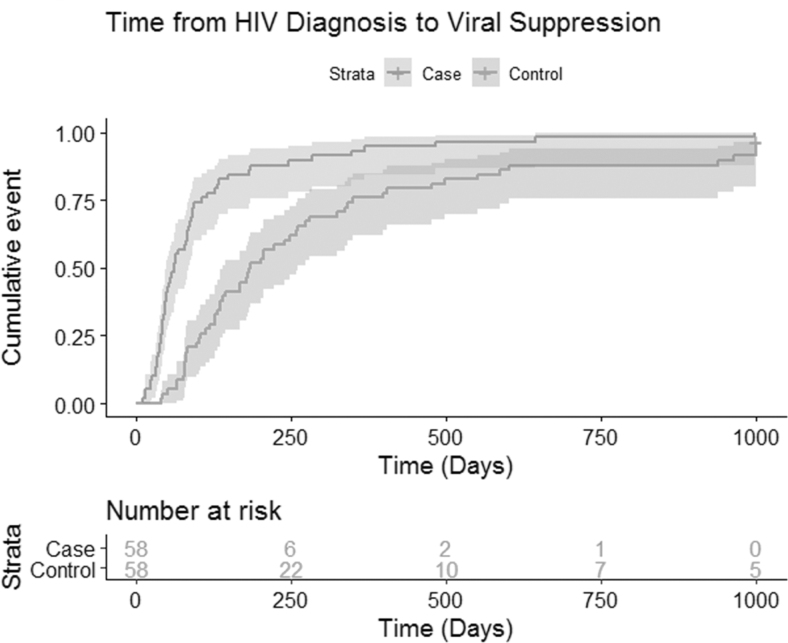
The Kaplan–Meier curve with the proportion of patients reaching VS (HIV RNA <200 copies) through the end of follow-up from diagnosis is displayed in Fig. 3. The adjusted Cox proportional hazards model can be found in Table 2. Time to suppression for the Rapid Start cohort was significantly shorter than patients under previous clinic standard of care (pre-rapid start following universal ART guidelines). Compared with controls, the Rapid Start patients were 2.65 [95% confidence interval (CI) 1.69, 4.16; p < 0.001] times more likely to achieve VS at any given time point during the study period (Table 2) when controlling for baseline viral load, integrase inhibitor usage, age, race, and gender (p < 0.001). Proportional hazard assumptions were checked through log–log plots and Schoenfeld residuals and were not severely violated by the model. The sensitivity analysis with excluded patients yielded a hazard ratio for the Rapid Start group of 2.86 (95% CI 1.85, 4.43; p < 0.001) and thus did not change enough from the main model (hazard ratio = 2.65) to have a substantial impact on any conclusions.
FIG. 3.
Time to VS among newly enrolling patients with HIV at the Atlanta VA Medical Center IDC. The Kaplan–Meier curves show the proportion of patients with HIV RNA <200 copies/mL over time from diagnosis. Time to VS for patients in the Rapid Start group was shorter than for the historical controls. RNA, ribonucleic acid; VS, viral suppression.
Table 2.
Cox Proportional Hazard Models for Time from HIV Diagnosis to Viral Suppression
| Predictor | Hazard ratio (95% CI) | p |
|---|---|---|
| Case (vs. control) | 2.65 (1.69–4.16) | <0.001 |
| Black race (vs. White race) | 0.81 (0.46–1.43) | 0.47 |
| Female (vs. male) | 1.34 (0.52–3.46) | 0.55 |
| Agea | 0.99 (0.92–1.07) | 0.82 |
| Baseline VLb | 1.00 (1.00–1.00) | 0.57 |
| Integrase inhibitor use | 0.98 (0.60–1.59) | 0.93 |
Measured in 5-year increments.
Measured in 100 (copies/mL) increments.
CI, confidence interval; VL, viral load.
The Rapid Start group ART regimens comprised mostly integrase inhibitor based (98.3%, n = 57). The historical control group ART regimen comprised non-nucleoside reverse transcriptase inhibitors (50%, n = 29), integrase inhibitor based (39.7%, n = 23), and protease inhibitor based (10.3%, n = 6). During the observation period, most patients were changed to an integrase inhibitor-based regimen (92% overall, n = 106/116). There was a median of 1 (0–1.5) ART changes per patient appreciated during the observation period. Fidelity measures of the intervention group and control group were very high—100% of patients diagnosed had a first visit appointment with a subsequent follow-up visit and >95% were retained in care for both groups.
Discussion
In this study, we found that implementation of a protocol, including rapid initiation of ART in a large VA clinic using a multidisciplinary team resulted in a significant reduction in time from diagnosis, referral, and initial clinic visit to VS, and reduced the time from diagnosis and separately referral to first clinic visit and first ART prescription. These findings suggest that rapid ART can help compress the HIV care continuum from diagnosis to linkage to VS.
Our results are consistent with other analyses conducted in the United States, including studies performed locally. The San Francisco General Hospital HIV clinic initiated the Rapid ART Program for Individuals with an HIV Diagnosis (RAPID) intervention protocol, which reduced time from diagnosis to VS from 170 to 65 days, whereas our study decreased this time from 180.5 to 62 days.6 Locally, the Infectious Diseases Program Ponce Clinic of the Grady Health System, which serves a demographically similar population with comparable ancillary support units, launched a rapid entry to ART program in 2016. Their program resulted in a decrease in median time to VS from first appointment from 77 (62–96) days to 57 (41–70).7 Similarly, our protocol and intervention reduced the time from first appointment to VS from 123 to 45 days (p = 0.01, see Fig. 2 for shortened windows in treatment cascade). Given this, the Rapid Start program likely functions by accelerating the time from diagnosis to ART initiation and subsequent VS, based on the findings in the secondary Cox analysis.
The improvements observed in this study may have resulted from utilization of a streamlined HIV treatment initiation model, with multiple system-level changes to help facilitate linkage to care/clinic enrollment with subsequent rapid initiation of ART from the clinic and pharmacy. The intervention significantly reduced the median time from referral to first attended clinic visit (20 days to 1 day) and from first visit to ART initiation (27.5 days to 0 day). The intervention did not seem to negatively impact subsequent engagement in care. All patients in the intervention group achieved VS, but not all in the control group achieved VS. In addition, more deaths were noted in the control group, with a trend toward reduced mortality in the intervention group. Other studies have shown that increased rates of ART uptake and more rapid VS may lead to downstream increased rates of retention in care and sustained VS.12 Long-term benefits of rapid initiation of ART will need to be investigated in future studies.
In the treatment as prevention paradigm, population-level viral load suppression is strongly associated with decreases in HIV incidence.9,10 The ending of the HIV epidemic (EHE) initiative in the United States seeks to reduce the number of new HIV infections by at least 90% by 2030 and to increase VS to 90% nationally by 2030.18 Accelerating interruption of the cycle of transmission by achieving VS as early as possible is key, along with evidence-based strategies to identify, engage, and retain PWH in care. The findings from this study support the use of this Rapid Start Protocol for all VA facilities as a strategy to achieve EHE's goals.
Confirmation of the benefits of rapid initiation of ART in a VA treatment setting is of value since the VA is the largest national provider of HIV care and is an integrated managed care system that provides long-term follow-up for most of its enrollees. Our findings suggest PWH receiving care at the Atlanta VA Medical Center IDC have excellent rates of VS and retention in care. Other studies have demonstrated VA hospitals perform similarly or better than the non-VA system on most of the nationally recognized measures of inpatient and outpatient care quality.19,20 The VA is uniquely designed for rapid initiation of ART as cost of ART medication and insurance coverage are not a barrier, medications can be mailed to the patient quickly or picked up the same day. Furthermore, patients newly diagnosed at the VA are likely to receive all treatment and care within the VA system, which has a centralized EHR system.
The clinic used a multidisciplinary approach for care coupled with selection of the most appropriate and tolerated ART regimen, adherence counseling, education, and psychosocial support to ensure successful ART initiation and maintenance over time. The multidisciplinary approach is necessary for this population as the mental health burden in the cohort was higher than other cohorts in the literature, and further linkage to care and services needs to be prioritized. However, immediately starting ART required additional time and effort from the multidisciplinary care team, as many patients had immediate housing needs, substance use, or mental health concerns. The Rapid Start Protocol allows for care to be initiated while also simultaneously addressing other aspects of care such as management of housing and substance use.
Implementation of rapid ART programs can be challenging. Buy-in is necessary at multiple levels, including funders, local health systems, clinic administration, and clinic staff. To implement and sustain rapid ART programs, necessary resources include (1) a dedicated point of contact for efficient and reliable notification of new diagnosis of HIV, (2) peers or navigators to assist through clinic and rapid ART process, (3) training the staff to assist with pharmaceutical assistance program applications, and (4) a multidisciplinary team that includes a social worker, eligibility/insurance specialist, and a dedicated medical provider that can quickly mobilize to see rapid ART patients on a same-day basis.7 While VA facilities bypass some logistical challenges with insurance or medication coverage, our clinic's ancillary support team felt understaffed with a case manager, social worker, psychologist, clinical pharmacist, and several schedulers, nurses, and clinicians for an entire high-volume clinic serving populations disproportionately impacted by HIV.
Successful health systems with rapid ART programs should have a network of participating health care facilities to accommodate referrals depending on insurance coverage and patient preference. Programs should also have experience addressing barriers to linkage to care such as patient transportation needs and insurance issues relayed to payment or coverage. Before implementation, educating clinical staff on the benefits of rapid ART initiation without comprehensive laboratory data should occur with subsequent patient education regarding ART teaching, adherence counseling, and provision of extra assistance such as pillboxes.
Our study has several limitations. First, there is the possibility of selection bias among the historical control population; however, the two populations were remarkably similar on important characteristics which were controlled for in the analysis. Several structural variables were not readily available and could not be used as covariates, such as education and poverty levels, housing status, and distance to clinic. Second, this was a single-center study, with a predominantly male population, which may limit the generalizability of the findings.4 Third, the use of first recorded viral load suppression may represent time-to-lab measurement, rather than when exactly viral load suppression occurred, which could have occurred sooner. Although this concern for a monitoring bias exists, the time difference between viral load testing after initial diagnosis and after ART initiation for each group was not statistically significant.
Lastly, retrospective cohort surveillance data, although systematic and complete, cannot truly eliminate complex residual confounders, such as those willing to undergo rapid ART initiation, might possess attributes such as increased self-efficacy and trust that are associated with medication adherence and VS. An important example in the present study arises from differences in ART regimens between the control period and the study period, which could have influenced uptake of recommended treatment or less likely potency. Likewise, guideline recommendations were not as firmly linked to policy during the control period, which may have affected patient and provider behaviors.
In summary, these results provide evidence that prioritizing immediate ART initiation can help compress the HIV care continuum from diagnosis to linkage to VS. Shorter times to VS can have long-term clinical benefits not only to the patient, but also prevention benefits for the community. The Rapid Start Protocol should be considered for implementation at all VA facilities providing HIV care.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This research is supported by the Emory cFAR–Emory Center for AIDS Research (P30 AI050409).
References
- 1. U.S. Department of Health and Human Services. Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2020. Available at: https://aidsinfo.nih.gov/contentfiles/adultandadolescentgl.pdf (Last accessed June 1, 2020).
- 2. Mateo-Urdiales A, Johnson S, Smith R, et al. Rapid initiation of antiretroviral therapy for people living with HIV. Cochrane Database Syst Rev 2019;6:CD012962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coffey S, Bacon O. Immediate ART Initiation & Restart: Guide for Clinicians. AETC National Coordinating Resource Center. 2021. Available at: https://aidsetc.org/resource/immediate-art-initiation-restart-guide-clinicians (Last accessed September 2, 2021).
- 4. Koenig SP, Dorvil N, Dévieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med 2017;14:e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: The CASCADE randomized clinical trial. JAMA 2018;319:1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr 2017;74:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Colasanti J, Sumitani J, Mehta CC, et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the Southern United States. Open Forum Infect Dis 2018;5:ofy104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grabowski MK, Serwadda DM, Gray RH, et al. HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med 2017;377:2154–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solomon SS, Mehta SH, McFall AM, et al. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: A cross-sectional, comparative study. Lancet HIV 2016;3:e183–e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansoti B, Mwinnyaa G, Hahn E, et al. Targeting the HIV epidemic in South Africa: The need for testing and linkage to care in emergency departments. EClin Med 2019;15:14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Halperin J, Conner K, Butler I, et al. A care continuum of immediate ART for newly diagnosed patients and patients presenting later to care at a federally qualified health center in New Orleans. Open Forum Infect Dis 2019;6:ofz161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coffey S, Bacchetti P, Sachdev D, et al. RAPID antiretroviral therapy: High virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population [published correction appears in AIDS]. AIDS 2019;33:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bacon OML, Coffey SC, Hsu LC, et al. Development of a citywide rapid antiretroviral therapy initiative in San Francisco. Am J Prev Med 2021;61:S47–S54. [DOI] [PubMed] [Google Scholar]
- 14. Guidelines for Managing Advanced HIV Disease and Rapid Initiation of Antiretroviral Therapy. Geneva: World Health Organization; 2017. PMID: . [PubMed] [Google Scholar]
- 15. Dombrowski JC, Simoni JM, Katz DA, Golden MR. Barriers to HIV care and treatment among participants in a public health HIV care relinkage program. AIDS Patient Care STDS 2015;29:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gomillia CES, Backus KV, Brock JB, et al. Rapid antiretroviral therapy (ART) initiation at a community-based clinic in Jackson, MS. AIDS Res Ther 2020;17:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sigel K, Park L, Justice A. HIV and cancer in the Veterans health administration system. Semin Oncol 2019;46:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fauci AS, Redfield RR, Sigounas G, et al. Ending the HIV epidemic: A plan for the United States. JAMA 2019;321:844–845. [DOI] [PubMed] [Google Scholar]
- 19. Mangal JP, Rimland D, Marconi VC. The continuum of HIV care in a Veterans' Affairs clinic. AIDS Res Hum Retroviruses 2014;30:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anhang Price R, Sloss EM, Cefalu M, et al. Comparing quality of care in veterans affairs and non-veterans affairs settings. J Gen Intern Med 2018;33:1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]