Abstract
Background:
We recently reported that use of an “advanced” hybrid closed-loop system reduced hyperglycemia without increasing hypoglycemia compared to a first-generation system. The aim of this analysis was to evaluate whether this improved performance was specifically related to better mealtime glycemic control.
Methods:
We conducted a secondary analysis of postprandial glycemic control in an open-label, multinational, randomized crossover trial of 112 participants with type 1 diabetes, aged 14–29, of the Medtronic MiniMed™ 670G hybrid closed-loop system (670G) versus the Medtronic advanced hybrid closed-loop (AHCL) system, for 12 weeks each. We compared glycemic and insulin delivery metrics over a 3 h horizon across all meals to assess system performance and outcomes.
Results:
Overall meal size and premeal insulin on board were similar during run-in and between 670G and AHCL arms. Compared with 670G arm, premeal, peak, and mean glucose levels were numerically lower in the AHCL arm (167 ± 23, 231 ± 23, and 177 ± 20 mg/dL vs. 175 ± 23, 235 ± 23, and 180 ± 19 mg/dL, respectively), with a trend to lower hyperglycemia level 2 in AHCL arm. Adjusting for premeal glucose level, all postmeal outcomes between 670G and AHCL were statistically similar. Prandial insulin delivery also was similar in both treatment arms (21 ± 9 vs. 23 ± 10 U), with a shift in basal/bolus ratio from 28%/71% in 670G arm to 20%/80% in AHCL arm.
Conclusions:
Reduced hyperglycemia with AHCL compared to 670G was not related to early postprandial glycemic excursions after adjusting for premeal glucose level (<3 h after meal), but likely to later (>3 h) postprandial or overnight improvements. Further refinements to mealtime bolus algorithms and strategies may more optimally control prandial glycemic excursions.
Keywords: Postprandial blood glucose, Closed-loop system, Adolescents, Young adults
Introduction
Improvements in insulin pumps, continuous glucose sensors, and control algorithms have led to their integration into advanced systems that automatically adjust insulin delivery based on glucose sensor values and trends. These closed-loop systems are still considered “hybrid,” as their intended function is to adjust insulin delivery automatically while still requiring user input of carbohydrate intake and manual triggering of insulin bolus at mealtimes. The first two systems to achieve regulatory approval in the United States, the Medtronic MiniMed 670G (670G) hybrid closed-loop system (Medtronic, Northridge, CA) and the t:slim X2 with Control-IQ technology (Tandem, San Diego, CA) have been shown in large-scale trials to improve A1c levels and time in range and to reduce hyperglycemia and hypoglycemia in both pediatric and adult age ranges.1–4 A third system, in development, but not yet commercially available, (Omnipod5; Insulet, Acton, MA) has demonstrated similar results in a recently completed clinical study.5
The 670G, as the first commercial hybrid closed-loop system, used a conservative approach to glucose control, modifying basal insulin delivery in response to glucose levels and choosing a fixed target glucose setpoint of 120 mg/dL. An updated version of this algorithm, the “advanced hybrid closed-loop system” (AHCL), was subsequently released by Medtronic. It incorporates some features developed in the MD-Logic automated insulin delivery system (Dreamed, Petah Tikva, Israel), including selectable target glucose setpoints of 100 or 120 mg/dL, and additional algorithmic enhancements designed to improve performance around meals, including an autobolus module that delivers correction doses automatically and an automated meal-detection algorithm, which when triggered, enables the system to deliver more aggressive autocorrection boluses.6–8
We previously reported the results of a randomized crossover trial comparing glycemic outcomes during 12 weeks of treatment with the 670G to 12 weeks of the AHCL System in 113 adolescents and young adults with type 1 diabetes (T1D). Use of the advanced hybrid closed-loop (AHCL) was associated with less daytime (6 AM–11:59 PM) hyperglycemia (time >180 mg/dL was 34% with AHCL vs. 37% with 670G), with no increase in hypoglycemia. AHCL use was also associated with improved mean glucose 159 ± 13 vs. 166 ± 13 mg/dL) and 24 h time in range (67% ± 8% vs. 63% ± 8%).9
These improvements were accompanied by an average 10% increase in total insulin dose in AHCL arm compared to 670G arm and shift of the basal/bolus ratio toward greater percentage of insulin delivered as bolus (64% for AHCL compared to 50% for 670G). Approximately 1/3 of the bolus insulin in the AHCL arm was provided by automated correction boluses.
Given the improved daytime glucose control (when most meals occur), the increased insulin delivery, and the proportion of insulin boluses delivered by automated correction in the AHCL arm compared to the 670G arm, we sought to examine more closely the glycemic dynamics and insulin delivery characteristics specifically related to mealtimes during the Fuzzy Logic Automated Insulin Regulation (FLAIR) study.
Methods
The FLAIR study compared the 670G system and the AHCL system in adolescents and young adults with T1D. The study was conducted at seven endocrinology practices: four in the United States and one each in Germany, Israel, and Slovenia. The protocol and informed consent/assent forms were approved by the appropriated institutional review boards and ethics committees, and regulatory approval to conduct the study was obtained in all four countries. The protocol is available online at https://public.jaeb.org/datasets and details of the trial and methods have been published elsewhere9 and summarized here.
Of the 113 participants randomized in the study, mean age was 19 ± 4 years (range: 14–29 years), mean HbA1c at baseline was 7.9% ± 0.7% (range: 7.0%–10.9%), 62% were female, 20% were not using a pump, and 38% were not using a continuous glucose monitor (CGM). Key exclusion criteria were concomitant disease that affects metabolic control or HbA1c levels, one or more episodes of severe hypoglycemia requiring treatment by another person or ketoacidosis requiring admission to the hospital within 6 months before screening, and clinically significant nephropathy or on dialysis.
Eligible participants were entered in a run-in phase where participants were trained to use a study pump without automated insulin delivery and a CGM. Participants already using a 670G system in auto mode or using an insulin pump and CGM were able to skip the run-in training. All other participants were started on the study pump for ∼14 days and then used the study CGM for ∼14 days (with an opportunity to repeat). Participants who had 80% of CGM data over the possible 14 days and an average of ≥3 blood glucose meter tests per day were entered into the randomized trial.
Participants were randomly assigned to receive 12 weeks of 670G followed by 12 weeks of AHCL or vice versa. There was no washout period in-between. At the beginning of each 12-week period, participants, and a parent or guardian when applicable, were trained on the assigned closed-loop system. The AHCL was started with an auto mode target glucose setpoint of 120 mg/dL (6.7 mmol/L) and an active insulin time of 3–4 h. These could be adjusted to a target glucose setpoint of 100 mg/dL (5.5 mmol/L) and an active insulin time down to 2 h based on assessment visits conducted every 2 weeks. The 670G had a fixed target glucose setpoint of 120 mg/dL.
Participants entered carbohydrate intake before each meal to enable an appropriate meal-time insulin bolus. Two participants dropped out during period 1: one while using AHCL and one while using 670G. Both periods that were started met the prespecified requirement of at least 72 h of postprandial CGM data and insulin data.
Statistical Methods
Meals were defined as the three largest carbohydrate amounts entered into the pump on each day. If fewer than three meals were entered in a day, only the meals available from that day were included. The start of the meal is the time when the participants entered carbs into the pump. The postmeal period consisted of the 3 h after the start of a meal, or until start of the next meal. Postprandial CGM outcomes were calculated pooling all CGM readings during all the postmeal periods. Baseline glucose was defined as the most recent CGM value within 15 min before the start of the meal. The nadir, peak, excursion (peak minus baseline), and time to peak is computed for each meal and then averaged across all meals in a period.
These outcomes were only calculated from meals with at least 2 h of CGM data in the 3-h window. Glycemic outcomes during the run-in phase are only reported from participants not using 670G in auto mode to represent a baseline for participants without previous automated insulin delivery system use. In classifying meals as breakfast, lunch, or dinner, any meal starting between 5 AM and 10:59 AM was considered breakfast, between 11 AM and 4:59 PM was considered lunch, and between 5 PM and 9:59 PM dinner (postprandial analysis was defined as the 3 h window after the start of that meal). Overnight glycemia was defined as 12 AM–5:59 AM. Calculation of insulin on board at mealtime includes any bolus given within 15 min of the start of the meal.
The mean ± standard deviation is reported for continuous outcomes. Time spent with glucose levels below 54 mg/dL (3.0 mmol/L) and insulin on board at the time of the meal were winsorized at the 10th and 90th percentiles to account for skewness. This process caps values at the 10th and 90th percentiles of the distribution so that analyses are more robust to outliers. A repeated measures least squares regression model with an unstructured covariance structure compared postprandial glycemia between AHCL and 670G adjusting for baseline glucose at start of the meal, period, prestudy 670G use, and HbA1c at randomization.
The model included three timepoints: run-in phase, AHCL period, and 670G period. A sensitivity analysis replicated treatment group comparisons using 4 h after the start of a meal as the definition of a postprandial period. An additional analysis excluded meals with intervening boluses given during the postmeal period. The false discovery rate was controlled using the adaptive two stage Benjamini-Hochberg procedure.10 All P-values are two-tailed, and analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC).
Results
The demographics have been previously reported.9 The mean number of meals per participant was 229 meals in 670G period and in AHCL period.
Baseline glucose at start of meal was 181 ± 30 mg/dL in the run-in phase, 175 ± 23 mg/dL in the 670G arm, and 167 ± 23 mg/dL in the AHCL arm (Table 1). The meal size and insulin on board at the start of the meal were similar for the three groups: mean meal size was 46 ± 16 g during run-in, 46 ± 16 g in 670G, and 44 ± 15 g in AHCL and mean insulin on board was 9.1 ± 3.4 U, 9.1 ± 3.1 U, and 9.3 ± 3.1 U, respectively.
Table 1.
Glycemic Outcomes During Postmeal Periods
| Run-in | 670G arm | AHCL arm | Baseline-adjusted difference between AHCL and 670G (95% CI) [P-value]a |
|
|---|---|---|---|---|
| No. of participants | 98 | 112 | 112 | |
| No. of meals per participant | 67 ± 20 | 229 ± 29 | 229 ± 27 | |
| Meal size (g) | 46 ± 16 | 46 ± 16 | 44 ± 15 | |
| Baseline glucose at start of meal (mg/dL) | 181 ± 30 | 175 ± 23 | 167 ± 23 | |
| Insulin on board at start of meal (U)b | 9.1 ± 3.4 | 9.1 ± 3.1 | 9.3 ± 3.1 | |
| Peak glucose (mg/dL) | 241 ± 30 | 235 ± 23 | 231 ± 23 | |
| Time to peak (minutes) | 73 ± 15 | 78 ± 12 | 82 ± 12 | |
| Nadir glucose (mg/dL) | 128 ± 24 | 124 ± 14 | 121 ± 16 | |
| Glucose excursion (peak—baseline; mg/dL) | 59 ± 15 | 61 ± 13 | 64 ± 13 | 1.7 (−0.6 to 4.1) [0.10] |
| Overall glucose control | ||||
| Mean glucose (mg/dL) | 185 ± 27 | 180 ± 19 | 177 ± 20 | 1.8 (−0.4 to 4.0) [0.10] |
| % Time in the target range (70–180 mg/dL) | 49% ± 15% | 53% ± 11% | 54% ± 11% | −0.8% (−2.1% to 0.4%) [0.12] |
| % Time in the tight target range (70–140 mg/dL) | 28% ± 11% | 29% ± 8% | 30% ± 9% | −0.1% (−1.4% to 1.1%) [0.85] |
| Glycemic variability | ||||
| Coefficient of variation | 35% ± 5% | 36% ± 4% | 36% ± 3% | 0.0% (−0.7% to 0.7%) [0.96] |
| Hypoglycemia | ||||
| % Time <70 mg/dL | 2.2% ± 1.9% | 2.1% ± 1.4% | 2.1% ± 1.5% | −0.1% (−0.4% to 0.2%) [0.48] |
| % Time <54 mg/dLb | 0.46% ± 0.52% | 0.49% ± 0.39% | 0.46% ± 0.34% | −0.05% (−0.13% to 0.03%) [0.13] |
| Hyperglycemia | ||||
| % Time >180 mg/dL | 49% ± 16% | 45% ± 11% | 44% ± 12% | 1.0% (−0.4% to 2.3%) [0.12] |
| % Time >250 mg/dL | 18% ± 12% | 15% ± 9% | 14% ± 9% | 1.0% (0.0% to 1.9%) [0.05] |
Values are reported as mean ± SD.
Baseline-adjusted difference is the mean outcome in AHCL minus mean outcome in 670G estimated from a repeated measures least squares regression model adjusting for baseline glucose at start of meal, period, prestudy 670G system use, and HbA1c at randomization as fixed effects. The model includes three time points: (1) baseline, (2) period 1 outcome, and (3) period 2 outcome. Nominal (uncorrected) P-values were adjusted for multiple comparisons using the Two Stage Benjamini-Hochberg adaptive false discovery rate procedure.
Summary statistics winsorized at the 10th and 90th percentiles to account for skewness.
AHCL, advanced hybrid closed-loop; CI, confidence interval; SD, standard deviation.
The mean peak glucose was 241 ± 30 mg/dL during run-in, 235 ± 23 mg/dL in the 670G arm, and 231 ± 23 mg/dL in the AHCL arm (Table 1). Excursion was generally higher for meals with lower baseline glucose at start of meal, and the mean excursion was 59 ± 15 mg/dL during run-in, 61 ± 13 mg/dL in the 670G arm, and 64 ± 12 mg/dL in the AHCL arm (P = 0.10). Postprandial time in range was 49% ± 15% during run-in, 53% ± 11% in the 670G arm, and 54% ± 11% in the AHCL arm (P = 0.12). Postprandial euglycemic and hyperglycemic outcomes generally improved during follow-up when participants were using AHCL or 670G, while postprandial hypoglycemia was infrequent and similar during the prestudy baseline and two study periods.
All postprandial outcomes between AHCL and 670G after adjusting for baseline glucose at start of meal were similar with small effect sizes and narrow confidence intervals. A sensitivity analyses using 4 h postprandial periods yielded similar results (Supplementary Table S1). An additional sensitivity analyses using a 4 h postprandial period and excluding meals with intervening boluses given during the period also yielded similar results (Supplementary Table S2).
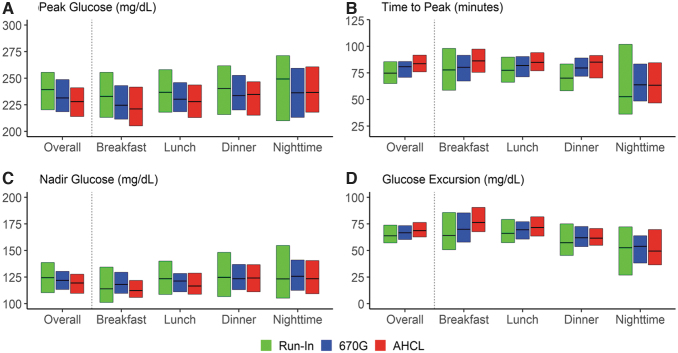
The peak, time to peak, nadir, and excursion overall and by type of meal are presented in Figure 1 and Table 2. The baseline glucose was lowest for breakfast with a mean of 166 mg/dL during run-in phase, 162 mg/dL in 670G arm, and 148 mg/dL in AHCL arm. Mean peak was smallest for breakfast and for AHCL, while mean excursion was highest for breakfast and for AHCL. Mean postprandial time in range at breakfast was 50% during run-in phase and increased to 55% in 670G and 59% in AHCL. Other postprandial euglycemic and hyperglycemic outcomes showed a slight improvement in favor of the AHCL arm likely due to the lower glucose at start of the meal. The mean baseline glucose at dinner was 189 mg/dL during run-in phase, 182 mg/dL in 670G, and 177 mg/dL in AHCL with postprandial time in range of 49%, 52%, and 52%, respectively.
FIG. 1.
Boxplots of peak, time to peak, nadir, and excursion by type of meal and treatment arm.
Table 2.
Glycemic Outcomes During Postmeal Periods by Time of Day
| Time of meal | Run-in (n = 98) | 670G arm (n = 112) | AHCL arm (n = 112) |
|---|---|---|---|
| Breakfast | |||
| No. of meals per participant | 13 ± 7 | 46 ± 25 | 45 ± 23 |
| Meal size (g) | 41 ± 16 | 41 ± 15 | 40 ± 15 |
| Baseline glucose (mg/dL) | 166 ± 30 | 162 ± 28 | 148 ± 23 |
| Peak glucose (mg/dL) | 233 ± 32 | 230 ± 29 | 225 ± 28 |
| Time to peak (minutes) | 79 ± 32 | 80 ± 21 | 87 ± 16 |
| Nadir glucose (mg/dL) | 120 ± 31 | 121 ± 18 | 115 ± 16 |
| Glucose excursion (mg/dL) | 67 ± 29 | 69 ± 23 | 76 ± 21 |
| Mean glucose (mg/dL) | 181 ± 32 | 178 ± 23 | 173 ± 22 |
| % TIR (70–180 mg/dL) | 50% ± 21% | 55% ± 15% | 59% ± 14% |
| % TIR (70–140 mg/dL) | 27% ± 16% | 29% ± 11% | 33% ± 10% |
| Coefficient of variation | 31% ± 9% | 33% ± 5% | 34% ± 5% |
| % Time <70 mg/dL | 2.7% ± 4.1% | 1.6% ± 1.4% | 1.8% ± 1.9% |
| % Time <54 mg/dLa | 0.41% ± 0.64% | 0.31% ± 0.40% | 0.33% ± 0.39% |
| % Time >180 mg/dL | 47% ± 21% | 44% ± 15% | 39% ± 14% |
| % Time >250 mg/dL | 15% ± 15% | 13% ± 12% | 12% ± 11% |
| Lunch | |||
| No. of meals per participant | 26 ± 9 | 87 ± 16 | 89 ± 16 |
| Meal size (g) | 47 ± 17 | 46 ± 17 | 45 ± 15 |
| Baseline glucose (mg/dL) | 177 ± 32 | 172 ± 25 | 165 ± 26 |
| Peak glucose (mg/dL) | 241 ± 37 | 235 ± 25 | 231 ± 25 |
| Time to peak (minutes) | 76 ± 21 | 81 ± 15 | 85 ± 15 |
| Nadir glucose (mg/dL) | 128 ± 29 | 122 ± 15 | 120 ± 18 |
| Glucose excursion (mg/dL) | 63 ± 21 | 64 ± 15 | 66 ± 15 |
| Mean glucose (mg/dL) | 186 ± 32 | 180 ± 20 | 177 ± 23 |
| % TIR (70–180 mg/dL) | 50% ± 18% | 53% ± 12% | 55% ± 13% |
| % TIR (70–140 mg/dL) | 28% ± 14% | 29% ± 9% | 30% ± 11% |
| Coefficient of variation | 34% ± 6% | 35% ± 4% | 35% ± 4% |
| % Time <70 mg/dL | 1.9% ± 2.0% | 2.1% ± 1.7% | 2.1% ± 1.8% |
| % Time <54 mg/dLa | 0.32% ± 0.49% | 0.47% ± 0.46% | 0.44% ± 0.38% |
| % Time >180 mg/dL | 49% ± 19% | 45% ± 12% | 43% ± 14% |
| % Time >250 mg/dL | 19% ± 15% | 15% ± 10% | 14% ± 10% |
| Dinner | |||
| No. of meals per participant | 23 ± 9 | 76 ± 17 | 76 ± 17 |
| Meal size (g) | 47 ± 17 | 47 ± 17 | 46 ± 16 |
| Baseline glucose (mg/dL) | 189 ± 39 | 182 ± 26 | 177 ± 24 |
| Peak glucose (mg/dL) | 243 ± 38 | 238 ± 26 | 234 ± 25 |
| Time to peak (minutes) | 70 ± 22 | 78 ± 13 | 80 ± 15 |
| Nadir glucose (mg/dL) | 129 ± 28 | 126 ± 19 | 125 ± 20 |
| Glucose excursion (mg/dL) | 53 ± 19 | 57 ± 15 | 57 ± 14 |
| Mean glucose (mg/dL) | 186 ± 32 | 182 ± 22 | 179 ± 23 |
| % TIR (70–180 mg/dL) | 49% ± 17% | 52% ± 13% | 52% ± 12% |
| % TIR (70–140 mg/dL) | 28% ± 15% | 28% ± 10% | 29% ± 11% |
| Coefficient of variation | 35% ± 6% | 36% ± 4% | 35% ± 4% |
| % Time <70 mg/dL | 2.5% ± 3.1% | 2.3% ± 1.9% | 2.2% ± 1.9% |
| % Time <54 mg/dL a | 0.42% ± 0.63% | 0.49% ± 0.45% | 0.44% ± 0.42% |
| % Time >180 mg/dL | 48% ± 18% | 46% ± 14% | 46% ± 13% |
| % Time >250 mg/dL | 19% ± 14% | 16% ± 10% | 15% ± 9% |
| Nighttime | |||
| no. of meals per participant | 7 ± 6 | 21 ± 19 | 20 ± 18 |
| Meal size (g) | 41 ± 18 | 41 ± 17 | 41 ± 17 |
| Baseline glucose (mg/dL) | 194 ± 53 | 196 ± 41 | 192 ± 42 |
| Peak glucose (mg/dL) | 246 ± 49 | 241 ± 36 | 241 ± 36 |
| Time to peak (minutes) | 68 ± 46 | 67 ± 34 | 65 ± 34 |
| Nadir glucose (mg/dL) | 138 ± 50 | 127 ± 24 | 127 ± 26 |
| Glucose excursion (mg/dL) | 47 ± 30 | 48 ± 29 | 49 ± 31 |
| Mean glucose (mg/dL) | 188 ± 48 | 184 ± 28 | 182 ± 30 |
| % TIR (70–180 mg/dL) | 47% ± 29% | 51% ± 18% | 50% ± 17% |
| % TIR (70–140 mg/dL) | 28% ± 25% | 28% ± 15% | 28% ± 14% |
| Coefficient of variation | 27% ± 12% | 34% ± 9% | 34% ± 8% |
| % Time <70 mg/dL | 1.8% ± 4.2% | 2.4% ± 3.6% | 2.7% ± 3.9% |
| % Time <54 mg/dLa | 0.15% ± 0.41% | 0.35% ± 0.53% | 0.40% ± 0.52% |
| % Time >180 mg/dL | 52% ± 29% | 47% ± 18% | 47% ± 18% |
| % Time >250 mg/dL | 19% ± 23% | 18% ± 15% | 16% ± 13% |
Values are reported as mean ± SD.
Summary statistics winsorized at the 10th and 90th percentiles to account for skewness.
TIR, time in range.
Discussion
In this secondary analysis of the FLAIR study, we have shown that despite the incorporation of several enhancements to the closed-loop system algorithms, including lower glucose set-point (100 vs. 120 mg/dL), automated correction boluses, and modified controller gains and integral action, the AHCL system performed similarly to the existing 670G hybrid closed-loop system with respect to the early (3-h) postprandial meal glucose control with a trend to lower hyperglycemia level 2 (P = 0.05).
Since the earliest studies of automated insulin delivery, meals have proven a significantly greater challenge to glucose control than the overnight period. Manual insulin boluses are needed to compensate for the slower pharmacokinetics of subcutaneous insulin delivery, hence the term “hybrid” closed-loop.11 Even with full meal boluses, significant postprandial elevations occur during closed-loop control that cannot be adequately addressed by automated incremental increases in basal insulin delivery by the 670G algorithm, resulting in time-in-range attainments of 70%–72% with this system. While this is an improvement over open loop insulin delivery, it still represents about 7 h per day with glucose levels >180 mg/dL.
Automated correction boluses for hyperglycemia were incorporated into second-generation systems in an attempt to mitigate these postmeal glucose elevations. The AHCL system includes a meal-detection module that, when triggered, enables the system to deliver automated correction boluses every 5 min. The AHCL system under study compares favorably to that of the Insulet Omnipod 5 system, which also incorporates autocorrection features into its algorithm. In that system, following meal challenges of ∼50 g carbohydrates (with full bolus for meals), postprandial glucose excursions over a 4 h analysis window still exceeded 100 mg/dL, with peak postprandial glucose levels of 229 mg/dL.12
The Tandem Control-IQ system also incorporates an automated correction dose algorithm, in which correction boluses of 60% of the calculated correction dose can be given automatically by the system once per hour. While formal meal studies of this system are not available for comparison, a recent report of a 6-month clinical trial of that system noted that the time in 70–180 mg/dL range in the 4 h following a meal bolus ranged from 54% to 62%.13 While this figure is slightly higher than the 54% in our current study, this type of postprandial analysis would likely favor a longer window, by allowing more time for correction of hyperglycemia. Indeed, in a feasibility study of the AHCL following dinner challenge of 40 g carbohydrate, time in range for the following 5 h postprandial was 63.5%.7
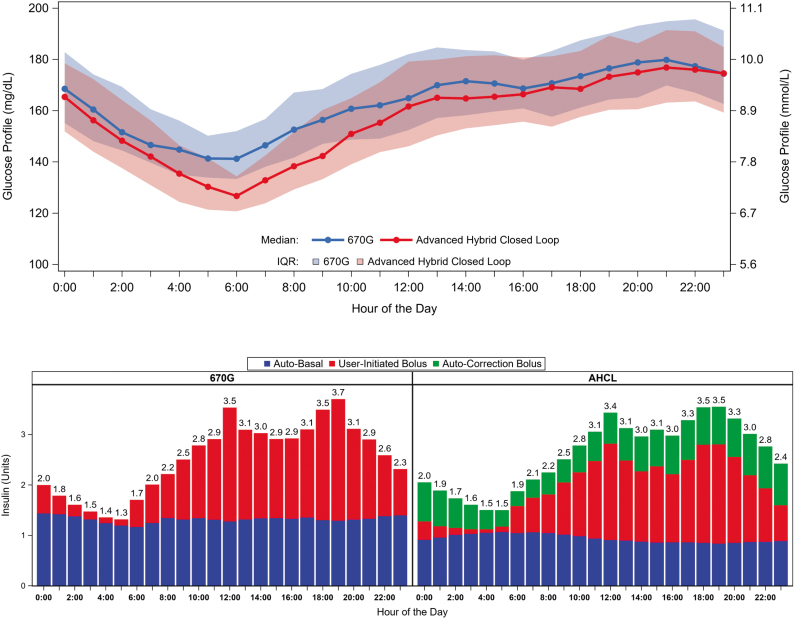
There are several possible explanations for why the automated correction bolus feature in the AHCL did not demonstrate markedly better performance than the standard 670G algorithm in the early postprandial period. Most obviously, as shown in Table 3 and Figure 2, the total amount of insulin delivered during the 3 h postprandial window was nearly identical in the two treatment conditions. While an additional 4 U of insulin was provided via autocorrection in the AHCL condition, there was a corresponding reduction in the programmed bolus and in auto-basal, resulting in a similar total insulin dose over the 3 h window; the only resulting major difference was an ∼8%–9% greater percentage of the total insulin delivered by bolus in the AHCL.
Table 3.
Insulin Metrics During Postmeal Periods by Time of Day
| 670G arm n = 112 |
AHCL arm n = 112 |
|
|---|---|---|
| Overall | ||
| Insulin on board (U),a mean ± SD | 9.1 ± 3.1 | 9.3 ± 3.1 |
| Total insulin (U), mean ± SD | 29 ± 12 | 29 ± 12 |
| Auto basal insulin (U), mean ± SD | 8 ± 4 | 6 ± 2 |
| Bolus insulin (U), mean ± SD | 21 ± 9 | 23 ± 10 |
| Autocorrection bolus (U), mean ± SD | NA | 4 ± 2 |
| Insulin from basal | 28% ± 6% | 20% ± 4% |
| Insulin from bolus | 71% ± 6% | 80% ± 4% |
| Breakfast | ||
| Insulin on board (U),a mean ± SD | 7.6 ± 2.7 | 7.7 ± 2.7 |
| Total insulin (U), mean ± SD | 12 ± 5 | 12 ± 5 |
| Auto basal insulin (U), mean ± SD | 4 ± 2 | 3 ± 1 |
| Bolus insulin (U), mean ± SD | 8 ± 4 | 9 ± 4 |
| Autocorrection bolus (U), mean ± SD | NA | 3 ± 1 |
| Insulin from basal | 32% | 23% |
| Insulin from bolus | 68% | 77% |
| Lunch | ||
| Insulin on board (U),a mean ± SD | 9.2 ± 3.1 | 9.3 ± 3.0 |
| Total insulin (U), mean ± SD | 15 ± 6 | 15 ± 6 |
| Auto basal insulin (U), mean ± SD | 4 ± 2 | 3 ± 1 |
| Bolus insulin (U), mean ± SD | 11 ± 5 | 12 ± 5 |
| Autocorrection bolus (U), mean ± SD | NA | 3 ± 1 |
| Insulin from basal | 27% | 20% |
| Insulin from bolus | 72% | 80% |
| Dinner | ||
| Insulin on board (U),a mean ± SD | 9.8 ± 3.4 | 10.1 ± 3.4 |
| Total insulin (U), mean ± SD | 14 ± 6 | 14 ± 6 |
| Auto basal insulin (U), mean ± SD | 3 ± 2 | 2 ± 1 |
| Bolus insulin (U), mean ± SD | 11 ± 5 | 11 ± 5 |
| Autocorrection bolus (U), mean ± SD | NA | 3 ± 1 |
| Insulin from basal | 25% | 18% |
| Insulin from bolus | 74% | 81% |
| Nighttime | ||
| Insulin on board (U),a mean ± SD | 8.4 ± 3.3 | 9.0 ± 3.2 |
| Total insulin (U), mean ± SD | 10 ± 6 | 8 ± 4 |
| Auto basal insulin (U), mean ± SD | 3 ± 2 | 2 ± 1 |
| Bolus insulin (U), mean ± SD | 7 ± 4 | 6 ± 3 |
| Autocorrection bolus (U), mean ± SD | NA | 3 ± 1 |
| Insulin from basal | 27% | 21% |
| Insulin from bolus | 75% | 78% |
Summary statistics winsorized at the 10th and 90th percentiles to account for skewness.
NA, not applicable.
FIG. 2.
Mean glucose by 24 h (top panel) and mean insulin delivery by hour (bottom panel).
It is possible that with increasing familiarity with the AHCL system, wearers tended to bolus less aggressively for meals, or not at all, after seeing performance of the system in real-world situations. Given the very low rates of time below range in the AHCL condition, more aggressive “tuning” of the autocorrections may be justified.
While the 670G and AHCL systems had similar early postprandial glucose control, the AHCL clearly demonstrated some benefit in improving daytime and nighttime control, as evidenced by the lower premeal glucose levels at each meal, a trend to lower postprandial time in hyperglycemia above 250 mg/dL, lower daytime glucose, and significantly improved overnight control, as evidenced by the lower 6 AM and prebreakfast glucose levels (Fig. 2).9 It is likely that the ability of the AHCL system to continue to deliver auto-boluses after 3 h (which is not captured in the current analysis) contributed to the improved control in this arm. The ability of the system to provide auto-boluses overnight, along with the lower glucose setpoint, achieved lower glucose levels to start the day with fewer user-initiated boluses overnight. This also resulted in more effective breakfast glycemic control.
It has been suggested that faster-acting insulin analogs might improve the performance of automated insulin delivery systems. Two studies comparing the newest generation faster-acting insulin aspart (Fiasp) with insulin aspart in the 670G reported similar results with both insulins, the one notable benefit of the faster insulin was seen in the first hour following the meal bolus.14,15 Peak postprandial excursions were similar to the present study. Most recently, a study of the AHCL system using Fiasp found that after a meal challenge of 40 g carbohydrate, time in 70–180 mg/dL range in a postprandial 4 h window was modestly greater compared to insulin aspart (73.6% vs. 72.1%).16 This time in range surpassed our present study, but with smaller magnitude of meal challenge.
The addition of adjunctive glucose-lowering agents with an automated insulin delivery system, using either rapid-or ultrarapid-acting insulin analogs, may improve performance further, as seen with recent pilot studies of dapagliflozin17 and pramlintide.18
It is difficult to compare postprandial performance of automated insulin delivery systems, given the variability in design of clinical studies, including meal size and content, definition of postprandial window, and the effect of prior residual insulin on board. It also bears noting that when analyzing postprandial glucose excursions, the common practice of reporting changes in mean glucose over time necessarily underestimates the true per-subject glucose excursion. Interindividual differences in time to peak glucose level will “smooth” out the curve and minimize the full impact of meal-related hyperglycemia. We suggest that a more standardized approach to these analyses should include calculation and reporting of mean per-subject glucose excursion (baseline to peak) and postprandial time in range, which will provide a more accurate description of system performance around meals.
Several important limitations of this study should be noted. We set a 3 h time horizon for the analysis of the postprandial periods. This specific duration was chosen to minimize the effect of interference of subsequent meal-induced glucose excursions: the longer duration of postprandial analysis, the fewer analyzable meals without this interference would have been available for comparison. A second limitation relates to the lack of standardization of meals and bolus strategies: because this was a free-living outpatient trial, there were no attempts made to standardize timing of the meal bolus with respect to the start of the meal, insulin action time, or meal content/size.
More rigorous control of these parameters would have potentially enabled us to draw more definitive conclusions about meal effects, but at the expense of the free-living design of the overall study. Finally, as noted earlier, the benefit of the auto-bolus functionality may have led some participants to forego or alter their behaviors in manual bolusing, which would have had the effect of mitigating the full benefit of the auto-bolus.
In conclusion, the superior glycemic outcomes of participants using the AHCL compared to 670G system demonstrated in the FLAIR study does not appear to stem from the early (first 3 h) postprandial glycemic excursion, but to later (after 3-h) portion of the postprandial period, as well as the improved overnight control. Continued refinements to auto-bolus tuning and/or meal-prediction algorithms, as well as further considerations of manual bolus parameters such as insulin-carbohydrate ratios, timing of meal boluses, and insulin-on-board calculations, are warranted to provide optimal meal control, particularly in the first several hours after the meal, in this closed-loop system.
Supplementary Material
Acknowledgments
Study Group Members—Protocol Chairs: Protocol Chairs and Co-principal investigator (PI): Richard M. Bergenstal, MD and Moshe Phillip, MD. Clinical Sites: A listing of the FLAIR sites with participating PI, coinvestigators (CI), subinvestigators (I), primary coordinator (PC), coordinators (C), study nurses (SN), research assistants (RA), project manager (PM), and administrative manager (AM) is included below. The number included in the randomized trial at each site is indicated in parenthesis after the sites' name. International Diabetes Center (23): Amy Criego (PI), Richard M. Bergenstal (PI), Anders Carlson (PI), Thomas Martens (I), Shannon Beasley (I), Mary L. Johnson (PM), Diane Whipple (PC), Jamie Hyatt (C), Alina Punel (C), Aimee Grieme (C), Lee Ann Thomas (RA), Amy LaFrance (AM), and Caitlin Hasledalen (RA). Joslin Diabetes Center (19): Lori Laffel (PI), Elvira Isganaitis (I), Emily Freiner (PC, I), Louise Ambler-Osborn (I), Hannah Desrochers (C), Christine Turcotte (C), Nisha Naik (C), Lindsay Roethke (C), and Margaret Fisher (C). Schneider Children's Hospital (17): Revital Nimri (PI), Michal Nevo (I), Rachel Bello (I), Alona Hamou (PC), Orna Hermon (PC), Orit Horesh (SN), Galit Shiovitch Mantzuri (SN), Irit Drotz (SN), Nava Yehiel (SN), and Rachel Naveh (C). University of Florida (17): Desmond Schatz (PI), Michael Haller (CI), Anastasia Albanese-O'Neill (CI), Eleni Sheehan (I), Julio Leey (I), Madison Smith (I), Laura Jacobsen (I), Janey Adams (PC), Jennifer Hosford (C), and Loren Whyte (C). University Medical Centre Ljubljana (16): Tadej Battelino (PI), Klemen Dovč (I), Nataša Bratina (I), Darja Šmigoc Schweiger (I), Urška Sever (C), Ana Gianini (C), Barbara Murn Berkopec (C), and Brigita Mali (C). Yale University (11): Stuart A. Weinzimer (PI), Kate Weyman (I), Lori Carria (PC), and Melinda Zgorski (C). Auf der Bult Centre for Children and Adolescents (10): Thomas Danne (PI), Torben Biester (PI), Thekla von dem Berge (I), Jantje Weiskorn (I), Olga Kordonouri (I), Sarah Biester (PC), Bärbel Aschemeier (C), Kerstin Remus (C), and Nicole Pisarek (C). Jaeb Center for Health Research Tampa, FL: Judy Sibayan, Roy W. Beck, Thomas Mouse, Julie Davis, Ryan J. Bailey, Amanda Hellmann, Nicole Reese, Peter Calhoun, Heidi Strayer, Nathan Cohen, Robert Henderson, Craig Kollman, Jennifer Kennedy, William Woodall, and Israel Mahr. Quality of Life Investigator: Korey Hood, Stanford University. National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK): Guillermo Arreaza-Rubin (Project Scientist), Thomas Eggerman (Program Officer), and Neal Green (PM). Central Laboratory—University of Minnesota Advanced Research and Diagnostic Laboratory: Robert Janicek, Deanna Gabrielson. Data and Safety Monitoring Board (DSMB): Steven H. Belle (Chair), Jessica Castle, Jennifer Green, Laurent Legault, Steven M. Willi, Carol Wysham, and Thomas Eggerman (DSMB Executive Secretary for NIDDK).
Contributor Information
for the FLAIR Study Group:
Richard M. Bergenstal, Moshe Phillip, Amy Criego, Richard M. Bergenstal, Anders Carlson, Thomas Martens, Shannon Beasley, Mary L. Johnson, Diane Whipple, Jamie Hyatt, Alina Punel, Aimee Grieme, Lee Ann Thomas, Amy LaFrance, Caitlin Hasledalen, Lori Laffel, Elvira Isganaitis, Emily Freiner, Louise Ambler-Osborn, Hannah Desrochers, Christine Turcotte, Nisha Naik, Lindsay Roethke, Margaret Fisher, Revital Nimri, Michal Nevo, Rachel Bello, Alona Hamou, Orna Hermon, Orit Horesh, Galit Shiovitch Mantzuri, Irit Drotz, Nava Yehiel, Rachel Naveh, Desmond Schatz, Michael Haller, Anastasia Albanese-O'Neill, Eleni Sheehan, Julio Leey, Madison Smith, Laura Jacobsen, Janey Adams, Jennifer Hosford, Loren Whyte, Tadej Battelino, Klemen Dovč, Nataša Bratina, Darja Šmigoc Schweiger, Urška Sever, Ana Gianini, Barbara Murn Berkopec, Brigita Mali, Stuart A. Weinzimer, Kate Weyman, Lori Carria, Melinda Zgorski, Thomas Danne, Torben Biester, Thekla von dem Berge, Jantje Weiskorn, Olga Kordonouri, Sarah Biester, Bärbel Aschemeier, Kerstin Remus, Nicole Pisarek, Judy Sibayan, Roy W. Beck, Thomas Mouse, Julie Davis, Ryan J. Bailey, Amanda Hellmann, Nicole Reese, Peter Calhoun, Heidi Strayer, Nathan Cohen, Robert Henderson, Craig Kollman, Jennifer Kennedy, William Woodall, Israel Mahr, Korey Hood, Guillermo Arreaza-Rubin, Thomas Eggerman, Neal Green, Robert Janicek, Deanna Gabrielson, Steven H. Belle, Jessica Castle, Jennifer Green, Laurent Legault, Steven M. Willi, Carol Wysham, and Thomas Eggerman
Collaborators: for the FLAIR Study Group
Authors' Contributions
S.A.W. drafted article. R.J.B. and P.C. performed the statistical analysis. R.M.B., R.N., R.W.B., D.S., L.A.-O., D.S.S., T.v.d.B., J.S., M.L.J., and M.P. reviewed and edited article.
Role of the Funder/Sponsor
The role of the Sponsor is specified for each of the following: Design and conduct of the study: There was no involvement from the National Institute of Diabetes and Digestive and Kidney Diseases or Medtronic in the design and conduct of the study. Collection, management, analysis, and interpretation of the data: There was no involvement from the National Institute of Diabetes and Digestive and Kidney Diseases or Medtronic in the collection, management, analysis, and interpretation of the data. Preparation, review, or approval of the article; and decision to submit the article for publication: The National Institute of Diabetes and Digestive and Kidney Diseases and Medtronic were not involved in the writing of the original article draft submitted. The National Institute of Diabetes and Digestive and Kidney Diseases and Medtronic were sent the article for review, but any revisions made based on their comments were at the discretion of the authors and permission for submitting content to journal was not required. There was no approval of the National Institute of Diabetes and Digestive and Kidney Diseases and Medtronic required or obtained for article submission.
Data Sharing Statement
Data will be made available on a public website (www.jaeb.org) at a later date. S.A.W. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
Medtronic MiniMed, Inc., provided the MiniMed 670G and AHCL systems, and infusion sets, used in the study. Medtronic, Inc.; provided study continuous glucose monitoring devices and sensors. S.A.W. reports grants from the National Institutes of Health, during the conduct of the study; personal fees from Medtronic, Insulet, Tandem, Eli Lilly, Sanofi, and Zealand, outside the submitted work. R.J.B. reports no conflicts of interest. R.M.B. reports grants and other from Abbott Diabetes Care, grants and other from Eli Lilly, outside the submitted work. R.N. reports grants from National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, nonfinancial support from Medtronic MiniMed, Inc., during the conduct of the study; grants, personal fees and other from DreaMed Diabetes Ltd., grants and nonfinancial support from Medtronic MiniMed, Inc., nonfinancial support from Dexcom, nonfinancial support from Insulet, grants, and personal fees from Eli Lilly, grants and personal fees from NovoNordisk, outside the submitted work. R.W.B. reports grants from National Institutes of Health, nonfinancial support from Medtronic during the conduct of the study; grants, nonfinancial support and study supplies from Dexcom, consulting fees paid to nonprofit employer from Bigfoot Biomedical, outside the submitted work. D.S. reports grants from the National Institutes of Health, during the conduct of the study; grants from the Leona M. and Harry B. Helmsley Charitable Trust, outside the submitted work. L.A.-O. reports no conflicts of interest. D.S.S. reports no conflicts of interest. T.v.d.B. reports grants and fees from NovoNordisk, Medtronic, Sanofi and Ypsomed outside the submitted work. J.S. reports no conflicts of interest. M.L.J. reports grants from the National Institutes of Health during the conduct of the study; grants from Abbott, Dexcom, Medtronic, Eli Lilly, NovoNordisk, Calibra, Insulet, Sanofi, the Leona M. and Harry B. Helmsley Charitable Trust,, and the Juvenile Diabetes Research Foundation, outside the submitted work. P.C. reports personal fees from Dexcom outside the submitted work. M.P. reports grants from the National Institutes of Health via Health Partners, during the conduct of the study; grants from Insulet, Dexcom, Medtronic, Roche, Eli Lilly, Lexicon, OPKO, and AstraZeneca, grants and personal fees from Novo Nordisk and Sanofi, grants, personal fees and owning stock from DreaMed Diabetes, personal fees from RSP Systems and Qulab Medical, grants and personal fees from Pfizer, grants and owning stock from Nutriteen Professional and NG Solutions, outside the submitted work. In addition, M.P. has a patent WO2010097796 issued to DreaMed Diabetes, a patent WO2011039741 issued to DraeMed Diabetes, and a patent WO2019077482 pending.
Funding Information
National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (IUC4DK10861); grant provided to Jaeb Center for Health Research and paid to each of the investigator's institutions.
Supplementary Material
References
- 1. Bergenstal RM, Garg S, Weinzimer SA, et al. : Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408. [DOI] [PubMed] [Google Scholar]
- 2. Brown SA, Kovatchev BP, Raghinaru D, et al. : i DCLTRG. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. : Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown SA, Forlenza GP, Bode BW, et al. : Omnipod 5 research G. multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44:1630–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Atlas E, Nimri R, Miller S, et al. : MD-logic artificial pancreas system: a pilot study in adults with type 1 diabetes. Diabetes Care 2010;33:1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nimri R, Muller I, Atlas E, et al. : MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 2014;37:3025–3032. [DOI] [PubMed] [Google Scholar]
- 8. Phillip M, Battelino T, Atlas E, et al. : Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833. [DOI] [PubMed] [Google Scholar]
- 9. Bergenstal RM, Nimri R, Beck RW, et al. : Group FS. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 2021;397:208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benjamini Y, Hochberg Y: On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat 2000;25:60–83. [Google Scholar]
- 11. Weinzimer SA, Steil GM, Swan KL, et al. : Fully automated closed-loop insulin delivery versus semiautomated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes care 2008;31:934–939. [DOI] [PubMed] [Google Scholar]
- 12. Buckingham BA, Christiansen MP, Forlenza GP, et al. : Performance of the Omnipod personalized model predictive control algorithm with meal bolus challenges in adults with type 1 diabetes. Diabetes Technol Ther 2018;20:585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Malley G, Messer LH, Levy CJ, et al. : i DCLTRG. Clinical management and pump parameter adjustment of the control-IQ closed-loop control system: results from a 6-month, multicenter, randomized clinical trial. Diabetes Technol Ther 2021;23:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsu L, Buckingham B, Basina M, et al. : Fast-acting insulin Aspart use with the MiniMed(TM) 670G system. Diabetes Technol Ther 2021;23:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ozer K, Cooper AM, Ahn LP, et al. : Fast acting insulin Aspart compared with insulin Aspart in the Medtronic 670G hybrid closed loop system in type 1 diabetes: an open label crossover study. Diabetes Technol Ther 2021;23:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee MH, Paldus B, Vogrin S, et al. : Fast-acting insulin Aspart versus insulin Aspart using a second-generation hybrid closed-loop system in adults with type 1 diabetes: a randomized, open-label, crossover trial. Diabetes Care 2021;dc210814 [Online ahead of print], DOI: 10.2337/dc21-0814. [DOI] [PubMed] [Google Scholar]
- 17. Biester T, Muller I, von dem Berge T, et al. : Add-on therapy with dapagliflozin under full closed loop control improves time in range in adolescents and young adults with type 1 diabetes: the DAPADream study. Diabetes Obes Metab 2021;23:599–608. [DOI] [PubMed] [Google Scholar]
- 18. Tsoukas MA, Majdpour D, Yale JF, et al. : A fully artificial pancreas versus a hybrid artificial pancreas for type 1 diabetes: a single-centre, open-label, randomised controlled, crossover, non-inferiority trial. Lancet Digit Health 2021;3:e723–e732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.