Abstract
Background
Atrial fibrillation detected after stroke (AFDAS) has a lower risk of ischemic stroke recurrence than known atrial fibrillation (KAF). While the benefit of oral anticoagulants (OAC) for preventing ischemic stroke recurrence in KAF is well established, their role in patients with AFDAS is more controversial. This study aimed to evaluate the association between OAC use and the risk of recurrent ischemic stroke in patients with AFDAS in a real-world setting.
Methods
This nationwide retrospective cohort study was conducted using the Taiwan National Health Insurance Research Database. Patients hospitalized with a first-ever ischemic stroke and AFDAS confirmed within 30 days after hospitalization were assigned to OAC and non-OAC cohorts. Inverse probability of treatment weighting was applied to balance the baseline characteristics of the cohorts. The primary outcome was ischemic stroke recurrence. Secondary outcomes were intracranial hemorrhage (ICH), death, and the composite outcome of “ischemic stroke recurrence, ICH, or death.” Multivariate Cox proportional hazard models were used to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI).
Results
A total of 4,508 hospitalized patients with stroke and AFDAS were identified. Based on OAC use, 2,856 and 1,652 patients were assigned to the OAC and non-OAC groups, respectively. During the follow-up period (median duration, 2.76 years), the OAC cohort exhibited a lower risk of ischemic stroke recurrence (aHR, 0.84; 95% CI, 0.70–0.99), death (aHR, 0.65; 95% CI, 0.58–0.73), and composite outcome (aHR, 0.70; 95% CI, 0.63–0.78) than did the non-OAC cohort. The risk of ICH (aHR, 0.96; 95% CI, 0.62–1.50) was not significantly different between the two cohorts.
Conclusion
OAC use in patients with AFDAS was associated with reduced risk of ischemic stroke recurrence, without an increased risk of ICH. This supports current guidelines recommending OACs for secondary stroke prevention in patients with AF, regardless of the time of diagnosis.
Keywords: atrial fibrillation, atrial fibrillation detected after stroke, anticoagulant, ischemic stroke, intracranial hemorrhage
Introduction
Stroke can be the initial clinical manifestation of previously undetected atrial fibrillation (AF) (1). Up to 58.7% of patients with AF-related acute ischemic stroke have AF detected after stroke (AFDAS) (2, 3). The prognosis and management of stroke patients with AFDAS have recently attracted more attention (3–9) owing to the increased utilization of advanced monitoring technology for AF screening after a stroke (10, 11). According to current guidelines (9, 12), newly detected AF in patients who suffered a stroke should prompt anticoagulation unless contraindicated. However, compared to patients with AF known before stroke (KAF), AFDAS seems to have a more benign profile (5, 6, 8, 13). A recent systematic review and meta-analysis showed that patients with AFDAS have a lower burden of risk factors, a lower CHA2DS2-VASc score, a smaller left atrium, and 26% lower risk of stroke recurrence than patients with KAF (14). Furthermore, another systematic review and meta-analysis of randomized controlled trials has shown that although prolonged cardiac monitoring in patients with stroke results increased AF detection and use of oral anticoagulants (OACs), it is not associated with reduced risk of stroke recurrence (15). These recent studies suggest that given the relatively benign risk profile of AFDAS, the use of OACs in these patients may not be as beneficial as it is for patients with KAF. However, to our knowledge, no prior randomized controlled trials or observational studies have confirmed the benefits of OACs in patients with AFDAS (16). Therefore, we conducted this nationwide population-based cohort study to examine the association between OAC use and ischemic stroke recurrence, as well as with intracranial hemorrhage (ICH) and death, in stroke patients with AFDAS.
Materials and methods
Data sources
The present study was conducted using data from Taiwan’s National Health Insurance Research Database (NHIRD) between 2000 and 2018. The NHIRD is derived from the electronic claims data of Taiwan’s National Health Insurance program, which enrolls more than 99% of the Taiwanese population (approximately 23.6 million). The NHIRD is currently stored and managed by the Health and Welfare Data Science Center of Taiwan’s Ministry of Health and Welfare (17). It provides comprehensive healthcare information, including medication prescriptions, medical device usage, and emergency, inpatient, or outpatient visits. Information on individual beneficiaries can be linked and longitudinally followed using an encrypted identification number. The study protocol was approved by the Institutional Review Board of Hualien Tzu Chi Hospital (IRB-107-152C). The requirement for obtaining informed consent was waived, as personal identifiers of patients were encrypted in the NHIRD.
Study design, population, and definitions
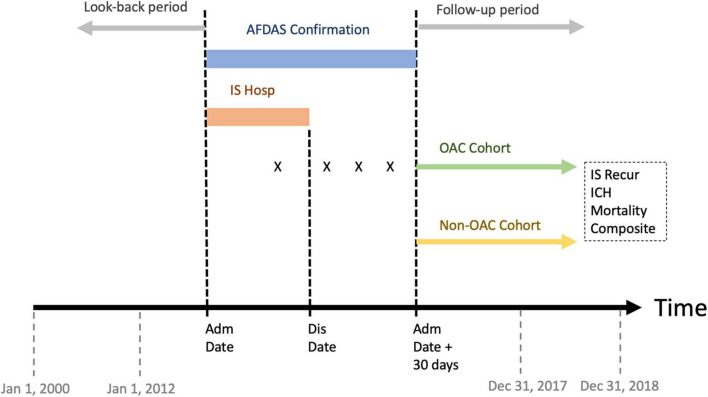
In this retrospective cohort study, we identified consecutive adult patients hospitalized due to first-ever ischemic stroke with AFDAS between 2012 and 2017 (Figure 1). Each patient’s index date and year were defined as the admission date and year of the index stroke event, respectively. Ischemic stroke was defined based on ICD-9-CM codes 433 and 434 before 2016, and ICD-10-CM code I63 thereafter (18–20). ICH was defined by applying ICD-9-CM codes 430, 431, and 432 before 2016 and ICD-10-CM codes I60, I61, and I62 thereafter (21). Only patients with available brain imaging during hospitalization for their index stroke event were included.
FIGURE 1.
Study design. AFDAS, atrial fibrillation detected after stroke; Hosp, hospitalization; X, prescription of oral anticoagulant; Adm, admission; Dis, discharge; OAC, oral anticoagulant; IS Recur, ischemic stroke recurrence; ICH, intracranial hemorrhage.
We established a 10-year lookback window to identify and exclude patients with a previous diagnosis of stroke or related cerebral vascular disease (ICD-9-CM codes 430–438 or ICD-10-CM codes I60–I69), in either inpatient and outpatient claims, to avoid reporting bias based on outcomes and indication bias based on anticoagulant use. AF was identified by using ICD-9-CM codes 427.31 and ICD-10-CM code I48.0–I48.2 or I48.9 (22, 23). AFDAS was defined as a new diagnosis of AF in either the inpatient or outpatient claims within 30 days after the index date. For this purpose, we applied the same 10-year look-back window before the index date to exclude patients with a previous diagnosis of AF. In Taiwan, prolonged cardiac monitoring is not reimbursed by the National Health Insurance, so the vast majority of the AFDAS diagnoses are made on admission electrocardiography (ECG) or 24-h Holter. The diagnostic codes for ischemic stroke (18–20) and AF (22, 23) have been previously validated In Taiwan’s NHIRD.
We excluded patients with a previous diagnosis of severe valvular heart disease such as rheumatic heart disease (ICD-9-CM codes 393–398 or ICD-10-CM codes I00-I09), congenital heart disease (ICD-9-CM codes 746–747 or ICD-10-CM codes Q20-Q28), or those who had undergone valvular replacement surgery (NHI procedure code: 68016B, 68017B, 68018B). We also excluded patients who died or had new ischemic stroke or ICH within 30 days after the index date, prolonged hospitalization beyond 30 days, or age younger than 20 years (Supplementary Figure 1).
Allocation of cohorts
The OAC cohort consisted of patients with first-ever ischemic stroke with AFDAS who received OACs within 30 days following the index date. The non-OAC cohort consisted of patients with AFDAS who never received OACs during the same 30-day period (Figure 1).
Covariates
The baseline characteristics of both cohorts were listed in Table 1. The monthly income was defined based on the insurance premium, which was income-dependent and recorded on a graduated scale. It was categorized as dependent, USD 567–1,076, USD 1,077–1,615, and > USD 1,615. Comorbidities were defined as diagnostic codes recorded in at least one inpatient diagnosis or at least two outpatient diagnoses within 1 year before the index stroke event (23). These variables were also used to calculate the pre-stroke CHA2DS2-VASc scores (24). The timing of AFDAS was categorized as during the inpatient (before discharge) or the outpatient period (after discharge). Stroke severity was determined using a claims-based stroke severity index, which was further transformed to the estimated National Institutes of Health Stroke Scale (eNIHSS) score (25). We categorized the eNIHSS as mild (≤ 5), moderate (≥ 6 and ≤ 13), and severe (> 13) (26, 27). Other important covariates regarding the index stroke included length of hospitalization, physician specialty (neurology or others), and hospital level (tertiary referral center or others). To investigate anticoagulant use in the OAC cohort, we further classified patients into those treated with non-vitamin K antagonist oral anticoagulants for ≥ 1 day within the 30 days following the index date, and the others were defined as being treated with warfarin. Antiplatelet use was defined as the use of antiplatelet therapy for ≥ 1 day within the 30 days following the index date. 24-h Holter monitoring was defined as whether the patients received 24-h Holter monitoring within the 30 days following the index date.
TABLE 1.
Baseline characteristics before and after IPTW.
| Original cohorts |
IPTW cohorts |
|||||
| OAC | Non-OAC | SMD | OAC | Non-OAC | SMD | |
| N = 2,856 | N = 1,652 | N = 2,496 | N = 1,434 | |||
| Age | ||||||
| Age, years * | 71.7 (11.7) | 75.2 (11.9) | 0.298 | 72.5 (10.8) | 73.9 (11.2) | 0.123 |
| < 65 | 762 (26.7) | 330 (20.0) | 0.159 | 598 (24.0) | 325 (22.7) | 0.030 |
| 65–75 | 823 (28.8) | 386 (23.4) | 0.124 | 739 (29.6) | 371 (25.9) | 0.083 |
| ≥ 75 | 1,271 (44.5) | 936 (56.7) | 0.245 | 1,160 (46.5) | 738 (51.4) | 0.100 |
| Sex | ||||||
| Male | 1,680 (58.8) | 863 (52.2) | 0.133 | 1,433 (57.4) | 790 (55.1) | 0.047 |
| Female | 1,176 (41.2) | 789 (47.8) | 0.133 | 1,063 (42.6) | 644 (44.9) | 0.047 |
| Index year † | ||||||
| 2012 | 389 (13.6) | 331 (20.0) | 0.172 | 363 (14.6) | 245 (17.1) | 0.070 |
| 2013 | 396 (13.9) | 318 (19.3) | 0.145 | 379 (15.2) | 237 (16.5) | 0.036 |
| 2014 | 454 (15.9) | 308 (18.6) | 0.073 | 427 (17.1) | 264 (18.4) | 0.035 |
| 2015 | 524 (18.4) | 273 (16.5) | 0.048 | 469 (18.8) | 271 (18.9) | 0.002 |
| 2016 | 541 (18.9) | 208 (12.6) | 0.175 | 424 (17.0) | 204 (14.2) | 0.076 |
| 2017 | 552 (19.3) | 214 (13.0) | 0.174 | 433 (17.4) | 213 (14.8) | 0.069 |
| Monthly income (USD) ‡ | ||||||
| Dependent | 762 (26.7) | 468 (28.3) | 0.037 | 676 (27.1) | 403 (28.1) | 0.023 |
| 567–1,076 | 1,364 (47.8) | 853 (51.6) | 0.077 | 1,229 (49.2) | 728 (50.7) | 0.030 |
| 1,077–1,615 | 373 (13.1) | 187 (11.3) | 0.053 | 323 (12.9) | 170 (11.9) | 0.032 |
| > 1,615 | 357 (12.5) | 144 (8.7) | 0.123 | 268 (10.8) | 133 (9.3) | 0.049 |
| Comorbidities | ||||||
| Hypertension | 1,513 (53.0) | 919 (55.6) | 0.053 | 1,334 (53.4) | 775 (54.1) | 0.013 |
| Diabetes mellitus | 580 (20.3) | 349 (21.1) | 0.020 | 514 (20.6) | 300 (20.9) | 0.008 |
| Dyslipidemia | 576 (20.2) | 296 (17.9) | 0.057 | 497 (19.9) | 264 (18.4) | 0.038 |
| CAD | 476 (16.7) | 282 (17.1) | 0.011 | 406 (16.3) | 236 (16.4) | 0.004 |
| CHF | 79 (2.8) | 25 (1.5) | 0.087 | 51 (2.0) | 22 (1.6) | 0.037 |
| MI | 37 (1.3) | 38 (2.3) | 0.075 | 28 (1.1) | 24 (1.6) | 0.044 |
| Pre-stroke CHA2DS2-VASc score § | ||||||
| Score* | 2.4 (1.4) | 2.7 (1.4) | 0.217 | 2.5 (1.4) | 2.6 (1.4) | 0.082 |
| Low risk‡ | 394 (13.8) | 168 (10.2) | 0.112 | 300 (12.0) | 166 (11.6) | 0.013 |
| Intermediate risk | 560 (19.6) | 244 (14.8) | 0.129 | 483 (19.3) | 253 (17.6) | 0.045 |
| High risk | 1,902 (66.6) | 1,240 (75.1) | 0.187 | 1,713 (68.6) | 1,016 (70.8) | 0.047 |
| Timing of AFDAS diagnosis | ||||||
| Inpatient | 2,541 (89.0) | 1,427 (86.4) | 0.079 | 2,219 (88.9) | 1,264 (88.2) | 0.024 |
| Outpatient | 315 (11.0) | 225 (13.6) | 0.079 | 277 (11.1) | 170 (11.8) | 0.024 |
| Stroke severity | | | ||||||
| eNIHSS* | 9.0 (6.1) | 10.9 (7.1) | 0.289 | 9.1 (6.0) | 9.9 (6.6) | 0.128 |
| Mild§ | 1,525 (53.4) | 741 (44.9) | 0.172 | 1,297 (52.0) | 718 (50.1) | 0.038 |
| Moderate | 666 (23.3) | 319 (19.3) | 0.098 | 606 (24.3) | 290 (20.2) | 0.098 |
| Severe | 665 (23.3) | 592 (35.8) | 0.278 | 593 (23.8) | 427 (29.8) | 0.136 |
| Length of hospitalization | ||||||
| Days* | 11.6 (7.5) | 12.3 (8.0) | 0.091 | 11.6 (7.4) | 12.0 (7.9) | 0.060 |
| Physician specialty | ||||||
| Neurology | 2,517 (88.1) | 1,348 (81.6) | 0.183 | 2,208 (88.5) | 1,228 (85.6) | 0.086 |
| Others | 339 (11.9) | 304 (18.4) | 0.183 | 288 (11.5) | 207 (14.4) | 0.086 |
| Hospital level | ||||||
| Tertiary center | 1,179 (41.3) | 554 (33.5) | 0.160 | 980 (39.3) | 518 (36.1) | 0.065 |
| others | 1,677 (58.7) | 1,098 (66.5) | 0.160 | 1,516 (60.7) | 916 (63.9) | 0.065 |
| Anticoagulant type | ||||||
| NOAC | 1,855 (65.0) | n/a | n/a | 1,585 (63.5) | n/a | n/a |
| Warfarin | 1,001 (35.1) | n/a | n/a | 912 (36.5) | n/a | n/a |
| Antiplatelet use | ||||||
| Yes | 1,687 (59.1) | 1,055 (63.9) | 0.099 | 1,492 (59.8) | 939 (65.4) | 0.117 |
| No | 1,169 (40.9) | 597 (36.1) | 0.099 | 1,004 (40.2) | 496 (34.6) | 0.117 |
| 24-h Holter monitoring | ||||||
| Yes | 1,226 (42.9) | 614 (37.2) | 0.118 | 1,055 (42.3) | 565 (39.4) | 0.059 |
| No | 1,630 (57.1) | 1,038 (62.8) | 1,441 (57.7) | 869 (60.6) | 0.059 | |
Data are expressed as n (%) unless otherwise indicated.
*Expressed as mean (SD).
†Index year: the year of admission for the index stroke event.
‡1 NTD = 0.036 USD as of Nov 2021.
§CHA2DS2-VASc score: low stroke risk was defined as a score of 1 or 0 for women and 0 for men; intermediate stroke risk was defined as a score of 2 for women and 1 for men; high stroke risk was defined as a score of ≥ 3 for women and ≥ 2 for men.
| |Severity of stroke: mild severity was defined as a score of ≤ 5; moderate severity was defined as a score of ≥ 6 and ≤ 13; severe severity was defined as a score of > 13.
AFDAS, atrial fibrillation detected after stroke; CAD, coronary artery disease; CHF, congestive heart failure; eNIHSS, estimated National Institutes of Health Stroke Scale; IPTW, inverse probability of treatment weighting; MI, myocardial infarction; NOAC, non-vitamin K antagonist oral anticoagulant; OAC, oral anticoagulant; SMD, standardized mean difference.
Follow-up and outcomes
The date of follow-up onset was defined as 30 days after the index date (Figure 1). This approach has been previously used (28) to avoid immortal time bias (29). That is, patients in both OAC and non-OAC cohorts have to survive up to the same starting time point to be included in the analysis of outcomes.
The primary outcome was ischemic stroke recurrence, defined as an inpatient diagnosis of ischemic stroke after an examination of brain imaging. The secondary outcomes included ICH, death, and a composite endpoint of “ischemic stroke recurrence, ICH, or death.” Death was defined by using the National Death Registry, linked to the Taiwan’s NHIRD (30).
Statistical analysis
Categorical variables were expressed as counts and percentages, while continuous variables were expressed as means and standard deviations (SD). To minimize the selection bias inherent to a non-randomized controlled study, we used propensity score (PS) matching with a stabilized IPTW approach to create more homogeneous OAC and non-OAC groups with balanced baseline characteristics to facilitate comparisons. We calculated the PS using the logistic regression model and including covariates of age, sex, monthly premium level, pre-stroke CHA2DS2-VASc score, timing of AFDAS diagnosis, eNIHSS, length of hospitalization, physician specialty, hospital level, and comorbidities (listed in Table 1). The weights for the stabilized IPTW approach were defined as Z/PS for OAC group and (1-Z)/(1-PS) for the non-OAC group. Z and 1-Z were the marginal prevalence of OAC and non-OAC in the overall population, respectively. To avoid extreme weights, we removed patients whose PS were < 5% or > 95% of the population. Using PS with the stabilized IPTW approach could generate two interchangeable groups with the same treatment assignment probabilities, thus allowing for comparisons based on the average treatment effects of the entire population (31). Standardized mean differences were used to determine differences in baseline characteristics between the two cohorts, and a value of < 0.1 was considered no difference.
The probability of ischemic stroke event-free was estimated using the Kaplan-Meier method, and the difference between the event-free curves was examined using the log-rank test. The association between OAC use and primary and secondary outcomes was evaluated by applying multivariate Cox proportional hazard models and reported as hazard ratios (HR) and 95% confidence intervals (CI) (32). Multivariate models were adjusted for age, sex, income, comorbidities listed in Table 1, pre-stroke CHA2DS2-VASc score, timing of AFDAS diagnosis, eNIHSS, length of hospitalization, specialty of the treating physician (neurology or others), and hospital level (tertiary center or others).
Two sensitivity analyses were performed. First, a time-varying analysis was performed to account for crossovers in treatment groups during follow-up. Second, the Fine and Gray competing risk model was applied to account for the competing risk of ICH and death (33). Additionally, stratified analyses for age, sex, pre-stroke CHA2DS2-VASc score, timing of AFDAS diagnosis, eNIHSS, physician specialty, or hospital level were performed to estimate their interaction with the association between OAC use and the primary outcome. Statistical significance was defined as a two-tailed probability value of < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and Stata version 14.0 (StataCorp, College Station, TX).
Results
Baseline characteristics
A total of 4,508 hospitalized patients with both stroke and AFDAS were identified. Based on OAC use, 2,856 and 1,652 patients were assigned to the OAC and non-OAC groups, respectively. Patients in the OAC group tended to be younger, to have higher incomes and lower pre-stroke CHA2DS2-VASc and eNIHSS scores, and were more likely to be male, and to receive medical care from a neurologist or at a tertiary center (Table 1). In the IPTW cohorts, the baseline characteristics were well balanced between the two groups, except that the OAC group tended to be younger, had lower eNIHSS scores, and lower proportions of severe stroke, antiplatelet use than did the non-OAC group.
Primary and secondary outcomes in IPTW cohorts
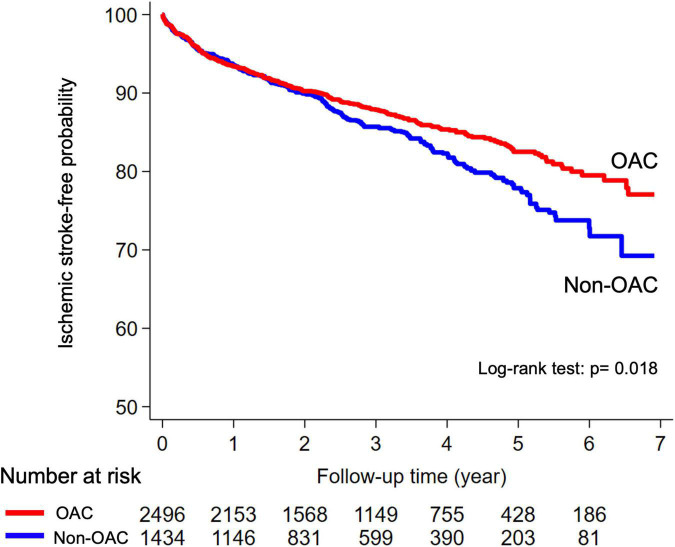
In the non-adjusted analysis, the risk of ischemic stroke recurrence was lower in the OAC cohort than in the non-OAC cohort (log-rank test, p = 0.018; Figure 2). At a median follow-up of 2.76 and 2.53 years, respectively (Table 2), the numbers (annualized event rates) of ischemic stroke recurrences in the OAC and non-OAC cohorts were 321 (4.29%) and 209 (5.33%), respectively. The univariate Cox proportion hazard model indicated a significantly lower risk of ischemic stroke recurrence in the OAC cohort than in the non-OAC cohort (HR, 0.81; 95% CI, 0.69–0.97; p = 0.018). This association remained significant in the multivariate model (adjusted HR, 0.84; 95% CI, 0.70–0.99; p = 0.042) (Table 2). Patients in the OAC cohort had a similar risk of ICH (adjusted HR, 0.96; 95% CI, 0.62–1.50; p = 0.864), and had a lower risk of death (adjusted HR, 0.65; 95% CI 0.58–0.73; p < 0.001) and the composite outcome (adjusted HR, 0.70; 95% CI, 0.63–0.78; p < 0.001), compared to patients in the non-OAC cohort.
FIGURE 2.
Kaplan–Meier curves for ischemic stroke event-free probability in the OAC and non-OAC cohorts among patients with AFDAS. AFDAS, atrial fibrillation detected after stroke; OAC, oral anticoagulant.
TABLE 2.
Risk of ischemic stroke and secondary outcomes in IPTW cohorts.
| Event | FU* | AER† | Univariate model |
Multivariate model‡ |
|||||
| HR | 95% CI | p | aHR | 95% CI | p | ||||
| Ischemic stroke | |||||||||
| OAC | 321 | 2.76 | 4.29 | 0.81 | 0.69–0.97 | 0.018 | 0.84 | 0.70–0.99 | 0.042 |
| Non-OAC | 209 | 2.53 | 5.33 | Ref. | |||||
| Intracranial hemorrhage | |||||||||
| OAC | 55 | 2.76 | 0.73 | 0.96 | 0.62–1.49 | 0.861 | 0.96 | 0.62–1.50 | 0.864 |
| Non-OAC | 30 | 2.53 | 0.76 | Ref. | |||||
| Death | |||||||||
| OAC | 600 | 3.11 | 7.29 | 0.57 | 0.51–0.64 | <0.001 | 0.65 | 0.58–0.73 | <0.001 |
| Non-OAC | 557 | 2.84 | 12.90 | Ref. | |||||
| Composite outcome§ | |||||||||
| OAC | 825 | 2.76 | 11.02 | 0.64 | 0.58–0.71 | <0.001 | 0.70 | 0.63–0.78 | <0.001 |
| Non-OAC | 680 | 2.53 | 17.29 | Ref. | |||||
*Expressed as median duration of follow-up (years).
†Expressed as annualized event rate (%).
‡Hazard ratios were calculated using multivariate Cox regression models with adjustment for age, sex, index year, monthly income, comorbidities listed in Table 1, pre-stroke CHA2DS2-VASc score, diagnosis of AFDAS, eNIHSS score, length of hospitalization, physician specialty, and hospital level.
§Composite outcome defined as development of ischemic stroke, intracranial hemorrhage, or mortality.
aHR, adjusted hazard ratio; AER: annualized event rate; CI, confidence interval; eNIHSS, estimated National Institutes of Health Stroke Scale; FU, follow-up; HR, hazard ratio; IPTW, inverse probability of treatment weighting; IR, incidence rate; OAC, oral anticoagulant.
Sensitivity analyses
In the time-varying sensitivity analysis accounting for treatment group crossovers, OAC use was associated with a nearly 50% lower risk of ischemic stroke recurrence (adjusted HR, 0.52; 95% CI, 0.43–0.63; p < 0.001) (Table 3). In Fine and Gray’s competing risk model, OAC use was also associated with a similar trend of lower risk of stroke recurrence compared with non-OAC use (adjusted HR, 0.91; 95% CI, 0.76–1.06; p = 0.305) (Table 3).
TABLE 3.
Sensitivity analyses in the risk of ischemic stroke in IPTW cohorts.
| Univariate model |
Multivariate model |
|||||
| HR | 95% CI | p | aHR† | 95% CI | p | |
| Sensitivity analysis A* | ||||||
| OAC | 0.55 | 0.47–0.66 | <0.001 | 0.52 | 0.43–0.63 | <0.001 |
| Non-OAC | Ref. | Ref. | ||||
| Sensitivity analysis B† | ||||||
| OAC | 0.90 | 0.76–1.07 | 0.240 | 0.91 | 0.76–1.09 | 0.3050 |
| Non-OAC | Ref. | Ref. | ||||
*Sensitivity analysis A: we used time-varying analysis to evaluate the effect of OAC on the primary outcome.
†Sensitivity analysis B: we used the Fine and Gray’s competing risk model to evaluate the effect of OAC on primary outcome.
aHR, adjusted hazard ratio; CI, confidence interval; HR, hazard ratio; IPTW, inverse probability of treatment weighting; OAC, oral anticoagulant.
Stratified analysis
In stratified analysis, there was no significant interaction for age, sex, pre-stroke CHA2DS2-VASc score, timing of AFDAS diagnosis, 24-h Holter monitoring, eNIHSS, physician specialty, or hospital level with the association between OAC and stroke recurrence (Supplementary Table 1).
Discussion
In this large population-based retrospective cohort study, the use of OACs in patients with first-ever ischemic stroke and AFDAS was associated with a 16% lower risk of ischemic stroke recurrence during a median follow-up of 2.76 years. Results were consistent in sensitivity analyses accounting for treatment group crossovers and the compering risk of ICH and death. There were no differences in the risk of ICH between treatment groups. There were no significant interactions identified for age, sex, CHA2DS2-VASc score, timing of AFDAS diagnosis, 24-h Holter monitoring, eNIHSS, physician specialty, or hospital level.
Currently, major guidelines suggest the use of OAC in patients with stroke and AF, without differentiating between KAF or AFDAS (9, 12). This is mainly based on the fact that AFDAS is a fairly novel concept (13, 15), and that there have not been any specific randomized clinical trials of OACs vs. antiplatelet agents or no antithrombotic therapy in patients with AFDAS. The results of the present real-world population-based study represent the closest possible approach to filling this knowledge gap, since a randomized controlled trial of OACs would be ethically unfeasible.
It is important to note that not all AFDAS have the same embolic risk. It has been proposed that AFDAS identified on the admission ECG or on short-term monitoring (e.g., 24-h Holter) may entail a higher burden and embolic risk, whereas lower-burden AFDAS detected on prolonged cardiac monitoring (e.g., 30-day external loop recorders or 2 or 3-year implantable loop recorders) may lower the risk of stroke recurrence (15). In the present study, AFDAS was diagnosed on admission with ECGs or 24-h Holter monitoring within 30 days after stroke in usual care settings. As a result, most AFDAS may have been high-burden and may have occurred asymptomatically before stroke occurrence. Although this assumption is hypothetical, the likely high-burden nature of most AFDAS in our cohorts may explain the association between OAC use and lower risk of stroke recurrence.
In sensitivity analysis, the time-varying analysis accounting for changes in OAC exposure during the follow-up period found that there was an even greater risk reduction (nearly 50% reduction in HR, p < 0.001) in ischemic stroke recurrence than there was in the main analysis (16% reduction, in HR, p = 0.042). However, this association was not statistically significant after taking into account the competing risks of ICH and death using Fine and Gray’s method in sensitivity analysis (9% reduction in HR, p = 0.305). This highlights the importance of adherence to OAC treatment for patients with AFDAS, and this information might provide physicians more confidence to initiate and maintain OAC treatment for post-stroke care in these patients. As only 37.1% and 39.3% of patients with stroke and newly confirmed AFDAS on serial ECGs or 24-h Holter monitoring, respectively, were prescribed with OACs at discharge (34), our real-world evidence lends support to current guidelines and indicates that physicians could prescribe OAC early with confidence once AFDAS has been confirmed.
Limitations
Our study has several limitations. First, the diagnosis of AFDAS in the present study was mainly based on ECGs at admission and 24-h Holter monitoring. As such, the results are not generalizable to patients with AFDAS on prolonged Holter monitoring or implantable loop recording, who may have a different (and probably lower) AF burden. Results are awaited from those ongoing randomized trials, such as the FIND-AF2 trial (35), which is expected to provide more definitive information on this subject. Second, the use of a limited time window (30 days after the index stroke event) to identify the OAC and non-OAC cohorts is a limitation of the current study, because there could be cross-overs between the specified time windows. Third, unmeasured confounders such as hemorrhagic transformation, the size of cerebral infarctions, cerebral microbleeds, or comorbidities associated with high embolic or hemorrhagic risk may have influenced the results. However, the application of IPTW, as well as the consistency of the results of multivariate models and sensitivity analyses, suggest that our results are unlikely to be explained by selection bias. Fourth, the proportion of severe stroke (eNIHSS > 13) was higher in the non-OAC group, even after the application of IPTW. Nevertheless, the p-value for this interaction was insignificant (p = 0.224) for the severe stroke subgroup (Supplementary Table 1). Fifth, the use of a 10-year lookback period to exclude patients with a previous stroke and/or a previous AF diagnosis may have led to misclassification. However, this risk might be negligible (5, 36). Sixth, it would be more accurate to consider a certain proportion of patients who were re-admitted within the first 30-day period after index stroke admission as experiencing a continuation of the same stroke episode, instead of having an early stroke recurrence. Excluding these patients from the current study may have caused a selection bias. Lastly, we did not apply a cut-off value for AF duration for it to be considered as clinically relevant. AF was identified retrospectively based on claims records (22, 23). Such AF was likely to be high burden, because it was diagnosed on admission ECGs or short-term monitoring in usual care; therefore, it was probably a fairly homogenous group of AFDAS from a prognostic perspective.
Conclusion
For acute patients with ischemic stroke with AFDAS, OAC initiation within 30 days after stroke was associated with a reduced risk of ischemic stroke recurrence but without a significantly increased risk of ICH. This finding might support current guidelines that recommend the use of OAC for secondary stroke prevention in patients with AF, regardless of AFDAS or KAF.
Data availability statement
Taiwan’s NHIRD is maintained and regulated by the Health and Welfare Data Science Center at the Ministry of Health and Welfare in Taiwan. The dataset only could be utilized in the division of the Health and Welfare Data Science Center. Researchers who are interested to analyze this dataset can request access to the Taiwan Ministry of Health and Welfare. Requests to access the datasets should be directed to Taiwan Ministry of Health and Welfare (website: https://dep.mohw.gov.tw/DOS/cp-2516-3591-113.html).
Ethics statement
The studies involving human participants were reviewed and approved by the Hualien Tzu Chi Hospital. Written informed consent for participation was not required for this study because personal identifiers of patients were encrypted in the NHIRD.
Author contributions
J-YH: manuscript preparation, study conception and design, data extraction, and interpretation. PP-SL: study design and data extraction statistical analysis. LAS and S-JL: critical revision of the manuscript. H-KH: study conception and design and data interpretation. A-BL: study conception and data interpretation. EC-CL: statistical consultation and data interpretation. C-YH: study conception and design, data interpretation, and critical revision of the manuscript. C-HL: study conception and design and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Health and Welfare Data Science Center of Taiwan’s Ministry of Health and Welfare for maintaining and processing the data and the Health and Welfare Data Science Center of Tzu Chi University for facilitating data extraction.
Funding
This work was supported by grants from Hualien Tzu Chi Hospital (Grant no. TCRD109-36) and Buddhist Tzu Chi Medical Foundation (Grant no. TCRD-A 110-04). The funders had no role in the study design, data collection, data analysis, data interpretation, writing of the report, decision to submit for publication, or approval of the manuscript for publication.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.929304/full#supplementary-material
References
- 1.Lubitz SA, Yin X, McManus DD, Weng LC, Aparicio HJ, Walkey AJ, et al. Stroke as the initial manifestation of atrial fibrillation: the Framingham heart study. Stroke. (2017) 48:490–2. 10.1161/STROKEAHA.116.015071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Higgins P, MacFarlane PW, Dawson J, McInnes GT, Langhorne P, Lees KR. Non-invasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke. (2013) 44:2525–31. 10.1161/STROKEAHA.113.001927 [DOI] [PubMed] [Google Scholar]
- 3.Sposato LA, Cipriano LE, Saposnik G, Vargas ER, Riccio PM, Hachinski V. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. (2015) 14:377–87. 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 4.Sposato LA, Riccio PM, Hachinski V. Poststroke atrial fibrillation: cause or consequence? Critical review of current views. Neurology. (2014) 82:1180–6. 10.1212/WNL.0000000000000265 [DOI] [PubMed] [Google Scholar]
- 5.Sposato LA, Cerasuolo JO, Cipriano LE, Fang J, Fridman S, Paquet M, et al. Atrial fibrillation detected after stroke is related to a low risk of ischemic stroke recurrence. Neurology. (2018) 90:e924–31. 10.1212/WNL.0000000000005126 [DOI] [PubMed] [Google Scholar]
- 6.Yang XM, Rao ZZ, Gu HQ, Zhao XQ, Wang CJ, Liu LP, et al. Atrial fibrillation known before or detected after stroke share similar risk of ischemic stroke recurrence and death. Stroke. (2019) 50:1124–9. 10.1161/STROKEAHA.118.024176 [DOI] [PubMed] [Google Scholar]
- 7.Lip GY, Hunter TD, Quiroz ME, Ziegler PD, Turakhia MP. Atrial fibrillation diagnosis timing, ambulatory ECG monitoring utilization, and risk of recurrent stroke. Circ Cardiovasc Qual Outcomes. (2017) 10:e002864. 10.1161/CIRCOUTCOMES.116.002864 [DOI] [PubMed] [Google Scholar]
- 8.Hsieh CY, Lee CH, Wu DP, Sung SF. Characteristics and outcomes of ischemic stroke in patients with known atrial fibrillation or atrial fibrillation diagnosed after stroke. Int J Cardiol. (2018) 261:68–72. 10.1016/j.ijcard.2017.11.047 [DOI] [PubMed] [Google Scholar]
- 9.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 10.Schnabel RB, Haeusler KG, Healey JS, Freedman B, Boriani G, Brachmann J, et al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN international collaboration. Circulation. (2019) 140:1834–50. 10.1161/CIRCULATIONAHA.119.040267 [DOI] [PubMed] [Google Scholar]
- 11.Haeusler KG, Gröschel K, Köhrmann M, Anker SD, Brachmann J, Böhm M, et al. Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin Res Cardiol. (2018) 107:871–80. 10.1007/s00392-018-1256-9 [DOI] [PubMed] [Google Scholar]
- 12.Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. (2021) 52:e364–467. 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 13.Cerasuolo JO, Cipriano LE, Sposato LA. The complexity of atrial fibrillation newly diagnosed after ischemic stroke and transient ischemic attack. Curr Opin Neurol. (2017) 30:28–37. 10.1097/WCO.0000000000000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fridman S, Jimenez-Ruiz A, Vargas-Gonzalez JC, Sposato LA. Differences between atrial fibrillation detected before and after stroke and TIA: a systematic review and meta-analysis. Cerebrovasc Dis. (2022) 51:152–7. 10.1159/000520101 [DOI] [PubMed] [Google Scholar]
- 15.Sposato LA, Chaturvedi S, Hsieh CY, Morillo CA, Kamel KH. Atrial fibrillation detected after stroke and TIA (AFDAS): a novel clinical concept challenging current views. Stroke. (2022) 53:e94–103. 10.1161/STROKEAHA.121.034777 [DOI] [PubMed] [Google Scholar]
- 16.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 17.Lin LY, Warren-Gash C, Smeeth L, Chen PC. Data resource profile: the National Health Insurance Research Database (NHIRD). Epidemiol Heal. (2018) 40:e2018062. 10.4178/epih.e2018062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsieh CY, Chen CH, Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. (2015) 114:254–9. 10.1016/j.jfma.2013.09.009 [DOI] [PubMed] [Google Scholar]
- 19.Cheng CL, Kao YHY, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. (2011) 20:236–42. 10.1002/pds.2087 [DOI] [PubMed] [Google Scholar]
- 20.Hsieh MT, Hsieh CY, Tsai TT, Wang YC, Sung SF. Performance of icd-10-cm diagnosis codes for identifying acute ischemic stroke in a national health insurance claims database. Clin Epidemiol. (2020) 12:1007–13. 10.2147/CLEP.S273853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh MT, Huang KC, Hsieh CY, Tsai TT, Chen LC, Sung SF. Validation of ICD-10-CM diagnosis codes for identification of patients with acute hemorrhagic stroke in a national health insurance claims database. Clin Epidemiol. (2021) 13:43–51. 10.2147/CLEP.S288518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai WC, Chen CY, Kuo HF, Wu MT, Tang WH, Chu CS, et al. Areca nut chewing and risk of atrial fibrillation in Taiwanese men: a nationwide ecological study. Int J Med Sci. (2013) 10:804–11. 10.7150/ijms.5998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung SF, Hsieh CY, Lin HJ, Chen YW, Yang YH, Li CY. Validation of algorithms to identify stroke risk factors in patients with acute ischemic stroke, transient ischemic attack, or intracerebral hemorrhage in an administrative claims database. Int J Cardiol. (2016) 215:277–82. 10.1016/j.ijcard.2016.04.069 [DOI] [PubMed] [Google Scholar]
- 24.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. (2010) 137:263–72. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 25.Barber M, Fail M, Shields M, Stott DJ, Langhorne P. Validity and reliability of estimating the scandinavian stroke scale score from medical records. Cerebrovasc Dis. (2004) 17:224–7. 10.1159/000075795 [DOI] [PubMed] [Google Scholar]
- 26.Sung SF, Hsieh CY, Lin HJ, Chen YW, Chen CH, Kao Yang YH, et al. Validity of a stroke severity index for administrative claims data research: a retrospective cohort study. BMC Health Serv Res. (2016) 16:509. 10.1186/s12913-016-1769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang HP, Sung SF, Yang HY, Huang WT, Hsieh CY. Associations between stroke type, stroke severity, and pre-stroke osteoporosis with the risk of post-stroke fracture: a nationwide population-based study. J Neurol Sci. (2021) 427:117512. 10.1016/j.jns.2021.117512 [DOI] [PubMed] [Google Scholar]
- 28.Hsieh CY, Huang HC, Wu DP, Li CY, Chiu MJ, Sung SF. Effect of rehabilitation intensity on mortality risk after stroke. Arch Phys Med Rehabil. (2018) 99:1042–8.e6. 10.1016/j.apmr.2017.10.011 [DOI] [PubMed] [Google Scholar]
- 29.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. (2008) 167:492–9. 10.1093/aje/kwm324 [DOI] [PubMed] [Google Scholar]
- 30.Hsieh CY, Su CC, Shao SC, Sung SF, Lin SJ, Kao Yang YH, et al. Taiwan’s national health insurance research database: past and future. Clin Epidemiol. (2019) 11:349–58. 10.2147/CLEP.S196293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. (2015) 34:3661–79. 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. (1999) 94:496–509. 10.1111/sjos.12167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCaw ZR, Claggett BL, Tian L, Solomon SD, Berwanger O, Pfeffer MA, et al. Practical recommendations on quantifying and interpreting treatment effects in the presence of terminal competing risks: a review. JAMA Cardiol. (2021) 7:450–6. 10.1001/jamacardio.2021.4932 [DOI] [PubMed] [Google Scholar]
- 34.Huang WY, Lee M, Sung SF, Tang SC, Chang KH, Huang YS, et al. Atrial fibrillation trial to evaluate real-world procedures for their utility in helping to lower stroke events: a randomized clinical trial. Int J Stroke. (2021) 16:300–10. 10.1177/1747493020938297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clinicaltrials. Intensive Rhythm Monitoring to Decrease Ischemic Stroke and Systemic Embolism - the Find-AF 2 Study. (2020). Available online at: https://clinicaltrials.gov/ct2/show/NCT04371055 (accessed December 2, 2021). [DOI] [PubMed] [Google Scholar]
- 36.Worthington JM, Gattellari M, Goumas C, Jalaludin B. Differentiating incident from recurrent Stroke using administrative data: the impact of varying lengths of look-back periods on the risk of misclassification. Neuroepidemiology. (2017) 48:111–8. 10.1159/000478016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Taiwan’s NHIRD is maintained and regulated by the Health and Welfare Data Science Center at the Ministry of Health and Welfare in Taiwan. The dataset only could be utilized in the division of the Health and Welfare Data Science Center. Researchers who are interested to analyze this dataset can request access to the Taiwan Ministry of Health and Welfare. Requests to access the datasets should be directed to Taiwan Ministry of Health and Welfare (website: https://dep.mohw.gov.tw/DOS/cp-2516-3591-113.html).