Abstract
Background:
Postpartum depression (PPD) is a major public health concern and has, at its core, a sense of maternal ‘disconnection’ – from the self, the infant, and the support system. While PPD bears similarities with MDD, there is increasing evidence for its distinct nature, especially with the unique aspect of the mother-infant relationship. Current treatment modalities for PPD, largely based on those used in major depressive disorder (MDD), have low remission rates with emerging evidence for treatment resistance. It is, therefore, necessary to explore alternative avenues of treatment for PPD.
Objective:
In this narrative review, we outline the potential therapeutic rationale for serotonergic psychedelics in the treatment of PPD, and highlight safety and pragmatic considerations for the use of psychedelics in the postpartum period.
Methods:
We examined the available evidence for the treatment of PPD and the evidence for psychedelics in the treatment of MDD. We explored safety considerations in the use of psychedelics in the postpartum period.
Results:
There is increasing evidence for safety, and encouraging signals for efficacy, of psilocybin in the treatment of MDD. Psilocybin has been shown to catalyse a sense of ‘reconnection’ in participants with MDD. This effect in PPD, by fostering a sense of ‘reconnection’ for the mother, may allow for improved mood and maternal sensitivity towards the infant, which can positively impact maternal role gratification and the mother-infant relationship.
Conclusion:
Psychedelic assisted therapy in PPD may have a positive effect on the mother-infant dyad and warrants further examination.
Keywords: Mother–infant relationship, postpartum depression, psilocybin, psychedelics
Introduction
The transition to motherhood, termed ‘matrescence’, is a transformative life event involving both positive and negative emotional processes and, with a complex interplay of hormonal, physical, psychological and social factors, is a significant risk factor for the development of postpartum mental illness (Davé et al., 2010; Raphael, 1975). Postpartum depression (PPD), in particular, is recognised as a significant public health concern (Bauer et al., 2014). With emerging evidence for treatment resistance, there is a need for innovative and efficacious treatment for PPD in order to improve wellbeing in the mother–infant dyad (Cepeda et al., 2019). Brexanolone, an analogue of pregnanolone and a positive allosteric modulator of the GABA-A receptor, is the only drug to be developed specifically for the treatment of PPD, and has gained regulatory approval for the treatment of PPD in the United States (Kanes et al., 2017; Meltzer-Brody et al., 2018). Aside from brexanolone, there are no licensed treatment options for PPD, highlighting a need for investigating alternative forms of treatment.
Prohibited from routine clinical use since 1971, research into the use of classic psychedelics, specifically psilocybin and lysergic acid diethylamide (LSD), has resumed since 2000. Early phase clinical trial evidence suggests safety and feasibility of psilocybin given with psychological support in the treatment of depression (Carhart-Harris et al., 2016; Davis et al., 2021), end-of-life distress (Griffiths et al., 2016; Grob et al., 2011; Ross et al., 2016), and substance use disorders (Bogenschutz et al., 2015; Johnson et al., 2014). There is growing evidence for the transdiagnostic applications of psychedelic therapy (Kelly et al., 2021). In this review, we outline the potential therapeutic rationale for serotonergic psychedelics in the treatment of PPD, and highlight safety and pragmatic considerations for the use of psychedelics in the postpartum period.
Postpartum depression
Perinatal depression is a common illness with prevalence rates ranging from 10% to 20%, with higher incidence across some cultures and in socially disadvantaged populations (Gavin et al., 2005; Jairaj et al., 2019; O’Hara and Swain, 1996; Shorey et al., 2018). Risk factors for developing PPD include a range of psychosocial and biological factors including antenatal depression, childbirth, past history of trauma, intimate partner violence, lack of social support, social deprivation, impaired mother–infant interaction, and adverse life events (Jairaj et al., 2020; O’Hara and McCabe, 2013; Righetti-Veltema et al., 1998; Robertson et al., 2004). In addition to its consequences on maternal mental and physical health, perinatal depression is associated with negative foetal and neonatal outcomes, impaired infant neurodevelopment and attachment, and increased risk of developing depression during adulthood (O’Leary et al., 2019, 2021; Oyetunji and Chandra, 2020; Plant et al., 2015; Slomian et al., 2019). The public health cost of a single case of perinatal depression was reported to be £74,000 in the United Kingdom, with £23,000 of the cost relating to the mother and £51,000 relating to the infant (Bauer et al., 2014). Bearing these protracted human and economic costs in mind, timely and appropriate management of PPD is essential for the well-being of the mother and her infant.
PPD has a heterogeneous presentation with high comorbidity with anxiety (Dennis et al., 2018). Women with PPD can present with pervasive low mood, impaired sleep, appetite, and concentration, feelings of inadequacy or guilt, and a sense of detachment from the infant. Suicidal ideation is common, seen in 20% of women with PPD (Wisner et al., 2013). Irritability and obsessional thoughts, particularly concerning the infant, and thoughts of harm to the infant are also reported in PPD (Spinelli, 2004; Stewart and Vigod, 2019).
The pathophysiology of PPD remains unclear but is thought to be multifactorial with neuroendocrine factors, neurotransmitter changes, inflammation, genetics, and environmental risk factors all playing a role. The abrupt withdrawal of hormones after delivery is thought to precipitate PPD in women with a vulnerability to developing the disorder (Bloch et al., 2000). Dysfunction of the hypothalamic-pituitary-adrenal axis and changes in GABA pathway signalling are implicated in the aetiology of PPD (Licheri et al., 2015; Meltzer-Brody et al., 2011). Low allopregnanolone levels of postpartum are thought to underlie the pathophysiology of PPD in some women, and this hypothesis formed the basis of the development of brexanolone for the treatment of PPD (Meltzer-Brody and Kanes, 2020).
It is debated that PPD is distinct to depression occurring outside the perinatal period, with differing aetiology and therapeutic needs to major depressive disorder (MDD), particularly because of the unique aspect of the mother–infant relationship (Batt et al., 2020; Di Florio and Meltzer-Brody, 2015). While there are clear similarities between PPD and MDD in some areas such as symptomatology, genetic risk factors, decreased activation in reward-related regions, and associations with early life and chronic psychosocial stress, there are also several significant differences (Couto et al., 2015; Elwood et al., 2019; Kimmel et al., 2020; Laurent and Ablow, 2012).
Hormonal factors, change in role to motherhood, and the responsibility of caring for a dependent infant are all specific to PPD. Genetic and hormonal factors are thought to predict the onset of PPD in the early postpartum period (the first 6–8 weeks postpartum), while the unique psychosocial stressors of parenting and infant care predict PPD in the late postpartum period (beyond the first 8 weeks postpartum) (Beck, 2001; Bloch et al., 2003; Figueiredo et al., 2015; Venkatesh et al., 2014). Infant-related symptoms, including feelings of maternal inadequacy and negative perceptions of the infant, are distinct to PPD (Lefkovics et al., 2018). Although research into the neurobiology of PPD is in the early stages, dampened amygdala response and corticolimbic activity, both in early and late PPD, is emerging as a distinct feature (Moses-Kolko et al., 2014; Stickel et al., 2019). While MDD involves a heightened amygdala response to negative stimuli (Fales et al., 2008), PPD is associated with a blunted amygdala response to negative (non-infant related) stimuli (Silverman et al., 2011), which predicts greater self-reported maternal hostility towards the infant (Moses-Kolko et al., 2010). Reduced activation of corticolimbic circuitry involved in emotional salience and threat processing is also a distinct feature of PPD, and is thought to underlie the decreased maternal sensitivity and increased self-reported hostility towards the infant seen in PPD (Moses-Kolko et al., 2014; Pechtel et al., 2013). Although PPD overlaps with MDD in some aspects, it has unique features that may explain why it does not respond adequately to treatment options for MDD in some women.
The therapeutic needs of PPD, while sharing the goals of symptom remission and improved quality of life with MDD, have the additional aim of improving maternal care and the quality of the mother–infant interaction (Batt et al., 2020; Brummelte and Galea, 2016; Di Florio and Meltzer-Brody, 2015). Current treatments for PPD are based on those used in the general adult population, and evidence for antidepressant treatment specific to PPD is limited. Selective serotonin reuptake inhibitors (SSRI) are commonly used, although systematic and Cochrane reviews suggest there is little evidence of efficacy for SSRIs in treating or preventing PPD (Molyneaux et al., 2014, 2018; Sharma and Sommerdyk, 2013). The tricyclic antidepressant nortriptyline was shown to be of similar efficacy to sertraline for treating PPD, although was not effective in preventing PPD (Wisner et al., 2001, 2006). Small studies of bupropion (n = 8) and venlafaxine (n = 15) in the treatment of PPD have shown positive results but these have not been replicated (Cohen et al., 2001; Nonacs et al., 2005). In one study of treatment outcomes in PPD, only 6.3% of women with PPD received adequate treatment and just 3.2% achieved remission (Cox et al., 2016). There is also evidence for treatment resistance in PPD (Cepeda et al., 2019; Cox et al., 2016). Current MDD treatments are, therefore, not adequately targeting the distinct features of PPD.
Brexanolone, developed specifically for the treatment of PPD, has shown promise in clinical trials. It is administered as an intravenous infusion over a 60-h period. It results in a rapid treatment response with a reduction in depressive symptoms from about 24 h after starting treatment (Meltzer-Brody et al., 2018). Phase 3 trials of brexanolone in PPD have demonstrated a significant Hamilton Depression Rating Scale score reduction of 17.7 to 19.5 points in brexanolone groups compared to 14 points in placebo groups (Meltzer-Brody et al., 2018). Response appears to be maintained for at least 30 days after the infusion. There are no studies evaluating response beyond this period. Adverse effects include excessive sedation and sudden loss of consciousness during drug administration, requiring admission to a medical ward for brexanolone treatment (Meltzer-Brody et al., 2018). The cost of administering brexanolone is reported to be US$34,000, not including hospital admission costs (Faden and Citrome, 2020). The high cost and prolonged period of admission required for brexanolone treatment are barriers to its accessibility and generalisability.
Postpartum maternal mental disorders can impact on infant development and future well-being, in part due to adverse effects on mother–infant interaction and attachment (Reck et al., 2018; Tronick and Reck, 2009). Maternal sensitivity, characterised by the mother’s ability to accurately perceive, interpret and respond to her infant’s signals, can be compromised in PPD, and can result in an impaired mother–infant relationship (Ainsworth et al., 1971). Antidepressants, although having a positive effect on mood in some women, have no effect on the mother–infant interaction, and the influence of PPD on the mother–infant relationship may continue beyond maternal symptom resolution (Batt et al., 2020; Murray and Cooper, 1997; Stein et al., 1991). Parent–infant psychotherapy has been developed to improve health outcomes in the parent–infant dyad. In addition to the management of parental postpartum psychological symptoms, parent–infant psychotherapy aims to establish stable parent–infant relationship patterns and improve parental sensitivity (Barlow et al., 2016; Mattheß et al., 2021). This intervention has been shown to be effective in alleviating depressive symptoms and improving infant attachment security (Barlow et al., 2015; Tsivos et al., 2015). Targeting maternal behaviour in the treatment of PPD is important not only for maternal role gratification and self-efficacy but also for ensuring optimal infant development (Batt et al., 2020; Kingston et al., 2012).
In summary, PPD, even in the late postpartum period, appears to have distinct features to MDD with dampened corticolimbic circuitry and amygdala response (Moses-Kolko et al., 2014; Silverman et al., 2011), in addition to the unique aspect of the mother–infant relationship. Current treatment options for PPD, with the exception of brexanolone, assume similarities with MDD and disregard the differences, leading to poor efficacy and treatment resistance. Persistent PPD can impact negatively on the mother–infant relationship, leading to suboptimal outcomes for the dyad. This highlights a rationale for exploring treatments with novel mechanisms for PPD.
Overview of psychedelic research
There is a long history of ritualistic use of the tryptamines psilocybin and N,N-dimethyltryptamine, the main psychedelic component of ayahuasca. The first synthetic psychedelic known to the Western world was LSD. There was a period of extensive research with suboptimal methodologies in the 1950s and 1960s into the use of classic psychedelics for the treatment of anxious and depressive disorders, substance use disorders, functional neurological disorders, and distress in terminal cancer, with results that (albeit clinician-judged) were often encouraging (Butler et al., 2020; Dyck, 2006; Grof and Halifax, 1977; Rucker et al., 2016; Weston et al., 2020). However, LSD, in particular, diffused from the clinic into recreational use. Concerns about serious adverse effects arose, and classic psychedelics like LSD and psilocybin were included in international conventions and subsequent national laws that prevented routine medical use (Rucker et al., 2018).
Since the turn of the millennium, clinical research with classic psychedelics (particularly psilocybin) has resumed despite legal restrictions remaining unchanged. Trials were initially conducted in healthy volunteers (Vollenweider et al., 1997) and patient trials commenced later (Moreno et al., 2006). A pooled analysis of eight double-blind placebo-controlled experimental studies conducted between 1999 and 2008 included 110 healthy subjects who received one to four oral doses of psilocybin (45–315 µg/kg body weight) (Studerus et al., 2011). Acute adverse drug events, characterised by strong dysphoria and/or anxiety/panic, occurring in a small proportion of subjects in the two largest dose groups, were managed with interpersonal support. Follow-up of participants suggested that there was no subsequent drug abuse, prolonged psychosis or long-term functional impairment. The largest randomised, double-blind, placebo-controlled study measuring cognitive safety of psilocybin in healthy volunteers completed in 2019 reported no negative impact on a range of cognitive scales, no serious adverse events in participants followed up to 12 weeks after psilocybin dosing, and no adverse events leading to participant withdrawal from the trial (Rucker et al., 2022). Although psilocybin has low abuse potential and is well tolerated, dysphoric experiences (‘bad trips’) can sometimes occur (Johnson et al., 2018; Nutt, 2015) and are minimised in psychedelic trials by excluding participants with a personal or family history of psychotic illness, ensuring the establishment of rapport with the guides or therapists before the dosing session and providing a safe environment for the session (Johnson et al., 2008).
Serotonergic psychedelics exert their action through numerous serotonin receptors (5-HTR), with psilocybin and LSD acting as partial agonists at the 5-HT1R, 5-HT2R, 5-HT6R and 5-HT7R (Nichols, 2004; Rickli et al., 2016). Agonism at the 5-HT2AR is thought to be necessary (but not sufficient) for subjective psychedelic effects. The 5-HT2AR plays a role in cognitive processing, and pre-clinical data suggest that 5-HT2A pathway signalling may be involved in mediating neural plasticity (Carhart-Harris and Nutt, 2017; Ly et al., 2018). However, lisuride, a drug with a high affinity for 5-HT2AR and 5-HT2CR, produces no psychedelic effects (Pieri et al., 1978). This suggests that the functionally selective effects of serotonergic psychedelics on the 5-HT2AR differentially activate downstream second messenger signalling pathways to mediate subjective effects (Flanagan and Nichols, 2018).
Serotonergic psychedelics enhance amygdala activity and reduce default mode network (DMN) and salience network connectivity (Barrett et al., 2020; Carhart-Harris et al., 2017; Lebedev et al., 2015; Roseman et al., 2018). It has been hypothesised that psilocybin increases DMN connectivity in the post-acute phase, followed by a ‘resetting’ and reduction in DMN connectivity, which is proposed to be associated with the therapeutic effects of psychedelics (Carhart-Harris et al., 2017). However, these functional connectivity effects are not specific to classic psychedelics, with 3,4-methylenedioxymethamphetamine and the SSRI sertraline also showing similar results (Klaassens et al., 2015; Müller et al., 2020). This does not exclude the possibility that these effects might be important for the potential therapeutic benefits of psychedelics, although it highlights the constraints of our comprehension of the mechanisms by which psychedelics exert their therapeutic action.
Psychedelic trials in depression
A systematic review of pre-prohibition studies of classic psychedelics in cases classed as ‘depressives’ or ‘depressive reactions’ reported that clinician-judged improvement occurred in 79.2% of patients (Rucker et al., 2016). Recent trials of psychedelics in MDD have demonstrated safety of psychedelics as a precursor to future randomised controlled trials (RCT), and report encouraging results, albeit with small sample sizes and single centre designs that do not allow generalisation of the results more widely (Table 1).
Table 1.
Psychedelic trials in depression.
| Condition | Study design | Sample size | Psychedelic | Outcomes | Adverse events | Authors, Date |
|---|---|---|---|---|---|---|
| Recurrent depression | Open-label trial | n = 6 | Ayahuasca |
Day 1: Statistically significant reduction in HAM-D scores by 62% Day 7: 72% reduction Day 21: enduring low scores, 45% below baseline |
Vomiting | de Osório et al. (2015) |
| Recurrent depression | Open-label trial | n = 17 | Ayahuasca | Day 21: Significantly lower HAM-D scores, with mean scores of 7.56 (SD = 4.7) | Vomiting, non-significantly increased blood pressure and heart rate | Sanches et al. (2016) |
| Treatment-resistant depression | Randomised placebo-controlled trial |
n = 14 (ayahuasca group) n = 15 (placebo group) |
Ayahuasca Placebo |
Days 1 and 2: Lower MADRS scores in ayahuasca group compared to placebo Day 7: Significantly lower MADRS scores with between-groups effect size of 1.49. Response rate 64% in ayahuasca group |
Transient nausea, vomiting and restlessness | Palhano-Fontes et al. (2019) |
| Treatment-resistant depression | Open-label feasibility trial | n = 12 | Psilocybin 10 mg and 25 mg, 7 days apart |
1 week: Response rate 67% (n = 8), and 7 of these patients met criteria for full remission. Depression scores decreased from 21.4 to 7.4, Hedges’ g effect size 3.1 3 months: 58% (n = 7) maintained response, 42% (n = 5) remained in complete remission. 6 months: Depression scores remained low with a 14.9 point mean difference from baseline, effect size 1.2 |
Transient anxiety, confusion, nausea, headache. | Carhart-Harris et al. (2016, 2018) |
| Major depressive disorder | Randomised, waiting list-controlled trial | n = 27 | Psilocybin 20 mg/70 kg and 30 mg/70 kg, 1 week apart. Control arm received same intervention delayed by 8 weeks |
Day 1 after dosing: rapid reduction in depression scores from 16.7 to 6.3. 1 week: depression scores remained low, effect size 2.2. 67% of participants had clinically significant response to intervention. 4 weeks: 71% had clinically significant response. |
Mild-to-moderate headache and challenging emotions limited to the duration of psilocybin sessions. | Davis et al. (2021) |
| Major depressive disorder | Double-blind randomised controlled trial comparing psilocybin with escitalopram |
n = 30 (psilocybin group) n = 29 (escitalopram group) |
Psilocybin group: Psilocybin 25 mg, 2 doses 3 weeks apart and daily oral placebo for 6 weeks Escitalopram group: Psilocybin 1 mg, 2 doses 3 weeks apart, 6 weeks of oral escitalopram 10–20 mg |
Primary outcome: Change in QIDS-SR scores – a non-significant between-group difference of two points. Secondary outcomes: All other depression rating scales favoured psilocybin but analyses were not corrected for multiple comparisons and no conclusions can be drawn from results. |
Similar incidence of adverse events in both groups. | Carhart et al. (2021) |
HAM-D: Hamilton Depression Rating Scale; MADRS: Montgomery Asberg Depression Rating Scale; QIDS-SR: Quick Inventory of Depressive Symptomatology (Self-Report).
In an open-label study of a single dose of ayahuasca in six patients with recurrent depression, a 72% reduction in depression scores by day 7 after dosing, with enduring lower scores up to day 21, was reported (de Osório et al., 2015). Another open-label study of 17 patients with recurrent depression dosed with ayahuasca found significantly lower depression scores up to day 21 after dosing (Sanches et al., 2016). Aside from vomiting and non-significantly increased blood pressure and heart rate, participants did not report any adverse events in either open-label trial. More recently, a randomised placebo-controlled trial of a single dose of ayahuasca in 29 participants with treatment-resistant depression was reported (Palhano-Fontes et al., 2019). Participants received either ayahuasca or placebo, a liquid designed to simulate the taste, smell, and consistency of ayahuasca, although without the subjective psychedelic effects. Following the dosing session lasting about 8 h, participants had a psychiatric evaluation, debriefed their experience, and returned home. A rapid antidepressant effect was noted in the ayahuasca group compared to the placebo group, with significantly lower depression scores up to 7 days after dosing and a between-groups effect size of 1.49 (Palhano-Fontes et al., 2019). Aside from transient nausea, vomiting and restlessness, no safety issues were noted in the ayahuasca group. Further research is warranted to demonstrate safety and efficacy of ayahuasca in the treatment of MDD.
An open-label study evaluating the feasibility of psilocybin assisted psychotherapy in 12 patients with moderate to severe treatment-resistant depression was the first modern-day depression trial conducted with psilocybin (Carhart-Harris et al., 2016). Participants, after an initial preparatory session with a therapist, received 10 mg and 25 mg of oral psilocybin 7 days apart. Psychological support was provided during the sessions, with further psychological input at the 1-week follow-up visit. One week after dosing, 67% of participants had achieved complete remission with depression scores dropping from 21.4 to 7.4, and a Hedges’ g effect size of 3.1. At 3-month follow-up, 58% of patients maintained response while 42% remained in complete remission, with an effect size of 2.0. Depression scores remained significantly reduced at the 6-month follow-up, with an effect size of 1.2 (Carhart-Harris et al., 2018). No major safety concerns were reported in this study. As this was an open-label pilot study, efficacy results are likely to be biased and should be treated with caution. However, this study demonstrated that the intervention was possible to deliver in a clinical trial setting, and laid a foundation for more rigorous forms of trial design.
A randomised, waiting list-controlled trial of psilocybin-assisted therapy in 27 participants with MDD aimed to investigate the effect of psilocybin therapy in MDD (Davis et al., 2021). Participants in one arm of this study received a moderate dose of psilocybin (20 mg/70 kg) followed by a high dose (30 mg/70 kg) 1 week later. The control arm received the same intervention delayed by 8 weeks, in order to differentiate effects of psilocybin intervention from spontaneous symptom improvement. Both groups received supportive psychotherapy. Depression scores showed rapid reduction on day 1 after the first psilocybin dosing, from 16.7 to 6.3. Following the second psilocybin dose 1 week later, depression scores remained low at 4 weeks, with a Cohen d effect size of 2.2. In the overall sample, 67% of participants had clinically significant response (defined as 50% or greater reduction in depression scores from baseline) to the intervention at week 1, increasing to 71% at week 4. These results suggest that psilocybin may produce a rapid antidepressant response with sustained remission up to 4 weeks after dosing. This was a single centre trial with carefully selected participants, and replication of these results in multi-centre RCTs is needed.
A phase 2, double-blind RCT comparing psilocybin with the SSRI escitalopram in a cohort of 59 participants with moderate-to-severe MDD was recently reported (Carhart-Harris et al., 2021). Patients assigned to the psilocybin group (n = 30) received two separate doses of psilocybin 25 mg 3 weeks apart, in addition to 6 weeks of daily oral placebo. Patients randomised to the escitalopram group (n = 29) received two separate doses of psilocybin 1 mg 3 weeks apart, in addition to 6 weeks of oral escitalopram (10–20 mg). Both groups received psychological support. The primary outcome was a change from baseline score on the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR-16). The incidence of adverse events was similar among the two groups. The mean change in QIDS-SR-16 scores from baseline to week 6 were −8.0 ± 1.0 in the psilocybin group and −6.0 ± 1.0 in the escitalopram group, with a between-group difference of two points (95% CI: −5.0 to 0.9). In comparison to previous trials with relatively large effect sizes for psilocybin in the treatment of MDD, this trial did not report a significant difference in the primary outcome among the two groups. Secondary outcomes with other depression rating scales favoured psilocybin to escitalopram, but the analyses were not corrected for multiple comparisons and no conclusions can be drawn from the results. More RCTs are needed to further evaluate the efficacy of psilocybin in MDD.
The safety profile of psilocybin in the treatment of MDD is becoming established. Multi-centre phase 2 trials for psilocybin in MDD are currently in progress (NCT03775200, NCT03866174, NCT04670081, NCT03429075) and will likely give a more robust indication of efficacy. COMPASS Pathways recently announced results from a multi-centre phase IIb trial of psilocybin therapy in 233 participants with treatment-resistant depression (COMPASS Pathways, 2021). These results are not yet independently verified or peer-reviewed. A single dose of psilocybin 25 mg was reported to result in a rapid and enduring reduction in depressive symptom severity after 3 weeks, and nearly 25% of the patients had sustained response at 12 weeks. The majority of treatment-emergent adverse events occurred and resolved on the day of psilocybin administration. Suicidal ideation and behaviours were reported, all occurring in participants who were non-responders to psilocybin. Taking into account the timing and circumstances of adverse events including suicidal behaviours, psilocybin was reported to be generally well-tolerated in this trial (COMPASS Pathways, 2021). In comparison with brexanolone in PPD, psilocybin therapy appears to have similar rapid reduction in depression scores, with enduring response at 6 months. Phase 3 trials of psilocybin will give a better indication of psilocybin efficacy in treatment of depression.
Psychedelics postpartum
There are no studies to our knowledge, animal or human, examining the safety of psychedelics postpartum, particularly in breastfeeding. In general, safety data for recreational drugs in breastfeeding is limited, with evidence largely coming from case reports (Anderson, 2018). The concentration of any drug in breast milk is influenced by factors such as maternal plasma concentration, maternal plasma protein binding, size of the drug molecule, degree of ionisation, and lipid solubility (Hotham and Hotham, 2015).
Breast milk drug concentration is found to be concordant with maternal plasma drug concentration (Hotham and Hotham, 2015). Peak plasma concentration of psilocin, the active metabolite of psilocybin, is reported to occur 105 ± 37 min after oral psilocybin administration (Hasler et al., 1997). The elimination half-life of psilocin is 3 h, indicating that, 48 h after oral administration of psilocybin, all but 0.0016% of psilocin will be eliminated (Brown et al., 2017; Passie et al., 2002). One study found psilocin to be undetectable in urine 24 h after oral administration (Hasler et al., 2002). Plasma protein binding also determines drug transfer into breast milk, with free unbound drugs diffusing readily (Hotham and Hotham, 2015). Psilocybin and psilocin both bind to human serum albumin, which suggests they are less likely to diffuse into breast milk (Khastar et al., 2020). Most drugs cross into breast milk in an unionised form, and milk, being slightly more acidic than plasma, attracts weak bases (Begg et al., 2002). Psilocin, with a pH of 5.2, is acidic and less likely to pass into breast milk (National Centre for Biotechnology Information, 2021).
Molecular size and lipid solubility also determine drug diffusion into breast milk. Low molecular weight drugs, such as psilocin, can cross readily into breast milk (Hotham and Hotham, 2015). Psilocin is more lipid-soluble than psilocybin, suggesting that it can diffuse readily into breast milk (Tylš et al., 2014). Psilocybin can transiently increase prolactin during peak effects, but levels return to baseline 5 h after oral administration, leaving no effect on breast milk production (Hasler et al., 2004).
Another factor determining the risk of infant adverse effects through drug exposure in breast milk is the age of the infant. About 78% of drug-related adverse effects occur in breastfeeding infants aged under 2 months, and only 4% of adverse effects are noted in infants older than 6 months (Anderson et al., 2003).
The pharmacokinetics of psilocybin and psilocin in breastmilk are yet to be examined in trials. However, current knowledge can be extrapolated to indicate that the lipophilicity of psilocin and its low molecular weight may allow for transfer into breast milk, while its acidity and binding to serum albumin suggest it is less likely to pass into breast milk. In the absence of pharmacokinetic evidence for psilocin in breast milk, maternal plasma concentration of psilocin may be the most useful indicator of breast milk concentration, with evidence to suggest that almost all psilocin will be eliminated by 48 h after administration. Including women beyond 6 months postpartum and advising abstention from breastfeeding for a 48-h period after psilocybin administration may reduce potential risks to the infant in future clinical trials.
Therapeutic rationale for the use of psychedelics in the treatment of PPD
PPD has, at its core, a profound sense of maternal ‘disconnection’ – from the self, from the infant, and from the support network (Knudson-Martin and Silverstein, 2009). Themes that emerge from qualitative analyses of PPD symptoms include a deep sense of isolation, detachment from the infant, withdrawal from family and friends, an overwhelming sense of shame and guilt, and feelings of inadequacy as a mother (Beck, 2020; Holopainen and Hakulinen, 2019; Knudson-Martin and Silverstein, 2009). Reduced self-compassion, decreased maternal role gratification, increased self-criticism, maladaptive beliefs about motherhood, and negative perceptions of the infant are often associated with PPD (Felder et al., 2016; Sockol and Battle, 2015; Sockol et al., 2011). Bearing in mind these negative cognitions, PPD is associated with lower maternal sensitivity towards the infant (Bernard et al., 2018), which can impact on infant development, behaviour and mental health in later years (Bernier et al., 2019; Deans, 2020).
Psychedelics induce altered states of consciousness and can, in a dose-dependent manner, elicit mystical-type experiences, feelings of ‘oneness’ or interconnectedness, feelings of sacredness, transcendence of space and time, and a strong positive mood (Griffiths et al., 2006, 2018; James, 1902; MacLean et al., 2011). A core feature of the mystical-type experience is a sense of unity, an experience of becoming one with all that exists (Stace, 1960). Mystical-type experiences are associated with enduring improvements in depression and anxiety outcome measures in psilocybin trials and are thought to mediate these positive effects (Griffiths et al., 2016; Ross et al., 2016), although contrasting views also exist (Olson, 2020).
Along with this sense of unity and spirituality, a number of themes that emerge from qualitative studies of psychedelic experiences in participants with mental ill-health bear relevance to PPD. Acquiring insights into oneself, heightened self-awareness and a greater insight into one’s relationship with others are commonly cited by participants with anxiety and depression (Breeksema et al., 2020). Participants with treatment-resistant depression described a narrative of transformation from a pre-session ‘disconnection’ (from self, others and the world) to a renewed sense of ‘connectedness’ (to close family and friends, and all humanity) after psilocybin dosing (Belser et al., 2017; Watts et al., 2017). In the same cohort, participants reported gaining a fresh perspective on their lives, with increased self-compassion, an improved sense of self-worth, and a transition from emotional avoidance to acceptance (Watts et al., 2017). This sense of openness and acceptance was reported to persist for months in some participants (Watts et al., 2017). Increased self-compassion and self-acceptance have also been reported in psychedelic trials of participants with cancer and alcohol use disorder (Bogenschutz et al., 2018; Malone et al., 2018).
In addition to enhancing the experience of connectedness, LSD is associated with an increase in oxytocin levels in healthy participants, thought to be mediated through 5-HT2AR agonism (Holze et al., 2021). A systematic review examining the role of oxytocin in parent-infant attachments in infancy reported a positive correlation between maternal oxytocin levels and mother–infant interactions, and mothers with higher oxytocin levels were reported to have higher sensitivity in their infant interactions (Scatliffe et al., 2019). This hormonal effect of psychedelics, in conjunction with their psychological effects, may have a role in improving maternal sensitivity and the mother–infant relationship. Trials examining the relationship between psilocybin and oxytocin levels would be helpful in this regard.
An important aspect of psychedelic treatment is psychedelic-assisted therapy, both for ensuring safety and maximal benefit of the treatment for the patient. Psychedelic-assisted therapy involves preparation sessions with the therapist before the dosing session and support during the dosing session, followed by integration sessions after dosing. Preparation sessions are important for maximising the potential benefits of a psychedelic experience and integration is thought to prolong the positive effects of psychological transformation (Watts and Luoma, 2020). Contemporary models of psychedelic-assisted therapy are, in part, grounded in the principles of Acceptance and Commitment Therapy (ACT), a third-wave behavioural therapy emphasising acceptance of internal events in order to promote psychological flexibility and reduce experiential avoidance (Hayes et al., 2006). ACT has also shown promise in the treatment of perinatal mood and anxiety disorders (Bonacquisti et al., 2017; Waters et al., 2020).
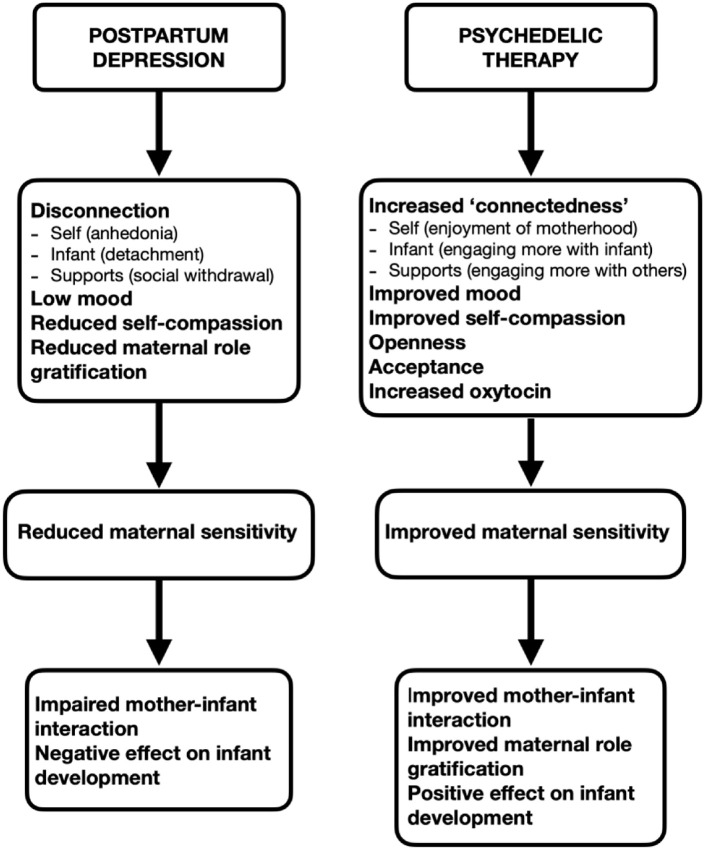
Taken together, psychedelics may, through mystical-type experiences, promote a sense of ‘reconnection’ for the mother in PPD. This increased sense of ‘connectedness’ can improve her connection with herself, her infant, and her support systems, which can have a positive effect on the mother–infant relationship [Figure 1]. Increase in oxytocin levels with psychedelic therapy may have a role in improving maternal sensitivity and mother–infant attachment. Furthermore, psychedelics, through increasing a sense of openness, acceptance and self-compassion, can foster an improvement in maternal self-compassion and role gratification, which can in turn enhance maternal sensitivity and the mother’s relationship with her infant (Fernandes et al., 2021).
Figure 1.
Therapeutic rationale for psilocybin therapy in the treatment of postpartum depression.
Discussion
There is increasing evidence for PPD being a distinct disorder to MDD in aetiology and neurobiology, although with some overlapping features. Poor response to treatment and treatment resistance is seen with conventional MDD treatment in PPD, highlighting its distinct therapeutic needs. Exploration of treatment with novel mechanisms is necessary to improve the mental health and wellbeing of women with PPD and their infants.
Psilocybin, with a favourable safety profile and propensity to promote openness, acceptance, and ‘connectedness’, in addition to positive effects on mood, may be a useful adjunct in the treatment of PPD. Maternal loss of connection, a core feature of PPD, can lead to a deep sense of isolation, lack of self-compassion and low mood, all of which can affect maternal sensitivity and infant development and mental health in later life. Psilocybin assisted therapy may have a role in addressing this ‘disconnection’ while improving maternal mood and maternal role gratification. Restoration of the mother’s connection with herself and her infant, along with an increased sense of acceptance, can help encourage self-compassion and address maladaptive beliefs about motherhood. Psychedelic-assisted therapy can act as a ‘container’ for this process of ‘reconnection’ to occur safely. It can also mitigate any adverse events in response to psilocybin and provide the necessary support and encouragement to take any lessons learned during the dosing session into the real world, practising them until they become habitual. The sense of acceptance and openness may, therefore, promote enduring positive change in the well-being of the mother–infant dyad.
The favourable safety profile of psilocybin, when administered in a therapeutic setting, is becoming evident in healthy participants and those with MDD (Carhart-Harris et al., 2016; Davis et al., 2021; Rucker et al., 2019). However, there is little research on the safety of psilocybin or other psychedelics in the perinatal period. Although the teratogenic potential of classic psychedelics appears low from observational studies (Cohen and Shiloh, 1977; Dishotsky et al., 1971; Long, 1972), in the absence of clinical trials of teratogenicity and in common with almost all other clinical drug trials, trials with classic psychedelics continue to exclude women who are pregnant, intending to become pregnant, or breastfeeding from participating.
There is no research into the safety of psilocybin in breastfeeding women, largely due to ethical concerns. However, in recent years, there has been a shift from systematic exclusion of breastfeeding women from clinical drug trials towards a more reasoned approach of inclusion while taking infant safety into consideration. The Council of International Organisations of Medical Sciences International Ethical Guideline for Health-related Research Involving Humans now promotes research designed to obtain knowledge relevant to the health needs of breastfeeding women, initiated after consideration of the best available data (Council for International Organisations of Medical Sciences, 2016). The latest International Council of Harmonisation Good Clinical Practice draft guideline gives consideration to the inclusion of breastfeeding women in clinical trials, highlighting that excretion of the investigational drug or its metabolites into breastmilk should be examined where applicable and feasible, with infants monitored for the effects of the drug (ICH Harmonised Guideline, 2019). Phase 2 trials continue to demonstrate the safety of psilocybin in the general population, and bearing in mind the potential benefit of psilocybin therapy on maternal wellbeing and the mother–infant relationship, there is a case for examining safety and feasibility of psilocybin in breastfeeding women. This would allow for broader inclusion of breastfeeding women in future psychedelic trials.
We propose that psilocybin may have a role in the treatment of PPD, particularly in engendering ‘reconnection’ of the mother to herself, her infant, and her support structures, and promoting positive enduring changes in the mother–infant relationship. We propose a pilot study to evaluate the safety and efficacy of psilocybin in the treatment of PPD. The increasing evidence base for safety of psilocybin in both healthy adults and those with MDD lays the foundation for pilot studies of psilocybin in the treatment of PPD, with appropriate precautionary and safety measures in place.
Safety considerations
There are no studies, to our knowledge, of psilocybin use in breastfeeding. Excluding breastfeeding women from any future psilocybin trials in PPD would potentially disregard a large proportion of women with PPD, who are equally deserving of evidence-based treatments as any other group. As psilocybin is given as a single dose and found to be undetectable in urine by 24 h after administration, women may be advised to abstain from breastfeeding for a period of 48 h after psilocybin administration. By that stage, with an elimination half-life of 3 h, over 99.99% of psilocybin will have been eliminated from the maternal system (Brown et al., 2017). In addition, only 4% of adverse effects of breast milk drug exposure occur in infants older than 6 months (Anderson et al., 2003). Infant exposure and risk can be reduced by excluding breastfeeding women in the first 6 months postpartum, and advising women to abstain from breastfeeding for a 48-h period post-dose. These measures will reduce infant exposure to psilocybin and will allow breastfeeding women to participate in psilocybin trials.
Limitations
A limitation of this review is that much of the evidence on psychedelics presented relates to the examination of psilocybin in the treatment of MDD. The evidence is derived from single-centre phase 2 trials with small sample sizes. Although these trials show signals of efficacy, larger multi-centre RCTs are awaited. There is no substantial data on the safety of psilocybin in the postpartum period, although safety of psilocybin has been demonstrated in healthy adults and those with MDD.
Conclusion
Psychedelic-assisted therapy may be beneficial in treating one of the core features of PPD – a maternal loss of connection with herself and her infant – and can engender a sense of acceptance, which can promote enduring positive changes for the mother–infant dyad. It is reasonable, given the safety of psilocybin in adults and in MDD when used in a therapeutic setting, to examine safety of psilocybin in the postpartum period with a pilot study, which can then inform future studies of psilocybin in PPD.
Footnotes
Authors’ note: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.R. is an honorary consultant psychiatrist at The South London & Maudsley NHS Foundation Trust, a consultant psychiatrist at Sapphire Medical Clinics and an NIHR Clinician Scientist Fellow at the Centre for Affective Disorders at King’s College London. J.R.’s salary is funded by a fellowship (CS-2017-17-007) from the National Institute for Health Research (NIHR). J.R. leads the Psychedelic Trials Group with Professor Allan Young at King’s College London. King’s College London receives grant funding from COMPASS Pathways PLC and Beckley PsyTech to undertake phase 1 and phase 2 trials with psychedelic compounds. COMPASS Pathways PLC has paid for J.R. to attend trial related meetings and conferences to present the results of research using psilocybin. No funder had influence over the content of this article. J.R. has undertaken paid consultancy work for Beckley PsyTech and Clerkenwell Health. Payments for consultancy work are received and managed by King’s College London. J.R. does not benefit personally. J.R. has no shareholdings in pharmaceutical companies. C.J. reports no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work presents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
ORCID iD: Chaitra Jairaj  https://orcid.org/0000-0002-4473-5303
https://orcid.org/0000-0002-4473-5303
James Rucker  https://orcid.org/0000-0003-4647-8088
https://orcid.org/0000-0003-4647-8088
References
- Ainsworth M, Bell S, Stayton D. (1971) Individual differences in strange-situational behaviour of one-year-olds. In: Schaffer H. (ed.) The Origins of Human Social Relations. Oxford: Academic Press, pp.17–57. [Google Scholar]
- Anderson PO. (2018) Drugs of abuse during breastfeeding. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine 13(6): 405–407. [DOI] [PubMed] [Google Scholar]
- Anderson PO, Pochop SL, Manoguerra AS. (2003) Adverse drug reactions in breastfed infants: Less than imagined. Clinical Pediatrics 42(4): 325–340. [DOI] [PubMed] [Google Scholar]
- Barlow J, Bennett C, Midgley N, et al. (2015) Parent-infant psychotherapy for improving parental and infant mental health. Cochrane Database of Systematic Reviews 1(1): CD010534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J, Bennett C, Midgley N, et al. (2016) Parent–infant psychotherapy: A systematic review of the evidence for improving parental and infant mental health. Journal of Reproductive and Infant Psychology 34(5): 464–482. [Google Scholar]
- Barrett FS, Doss MK, Sepeda ND, et al. (2020) Emotions and brain function are altered up to one month after a single high dose of psilocybin. Scientific Reports 10(1): 2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batt MM, Duffy KA, Novick AM, et al. (2020) Is postpartum depression different from depression occurring outside of the perinatal period? A review of the evidence. Focus 18(2): 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A, Parsonage M, Knapp M, et al. (2014) Costs of Perinatal Mental Health Problems. London: London School of Economics and Political Science. Available at: http://eprints.lse.ac.uk/59885/ [Google Scholar]
- Beck CT. (2001) Predictors of postpartum depression: An update. Nursing Research 50(5): 275–285. [DOI] [PubMed] [Google Scholar]
- Beck CT. (2020) Postpartum depression: A metaphorical analysis. Journal of the American Psychiatric Nurses Association. Epub ahead of print 22 September. DOI: 10.1177/1078390320959448. [DOI] [PubMed] [Google Scholar]
- Begg EJ, Duffull SB, Hackett LP, et al. (2002) Studying drugs in human milk: Time to unify the approach. Journal of Human Lactation: Official Journal of International Lactation Consultant Association 18(4): 323–332. [DOI] [PubMed] [Google Scholar]
- Belser AB, Agin-Liebes G, Swift TC, et al. (2017) Patient experiences of psilocybin-assisted psychotherapy: An interpretative phenomenological analysis. Journal of Humanistic Psychology 57(4): 354–388. [Google Scholar]
- Bernard K, Nissim G, Vaccaro S, et al. (2018) Association between maternal depression and maternal sensitivity from birth to 12 months: A meta-analysis. Attachment & Human Development 20(6): 578–599. [DOI] [PubMed] [Google Scholar]
- Bernier A, Dégeilh F, Leblanc É, et al. (2019) Mother–infant interaction and child brain morphology: A multidimensional approach to maternal sensitivity. Infancy: The Official Journal of the International Society on Infant Studies 24(2): 120–138. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. (2003) Endocrine factors in the etiology of postpartum depression. Comprehensive Psychiatry 44(3): 234–246. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, et al. (2000) Effects of gonadal steroids in women with a history of postpartum depression. The American Journal of Psychiatry 157(6): 924–930. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Forcehimes AA, Pommy JA, et al. (2015) Psilocybin-assisted treatment for alcohol dependence: A proof-of-concept study. Journal of Psychopharmacology 29(3): 289–299. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Podrebarac SK, Duane JH, et al. (2018) Clinical interpretations of patient experience in a trial of psilocybin-assisted psychotherapy for alcohol use disorder. Frontiers in Pharmacology 9: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonacquisti A, Cohen MJ, Schiller CE. (2017) Acceptance and commitment therapy for perinatal mood and anxiety disorders: Development of an inpatient group intervention. Archives of Women’s Mental Health 20(5): 645–654. [DOI] [PubMed] [Google Scholar]
- Breeksema JJ, Niemeijer AR, Krediet E, et al. (2020) Psychedelic treatments for psychiatric disorders: A systematic review and thematic synthesis of patient experiences in qualitative studies. CNS Drugs 34(9): 925–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RT, Nicholas CR, Cozzi NV, et al. (2017) Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clinical Pharmacokinetics 56(12): 1543–1554. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. (2016) Postpartum depression: Etiology, treatment and consequences for maternal care. Hormones and Behavior 77: 153–166. [DOI] [PubMed] [Google Scholar]
- Butler M, Seynaeve M, Nicholson TR, et al. (2020) Psychedelic treatment of functional neurological disorder: A systematic review. Therapeutic Advances in Psychopharmacology 10: 2045125320912125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Nutt DJ. (2017) Serotonin and brain function: A tale of two receptors. Journal of Psychopharmacology 31(9): 1091–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Day CMJ, et al. (2018) Psilocybin with psychological support for treatment-resistant depression: Six-month follow-up. Psychopharmacology 235(2): 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris RL, Bolstridge M, Rucker J, et al. (2016) Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. The Lancet Psychiatry 3(7): 619–627. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Giribaldi B, Watts R, et al. (2021) Trial of psilocybin versus escitalopram for depression. New England Journal of Medicine 384(15): 1402–1411. [DOI] [PubMed] [Google Scholar]
- Carhart-Harris RL, Roseman L, Bolstridge M, et al. (2017) Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Scientific Reports 7(1): 13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda MS, Kern DM, Nicholson S. (2019) Treatment resistant depression in women with peripartum depression. BMC Pregnancy and Childbirth 19(1): 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LS, Viguera AC, Bouffard SM, et al. (2001) Venlafaxine in the treatment of postpartum depression. The Journal of Clinical Psychiatry 62(8): 592–596. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Shiloh Y. (1977) Genetic toxicology of lysergic acid diethylamide (LSD-25). Mutation Research/Reviews in Genetic Toxicology 47(3–4): 183–209. [DOI] [PubMed] [Google Scholar]
- COMPASS Pathways (2021) Treatment resistant depression and clinical trials. Available at: https://ir.compasspathways.com/news-releases/news-release-details/compass-pathways-announces-further-positive-results (accessed 29 December 2021).
- Council for International Organisations of Medical Sciences (2016) International Ethical Guidelines for Health-Related Research Involving Humans. Geneva: Council for International Organisations of Medical Sciences. [Google Scholar]
- Couto TC, e Brancaglion MYM, Alvim-Soares A, et al. (2015) Postpartum depression: A systematic review of the genetics involved. World Journal of Psychiatry 5(1): 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. (2016) The perinatal depression treatment cascade: Baby steps towards improving outcomes. The Journal of Clinical Psychiatry 77(9): 1189–1200. [DOI] [PubMed] [Google Scholar]
- Davé S, Petersen I, Sherr L, et al. (2010) Incidence of maternal and paternal depression in primary care: A cohort study using a primary care database. Archives of Pediatrics & Adolescent Medicine 164(11): 1038–1044. [DOI] [PubMed] [Google Scholar]
- Davis AK, Barrett FS, May DG, et al. (2021) Effects of psilocybin-assisted therapy on major depressive disorder. JAMA Psychiatry 78(5): 481–489. Available from: https://jamanetwork.com/journals/jamapsychiatry/fullarticle/2772630 (accessed 8 November 2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Osório L, Sanches RF, Macedo LR, et al. (2015) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Revista Brasileira de Psiquiatria 37(1): 13–20. [DOI] [PubMed] [Google Scholar]
- Deans CL. (2020) Maternal sensitivity, its relationship with child outcomes, and interventions that address it: A systematic literature review. Early Child Development and Care 190(2): 252–275. [Google Scholar]
- Dennis CL, Brown HK, Wanigaratne S, et al. (2018) Prevalence, incidence, and persistence of postpartum depression, anxiety, and comorbidity among Chinese immigrant and nonimmigrant women: A longitudinal cohort study. Canadian Journal of Psychiatry 63(1): 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Florio A, Meltzer-Brody S. (2015) Is postpartum depression a distinct disorder? Current Psychiatry Reports 17(10): 76. [DOI] [PubMed] [Google Scholar]
- Dishotsky NI, Loughman WD, Mogar RE, et al. (1971) LSD and genetic damage. Science 172(3982): 431–440. [DOI] [PubMed] [Google Scholar]
- Dyck E. (2006) ‘Hitting highs at rock bottom’: LSD treatment for alcoholism, 1950–1970. Social History of Medicine 19(2): 313–329. [Google Scholar]
- Elwood J, Murray E, Bell A, et al. (2019) A systematic review investigating if genetic or epigenetic markers are associated with postnatal depression. Journal of Affective Disorders 253: 51–62. [DOI] [PubMed] [Google Scholar]
- Faden J, Citrome L. (2020) Intravenous brexanolone for postpartum depression: What it is, how well does it work, and will it be used? Therapeutic Advances in Psychopharmacology 10: 2045125320968658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, et al. (2008) Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry 63(4): 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder JN, Lemon E, Shea K, et al. (2016) Role of self-compassion in psychological well-being among perinatal women. Archives of Women’s Mental Health 19(4): 687–690. [DOI] [PubMed] [Google Scholar]
- Fernandes DV, Canavarro MC, Moreira H. (2021) The role of mothers’ self-compassion on mother-infant bonding during the COVID-19 pandemic: A longitudinal study exploring the mediating role of mindful parenting and parenting stress in the postpartum period. Infant Mental Health Journal 42(5): 621–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo FP, Parada AP, Araujo LF, et al. (2015) The influence of genetic factors on peripartum depression: A systematic review. Journal of Affective Disorders 172: 265–273. [DOI] [PubMed] [Google Scholar]
- Flanagan TW, Nichols CD. (2018) Psychedelics as anti-inflammatory agents. International Review of Psychiatry 30(4): 363–375. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lorh KN, et al. (2005) Perinatal depression: A systematic review of prevalence and incidence. Obstetrics and Gynecology 106(5): 1071–1083. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Carducci MA, et al. (2016) Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of Psychopharmacology 30(12): 1181–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Johnson MW, Richards WA, et al. (2018) Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. Journal of Psychopharmacology 32(1): 49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Richards WA, McCann U, et al. (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187(3): 268–283; discussion 284–292. [DOI] [PubMed] [Google Scholar]
- Grob CS, Danforth AL, Chopra GS, et al. (2011) Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. Archives of General Psychiatry 68(1): 71–78. [DOI] [PubMed] [Google Scholar]
- Grof S, Halifax J. (1977) The history of psychedelic therapy with the dying. In: Grof S, Halifax J. (eds) The Human Encounter with Death (1st edn). New York: E. P Dutton. Available at: http://www.psychedelic-library.org/dying.htm [Google Scholar]
- Hasler F, Bourquin D, Brenneisen R, et al. (1997) Determination of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmacokinetic profiles of oral and intravenous psilocybin in man. Pharmaceutica Acta Helvetiae 72(3): 175–184. [DOI] [PubMed] [Google Scholar]
- Hasler F, Bourquin D, Brenneisen R, et al. (2002) Renal excretion profiles of psilocin following oral administration of psilocybin: A controlled study in man. Journal of Pharmaceutical and Biomedical Analysis 30(2): 331–339. [DOI] [PubMed] [Google Scholar]
- Hasler F, Grimberg U, Benz MA, et al. (2004) Acute psychological and physiological affects of psilocybin in healthy humans: A double-blind, placebo-controlled dose-effect study. Psychopharmacology 172(2): 145–156. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, et al. (2006) Acceptance and commitment therapy: Model, processes and outcomes. Behaviour Research and Therapy 44(1): 1–25. [DOI] [PubMed] [Google Scholar]
- Holopainen A, Hakulinen T. (2019) New parents’ experiences of postpartum depression. JBI Database of Systematic Reviews and Implementation Reports 17(9): 1731–1769. [DOI] [PubMed] [Google Scholar]
- Holze F, Avedisian I, Varghese N, et al. (2021) Role of the 5-HT2A receptor in acute effects of LSD on empathy and circulating oxytocin. Frontiers in Pharmacology 12: 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotham N, Hotham E. (2015) Drugs in breastfeeding. Australian Prescriber 38(5): 156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH Harmonised Guideline (2019) General considerations for clinical studies E8(R1) – Draft guideline. Available at: https://database.ich.org/sites/default/files/E8-R1_EWG_Draft_Guideline.pdf (accessed 24 June 2021).
- Jairaj C, Fitzsimons CM, McAuliffe FM, et al. (2019) A population survey of prevalence rates of antenatal depression in the Irish obstetric services using the Edinburgh Postnatal Depression Scale (EPDS). Archives of Women’s Mental Health 22(3): 349–355. [DOI] [PubMed] [Google Scholar]
- Jairaj C, O’Leary N, Doolin K, et al. (2020) The hypothalamic-pituitary-adrenal axis in the perinatal period: Its relationship with major depressive disorder and early life adversity. The World Journal of Biological Psychiatry 21(7): 552–563. [DOI] [PubMed] [Google Scholar]
- James W. (1902) The Varieties of Religious Experience. New York: Penguin Books. Available at: https://www.amazon.co.uk/Varieties-Religious-Experience-Study-Nature/dp/1439297274 [Google Scholar]
- Johnson MW, Garcia-Romeu A, Cosimano MP, et al. (2014) Pilot study of the 5-HT2AR agonist psilocybin in the treatment of tobacco addiction. Journal of Psychopharmacology 28(11): 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Griffiths RR, Hendricks PS, et al. (2018) The abuse potential of medical psilocybin according to the 8 factors of the Controlled Substances Act. Neuropharmacology 142: 143–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Richards W, Griffiths R. (2008) Human hallucinogen research: Guidelines for safety. Journal of Psychopharmacology 22(6): 603–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S, Colquhoun H, Gunduz-Bruce H, et al. (2017) Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. The Lancet 390(10093): 480–489. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Gillan CM, Prenderville J, et al. (2021) Psychedelic therapy’s transdiagnostic effects: A research domain criteria (RDoC) perspective. Frontiers in Psychiatry 12: 800072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khastar H, Foroughi K, Shahrokh Aghayan S, et al. (2020) Molecular docking and binding interaction between psychedelic drugs and human serum albumin. Computational Biology and Bionanotechnology 101(2): 109–116. [Google Scholar]
- Kimmel MC, Bauer A, Meltzer-Brody S. (2020) Toward a framework for best practices and research guidelines for perinatal depression research. Journal of Neuroscience Research 98(7): 1255–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D, Tough S, Whitfield H. (2012) Prenatal and postpartum maternal psychological distress and infant development: A systematic review. Child Psychiatry and Human Development 43(5): 683–714. [DOI] [PubMed] [Google Scholar]
- Klaassens BL, van Gorsel HC, Khalili-Mahani N, et al. (2015) Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. NeuroImage 122: 440–450. [DOI] [PubMed] [Google Scholar]
- Knudson-Martin C, Silverstein R. (2009) Suffering in silence: A qualitative meta-data-analysis of postpartum depression. Journal of Marital and Family Therapy 35(2): 145–158. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC. (2012) A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive and Affective Neuroscience 7(2): 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedev AV, Lövdén M, Rosenthal G, et al. (2015) Finding the self by losing the self: Neural correlates of ego-dissolution under psilocybin. Human Brain Mapping 36(8): 3137–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkovics E, Rigó J, Kovács I, et al. (2018) Effect of maternal depression and anxiety on mother’s perception of child and the protective role of social support. Journal of Reproductive and Infant Psychology 36(4): 434–448. [DOI] [PubMed] [Google Scholar]
- Licheri V, Talani G, Gorule AA, et al. (2015) Plasticity of GABAA receptors during pregnancy and postpartum period: From gene to function. Neural Plasticity 2015: 170435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SY. (1972) Does LSD induce chromosomal damage and malformations? A review of the literature. Teratology 6(1): 75–90. [DOI] [PubMed] [Google Scholar]
- Ly C, Greb AC, Cameron LP, et al. (2018) Psychedelics promote structural and functional neural plasticity. Cell Reports 23(11): 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean KA, Johnson MW, Griffiths RR. (2011) Mystical experiences occasioned by the hallucinogen psilocybin lead to increases in the personality domain of openness. Journal of Psychopharmacology 25(11): 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone TC, Mennenga SE, Guss J, et al. (2018) Individual experiences in four cancer patients following psilocybin-assisted psychotherapy. Frontiers in Pharmacology 9: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattheß J, Eckert M, Becker O, et al. (2021) Potential efficacy of parent-infant psychotherapy with mothers and their infants from a high-risk population: A randomized controlled pilot trial. Pilot and Feasibility Studies 7(1): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Kanes SJ. (2020) Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiology of Stress 12: 100212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. (2018) Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet 392(10152): 1058–1070. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Stuebe A, Dole N, et al. (2011) Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD). The Journal of Clinical Endocrinology and Metabolism 96(1): E40–E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux E, Howard LM, McGeown HR, et al. (2014) Antidepressant treatment for postnatal depression. Cochrane Database of Systematic Reviews 9: CD002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux E, Telesia LA, Henshaw C, et al. (2018) Antidepressants for preventing postnatal depression. Cochrane Database of Systematic Reviews 4: CD004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno FA, Wiegand CB, Taitano EK, et al. (2006) Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. The Journal of Clinical Psychiatry 67(11): 1735–1740. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, et al. (2014) In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. Journal of Neuroendocrinology 26(10): 665–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, et al. (2010) Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. The American Journal of Psychiatry 167(11): 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, Holze F, Dolder P, et al. (2020) MDMA-induced changes in within-network connectivity contradict the specificity of these alterations for the effects of serotonergic hallucinogens. Neuropsychopharmacology 46: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Cooper PJ. (1997) Effects of postnatal depression on infant development. Archives of Disease in Childhood 77(2): 99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Centre for Biotechnology Information (2021) PubChem Compound summary for CID 10624: Psilocybine. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Psilocybine (accessed 20 February 2021).
- Nichols DE. (2004) Hallucinogens. Pharmacology and Therapeutics 101(2): 131–181. [DOI] [PubMed] [Google Scholar]
- Nonacs RM, Soares CN, Viguera AC, et al. (2005) Bupropion SR for the treatment of postpartum depression: A pilot study. The International Journal of Neuropsychopharmacology 8(3): 445–449. [DOI] [PubMed] [Google Scholar]
- Nutt D. (2015) Illegal drugs laws: Clearing a 50-year-old obstacle to research. PLoS Biology 13(1): e1002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara MW, McCabe JE. (2013) Postpartum depression: Current status and future Directions. Annual Review of Clinical Psychology 9: 379–407. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. (1996) Rates and risk of postpartum depression – A meta-analysis. International Review of Psychiatry 8(1): 37–54. [Google Scholar]
- O’Leary N, Jairaj C, Molloy EJ, et al. (2019) Antenatal depression and the impact on infant cognitive, language and motor development at six and twelve months postpartum. Early Human Development 134: 41–46. [DOI] [PubMed] [Google Scholar]
- O’Leary N, Jairaj C, Nixon E, et al. (2021) Antenatal depression and maternal infant directed speech during the first postnatal year. Infant Behavior and Development 64: 101605. [DOI] [PubMed] [Google Scholar]
- Olson DE. (2020) The subjective effects of psychedelics may not be necessary for their enduring therapeutic effects. ACS Pharmacology and Translational Science 4(2): 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyetunji A, Chandra P. (2020) Postpartum stress and infant outcome: A review of current literature. Psychiatry Research 284: 112769. [DOI] [PubMed] [Google Scholar]
- Palhano-Fontes F, Barreto D, Onias H, et al. (2019) Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: A randomized placebo-controlled trial. Psychological Medicine 49(4): 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passie T, Seifert J, Schneider U, et al. (2002) The pharmacology of psilocybin. Addiction Biology 7(4): 357–364. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Murray LMM, Brumariu LE, et al. (2013) Reactivity, regulation, and reward responses to infant cues among mothers with and without psychopathology: An fMRI review. Translational Developmental Psychiatry 1(1): 19673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieri L, Keller HH, Burkard W, et al. (1978) Effects of lisuride and LSD on cerebral monoamine systems and hallucinosis. Nature 272(5650): 278–280. [DOI] [PubMed] [Google Scholar]
- Plant DT, Pariante CM, Sharp D, et al. (2015) Maternal depression during pregnancy and offspring depression in adulthood: Role of child maltreatment. The British Journal of Psychiatry: The Journal of Mental Science 207(3): 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael D. (1975) Being Female: Reproduction, Power, and Change. Berlin: De Gruyter Mouton. Available at: https://books.google.co.uk/books/about/Being_Female.html?id=84hyfRRHeakC&redir_esc=y [Google Scholar]
- Reck C, Tietz A, Müller M, et al. (2018) The impact of maternal anxiety disorder on mother-infant interaction in the postpartum period. PLoS ONE 13(5): e0194763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickli A, Moning OD, Hoener MC, et al. (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology 26(8): 1327–1337. [DOI] [PubMed] [Google Scholar]
- Righetti-Veltema M, Conne-Perréard E, Bousquet A, et al. (1998) Risk factors and predictive signs of postpartum depression. Journal of Affective Disorders 49(3): 167–180. [DOI] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, et al. (2004) Antenatal risk factors for postpartum depression: A synthesis of recent literature. General Hospital Psychiatry 26(4): 289–295. [DOI] [PubMed] [Google Scholar]
- Roseman L, Demetriou L, Wall MB, et al. (2018) Increased amygdala responses to emotional faces after psilocybin for treatment-resistant depression. Neuropharmacology 142: 263–269. [DOI] [PubMed] [Google Scholar]
- Ross S, Bossis A, Guss J, et al. (2016) Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. Journal of Psychopharmacology 30(12): 1165–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJ, Jelen LA, Flynn S, et al. (2016) Psychedelics in the treatment of unipolar mood disorders: A systematic review. Journal of Psychopharmacology 30(12): 1220–1229. [DOI] [PubMed] [Google Scholar]
- Rucker JJ, Marwood L, Ajantaival RJ, et al. (2022) The effects of psilocybin on cognitive and emotional functions in healthy participants: Results from a phase 1, randomised, placebo-controlled trial involving simultaneous psilocybin administration and preparation. Journal of Psychopharmacology 36(1): 114–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucker JJ, Young A, Williams S, et al. (2019) Psilocybin administration to healthy participants: Safety and feasibility in a placebo-controlled study. Neuropsychopharmacology 44(Suppl. 1): 443–444.30038413 [Google Scholar]
- Rucker JJH, Iliff J, Nutt DJ. (2018) Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology 142: 200–218. [DOI] [PubMed] [Google Scholar]
- Sanches RF, De Lima Osório F, Santos RGD, et al. (2016) Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression a SPECT Study. Journal of Clinical Psychopharmacology 36(1): 77–81. [DOI] [PubMed] [Google Scholar]
- Scatliffe N, Casavant S, Vittner D, et al. (2019) Oxytocin and early parent-infant interactions: A systematic review. International Journal of Nursing Sciences 6(4): 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Sommerdyk C. (2013) Are antidepressants effective in the treatment of postpartum depression? A systematic review. The Primary Care Companion for CNS Disorders 15(6): PCC.13r01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey S, Chee CYI, Ng ED, et al. (2018) Prevalence and incidence of postpartum depression among healthy mothers: A systematic review and meta-analysis. Journal of Psychiatric Research 104: 235–248. [DOI] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Liu X, et al. (2011) The neural processing of negative emotion postpartum: A preliminary study of amygdala function in postpartum depression. Archives of Women’s Mental Health 14(4): 355–359. [DOI] [PubMed] [Google Scholar]
- Slomian J, Honvo G, Emonts P, et al. (2019) Consequences of maternal postpartum depression: A systematic review of maternal and infant outcomes. Women’s Health 15: 1745506519844044. Available from: http://journals.sagepub.com/doi/10.1177/1745506519844044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockol LE, Battle CL. (2015) Maternal attitudes, depression, and anxiety in pregnant and postpartum multiparous women. Archives of Women’s Mental Health 18(4): 585–593. [DOI] [PubMed] [Google Scholar]
- Sockol LE, Epperson CN, Barber JP. (2011) A meta-analysis of treatments for perinatal depression. Clinical Psychology Review 31(5): 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli MG. (2004) Maternal infanticide associated with mental illness: Prevention and the promise of saved lives. The American Journal of Psychiatry 161(9): 1548–1557. [DOI] [PubMed] [Google Scholar]
- Stace W. (1960) The Teachings of the Mystics. New York: New American Library. [Google Scholar]
- Stein A, Gath DH, Bucher J, et al. (1991) The relationship between post-natal depression and mother-child interaction. The British Journal of Psychiatry: The Journal of Mental Science 158: 46–52. [DOI] [PubMed] [Google Scholar]
- Stewart DE, Vigod SN. (2019) Postpartum depression: Pathophysiology, treatment, and emerging therapeutics. Annual Review of Medicine 70: 183–196. [DOI] [PubMed] [Google Scholar]
- Stickel S, Wagels L, Wudarczyk O, et al. (2019) Neural correlates of depression in women across the reproductive lifespan – An fMRI review. Journal of Affective Disorders 246: 556–570. [DOI] [PubMed] [Google Scholar]
- Studerus E, Kometer M, Hasler F, et al. (2011) Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. Journal of Psychopharmacology 25(11): 1434–1452. [DOI] [PubMed] [Google Scholar]
- Tronick E, Reck C. (2009) Infants of depressed mothers. Harvard Review of Psychiatry 17(2): 147–156. [DOI] [PubMed] [Google Scholar]
- Tsivos ZL, Calam R, Sanders MR, et al. (2015) Interventions for postnatal depression assessing the mother–infant relationship and child developmental outcomes: A systematic review. International Journal of Women’s Health 7: 429–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylš F, Páleníček T, Horáček J. (2014) Psilocybin – Summary of knowledge and new perspectives. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology 24(3): 342–356. [DOI] [PubMed] [Google Scholar]
- Venkatesh KK, Phipps MG, Triche EW, et al. (2014) The relationship between parental stress and postpartum depression among adolescent mothers enrolled in a randomized controlled prevention trial. Maternal and Child Health Journal 18(6): 1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Leenders KL, Scharfetter C, et al. (1997) Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology 16(5): 357–372. [DOI] [PubMed] [Google Scholar]
- Waters CS, Annear B, Flockhart G, et al. (2020) Acceptance and commitment therapy for perinatal mood and anxiety disorders: A feasibility and proof of concept study. The British Journal of Clinical Psychology 59(4): 461–479. [DOI] [PubMed] [Google Scholar]
- Watts R, Luoma JB. (2020) The use of the psychological flexibility model to support psychedelic assisted therapy. Journal of Contextual Behavioral Science 15: 92–102. [Google Scholar]
- Watts R, Day C, Krzanowski J, et al. (2017) Patients’ accounts of increased ‘connectedness’ and ‘acceptance’ after psilocybin for treatment-resistant depression. Journal of Humanistic Psychology 57(5): 520–564. [Google Scholar]
- Weston NM, Gibbs D, Bird CIV, et al. (2020) Historic psychedelic drug trials and the treatment of anxiety disorders. Depression and Anxiety 37(12): 1261–1279. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Hanusa BH, Perel JM, et al. (2006) Postpartum depression: A randomized trial of sertraline versus nortriptyline. Journal of Clinical Psychopharmacology 26(4): 353–360. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Perel JM, Peindl KS, et al. (2001) Prevention of recurrent postpartum depression: A randomized clinical trial. The Journal of Clinical Psychiatry 62(2): 82–86. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, McShea MC, et al. (2013) Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 70(5): 490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]