Abstract
Background:
Nitrous oxide (N2O) is a frequently used anaesthetic. Since the year 2000, recreational use of N2O, also known as ‘laughing gas’, became popular as a recreational drug due to its mild psychedelic effect. In the 1980s, several reports warned against N2O-induced reproductive risks among healthcare personnel, questioning the occupational safety of N2O in health care.
Methods:
Data about the reproductive risks of N2O were collected from literature.
Results:
Particularly in the past, professionals working in dental and midwifery practices, operating theatres and ambulance transport were exposed to high levels of N2O. Adverse reproduction effects included congenital anomalies, spontaneous abortion and reduced fertility rates in females. Following occupational measures, like maximal exposure limits for ambient N2O, this occupational risk was considerably reduced. Recreational users of N2O, however, voluntarily and repeatedly expose themselves to (very) high doses of N2O. As such, they exceed the health exposure limits some hundred times, but they are fully unaware of the related reproductive risks.
Conclusion:
We advocate to increase the awareness in recreational N2O-users about its potential reproductive risks, especially in heavy users, pregnant users or those who intend to become pregnant.
Keywords: Nitrous oxide, laughing gas, recreative drugs, reproduction, congenital anomalies, abortion
Introduction
Nitrous oxide (N2O; ‘laughing gas’) is a widely used anaesthetic. For over 150 years, N2O is used as an anaesthetic and anxiolytic in operating theatres, ambulance transport, dentistry and during labour and delivery. N2O has been advocated as a safe standard technique for dentistry and is particularly suitable to treat paediatric patients who are terrified of the dentist (Holroyd and Roberts, 2000; National Institute for Health and Clinical Excellence (NICE), 2010). In the United States, 87–89% of all paediatric dentists have used N2O (Mathers, 2018).
In addition to clinical use, recreational N2O use has been known since the late 19th century but recently a serious increase in recreational use emerged in many countries (Kaar et al., 2016; van Amsterdam et al., 2022). In the United Kingdom, N2O was second only to cannabis in drug use among those aged 16–24 in 2019 (April 2019–March 2020) (ONS, 2020). Recreational N2O use became particularly popular in youngsters and adolescents because it is widely available, cheap, legal and most users are convinced that N2O is a safe drug (Nabben et al., 2017). For instance, the majority (77%) of N2O users in the Netherlands was unaware of the drug’s harmful effects (Kaar et al., 2016). The use of hundreds of bulbs daily for prolonged periods of time and continuous filling and inhaling N2O balloons using large 2 kg N2O-tanks is emerging (van Riel et al., 2022), though fortunately, this is still an exceptional practice among recreational N2O users. These new practices are of concern, because repeated exposure to high doses of N2O for a prolonged time is known to induce vitamin B12 deficiency and related neurological damage, such as (irreversible) neuropathy and paralysis (for references, see van Amsterdam et al., 2022).
Reproductive risks of N2O
While describing the safety profile of N2O, our attention was drawn to reports dating back to the 1980s describing N2O-induced reproductive damage among healthcare personnel exposed to N2O. However, most of these professionals were also exposed to other general anaesthetics, like sevoflurane, and these also have reproductive risks (Schifilliti et al., 2011). Therefore, we focussed on N2O-related reproductive adverse effects in experimental animal studies and in observational studies in dental staff and midwives, that is, professionals that are generally not exposed to a mixture of anaesthetic gases. However, the results in early studies in dental assistants may be biased by exposure to amalgam, known to impair reproduction (Schuurs, 1999).
Rodents exposed to N2O showed a variety of developmental effects (increased resorptions, decreased foetal weight and malformations, like cleft palate, limb defects, gut herniation) (Dutch Health Council (DHC), 2000). More specifically, rodents exposed for 2–4 days to N2O (20–30% mixed with 20% O2 and 50–60% N2) showed decreased testis weight, impaired spermatogenesis and injury of the seminiferous tubules (Kripke et al., 1976), disrupted cycles after exposure of females and decreased fertility (Kugel et al., 1990). In another study, rodents exposed for 30 days to 0.5% N2O showed reduced litter size and smaller offspring in females mated with exposed males (Vieira et al., 1983). However, other studies failed to detect adverse effects of N2O on fertility. For a review on animal reproduction toxicity of N2O, see DHC (2000) and Menon et al. (2021).
Occupational exposure of dental assistants and midwifes to N2O appeared to be associated with congenital anomalies (Cohen et al., 1980), spontaneous abortion (Axelsson et al., 1996; Cohen et al., 1980; Nixon et al., 1979; Rowland et al., 1995) and reduced fertility rates (Ahlborg et al., 1996; Bennett, 2001; Rowland et al., 1992). For instance, in the study of Cohen et al. (1980) among 20,000 female dental assistants, exposure to N2O was associated with a 1.7–2.3 time increased risk for spontaneous abortion. A previous mail survey by Cohen et al. (1975) showed that spouses of male dentists (n = 7439), who were exposed to N2O in the year before conception, reported a 50% greater incidence of spontaneous abortion. A meta-analysis of 19 studies between 1971 and 1995 found a relative risk of spontaneous abortion after N2O exposure of 1.48 (95% confidence interval (CI): 1.41–1.58). In a selection of studies with the strongest design, the relative risk increased to 1.90 (95% CI: 1.72–2.09) (Boivin, 1997). Interestingly, foetal loss became only apparent when dental assistants were exposed to N2O for more than 3–5 working hours per week (Rowland et al., 1995), whereas reduced female fertility became evident in women with 5 or more hours of exposure per week (Rowland et al., 1992). The observed thresholds imply that spontaneous abortion and reduced fertility may occur only above a certain cumulative dose per week or above a certain minimum time of exposure (>3 h and >5 h per week, respectively).
The association between occupational N2O exposure of dentists and male infertility was not specifically studied. For a review on the effects of N2O exposure on reproductive outcomes in humans, see DHC (2000) and Olfert (2006).
While in the past, exposure levels up to 7500 ppm N2O (13,500 mg/m3) were measured in ambulances (Ancker et al., 1980) and dentist practices (Hillman et al., 1981), such high levels are no longer seen. Awareness about the reproductive harm of N2O has increased since occupational exposure limits (OELs) were set worldwide to protect healthcare personnel from unwanted exposure to ambient N2O (Sanders et al., 2008). The Dutch Health Council (DHC, 2000) has recommended to classify N2O as a substance that causes concern for human fertility (possible risk for impaired fertility) and development (possible risk of harm to the unborn child). Based on all available evidence for reproductive toxicity and adverse developmental outcomes in animals exposed to N2O, a health-based OEL of 20 mg/m3 (11 ppm) as 8 h time-weighted average was derived (Menon et al., 2021). In the United States, the NIOSH recommended a somewhat higher threshold limit value of 25 ppm or 46 mg/m3 (National Institute for Occupational Safety and Health (NIOSH), 2019).
Biochemical basis of the reproductive risks of N2O
The pathogenesis of impaired reproduction by most volatile anaesthetics, like isoflurane and halothane, remains unclear and is likely to involve numerous membrane proteins and ion channels. In contrast, the mechanism of N2O-induced harm is well known as it inactivates vitamin B12 (cobalamin) (Sanders et al., 2008). Operating theatre nurses exposed to N2O (36–1502 mg/m3; exceeding the OEL) showed lower vitamin B12 plasma levels (437 vs 373 pM; p = 0.001) and higher homocysteine levels (11.2 vs 8.9 mM, p = 0.006) compared to medical personnel not exposed to N2O and these effects were N2O exposure level-dependent (Krajewski et al., 2007). It should, however, be noted that most of the subjects in the N2O-exposed group were simultaneously exposed to low levels of other volatile anaesthetics, for example, sevoflurane (0.2–21 mg/m3), but the exposure levels to these other anaesthetics were well below existing OELs, which makes confounding of the negative effect of N2O exposure on vitamin B12 levels by simultaneous exposure to the other anaesthetics unlikely.
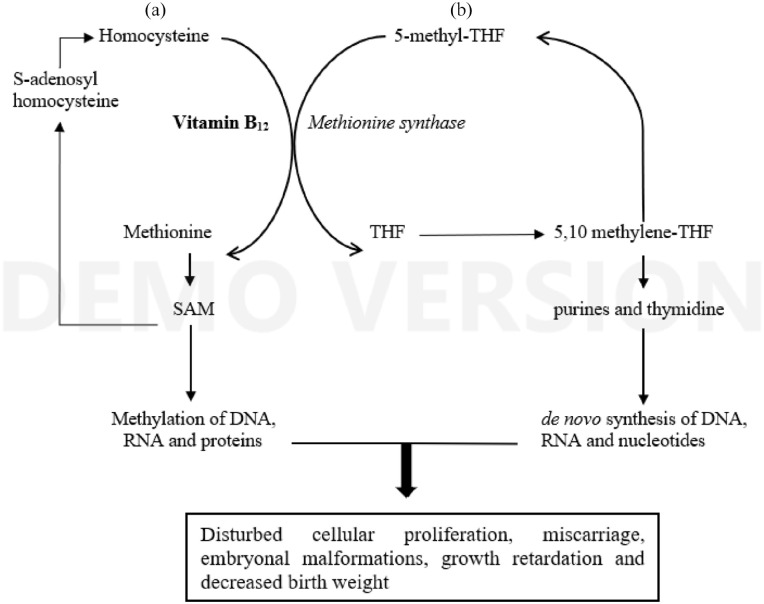
Vitamin B12 is an essential cofactor for methionine synthetase which plays a critical role in two key metabolic cycles in cell proliferation: the methylation cycle and the folate cycle (cf. Figure 1). In continuous recycling of homocysteine to methionine, catalysed by methionine synthetase, the methyl group of the substrate 5-methyl-tetrahydrofolate (5-methyl-THF; methyl folate) is transferred to homocysteine. The methionine formed is required for the biosynthesis of s-adenosylmethionine, an essential methyl donor in the synthesis of DNA, RNA, myelin and other proteins that contribute to gene expression and cell proliferation (Sanders et al., 2008; Vallejo and Zakowski, 2019). The folate cycle serves the de novo biosynthesis of thymidylate and purine, basic elements of DNA. Clearly, both cycles can be disrupted either by folate deficiency or inhibition (or deficiency) of vitamin B12, which results via DNA synthesis and impaired cellular proliferation and organogenesis, in genotoxicity and impaired reproductive functions, respectively. Indeed, in humans, exposure to N2O was found to be dose-dependently associated with DNA damage (Pasquini et al., 2001; Wrońska-Nofer et al., 2012). However, N2O-induced genotoxicity seems to be reversible due to DNA repair, since the increase of sister chromatid exchanges (marker of genotoxicity) reversed to normal values after a 2-month period of non-exposure (Eroglu et al., 2006). For a review on genotoxicity, see Schifilliti et al. (2011).
Figure 1.
N2O inactivates vitamin B12, the essential cofactor of the enzyme methionine synthase: (a) the methylation cycle synthesizes S-adenosylmethionine (SAM), the universal methyl donor for transmethylation of DNA and other non-genomic methylation reactions; (b) the folate cycle serves, indirectly via tetrahydrofolate (THF) and 5,10 methylene-THF, the de novo synthesis of purines and thymidine, building blocks of nucleotides, DNA and RNA.
It is generally known that folate deficiency results in pregnancy-related complications and abnormal embryonic development with congenital defects, like neural tube defects (NTDs). However, low vitamin B12 status was also found to be associated with the risk of NTDs (Molloy et al., 2009), cleft lip/palate (Munger et al., 2021), early recurrent abortion (Reznikoff-Etiévant et al., 2002), preterm birth and low birth weight (Rogne et al., 2017). For review of vitamin B12 deficiency in pregnancy, see Van Sande et al. (2013). Effects of N2O on male reproductivity in rodents (Kripke et al., 1976) have already been described. In addition, it is relevant to mention the importance of vitamin B12 in male fertility. Vitamin B12 plays a substantial role in spermatogenesis, and hence in semen quality, because (a) vitamin B12 deficiency lowers testicular function leading to aplasia of sperms and impaired spermatogenesis in rodents (Kawata et al., 1992) and (b) human data showed that total vitamin B12 concentration in seminal plasma was significantly correlated with the sperm concentration (r = 0.42; p < 0.001) (Boxmeer et al., 2007). The positive effects of vitamin B12 treatment on sperm parameters have been reviewed by Banihani (2017).
Another route to reproduction disorders induced by N2O exposure is low vitamin B12-related megaloblastic anaemia (Amess et al., 1978) (cf. Figure 1). Severe anaemia (Hb < 6 g/dL) is associated with placenta previa, abruptio placenta, operative delivery and post-partum bleeding (Flessa, 1974) in the mother and with prematurity, spontaneous abortions, low birth weight and foetal deaths (Sifakis and Pharmakides, 2000).
Relevance for recreational N2O users
It is widely accepted that the incidental recreational use of N2O elicits virtually no harm, although the genotoxic and the (related) reproductive risks have in this respect so far been neglected. Particularly, heavy use of hundreds of bulbs (one bulb contains 10 mL or 8000 mg of N2O in a pressurized liquid form) may be quite harmful.
Occupational exposure results from inhaling waste N2O that escapes from the patient’s mask to the work area. On average, one inhales over one 8 h day (480 min, at a frequency of 15 inhalations per minute and 0.5 L per inhalation) a volume of 3.6 m3 of air. Based on an OEL value of 20 mg/m3 as 8 h time-weighted average (Menon et al., 2021), the daily allowed inhaled dose of N2O for an employee is on average 3.6 × 20 mg N2O = 72 mg N2O. In recreational use, the inhalation of one bulb represents a dose of 8000 mg (8 g) of N2O, which is about 100 times the allowed daily occupational dose. Note that a recreational user fills one balloon with 8000 mg N2O (the filled balloon has a volume of 3.2 L) which is self-administered via 4–5 inhalations from the balloon. Although acute exposure to a high dose does not compare well with long-term occupational exposure to relatively low doses, this 100-fold difference in exposure is significant. Even if one realizes that an uncertainty factor of 50 was used to account for extrapolation from rats to humans (factor 10) and interindividual variability within the population (factor 5) to calculate the OEL (Menon et al., 2021), this 100-fold exposure is relevant within this comparison. Moreover, one should apply an addition uncertainty factor to account for the young age of many recreational users and their unfinished maturation. On the contrary, one-year occupational exposure to the maximally allowed value (OEL value) would lead to a cumulative dose of 16,000 mg (based on 220 full time working days per year) which equals the recreational dose of only two bulbs of 8000 mg. Moreover, medical treatment with N2O in dentistry or pregnancy can lead to relative high acute exposures, though an estimated volume of 15–25 L of N2O is routinely sufficient for clinical sedation which equals 5–8 balloons as used recreationally. However, such patients are at low risk, considering their low cumulative annual dose since they receive only 1–2 treatments per year. However, the threshold found with respect to reproductive harm, as reported by Rowland et al. (1992, 1995), seems irrelevant for heavy recreational users. First, because they are exposed to much higher levels of N2O. Second, it is evident that repeated inhalation of high levels of N2O from balloons for less than 3–5 h per week will not avoid the presumed reproductive harm.
It should be emphasized that this review provides only evidence for an association, but not for a causal relation, between N2O and impaired reproduction in humans. In addition, one may question how long-term, but intermittent exposure (occupational exposure) compares with repeated exposure to very high doses (compared to those occupational exposure; recreational exposure) with respect to reproductive harm. This is still a gap of knowledge to be further investigated. Furthermore, impaired reproduction in female recreational users of N2O is not yet reported. Finally, it seems that the risk for occasional users of N2O is very limited. In conclusion, the recreational user who frequently uses high doses of N2O should be aware of its potential genotoxic and reproductive risks, particularly when the exposed person is pregnant or intends to become pregnant. It is advocated to raise awareness of these risks among medical professionals through the medical curriculum and among recreational N2O users via public health campaigns.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iD: Jan van Amsterdam  https://orcid.org/0000-0002-8847-4387
https://orcid.org/0000-0002-8847-4387
References
- Ahlborg G, Jr, Axelsson G, Bodin L. (1996) Shift work, nitrous oxide exposure and subfertility among Swedish midwives. International Journal of Epidemiology 25: 783–790. [DOI] [PubMed] [Google Scholar]
- Amess JA, Burman JF, Rees GM, et al. (1978) Megaloblastic haemopoiesis in patients receiving nitrous oxide. The Lancet 2: 339–342. [DOI] [PubMed] [Google Scholar]
- Ancker K, Halldin M, Göthe CJ. (1980) Nitrous-oxide analgesia during ambulance transportation: Airborne levels of nitrous oxide. Acta Anaesthesiologica Scandinavica 24: 497–500. [DOI] [PubMed] [Google Scholar]
- Axelsson G, Ahlborg G, Jr, Bodin L. (1996) Shift work, nitrous oxide exposure, and spontaneous abortion among Swedish midwives. Occupational and Environmental Medicine 53: 374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banihani SA. (2017) Vitamin B(12) and semen quality. Biomolecules 7: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. (2001) Vitamin B12 deficiency, infertility and recurrent fetal loss. The Journal of Reproductive Medicine 46: 209–212. [PubMed] [Google Scholar]
- Boivin JF. (1997) Risk of spontaneous abortion in women occupationally exposed to anaesthetic gases: A meta-analysis. Occupational and Environmental Medicine 54: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxmeer JC, Smit M, Weber RF, et al. (2007) Seminal plasma cobalamin significantly correlates with sperm concentration in men undergoing IVF or ICSI procedures. Journal of Andrology 28: 521–527. [DOI] [PubMed] [Google Scholar]
- Cohen EN, Brown BW, Jr, Bruce DL, et al. (1975) A survey of anesthetic health hazards among dentists. Journal of the American Dental Association 90: 1291–1296. [DOI] [PubMed] [Google Scholar]
- Cohen EN, Gift HC, Brown BW, et al. (1980) Occupational disease in dentistry and chronic exposure to trace anesthetic gases. Journal of the American Dental Association 101: 21–31. [DOI] [PubMed] [Google Scholar]
- Dutch Health Council (DHC) (2000) Nitrous oxide: Evaluation of the effects on reproduction, recommendation for classification. Available at: https://www.gezondheidsraad.nl/binaries/gezondheidsraad/documenten/adviezen/2000/05/03/lachgas/advies-lachgas.pdf
- Eroglu A, Celep F, Erciyes N. (2006) A comparison of sister chromatid exchanges in lymphocytes of anesthesiologists to nonanesthesiologists in the same hospital. Anesthesia & Analgesia 102: 1573–1577. [DOI] [PubMed] [Google Scholar]
- Flessa HC. (1974) Hemorrhagic disorders and pregnancy. Clinical Obstetrics and Gynecology 17: 236–249. [DOI] [PubMed] [Google Scholar]
- Hillman KM, Saloojee Y, Brett II, et al. (1981) Nitrous oxide concentrations in the dental surgery, atmospheric and blood concentrations of personnel. Anaesthesia 36: 257–262. [DOI] [PubMed] [Google Scholar]
- Holroyd I, Roberts GJ. (2000) Inhalation sedation with nitrous oxide: A review. Dental Update 27: 141–142, 144, 146. [DOI] [PubMed] [Google Scholar]
- Kaar SJ, Ferris J, Waldron J, et al. (2016) Up: The rise of nitrous oxide abuse – An international survey of contemporary nitrous oxide use. Journal of Psychopharmacology 30: 395–401. [DOI] [PubMed] [Google Scholar]
- Kawata T, Takada T, Morimoto F, et al. (1992) Effects of vitamin B12-deficiency on testes tissue in rats. Journal of Nutritional Science and Vitaminology 38: 305–316. [DOI] [PubMed] [Google Scholar]
- Krajewski W, Kucharska M, Pilacik B, et al. (2007) Impaired vitamin B12 metabolic status in healthcare workers occupationally exposed to nitrous oxide. British Journal of Anaesthesia 99: 812–818. [DOI] [PubMed] [Google Scholar]
- Kripke BJ, Kelman AD, Shah NK, et al. (1976) Testicular reaction to prolonged exposure to nitrous oxide. Anesthesiology 44: 104–113. [DOI] [PubMed] [Google Scholar]
- Kugel G, Letelier C, Zive MA, et al. (1990) Nitrous oxide and infertility. Anesthesia Progress 37: 176–180. [PMC free article] [PubMed] [Google Scholar]
- Mathers FG. (2018) Lachgas in der zahnärztlichen Praxis [Laughing gas in the dental practice]. ZWR-Das Deutsche Zahnärzteblatt 127: 150–159. [Google Scholar]
- Menon JML, van Luijk J, Swinkels J, et al. (2021) A health-based recommended occupational exposure limit for nitrous oxide using experimental animal data based on a systematic review and dose-response analysis. Environmental Research 201: 111575. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Kirke PN, Troendle JF, et al. (2009) Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic acid fortification. Pediatrics 123: 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger RG, Kuppuswamy R, Murthy J, et al. (2021) Maternal vitamin B(12) status and risk of cleft lip and cleft palate birth defects in Tamil Nadu State, India. The Cleft Palate-Craniofacial Journal 58: 567–576. [DOI] [PubMed] [Google Scholar]
- Nabben T, van der Pol P, Korf D. (2017) Roes met een luchtje. Gebruik, gebruikers en markt van lachgas [Fuddle With a Smell. Use, Users and Market of Nitrous Oxide]. Amsterdam: Rozenberg. Available at: https://pure.uva.nl/ws/files/42253871/22059312.pdf [Google Scholar]
- National Institute for Health and Clinical Excellence (NICE) (2010) Sedation in Children and Young People: Sedation for Diagnostic and Therapeutic Procedures in Children and Young People. London: National Clinical Guideline Centre. Available at: https://www.nice.org.uk/guidance/cg112/evidence/full-guideline-136287325 [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) (2019) Nitrous oxide. Available at: https://www.cdc.gov/niosh/npg/npgd0465.html
- Nixon GS, Helsby CA, Gordon H, et al. (1979) Pregnancy outcome in female dentists. British Dental Journal 146: 39–42. [DOI] [PubMed] [Google Scholar]
- Olfert SM. (2006) Reproductive outcomes among dental personnel: A review of selected exposures. Journal of the Canadian Dental Association 72: 821–825. [PubMed] [Google Scholar]
- ONS (2020) Drug misuse in England and Wales: Year ending March 2020 – An overview of the extent and trends of illicit drug use for the year ending March 2020. Data are from the Crime Survey for England and Wales. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/crimeandjustice/articles/drugmisuseinenglandandwales/yearendingmarch2020/pdf
- Pasquini R, Scassellati-Sforzolini G, Fatigoni C, et al. (2001) Sister chromatid exchanges and micronuclei in lymphocytes of operating room personnel occupationally exposed to enfluorane and nitrous oxide. Journal of Environmental Pathology, Toxicology, and Oncology 20: 119–126. [PubMed] [Google Scholar]
- Reznikoff-Etiévant MF, Zittoun J, Vaylet C, et al. (2002) Low vitamin B(12) level as a risk factor for very early recurrent abortion. European Journal of Obstetrics & Gynecology and Reproductive Biology 104: 156–159. [DOI] [PubMed] [Google Scholar]
- Rogne T, Tielemans MJ, Chong MF-F, et al. (2017) Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: A systematic review and meta-analysis of individual participant data. American Journal of Epidemiology 185: 212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland AS, Baird DD, Shore DL, et al. (1995) Nitrous oxide and spontaneous abortion in female dental assistants. American Journal of Epidemiology 141: 531–538. [DOI] [PubMed] [Google Scholar]
- Rowland AS, Baird DD, Weinberg CR, et al. (1992) Reduced fertility among women employed as dental assistants exposed to high levels of nitrous oxide. The New England Journal of Medicine 327: 993–997. [DOI] [PubMed] [Google Scholar]
- Sanders RD, Weimann J, Maze M. (2008) Biologic effects of nitrous oxide: A mechanistic and toxicologic review. Anesthesiology 109: 707–722. [DOI] [PubMed] [Google Scholar]
- Schifilliti D, Mondello S, D’Arrigo MG, et al. (2011) Genotoxic effects of anesthetic agents: An update. Expert Opinion on Drug Safety 10: 891–899. [DOI] [PubMed] [Google Scholar]
- Schuurs AH. (1999) Reproductive toxicity of occupational mercury: A review of the literature. Journal of Dentistry 27: 249–256. [DOI] [PubMed] [Google Scholar]
- Sifakis S, Pharmakides G. (2000) Anemia in pregnancy. Annals of the New York Academy of Sciences 900: 125–136. [DOI] [PubMed] [Google Scholar]
- Vallejo MC, Zakowski MI. (2019) Pro-con debate: Nitrous oxide for labor analgesia. BioMed Research International 2019: 4618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amsterdam J, Brunt TM, Nabben T, et al. (2022) Recreational N2O use: Just laughing or really bad news? Addiction 117: 268–269. [DOI] [PubMed] [Google Scholar]
- van Riel A, Hunault CC, van den Hengel-Koot IS, et al. (2022) Alarming increase in poisonings from recreational nitrous oxide use after a change in EU-legislation, inquiries to the Dutch Poisons Information Center. International Journal of Drug Policy 100: 103519. [DOI] [PubMed] [Google Scholar]
- Van Sande H, Jacquemyn Y, Karepouan N, et al. (2013) Vitamin B12 in pregnancy: Maternal and fetal/neonatal effects – A review. Open Journal of Obstetrics and Gynecology 3: 37330. [Google Scholar]
- Vieira E, Cleaton-Jones P, Moyes D. (1983) Effects of intermittent 0.5% nitrous oxide/air (v/v) on the fertility of male rats and the post-natal growth of their offspring. Anaesthesia 38: 319–323. [DOI] [PubMed] [Google Scholar]
- Wrońska-Nofer T, Nofer JR, Jajte J, et al. (2012) Oxidative DNA damage and oxidative stress in subjects occupationally exposed to nitrous oxide (N(2)O). Mutation Research 731: 58–63. [DOI] [PubMed] [Google Scholar]