Abstract
Background:
No consensus has been reached regarding the optimal surgical treatment for focal chondral defects of the talus.
Purpose:
A Bayesian network meta-analysis was conducted to compare the clinical scores and complications of mosaicplasty, osteochondral auto- and allograft transplant, microfracture, matrix-assisted autologous chondrocyte transplant, and autologous matrix-induced chondrogenesis (AMIC) for chondral defects of the talus at midterm follow-up.
Study Design:
Bayesian network meta-analysis; Level of evidence, 4.
Methods:
This Bayesian network meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions. PubMed, Embase, Google Scholar, and Scopus databases were accessed in February 2021. All clinical trials comparing 2 or more surgical interventions for the management of chondral defects of the talus were accessed. The outcomes of interest were visual analog scale (VAS) score, American Orthopaedic Foot and Ankle Society (AOFAS) score, rate of failure, and rate of revision surgery. The network meta-analysis were performed through the routine for Bayesian hierarchical random-effects model analysis. The log odds ratio (LOR) effect measure was used for dichotomous variables, and the standardized mean difference (SMD) was used for continuous variables.
Results:
Data from 13 articles (521 procedures) were retrieved. The median length of the follow-up was 47.8 months (range, 31.7-66.8 months). Analysis of variance revealed no difference between the treatment groups at baseline in terms of age, sex, body mass index, AOFAS score, VAS score, and mean number of defects. AMIC demonstrated the greatest AOFAS score (SMD, 11.27) and lowest VAS score (SMD, –2.26) as well as the lowest rates of failure (LOR, 0.94) and revision (LOR, 0.94). The test for overall inconsistency was not significant.
Conclusion:
At approximately 4 years of follow-up, the AMIC procedure for management of focal chondral defects of the talus produced the best outcome.
Keywords: talus, chondral defect, AMIC, OAT
Focal chondral defects of the talus are common. 35 Given the limited intrinsic regeneration capability of cartilage, surgical intervention can be necessary.15,30 If left untreated, patients can experience increasing pain, reduced quality of life, reduced sporting activity, and early osteoarthritis. 29 Traditionally, microfracture has been considered the first-line intervention for focal chondral defects of the talus. 13 Several osteochondral transplant procedures have been proposed. With osteochondral autograft or allograft transplant (OAT), an osteochondral graft is harvested from a nonweightbearing zone of the knee and transplanted to fill the chondral defect of the talus.1,16 With mosaicplasty, several osteochondral grafts or plugs are harvested from a nonweightbearing zone of the knee to produce a mosaic-like structure. 37 In matrix-assisted autologous chondrocyte transplantion (MACT), chondrocytes harvested from a nonweightbearing area of the knee are cultivated, expanded, and implanted into a membrane.27,28 In a second surgical session, the chondrocyte-loaded membrane is transplanted into the defect with custom-made instruments in a full arthroscopic fashion. 34 Recently, autologous matrix-induced chondrogenesis (AMIC) has been proposed for the management of chondral defects of the talus.3,5 AMIC exploits the regenerative potential of bone marrow–derived cells.9,17
Despite all these options, it is unclear which is the optimal surgical treatment for focal chondral defects of the talus, and no consensus has been reached. To the best of our knowledge, no network meta-analysis has been conducted previously to evaluate the treatments for talar chondral defects. Therefore, a Bayesian network meta-analysis was conducted to compare these strategies for the surgical management of chondral defects of the talus at midterm follow-up. The present study compared the efficacy of these strategies in terms of clinical scores and complications.
Methods
Search Strategy
This Bayesian network meta-analysis followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions. 25 Before the literature search was conducted, the PICOT framework was established as follows:
P (Problem): focal chondral defect of the talus
I (Intervention): surgical management
C (Comparison): AMIC, OAT, microfracture, mosaicplasty, MACT
O (Outcomes): clinical scores and complications
T (Timing): ≥18 months of follow-up
Data Source
Two authors (F.M., A.B.) conducted the literature search independently. PubMed, Embase, Google Scholar, and Scopus databases were accessed in February 2021. The following keywords were used in combination: ankle, talus, cartilage, damage, chondral, articular, injury, chondropathy, focal, defect, pain, membrane, autologous matrix-induced chondrogenesis, matrix-assisted autologous chondrocyte transplantation, MACT, AMIC, OAT, cylinder, osteochondral, transplantation, outcomes, microfractures, failures, surgery, management, allograft, autograft, failure, revision. The same authors independently screened the resulting articles. The full-text versions of the articles of interest were accessed, and a cross-reference of the bibliographies was conducted. Divergences were solved by a third author (N.M.).
Eligibility Criteria
All clinical trials that compared 2 or more surgical interventions for the management of talar chondral defects were accessed. Given the authors’ language abilities, articles in English, German, Italian, French, and Spanish were eligible. Only studies of level I to IV of evidence, according to the Oxford Centre of Evidence-Based Medicine, 24 were considered. Other eligibility criteria were studies that focused on AMIC, OAT, microfracture, MACT, and mosaicplasty; involved patients with a focal lesion of the talus; reported data on a minimum of 10 patients; clearly stated the type of intervention; had a minimum of 18 months of follow-up; and reported quantitative data under the outcomes of interest. Not eligible were studies involving patients with end-stage joint osteoarthritis or patients with kissing lesions; animal, computational, or biomechanical studies; and studies augmenting the procedures with less committed cells (eg, mesenchymal stem cells).
Data Extraction
Two authors (F.M., A.B.) separately performed data extraction. Study details such as author, year, journal, study design, and length of follow-up were retrieved. Data regarding patient characteristics at baseline were collected: number of procedures, defect size, patients’ mean age and body mass index (BMI), and patients’ sex. Also retrieved were visual analog scale (VAS) and American Orthopaedic Foot and Ankle Society (AOFAS) 41 scores and the rates of failure and revision surgeries. Failure was defined as recurrence of pain and/or catching symptoms, graft hypertrophy, and/or partially or completely displaced delamination seen on magnetic resonance imaging (MRI) or arthroscopic examination.23,31,32
Methodological Quality Assessment
Two independent authors assessed methodological quality (F.M., A.B.) using the risk of bias graph tool of the Review Manager software (The Nordic Cochrane Collaboration). The following factors pertaining to risk of bias were evaluated: selection, detection, reporting, attrition, and other source of bias.
Statistical Analysis
The statistical analysis was performed by the main author (F.M.), using STATA software/MP (Stata Corporation). To assess baseline data, the Shapiro-Wilk test was performed to investigate data distribution. For parametric data, mean and standard deviation were evaluated. Baseline comparability of parametric data was assessed using analysis of variance (ANOVA), with P > .1 considered satisfactory. For nonparametric data, median and interquartile range were evaluated. The baseline comparability of nonparametric data was assessed by the Kruskal-Wallis test, with P > .1 considered satisfactory. Network meta-analysis were performed through the STATA routine for Bayesian hierarchical random-effects model analysis. The inverse variance method was used for all of the comparisons. The log odds ratio (LOR) effect measure was used for dichotomous variables, whereas the standardized mean difference (SMD) was used for continuous variables. Overall inconsistency was evaluated through the equation for global linearity via the Wald test. If the P value was >.1, the null hypothesis could not be rejected, and the consistency assumption was accepted at the overall level of each treatment. All variables were compared in the network analyses against a fictitious group control: no event for binary comparisons and the maximal value of a score for continuous endpoints. For each outcome of interest, we constructed edge, interval, and funnel plots. Edge plots demonstrate the amount of a direct comparison and its weight in the overall network analysis. Interval plots rank the estimate effect related to each treatment resulting from the network comparisons. For the interval plots, both confidence intervals and percentile intervals were set at 95%. Funnel plots investigate the risk of bias of the outcome of interest considered; greater asymmetry in the plot was associated with a greater risk of bias.
Results
Search Result
The literature search resulted in 905 articles; of them, 274 were duplicates. A further 610 articles were not compatible with the eligibility criteria: noncomparative study (n = 309), not focused on talus (n = 192), short follow-up (n = 8), included kissing lesion (n = 7), not focused on focal defect (n = 12), combined with stem cells (n = 10), language limitations (n = 2), reported on other surgical interventions (n = 51), and other (n = 19). A further 8 studies did not report quantitative data under the outcomes of interest. This left 13 articles for inclusion in the present study. The literature search results are shown in Figure 1.
Figure 1.
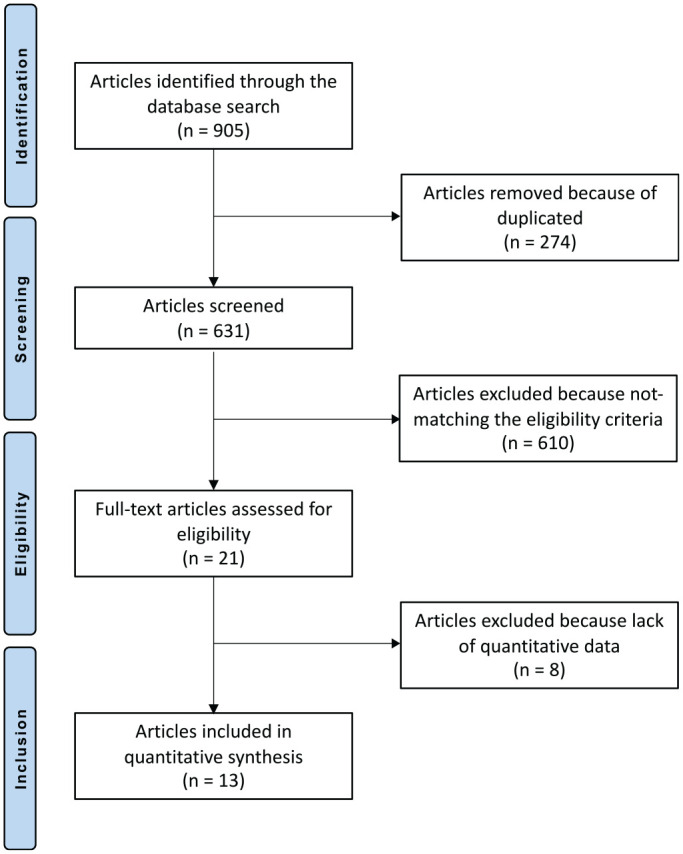
Flowchart of the literature search using the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines.
Methodological Quality Assessment
Given the large number of retrospective comparative studies (10/13), the risk of selection bias was high. Assessor blinding was performed in 10 of the 13 studies, resulting in a low risk of detection bias. The risks of attrition bias and reporting bias were moderate to low, as were the risks of other bias. The overall score for risk of bias was low, attesting that this study had moderate to good methodological quality. The risk of bias graph is shown in Figure 2.
Figure 2.
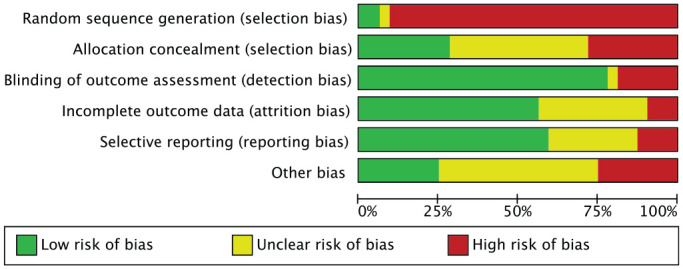
Methodological quality assessment.
Patient Characteristics
Data on 521 procedures were retrieved. The median length of follow-up was 47.8 months (range, 31.7-66.8 months). Of all patients, 42.4% (221/521) were women. The mean ± SD age of the patients was 35.0 ± 8.0 years, and the mean BMI was 25.1 ± 1.1. The mean defect size was 2.0 ± 1.6 cm2. At baseline, the mean VAS score was 7.4. ± 0.9 and the mean AOFAS score was 47.0 ± 8.2. The ANOVA revealed no difference between the treatment groups at baseline in terms of mean age and BMI, patient sex, defect size, and VAS and AOFAS scores (P > .1). Details of the studies are shown in Table 1.
Table 1.
Details of the Included Studies a
| Lead Author (Year) | Journal | Design | Treatment | Follow- up, mo | Procedures, n | Female Patients, % | Patients’ Mean Age, y |
|---|---|---|---|---|---|---|---|
| Ahmad 2 (2016) | Foot Ankle Int | Randomized | OAT: allograft | 40.5 | 16 | 37.5 | 39.7 |
| OAT: autograft | 35.2 | 20 | 45.0 | 41.3 | |||
| Apprich 4 (2012) | Osteoarthritis Cartilage | Retrospective | MACT | 48.0 | 10 | 60.0 | 31.0 |
| MFX | 59.6 | 10 | 40.0 | 32.4 | |||
| Becher 7 (2019) | Knee Surg Sports Traumatol Arthrosc | Retrospective | MFX | 67.2 | 16 | 56.3 | 33.3 |
| AMIC | 68.4 | 16 | 56.3 | 32.4 | |||
| D’Ambrosi 9 (2017) | Arthroscopy | Retrospective | AMIC | 27.0 | 17 | 52.9 | 25.0 |
| AMIC | 14 | 26.0 | 47.0 | ||||
| Domayer 12 (2012) | Osteoarthritis Cartilage | Retrospective | MFX | 113.8 | 10 | 55.6 | 30.8 |
| MACT | 65.4 | 10 | 77.8 | 25.4 | |||
| Gobbi 18 (2006) | Arthroscopy | Prospective | MFX | 53.0 | 10 | 40.0 | 24.0 |
| Control group | 11 | 45.5 | 32.0 | ||||
| OAT: autograft | 12 | 33.3 | 27.8 | ||||
| Gül 20 (2016) | J Foot Ankle Surg | Retrospective | OAT: autograft | 30.5 | 15 | 33.3 | 32.6 |
| OAT: autograft | 28.9 | 13 | 8.3 | 36.7 | |||
| Guney 21 (2016) | Knee Surg Sports Traumatol Arthrosc | Prospective | MFX | 47.3 | 19 | 37.4 | 47.4 |
| Control group | 40.4 | 22 | 43.9 | 50.0 | |||
| Mosaicplasty | 30.1 | 13 | 37.6 | 15.4 | |||
| Haleem 22 (2014) | Am J Sports Med | Retrospective | OAT: autograft | 93.0 | 14 | 50.0 | 42.8 |
| OAT: autograft | 85.3 | 28 | 39.3 | 44.1 | |||
| Park 33 (2018) | Am J Sports Med | Retrospective | OAT: autograft | 71.4 | 18 | 41.6 | |
| OAT: autograft | 28 | ||||||
| Shimozono 38 (2018) | Am J Sports Med | Retrospective | OAT: autograft | 52.0 | 63 | 42.9 | 36.0 |
| OAT: autograft | 45.0 | 31 | 32.3 | 34.0 | |||
| Shimozono 38 (2018) | Bone Joint Surg Am | Retrospective | OAT: autograft | 26.3 | 25 | 64.0 | 38.4 |
| OAT: allograft | 22.3 | 16 | 37.5 | 43.6 | |||
| Yoon 44 (2014) | Am J Sports Med | Retrospective | OAT: autograft | 45.0 | 22 | 31.8 | 37.1 |
| MFX | 50.0 | 22 | 18.2 | 41.6 |
AMIC, autologous matrix-induced chondrogenesis; MACT, matrix-assisted autologous chondrocyte transplant; MFX, microfracture; OAT, osteochondral autograft or allograft transplant.
Outcomes of Interest
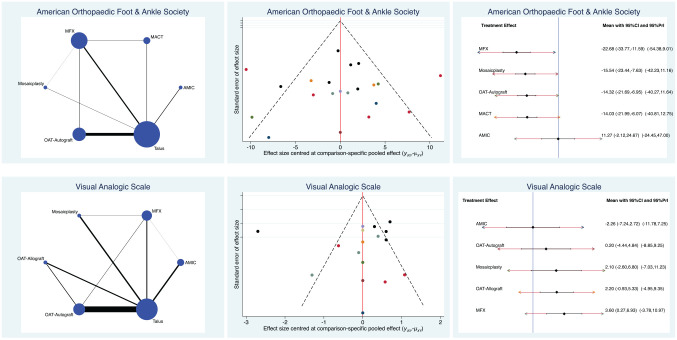
AMIC produced the highest AOFAS scores (SMD, 11.27; 95% CI, –2.12 to 24.67) and lowest VAS scores (SMD, –2.26; 95% CI, –7.24 to 2.72). The test for overall inconsistency was not significant (P = .6). Edge, funnel, and interval plots of the scores are shown in Figure 3.
Figure 3.
Edge, funnel, and interval plots of scores: American Orthopaedic Foot and Ankle Society and visual analog scale. AMIC, autologous matrix-induced chondrogenesis; MACT, matrix-assisted autologous chondrocyte transplantation; MFX, microfracture; OAT, osteochondral autograft or allograft transplant; PrI, percentile interval.
Complications
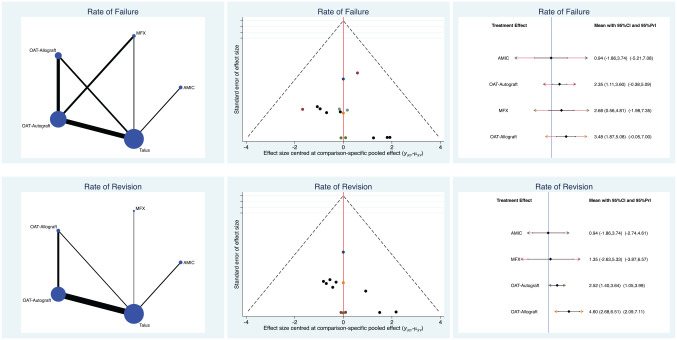
AMIC demonstrated the lowest rates of failure (LOR, 0.94; 95% CI, –1.86 to 3.74) and revision (LOR, 0.94; 95% CI, –1.86 to 3.74). OAT evidenced the highest rates of failure (LOR, 3.48; 95% CI, 1.87 to 5.08) and revision (LOR, 4.60; 95% CI, 2.68 to 6.51). The test for overall inconsistency was not significant (P = .8). Edge, funnel, and interval plots of complications are shown in Figure 4.
Figure 4.
Edge, funnel, and interval plots of complications: failure and revision. AMIC, autologous matrix-induced chondrogenesis; MFX, microfracture; OAT, osteochondral autograft or allograft transplant; PrI, percentile interval.
Discussion
The present Bayesian network meta-analysis demonstrated that AMIC for the management of focal osteochondral lesions of the talus performed better overall at approximately 4 years of follow-up. Patients undergoing OAT with allograft experienced the highest rate of complications, whereas microfracture resulted in the lowest values of patient-reported outcome measures.
To the best of our knowledge, this is the first Bayesian network meta-analysis to rank the treatments for talar chondral defects. AMIC exploits the potential of autologous bone marrow–derived mesenchymal stem cells, avoiding the harvesting of nonweightbearing cartilage, cell culture, and expansion. Moreover, being a single-stage procedure, it may present an attractive option for both patients and surgeons. The available scientific literature lacks head-to-head studies that compare AMIC with other surgical techniques for the management of knee chondral defects. Becher et al 7 compared AMIC versus microfracture in a cohort of 32 patients (16 patients each group) with a minimum follow-up of 5 years, evidencing no differences in outcome. D’Ambrosi et al, 9 using AMIC in a cohort of 17 young versus 14 old patients, concluded that AMIC was a reliable procedure regardless of patient age and that clinical outcome may depend on the preoperative conditions of the ankle. Walther et al 42 performed a systematic review and meta-analysis including 12 studies (492 procedures). Although their analysis was affected by a high grade of heterogeneity, they evidenced a statistically significant improvement of AOFAS, VAS, and Foot Function Index scores. 8 Given the lack of quantitative data, return to sports was not included in the network comparisons. D’Ambrosi et al 10 retrospectively analyzed return to sports in a cohort of patients after AMIC: 80.8% (21/26) of patients returned to sports within 42.6 months.
Our network comparison indicated that autografts for osteochondral transplant performed better than allografts. These observations agree with previous head-to-head studies that compared the 2 grafts. Ahmad and Jones 2 compared autograft versus allograft in a cohort of 40 patients in a randomized clinical trial. The results were comparable, but allografts had lower healing rates. Shimozono et al 38 found better clinical and MRI outcomes and a lower rate of failure in the allograft group in a cohort of 25 patients. Allografts have been introduced to avoid harvesting morbidity, with initially promising results. 14 However, allografts may deteriorate over time. 43 MRI studies show that allografts are not properly incorporated in some patients, and cartilage fissures or cysts close to the host-graft interface are more frequent when allografts are used versus autografts.14,19 Further studies are required to clearly establish the pros and cons of both type of osteochondral transplant.
The present network meta-analysis is not without limitations. The most important limitations are the retrospective nature of the design and the overall poor quality of many of the included studies. The analyses were performed regardless of the surgical approach (arthroscopy, mini-arthrotomy, arthrotomy), the nature of the membrane (collagen or hyaluronic acid), the fixation methods (glue, fibrin, both, none), and the location of the lesion on the articular surface. Given the lack of comparative studies, autologous chondrocyte implant and particulated juvenile articular cartilage techniques were not included for analysis. Because of the limited available data, articles were considered regardless of the cause of the chondral defect, and further differentiation between primary and revision settings was not possible. The lack of quantitative data prevented comparison of the time to return to sports. Most authors reported data of interventions combined with other surgical procedures, such as osteotomy or ligament repair. Given the lack of data concerning complications, it was not possible to separately analyze the causes of failure. The heterogeneous procedures and the limited available data precluded evaluation of the use of mesenchymal stem cells.
Regenerative medicine is rapidly evolving through the use of mesenchymal stem cells and via better understanding of the cellular and molecular basis of hyaline cartilage healing. Future studies should overcome the current obstacles to clinical translation—namely, cell source, cell isolation, and expansion and differentiation methods. In the past few years, several protocols for cell processing have been developed, but no consensus has been reached. Deeper understanding of the interactions between mesenchymal stem cells and the microenvironment, related signaling patterns, and influence on the regenerative cascade is required to develop appropriate therapeutic protocols. Given these controversies, studies using mesenchymal stem cells were not included. The AOFAS score is one of the most commonly used scores to assess foot and ankle ailments.36,40 However, whether the AOFAS score is a valid and reliable measure for assessment is controversial.6,11,26,39 In view of these limitations, results must be interpreted with caution.
Conclusion
At approximately 4 years of follow-up, AMIC displayed the most reliable results for the management of focal chondral defects of the talus.
Footnotes
Submitted December 7, 2020; accepted March 10, 2021.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
An online CME course associated with this article is available for 1 AMA PRA Category 1 Credit™ at http://www.sportsmed.org/aossmimis/Members/Education/AJSM_Current_Concepts_Store.aspx. In accordance with the standards of the Accreditation Council for Continuing Medical Education (ACCME), it is the policy of The American Orthopaedic Society for Sports Medicine that authors, editors, and planners disclose to the learners all financial relationships during the past 12 months with any commercial interest (A ‘commercial interest’ is any entity producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients). Any and all disclosures are provided in the online journal CME area which is provided to all participants before they actually take the CME activity. In accordance with AOSSM policy, authors, editors, and planners’ participation in this educational activity will be predicated upon timely submission and review of AOSSM disclosure. Noncompliance will result in an author/editor or planner to be stricken from participating in this CME activity.
ORCID iDs: Filippo Migliorini  https://orcid.org/0000-0001-7220-1221
https://orcid.org/0000-0001-7220-1221
Nicola Maffulli  https://orcid.org/0000-0002-5327-3702
https://orcid.org/0000-0002-5327-3702
References
- 1. Adams SB, Dekker TJ, Schiff AP, et al. Prospective evaluation of structural allograft transplantation for osteochondral lesions of the talar shoulder. Foot Ankle Int. 2018;39(1):28-34. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad J, Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent or large talar osteochondral lesions. Foot Ankle Int. 2016;37(1):40-50. [DOI] [PubMed] [Google Scholar]
- 3. Albano D, Martinelli N, Bianchi A, et al. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet Disord. 2017;18(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Apprich S, Trattnig S, Welsch GH, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthritis Cartilage. 2012;20(7):703-711. [DOI] [PubMed] [Google Scholar]
- 5. Baumfeld T, Baumfeld D, Prado M, Nery C. All-arthroscopic AMIC (AT-AMIC) for the treatment of talar osteochondral defects: a short follow-up case series. Foot (Edinb). 2018;37:23-27. [DOI] [PubMed] [Google Scholar]
- 6. Baumhauer JF, Nawoczenski DA, DiGiovanni BF, Wilding GE. Reliability and validity of the American Orthopaedic Foot and Ankle Society Clinical Rating Scale: a pilot study for the hallux and lesser toes. Foot Ankle Int. 2006;27(12):1014-1019. [DOI] [PubMed] [Google Scholar]
- 7. Becher C, Malahias MA, Ali MM, Maffulli N, Thermann H. Arthroscopic microfracture vs. arthroscopic autologous matrix-induced chondrogenesis for the treatment of articular cartilage defects of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2731-2736. [DOI] [PubMed] [Google Scholar]
- 8. Budiman-Mak E, Conrad KJ, Mazza J, Stuck RM. A review of the foot function index and the foot function index–revised. J Foot Ankle Res. 2013;6(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. D’Ambrosi R, Maccario C, Serra N, Liuni F, Usuelli FG. Osteochondral lesions of the talus and autologous matrix-induced chondrogenesis: is age a negative predictor outcome? Arthroscopy. 2017;33(2): 428-435. [DOI] [PubMed] [Google Scholar]
- 10. D’Ambrosi R, Villafane JH, Indino C, et al. Return to sport after arthroscopic autologous matrix-induced chondrogenesis for patients with osteochondral lesion of the talus. Clin J Sport Med. 2019;29(6): 470-475. [DOI] [PubMed] [Google Scholar]
- 11. De Boer AS, Meuffels DE, Van der Vlies CH, et al. Validation of the American Orthopaedic Foot and Ankle Society Ankle-Hindfoot Scale Dutch language version in patients with hindfoot fractures. BMJ Open. 2017;7(11):e018314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Domayer SE, Apprich S, Stelzeneder D, et al. Cartilage repair of the ankle: first results of T2 mapping at 7.0 T after microfracture and matrix associated autologous cartilage transplantation. Osteoarthritis Cartilage. 2012;20(8):829-836. [DOI] [PubMed] [Google Scholar]
- 13. Duramaz A, Baca E. Microfracture provides better clinical results than debridement in the treatment of acute talar osteochondral lesions using arthroscopic assisted fixation of acute ankle fractures. Knee Surg Sports Traumatol Arthrosc. 2018;26(10):3089-3095. [DOI] [PubMed] [Google Scholar]
- 14. El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93(17):1634-1640. [DOI] [PubMed] [Google Scholar]
- 15. Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E. Stem cells in articular cartilage regeneration. J Orthop Surg Res. 2016;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1272-1279. [DOI] [PubMed] [Google Scholar]
- 17. Galla M, Duensing I, Kahn TL, Barg A. Open reconstruction with autologous spongiosa grafts and matrix-induced chondrogenesis for osteochondral lesions of the talus can be performed without medial malleolar osteotomy. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2789-2795. [DOI] [PubMed] [Google Scholar]
- 18. Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22(10):1085-1092. [DOI] [PubMed] [Google Scholar]
- 19. Gomoll AH, Madry H, Knutsen G, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gül M, Cetinkaya E, Aykut US, et al. Effect of the presence of subchondral cysts on treatment results of autologous osteochondral graft transfer in osteochondral lesions of the talus. J Foot Ankle Surg. 2016;55(5):1003-1006. [DOI] [PubMed] [Google Scholar]
- 21. Guney A, Yurdakul E, Karaman I, et al. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1293-1298. [DOI] [PubMed] [Google Scholar]
- 22. Haleem AM, Ross KA, Smyth NA, et al. Double-plug autologous osteochondral transplantation shows equal functional outcomes compared with single-plug procedures in lesions of the talar dome: a minimum 5-year clinical follow-up. Am J Sports Med. 2014;42(8):1888-1895. [DOI] [PubMed] [Google Scholar]
- 23. Hoburg A, Loer I, Korsmeier K, et al. Matrix-associated autologous chondrocyte implantation is an effective treatment at midterm follow-up in adolescents and young adults. Orthop J Sports Med. 2019;7(4):2325967119841077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine. Accessed September 2020. https://wwwcebmnet/indexaspx?o=5653
- 25. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-784. [DOI] [PubMed] [Google Scholar]
- 26. Ibrahim T, Beiri A, Azzabi M, et al. Reliability and validity of the subjective component of the American Orthopaedic Foot and Ankle Society clinical rating scales. J Foot Ankle Surg. 2007;46(2):65-74. [DOI] [PubMed] [Google Scholar]
- 27. Kon E, Filardo G, Condello V, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39(8):1668-1675. [DOI] [PubMed] [Google Scholar]
- 28. Kon E, Gobbi A, Filardo G, et al. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37(1):33-41. [DOI] [PubMed] [Google Scholar]
- 29. Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mei-Dan O, Carmont MR, Laver L, et al. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534-541. [DOI] [PubMed] [Google Scholar]
- 31. Niemeyer P, Laute V, Zinser W, et al. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7(7):2325967119854442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ogura T, Merkely G, Bryant T, Winalski CS, Minas T. Autologous chondrocyte implantation “segmental-sandwich” technique for deep osteochondral defects in the knee: clinical outcomes and correlation with magnetic resonance imaging findings. Orthop J Sports Med. 2019;7(5):2325967119847173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park KH, Hwang Y, Han SH, et al. Primary versus secondary osteochondral autograft transplantation for the treatment of large osteochondral lesions of the talus. Am J Sports Med. 2018;46(6):1389-1396. [DOI] [PubMed] [Google Scholar]
- 34. Quirbach S, Trattnig S, Marlovits S, et al. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skeletal Radiol. 2009;38(8):751-760. [DOI] [PubMed] [Google Scholar]
- 35. Rolf CG, Barclay C, Riyami M, George J. The importance of early arthroscopy in athletes with painful cartilage lesions of the ankle: a prospective study of 61 consecutive cases. J Orthop Surg Res. 2006;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider W, Jurenitsch S. Normative data for the American Orthopedic Foot and Ankle Society ankle-hindfoot, midfoot, hallux and lesser toes clinical rating system. Int Orthop. 2016;40(2):301-306. [DOI] [PubMed] [Google Scholar]
- 37. Sherman SL, Thyssen E, Nuelle CW. Osteochondral autologous transplantation. Clin Sports Med. 2017;36(3):489-500. [DOI] [PubMed] [Google Scholar]
- 38. Shimozono Y, Hurley ET, Nguyen JT, Deyer TW, Kennedy JG. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2018;100(21):1838-1844. [DOI] [PubMed] [Google Scholar]
- 39. SooHoo NF, Shuler M, Fleming LL; American Orthopaedic Foot and Ankle Society. Evaluation of the validity of the AOFAS clinical rating systems by correlation to the SF-36. Foot Ankle Int. 2003;24(1):50-55. [DOI] [PubMed] [Google Scholar]
- 40. Stuber J, Zech S, Bay R, Qazzaz A, Richter M. Normative data of the Visual Analogue Scale Foot and Ankle (VAS FA) for pathological conditions. Foot Ankle Surg. 2011;17(3):166-172. [DOI] [PubMed] [Google Scholar]
- 41. Van Lieshout EM, De Boer AS, Meuffels DE, et al. American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Score: a study protocol for the translation and validation of the Dutch language version. BMJ Open. 2017;7(2):e012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walther M, Valderrabano V, Wiewiorski M, et al. Is there clinical evidence to support autologous matrix-induced chondrogenesis (AMIC) for chondral defects in the talus? A systematic review and meta-analysis. Foot Ankle Surg. 2021;27(3):236-245. [DOI] [PubMed] [Google Scholar]
- 43. Williams SK, Amiel D, Ball ST, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85(11):2111-2120. [DOI] [PubMed] [Google Scholar]
- 44. Yoon HS, Park YJ, Lee M, Choi WJ, Lee JW. Osteochondral autologous transplantation is superior to repeat arthroscopy for the treatment of osteochondral lesions of the talus after failed primary arthroscopic treatment. Am J Sports Med. 2014;42(8):1896-1903. [DOI] [PubMed] [Google Scholar]